Abstract
Purpose
The purpose of the present study was to assess the internal rotation of the tibia on Magnetic Resonance Imaging (MRI) in a series of consecutive athletes with Anterior cruciate Ligament (ACL) tears.
Methods
Retrospective analysis of prospectively collected data was performed to include all consecutive patients who had undergone primary ACL reconstruction between January 2022 and June 2022. The angle between surgical epicondylar axes (SEA) of the knee and posterior tibial condyles (PTC) was measured. A negative value was defined as internal torsion. KFs and ALL injuries were reported. Analysis of covariance (ANCOVA) was performed to examine the independent associations between SEA-PTC angle and injuries of KFs and ALL adjusted for physical variables (age, gender and body mass index [BMI]). Statistical significance was set at a p-value of < 0.05.
Results
A total of 83 eligible patients were included. The result of multiple linear regression analysis showed that internal tibial rotation was associated with KFs and ALL injuries. The estimated average of SEA-PTC angle in relation to ALL injuries controlling the other variables was -5.49 [95%CI -6.79 – (-4.18)] versus -2.99 [95%CI -4.55 – (-1.44)] without ALL injuries. On the other hand, the estimated average of SEA-PTC angle in relation to KFs lesions controlling the other variables was -5.73 [95%CI -7.04 – (-4.43)] versus -2.75 [95%CI -4.31 – (-1.18)] without KFs injuries.
Conclusions
KFs and ALL injuries were associated with an increased intra-articular internal tibial rotation in ACL-deficient knees. The measurement of femorotibial rotation on axial MRI could be useful to detect indirect signs of anterolateral complex (ALC) injuries.
Similar content being viewed by others
Introduction
Anterior cruciate ligament (ACL) rupture represents a serious injury for athletes [10]. It could be associated to meniscal, cartilage and collateral ligament lesions and represent a common cause of rotatory knee instability [9, 13, 33]. The ACL provides restraint to anterior translation and internal rotation of the tibia relative to the femur [16, 38, 50]. Vassalou et al. reported that patients with acute and chronic ACL tears had an internal tibial rotation measurements of 10.7 and 11 degrees respectively [50]. Moreover, Mitchell et al. found a significant increase in internal tibial rotation in ACL-deficient knees compared to intact knees in the adolescent population [38]. Hong et al. reported that aged patients with ACL tears exhibited significantly greater tibial internal rotation compared to younger patients (5.6° vs 4.2°) hypothesizing that older patients might have a higher incidence of associated injuries [26]. With the spread of Magnetic Resonance Imaging (MRI), several findings have been proposed to indicate ACL tears and static signs of anterolateral rotatory instability such as anterior tibial translation [6, 36], bone kissing contusions [47], and internal tibia rotation [38, 50]. Associated injuries that could lead to internal rotation of the tibia in the setting of ACL injury need to be clarified. Previous controlled laboratory studies reported that injuries of the anterolateral complex (ALC) such as Kaplan Fibers (KFs) and anterolateral ligament (ALL) injuries result in increased internal rotation of the tibia in ACL-deficient knees [18, 31, 42, 45].
The purpose of the present study was to assess the internal rotation of the tibia on MRI images of a series of consecutive athletes with ACL tears. We hypothesized that KFs and ALL injuries were associated with greater internal rotation on pre-operative MRI.
Material and methods
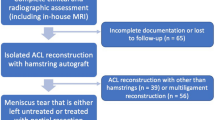
The present retrospective study was conducted following the Declaration of Helsinki Ethical Principles and Good Clinical Practices and was approved by the ethical committee of the Medical University of Innsbruck (AN2015-0050 346/4.28). A retrospective analysis of prospectively collected data from the database of a specialized joint surgery clinic was conducted. All consecutive patients who underwent arthroscopic primary ACL reconstruction (ACLR) between January 2022 and June 2022 were considered for study eligibility. Inclusion and exclusion criteria are listed in Fig. 1. Two senior surgeons (CF and CH) performed all the surgeries in both groups. Preoperatively, all patients had sustained an ACL tear, diagnosed based on clinical examination, MRI and arthroscopically.
MRI examinations were performed using the department protocol with the patient in the supine position and the knee extended (a maximum of 10° of slight flexion of the knee was allowed in case of marked effusion) on a 3-T whole-body scanner (Skyra, Siemens Healthineers, Er- langen, Germany) using a 6-channel flex coil. MRI was performed within 3 weeks from trauma. Three plane (sagittal, coronal, and axial) sequences using both proton density– and fat-suppressed proton density–weighted images were performed with repetition time (TR) between 3000 and 4000 ms, echo time (TE) between 33 and 35 ms, matrix between 320 × 320 and 384 × 384 (phase x frequency) with 3-mm slice thickness, and a total field of view of 130 mm.
Axial femorotibial alignment
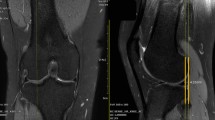
Two sports knee surgery fellows (LF and AM) independently analyzed all MRI images obtained before surgery. To measure the axial alignment of the distal femur and proximal tibia, two sections were identified from each MRI as described by previous studies [11, 12]. The first slice was taken in the midthrochlear region of the femoral condyle, identified by the Roman arch appearance of the intercondylar groove with the apex of the Roman arch corresponding to 1/3 of the height of the condyle. The surgical epicondylar axes (SEA) from the lateral epicondyle and medial sulcus were delineated. The second slice was taken in correspondence with the proximal tibial plateau above the end of the proximal tibiofibular joint where the semimembranosus tendon inserts into the tibial bone. The tangent line of the posterior tibial condyles (PTC) was delineated. The angle between SEA-PTC was measured (Fig. 2). A negative value was defined as internal torsion and a positive value as external torsion of the distal segment.
Kaplan fiber complex identification
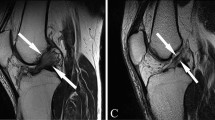
All scans were reviewed in all 3 planes (axial, coronal, and sagittal) as previously described by studies of Batty et al. [1, 2] and Godin et al. [19]. As a routine, the sagittal images were assessed first with the lateral gastrocnemius origin and superior lateral geniculate artery used as a reference to easily localize the region [37]. Indeed, the KFs were identified proximal to the lateral femoral condyle, adjacent to the branches of the superior lateral genicular artery [3]. Once identified, further assessment using proton density sequences was preferable to visualize KFs with greater clarity. The morphology of the femoral insertion could be different, ranging from a single thick linear insertion to the appearance of multiple smaller strands inserting individually from the posterolateral femur to the epicondylar region. In accordance with Batty et al. [2], KFs were classified as injured if there were: (1) direct signs of injury such as a clear discontinuity in the KFs or a femoral avulsion was visible or (2) there were indirect signs of injury such as thickening and/or intra-substance signal change of the KFs, focal bone marrow oedema at KFs insertion site to the femur, soft tissue oedema in the region of KFs or a wavy appearance to the KFs (Fig. 3).
a Coronal proton density, b Sagittal proton density, and c axial fat-suppressed T2 magnetic resonance imaging sections of knee depicting the integrity of distal Kaplan fibers (KFs) complex (white arrow). d Coronal fat-suppressed T2, e Sagittal proton density, and f axial fat-suppressed T2 magnetic resonance imaging sections of knee depicting the injuries of distal Kaplan fibers (KFs) complex (white arrow). The wavy appearance of KFs, discontinuity of KFs and soft tissue oedema in the region of KFs are visualized respectively in (d, e and f)
Anterolateral ligament identification
All scans were reviewed in all 3 planes (axial, coronal, and sagittal) as previously described by Helito et al. [20,21,22,23]. The ALL was evaluated by the use of T2-weighted coronal images, with the axial and sagittal planes used mainly for anatomic orientation [15, 34]. The ALL was defined as the low signal band originating from the posterior-proximal region of the lateral epicondyle of the femur to its tibial insertion between the Gerdy’s tubercle and the fibular head [44]. Specifically, the ALL was divided into three parts (femoral, meniscal and tibial portion) based on previous anatomic studies (Fig. 4a) [20, 21]. The fibers were considered injured in case of Segond fracture or when they presented irregular contours, a wavy aspect, or areas of discontinuity [15, 25]. The ALL was defined as injured if at least one of its portions resulted in tearing (Fig. 4).
a Coronal T2-weighted images with fat saturation shows the normal femoral portion (arrowhead), meniscal portion (dotted arrow) and tibial portion (arrow) of anterolateral ligament; popliteus tendon (star); b Segond avulsed bone fragment (arrow); c femoral portion of ALL presenting abnormal signal and irregular aspect of its fibers (arrow); d meniscal portion of ALL presenting abnormal signal and irregular aspect of its fibers (arrow)
Statistical analysis
Data were collected and analyzed using respectively Excel (Microsoft, Redmond, WA, USA) and XLSTAT statistical software packages (Addinsoft LLC, Paris, France). Categorical variables were expressed in numbers and percentages (%). The distribution of continuous variables was assessed by Shapiro–Wilk test. Mean and standard deviation (SD) or Median and interquartile ranges (IQR) were used to summarize the continuous variables according to their distribution. A 2-way random interclass correlation coefficient (ICC) was used to assess the interobserver reliability of the KFs and ALL injuries. ICC values were calculated for absolute agreement and consistency of agreement. ICC values were graded as follows: < 0.4 poor reliability, 0.4–0.75 moderate reliability, and > 0.75 excellent reliability [32]. An F-test was used to determine the statistical significance of the correlations. Analysis of covariance (ANCOVA) was performed to examine the independent associations between SEA-PTC angle and explanatory variables as ALL and KFs injuries adjusted for physical variables (age, gender and body mass index [BMI]). Mann–Whitney or Kruskall-Wallis test was used to assess significance between groups of continuous variables. Statistical significance was set at a p-value of < 0.05.
Results
The flowchart of the study is presented in Fig. 1. A total of 83 eligible patients were included in the study. The mean age of patients was 24.8 (range 18–53) and 41 were males (49.3%) (Table 1). Interrater reliability analysis revealed an ICC of 0.802 (95% CI 0.711 – 0.864), p < .0001 and 0.857 (95% CI 0.778—0.908), p < .0001 respectively for KFs and ALL injuries. The prevalence of ALL and KFs injuries in our cohort was 59.0% (Table 2). The result of multiple linear regression analysis adjusted for physical variables showed that internal tibial rotation was associated with KFs and ALL injuries with an odds ratio respectively of 1.36 (95% CI 1.10 – 1.67, p = 0.005) and 1.29 (95% CI 1.05 – 1.59, p = 0.017) (Table 3). Hence, the estimated average of SEA-PTC angle in relation to ALL injuries controlling the other variables was -5.49 [95%CI -6.79 – (-4.18)] versus -2.99 [95%CI -4.55 – (-1.44)] without ALL injuries (Fig. 5). On the other hand, the estimated average of SEA-PTC angle in relation to KFs lesions controlling the other variables was -5.73 [95%CI -7.04 – (-4.43)] versus -2.75 [95%CI -4.31 – (-1.18)] without KFs injuries (Fig. 5). Furthermore, the value of SEA-PTC angle variable was analyzed considering ALL and KFs injuries (Table 4). Hence, four groups were constituted: ALL / KFs injured (n = 32); ALL injured / KFs not injured (n = 17); ALL not injured / KFs injured (n = 17) and ALL / KFs not injured (n = 17) (Table 4). The mean of SEA-PTC differs significantly between groups (p < 0.001, Kruskal–Wallis test). Specifically, ALL / KFs injured group reported the greatest internal tibial rotation with a SEA-PTC angle that differs significantly from ALL / KFs not injured group (p < .001, Mann–Whitney U test). Considering the groups without KFs injuring, the presence of ALL tears was associated with significant greater internal tibial rotation (p = .011). Similarly, the presence of KFs tears in groups without ALL injuring, was associated with significant greater internal tibia rotation (p = .004) (Fig. 6).
Discussion
The most important finding of the present study was that KFs and ALL injuries were associated with an increased intra-articular internal tibia rotation in ACL-deficient knees based on high-field MRI. The ALC has been described as including the iliotibial band (ITB), the KFs complex, the capsule-osseous layers of ITB, the anterolateral capsule and ALL even though debating and conflicting results are present in literature due to the complexity of lateral knee anatomy and various dissecting techniques [39, 40]. Biomechanical studies have shown that the ALC has a role as secondary stabilizer to the ACL in opposing anterior tibial translation and internal tibial rotation [27, 31, 39, 41,42,43, 46, 48]. Moreover, additional investigations demonstrated that ACL reconstruction alone in the setting of combined ACL and ALC injuries failed to restore native knee kinematics [28, 31].
Previous studies by Van Dyck et al. [49] and Khanna et al. [30] reported respectively a rate of KFs complex injuries of 33% and 82%, whereas Batty et al. [2] reported a lower rate of KF injuries (18.6%) in patients with ACL-deficient knees. In the former studies the determination of injury was based on the presence of any altered signal within the ligament, periligamentous oedema and/or disruption of the fibers whereas in the latter probably more restrictive criteria were used [2]. From a biomechanical point of view, KFs complex has been described as an important factor controlling anterolateral rotatory stability [17, 31, 35]. Specifically, injuries to KFs complex concomitant to ACL deficit were associated to greater internal tibial rotation in laboratory studies [31]. Similarly, from our results a greater internal tibial rotation on MRI was associated to KFs complex injuries with Odds ratio of 1.36 (95%CI 1.10 – 1.67, p = .005).
From our cohort, we reported a rate of ALL injuries of 59% in ACL-deficient knee. Claes et al. [7] and Ferretti et al. [14] reported the prevalence of ALL abnormalities in the ACL-injured knee respectively in 80% and 90% of cases, but in others the rate was approximately 50% [5, 23, 24]. Biomechanical studies have shown that ALL injuries were associated with an increase internal rotation of the tibia in ACL-injured knee specially when knee flexion exceeds 35° [41, 43, 45]. In our study, we reported that injuries to KFs complex was associated with an increase internal tibial rotation with an odds ratio of 1.29 (95%CI 1.05 – 1.59, p = 0.017).
Considering the magnitude of internal tibial rotation in our cohort, patients with both ALL and KFs complex injuries were characterized by greatest internal tibial rotation compared to other groups assuming a synergistic effect of ALL and KFs complex in controlling anterolateral rotatory knee laxity [42]. In addition, analyzing the SEA-PTC variable in KFs injuries groups, we observed that ALL injuries did not significantly increase the internal rotation of the tibia (p = .219). A possible explanation could be that ALL has been demonstrated to act as a secondary stabilizer during internal rotation torque and simulated pivot-shift test in the ACL-deficient state over 30° of flexion whereas in our study the internal rotation was measured nearly in full extension due to MRI examination [44].
On the other hand, analyzing the SEA-PTC variable in KFs not injured groups, we observed that ALL injuries significantly increased the internal tibial rotation (4.4° vs 0.8°, p = .011). These results are similar with those of Spencer et al., who reported, after ALL sectioning in an ACL-sectioned knee, a significant increase in internal tibial rotation of only 2° at full knee extension. This amount was assumed by authors to be clinically undetectable and consistent with a secondary restraint to internal rotation [46].
Lateral extra-articular tenodesis (LET) and anterolateral ligament (ALL) reconstructions have been shown to restore knee kinematics in the setting of combined anterolateral instability and ACL injuries [8, 29, 31]. For these reasons, adding ALL reconstruction or LET should be considered in case of ALL and/or KFs complex injuries in order to decrease the chronic residual laxity after isolated ACLR [17, 28].
The present study has limitations that warrant disclosures. First, the ALL and KFs complex injuries were diagnosed on unvalidated diagnostic criteria and not confirmed by surgical exploration. There is undoubtedly variability and an element of subjectivity in evaluating the anterolateral structures on MRI. However, the ICC values showed excellent agreement and the methodology was the same as previous dedicated studies [2, 23, 26]. Only the distal fibers of KFs complex were considered in the present study because the most proximal fibers were outside the MRI field of view. Femoral anteversion, tibial torsion and contralateral femoral tibial rotation of the knee were not considered, representing limitations of our study. All MRIs were performed within 3 weeks from trauma, therefore the rate of KF injuries might be overestimated due to the presence of widespread oedema in acutely injured knees [2]. However, larger quantities of fluid inside the joint puts tension on the capsule and makes it easier to view the ALL compared to chronic cases [21]. The study was carried out by analyzing static measurement (static MRI of knee performed after trauma). For this reason, the different behavior of the various components of the ALC as the flexion angle of the knee increases, cannot be considered. Therefore, dynamic analysis is necessary to confirm the hypothesis. However, Carpenter et al. [4] in a study using three-dimensional MRI showed that knees with ACL reconstruction presented greater internal tibial rotation in going from extension to flexion than those with a native ACL. Authors hypothesized that reconstruction alone did not fully restore the kinematics of the knee maybe due to an undiagnosed and untreated ALC injury.
Conclusion
ALL and KFs injuries were associated with an increased internal tibial rotation in ACL-deficient knees on high-field MRI. The measurement of femorotibial rotation on axial MRI could be useful to detect indirect signs of ALC injuries which could help in the diagnosis and management of patients with these injuries. Further studies are required to assess and validate the measurement of femorotibial rotation on axial MRI as an indirect measure of rotatory instability in ACL-deficient knees.
References
Batty L, Murgier J, O’Sullivan R, Webster KE, Feller JA, Devitt BM (2019) The Kaplan fibers of the iliotibial band can be identified on routine knee magnetic resonance imaging. Am J Sports Med 47:2895–2903
Batty LM, Murgier J, Feller JA, O’Sullivan R, Webster KE, Devitt BM (2020) Radiological identification of injury to the Kaplan fibers of the iliotibial band in association with anterior cruciate ligament injury. Am J Sports Med 48:2213–2220
Berthold DP, Willinger L, Muench LN, Forkel P, Schmitt A, Woertler K, Imhoff AB, Herbst E (2020) Visualization of proximal and distal Kaplan fibers using 3-dimensional magnetic resonance imaging and anatomic dissection. Am J Sports Med 48:1929–1936
Carpenter RD, Majumdar S, Ma CB (2009) Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy 25:760–766
Cavaignac E, Faruch M, Wytrykowski K, Constant O, Murgier J, Berard E, Chiron P (2017) Ultrasonographic evaluation of anterolateral ligament injuries: correlation with magnetic resonance imaging and pivot-shift testing. Arthroscopy 33:1384–1390
Chang MJ, Chang CB, Choi J-Y, Je MS, Kim TK (2014) Can magnetic resonance imaging findings predict the degree of knee joint laxity in patients undergoing anterior cruciate ligament reconstruction? BMC Musculoskelet Disord 15:214
Claes S, Bartholomeeusen S, Bellemans J (2014) High prevalence of anterolateral ligament abnormalities in magnetic resonance images of anterior cruciate ligament-injured knees. Acta Orthop Belg 80:45–49
Delaloye JR, Hartog C, Blatter S, Schläppi M, Müller D, Denzler D, Murar J, Koch PP (2020) Anterolateral ligament reconstruction and modified Lemaire lateral extra-articular tenodesis similarly improve knee stability after anterior cruciate ligament reconstruction: a biomechanical study. Arthroscopy 36:1942–1950
Engebretsen L, Wijdicks CA, Anderson CJ, Westerhaus B, LaPrade RF (2012) Evaluation of a simulated pivot shift test: a biomechanical study. Knee Surg Sports Traumatol Arthrosc 20:698–702
Farinelli L, Abermann E, Meena A, Ueblacker P, Hahne J, Fink C (2023) Return to play and pattern of injury after ACL rupture in a consecutive series of elite UEFA soccer players. Orthop J Sport Med 11(3):23259671231153628
Farinelli L, Baldini M, Bucci A, Ulisse S, Carle F, Gigante A (2021) Axial and rotational alignment of lower limb in a Caucasian aged non-arthritic cohort. Eur J Orthop Surg Traumatol 31:221–228
Farinelli L, Baldini M, Faragalli A, Carle F, Ulisse S, Gigante A (2023) Surgical epicondylar axis of the knee and its relationship to the axial tibia alignment in knee osteoarthritis: the concept of proximal twist tibia. J Knee Surg 36(7):710–715
Farinelli L, Csapo R, Meena A, Abermann E, Hoser C, Fink C (2023) Concomitant injuries associated with ACL rupture in elite professional alpine ski racers and soccer players: a comparative study with propensity score matching analysis. Orthop J Sport Med 11:23259671231192130
Ferretti A, Monaco E, Fabbri M, Maestri B, De Carli A (2017) Prevalence and classification of injuries of anterolateral complex in acute anterior cruciate ligament tears. Arthroscopy 33:147–154
Ferretti A, Monaco E, Redler A, Argento G, De Carli A, Saithna A, Helito PVP, Helito CP (2019) High prevalence of anterolateral ligament abnormalities on MRI in knees with acute anterior cruciate ligament injuries: a case-control series from the SANTI study group. Orthop J Sport Med 7:2325967119852916
Frank JM, Moatshe G, Brady AW, Dornan GJ, Coggins A, Muckenhirn KJ, Slette EL, Mikula JD, LaPrade RF (2017) Lateral meniscus posterior root and meniscofemoral ligaments as stabilizing structures in the ACL-deficient knee: a biomechanical study. Orthop J Sport Med 5:2325967117695756
Geeslin AG, Chahla J, Moatshe G, Muckenhirn KJ, Kruckeberg BM, Brady AW, Coggins A, Dornan GJ, Getgood AM, Godin JA, LaPrade RF (2018) Anterolateral knee extra-articular stabilizers: a robotic sectioning study of the anterolateral ligament and distal iliotibial band Kaplan fibers. Am J Sports Med 46:1352–1361
Geeslin AG, Moatshe G, Chahla J, Kruckeberg BM, Muckenhirn KJ, Dornan GJ, Coggins A, Brady AW, Getgood AM, Godin JA, LaPrade RF (2018) Anterolateral knee extra-articular stabilizers: a robotic study comparing anterolateral ligament reconstruction and modified Lemaire lateral extra-articular tenodesis. Am J Sports Med 46:607–616
Godin JA, Chahla J, Moatshe G, Kruckeberg BM, Muckenhirn KJ, Vap AR, Geeslin AG, LaPrade RF (2017) A comprehensive reanalysis of the distal iliotibial band: quantitative anatomy, radiographic markers, and biomechanical properties. Am J Sports Med 45:2595–2603
Helito CP, Demange MK, Bonadio MB, Tírico LEP, Gobbi RG, Pécora JR, Camanho GL (2013) Anatomy and histology of the knee anterolateral ligament. Orthop J Sport Med 1:2325967113513546
Helito CP, Demange MK, Helito PVP, Costa HP, Bonadio MB, Pecora JR, Rodrigues MB, Camanho GL (2015) Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Ortop Brazil 50:214–219
Helito CP, Helito PVP, Costa HP, Bordalo-Rodrigues M, Pecora JR, Camanho GL, Demange MK (2014) MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol 43:1421–1427
Helito CP, Helito PVP, Costa HP, Demange MK, Bordalo-Rodrigues M (2017) Assessment of the anterolateral ligament of the knee by magnetic resonance imaging in acute injuries of the anterior cruciate ligament. Arthroscopy 33:140–146
Helito CP, Helito PVP, Leão RV, Demange MK, Bordalo-Rodrigues M (2017) Anterolateral ligament abnormalities are associated with peripheral ligament and osseous injuries in acute ruptures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 25:1140–1148
Helito PVP, Bartholomeeusen S, Claes S, Rodrigues MB, Helito CP (2020) Magnetic resonance imaging evaluation of the anterolateral ligament and the iliotibial band in acute anterior cruciate ligament injuries associated with Segond fractures. Arthroscopy 36:1679–1686
Hong CK, Lin YJ, Cheng TA, Chang CH, Hsu KL, Kuan FC, Su WR (2022) Adult patients with ACL tears have greater tibial internal rotation in MRI compared to adolescent patients. J Orthop Surg Res 17:17
Huser LE, Noyes FR, Jurgensmeier D, Levy MS (2017) Anterolateral ligament and iliotibial band control of rotational stability in the anterior cruciate ligament-intact knee: defined by tibiofemoral compartment translations and rotations. Arthroscopy 33:595–604
Inderhaug E, Stephen JM, Williams A, Amis AA (2017) Anterolateral tenodesis or anterolateral ligament complex reconstruction: effect of flexion angle at graft fixation when combined with ACL reconstruction. Am J Sports Med 45:3089–3097
Inderhaug E, Stephen JM, Williams A, Amis AA (2017) Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med 45:347–354
Khanna M, Gupte C, Dodds A, Williams A, Walker M (2019) Magnetic resonance imaging appearances of the capsulo-osseous layer of the iliotibial band and femoral attachments of the iliotibial band in the normal and pivot-shift ACL injured knee. Skeletal Radiol 48:729–740
Kittl C, El-Daou H, Athwal KK, Gupte CM, Weiler A, Williams A, Amis AA (2016) The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med 44:345–354
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Lian J, Diermeier T, Meghpara M, Popchak A, Smith CN, Kuroda R, Zaffagnini S, Samuelsson K, Karlsson J, Irrgang JJ, Musahl V (2020) Rotatory knee laxity exists on a continuum in anterior cruciate ligament injury. J Bone Joint Surg Am 102:213–220
Liebensteiner M, Runer A, Kranewitter C, Nachtigal P, Giesinger J, Dammerer D, Henninger B (2020) MRI visibility of the anterolateral ligament and the deep structures of the iliotibial tract. J Exp Orthop 7:25
Lutz C, Sonnery-Cottet B, Niglis L, Freychet B, Clavert P, Imbert P (2015) Behavior of the anterolateral structures of the knee during internal rotation. Orthop Traumatol Surg Res 101:523–528
Macchiarola L, Jacquet C, Dor J, Zaffagnini S, Mouton C, Seil R (2022) Side-to-side anterior tibial translation on monopodal weightbearing radiographs as a sign of knee decompensation in ACL-deficient knees. Knee Surg Sports Traumatol Arthrosc 30:1691–1699
Marom N, Greditzer HG 4th, Roux M, Ling D, Boyle C, Pearle AD, Marx RG (2020) The incidence of Kaplan fiber injury associated with acute anterior cruciate ligament tear based on magnetic resonance imaging. Am J Sports Med 48:3194–3199
Mitchell BC, Siow MY, Bastrom T, Bomar JD, Pennock AT, Parvaresh K, Edmonds EW (2021) Coronal lateral collateral ligament sign: a novel magnetic resonance imaging sign for identifying anterior cruciate ligament-deficient knees in adolescents and summarizing the extent of anterior tibial translation and femorotibial internal rotation. Am J Sports Med 49:928–934
Musahl V, Getgood A, Neyret P, Claes S, Burnham JM, Batailler C, Sonnery-Cottet B, Williams A, Amis A, Zaffagnini S, Karlsson J (2017) Contributions of the anterolateral complex and the anterolateral ligament to rotatory knee stability in the setting of ACL Injury: a roundtable discussion. Knee Surg Sports Traumatol Arthrosc 25:997–1008
Musahl V, Herbst E, Burnham JM, Fu FH (2018) The anterolateral complex and anterolateral ligament of the knee. J Am Acad Orthop Surg 26:261–267
Nitri M, Rasmussen MT, Williams BT, Moulton SG, Cruz RS, Dornan GJ, Goldsmith MT, LaPrade RF (2016) An in vitro robotic assessment of the anterolateral ligament, part 2: anterolateral ligament reconstruction combined with anterior cruciate ligament reconstruction. Am J Sports Med 44:593–601
Noyes FR, Huser LE, Levy MS (2017) Rotational knee instability in ACL-deficient knees: role of the anterolateral ligament and iliotibial band as defined by tibiofemoral compartment translations and rotations. J Bone Joint Surg Am 99:305–314
Rasmussen MT, Nitri M, Williams BT, Moulton SG, Cruz RS, Dornan GJ, Goldsmith MT, LaPrade RF (2016) An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med 44:585–592
Sonnery-Cottet B, Daggett M, Fayard J-M, Ferretti A, Helito CP, Lind M, Monaco E, de Pádua VBC, Thaunat M, Wilson A, Zaffagnini S, Zijl J, Claes S (2017) Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament - deficient knee. J Orthop Traumatol 18:91–106
Sonnery-Cottet B, Lutz C, Daggett M, Dalmay F, Freychet B, Niglis L, Imbert P (2016) The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med 44:1209–1214
Spencer L, Burkhart TA, Tran MN, Rezansoff AJ, Deo S, Caterine S, Getgood AM (2015) Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med 43:2189–2197
Terzidis IP, Christodoulou AG, Ploumis AL, Metsovitis SR, Koimtzis M, Givissis P (2004) The appearance of kissing contusion in the acutely injured knee in the athletes. Br J Sports Med 38:592–596
Thein R, Boorman-Padgett J, Stone K, Wickiewicz TL, Imhauser CW, Pearle AD (2016) Biomechanical Assessment of the anterolateral ligament of the knee: a secondary restraint in simulated tests of the pivot shift and of anterior stability. J Bone Joint Surg Am 98:937–943
Van Dyck P, De Smet E, Roelant E, Parizel PM, Heusdens CHW (2019) Assessment of anterolateral complex injuries by magnetic resonance imaging in patients with acute rupture of the anterior cruciate ligament. Arthroscopy 35:521–527
Vassalou EE, Klontzas ME, Kouvidis GK, Matalliotaki PI, Karantanas AH (2016) Rotational knee laxity in anterior cruciate ligament deficiency: an additional secondary sign on MRI. AJR Am J Roentgenol 206:151–154
Acknowledgements
The study group wants to thank all the participants of the study for their efforts.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualisation: LF, AM, CF, BSC; methodology, AM, LF, AE and CH; data curation and synthesis, LF, AM, EA, ST; writing—original draft preparation, LF, AM, TDV, EA; writing—review and editing, CP, BSC, ST, TDV and CF; supervision BSC and CF; all authors interpreted the data, critically reviewed the work, made important contributions to the manuscript with their suggestions for improvement, approved the published version and agreed to be responsible for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing interests
One of more authors has declared a potential conflict of interest as specified in the ICMJE conflict of interest statement.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Investigation performed at Gelenkpunkt–Sports and Joint Surgery, Innsbruck, Austria.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farinelli, L., Meena, A., Sonnery-Cottet, B. et al. Distal Kaplan fibers and anterolateral ligament injuries are associated with greater intra-articular internal tibial rotation in ACL-deficient knees based on magnetic resonance imaging. J EXP ORTOP 10, 113 (2023). https://doi.org/10.1186/s40634-023-00682-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-023-00682-0