Abstract
Purpose
To evaluate the effectiveness of dynamic intermittent compression cryotherapy (DICC) (CryoNov®) with an intravenous nefopam-based pain management protocol (DCIVNPP) in reducing post-operative pain following anterior cruciate ligament reconstruction (ACLR) compared to static compression cryotherapy (SCC) (Igloo®) and oral Nefopam.
Methods
This was a retrospective analysis of prospectively collected data including 676 patients who underwent primary ACLR in 2022. Patients were either in the DCIVNPP group or in the SCC (control group), and were matched for age, sex, and Lysholm and Tegner scores (338 per arm). The primary outcome was pain on the visual analogue scale (VAS), analyzed in relation to the minimal clinically important difference (MCID) and the Patient Acceptable Symptom State (PASS) thresholds for VAS. The secondary outcome was side effects.
Results
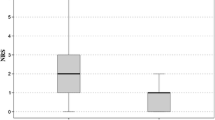
Postoperative pain in the DCIVNPP group was less severe on the VAS than in the control group (p < 0.05). The maximum difference in the VAS between groups was 0.57, which is less than the MCID threshold for VAS. The DCIVNPP group crossed the PASS threshold for VAS on Day 3, sooner than the control group. The side effect profiles were similar in both groups except for higher rates of dizziness and malaise in the DCIVNPP group, and higher rates of abdominal pain in the control group. Most of the side effects decreased over time in both groups, with no significant side effects after Day 3.
Conclusion
DCIVNPP effectively allows for faster pain recovery than in the control group. The difference in side effects between the protocols may be due to mode of administration of nefopam.
Level of evidence
III.
Similar content being viewed by others
Introduction
Anterior Cruciate Ligament (ACL) is the most frequently reconstructed knee ligament [1]. ACL reconstruction (ACLR) procedure is known to be associated with moderate to severe pain, that can negatively affect knee function and extend hospital stays if it is not properly managed [2, 3], with an early postoperative Visual analogue scale (VAS) ranging from 2.5 to 5.4 depending on the pain protocol [4, 5]. Arthrogenic Muscle Inhibition (AMI) is a recently identified event following ACLR surgery. In AMI pain, inflammation, and changes in knee joint receptors lead to central inhibition of the quadriceps causing knee extension lag and affecting ACLR outcomes [6]. This shows the importance of obtaining optimal pain management. It is associated with high levels of patient satisfaction and is crucial for early hospital discharge, helping to avoid unnecessary hospitalization [2]. Furthermore, optimal pain management and ambulatory surgery can significantly decrease total healthcare costs following primary ACLR [7]. More than 77 randomized clinical trials have evaluated the best pain protocols including regional nerve blocks, intraarticular injections, intravenous and intramuscular injections, multimodal regimens, cryotherapy, and oral medications [8]. These studies have compared the effectiveness of these protocols on the VAS using different methods, including the evaluation of the minimally clinically important difference (MCID) and the Patient Acceptable Symptom State (PASS) [9,10,11,12,13,14]. The MCID is the smallest clinically significant improvement identified by patients, and is typically set between 1 and 1.4 for the VAS, depending on the study [10,11,12]. The PASS is the point when patients consider themselves to be well and satisfied with treatment [9, 13, 14]. The PASS for the VAS is typically set at 3.4 to 4 [9, 13,14,15].
Cryotherapy, a non-pharmacological treatment that reduces the local metabolism to alleviate pain and inflammation, has been found to be effective in reducing postoperative pain following ACLR [16,17,18]. There are different types of cryotherapies including non-compression cryotherapy (NCC), static compression cryotherapy (SCC), and dynamic intermittent compression cryotherapy (DICC). Although several RCTs and metanalyses have shown that compression cryotherapy is better than traditional forms of cryotherapy for reducing pain [18,19,20], there are only two studies comparing DICC and SCC in the literature [21, 22]. The first experimental study by Holwerda et al. compared tissue temperature changes and cardiovascular response between DICC with the GameReady® device to those of SCC using elastic ice wrapping [21]. This study showed that neither of these techniques caused acute cardiovascular strain and that there were no significant differences in the intramuscular temperature changes. The second preliminary study by Murgier et al., in 2014, including 39 patients in a case–control design, suggested that DICC could be more effective than SCC, with a 1.15 difference in VAS, although this was not statistically significant [22].
The aim of this study was to compare these two cryotherapy techniques by analyzing the effect of two morphine-free multimodal pain management protocols on early post-operative pain following ACLR. The first protocol used DICC with an intravenous-nefopam pain management protocol (DCIVNPP), which was compared to a similar protocol that used oral nefopam and SCC (control group). Both protocols were morphine-free, and nefopam was chosen due to its analgesic properties and its common usage in France. Nefopam is a non-opioid tricyclic drug developed in the late 1960s and early 1970s as an antidepressant that possesses analgesic properties [23]. Studies have not shown superiority of one route of administration over the other, but rather a difference in the potential for side effects [23,24,25,26].
We hypothesized that DCIVNPP would result in significantly lower pain levels according to the VAS, and that these patients would recover more rapidly with no increase in adverse effects such as nausea, dizziness, and malaise.
Material and methods
Study design
This is a retrospective analysis of prospectively collected data that included all patients who underwent primary ACL reconstruction in 2022 at a referral center for sports surgery.
Patients who underwent primary ACLR were included, while those who underwent revision surgery, and/or refused to participate in the study or to fill the online questionnaires were excluded.
Patients were divided into the DCIVNPP and the control groups, based on the change in pain management protocol that occurred mid-2022 at our institution. The control group included patients who received the traditional pain management protocol prior to the change, while the DCIVNPP group received the new protocol. The control group included patients matched to the DCIVNPP group for age, sex, Lysholm score [27], and Tegner score [28].
Anesthesia, surgical procedures, and rehabilitation protocol
Surgery
Patients from both groups were operated on by 6 orthopedic surgeons specialized in sports surgery, using different ACLR techniques, mainly hamstring grafts (quadrupled semitendinosus, doubled gracilis and doubled semitendinosus, biofast technique [29] with or without lateral extraarticular procedure), and modified Macintosh procedures [30].
Anesthesia
Both groups received preoperative spinal anesthesia and an ultrasound-guided selective sensory nerve block of the saphenous nerve with ropivacaine in the adductor canal (20 mL ropivacaine 0.2%, equivalent of 40 mg [5, 31]). Both groups received peri-operative local ropivacaine injections (1 vial of ropivacaine 2%) at the incision site [5, 31].
Postoperative pain management
Both post-operative pain management protocols were detailed in Table 1.
Rehabilitation protocol
The postoperative rehabilitation protocol was the same for all patients. The patient wore a hinged brace in full passive extension for several days and total weight bearing was allowed. The rehabilitation protocol began several days after surgery including closed chain isometric and eccentric quadriceps strengthening, and isometric hamstring co-contraction.
Outcome measures
The primary outcome measure was the VAS on day 0, the first night, days 1, 2, and 3. The VAS results were compared between the groups and analyzed in relation to the MCID and PASS thresholds for the VAS [10,11,12,13].
Secondary outcomes included the rate of side effects such as nausea, malaise, dizziness, anxiety, and stomach pain.
The MCID threshold for VAS, typically ranging from 1 to 1.4 [10,11,12,13], and the PASS thresholds for the VAS, typically ranging from 3.2 to 4 [13, 15], were derived from relevant literature. In our analysis, we opted to utilize the highest reported values for MCID and lowest for PASS to facilitate a more conservative comparison between the groups as we did not find validation for these values in French population.
Collected data
The following information was collected: age, sex, body mass index (BMI), preoperative IKDC subjective score, Lysholm score [27], Tegner score [28], type of sport, type of ACLR, addition of anterolateral reconstruction to the procedure, presence of meniscal lesions, type of hospitalization, and delay to surgery. Data for this study was collected prospectively by the French Prospective Anterior Cruciate Ligament Reconstruction Cohort Study (FAST, ClinicalTrials.gov Identifier: NCT02511158) and was compiled using Websurvey® software. Surgeons completed entries for the medical history, physical examination findings, work-up, and follow-up, while patients completed the questionnaires and scores.
Ethical consideration
The study received approval from the center’s Ethics Committee (CPP-IDF-VI). All participating patients provided informed consent when they filled out the online survey.
Study size
A total of 1360 ACLR procedures were performed at our institution in 2022. Five hundred and forty-three of these were excluded from the study, 459 for failure to fill out the online questionnaire and 84 were revision ACLR. Thus, a total of 778 patients were eligible for the study, 360 in the DCIVNPP group and 418 in the control group (Fig. 1).
The power calculation was conducted based on the hypothesis that the new protocol would lead to a minimum 10% reduction in pain compared to a previous study by the same institution, with a post-operative VAS score of 5 [32]. Using the Mann–Whitney method, the sample size was estimated to be 668 patients (334 per arm) to detect a significant difference of approximately 0.5 (assuming a standard deviation of 2.4). This sample size was determined to achieve a statistical power of 80% and a type I error rate of 5%.
Selection bias was minimized in both treatment groups by matching the study population using a propensity score. Participants were matched in a 1:1 ratio using a logit scale with a caliper width equal to 0.2. The matched variables included age, gender as well as Lysholm score [27], Tegner score [28] before surgery.
Statistical analysis
Categorical variables were presented as percentages while continuous variables were presented as means and standard deviations. Comparisons were performed with the Fisher exact test or the Chi square test for categorical data and the Student t-test or Mann Whitney test for continuous variables, when appropriate.
A p value < 0.05 was considered to be statistically significant. All statistical analyses were performed using R software (version 4.2).
Results
Patient characteristics (Table 2)
Both groups were statistically comparable. Patients’ mean age was 30.1 ± 9.9 in the DCIVNPP group and 30.3 ± 10.8 in the control group (p = 0.80) and there were more males in both groups (58% for DCIVNPP, 60% for control). The BMI (24.4 vs 24.2), the reoperative subjective IKDC, the Lyshlom and Tegner scores were also comparable between the groups (59.7/58.6), (72.5/72.3), and (6.9/7.0) respectively. There were more outpatient surgeries in the DCIVNPP group (292/338, 86%) than in the control group (274/338, 81%) (p = 0.09). The level and type of sports were comparable. There was no difference in time to surgery between both groups.
The doubled semitendinous doubled gracilis (134, 40%) and the quadrupled semi-tendinous (143, 42%) were the most frequent grafts in the DCIVNPP group while the doubled semitendinous, doubled gracilis (163, 49%) and the Macintoch Fascia lata technique (94, 28%) (p < 0.0001) were the most frequent in the control group. Lateral stabilizing surgery was performed in 40% of the DCIVNPP group (135 patients) vs 21% (71 patients) in control group (p < 0.0001). An associated meniscal lesion was not significantly different between the DCIVNPP and control group: 45% (151 patients) and 39% (130), respectively (p = 0. 12).
Post-operative VAS (Tables 3 and 4)
Patients in the DCIVNPP group had statistically significant lower levels of post-operative pain on the VAS on Day 0, the first night and Days 1, 2, and 3 compared to the control group (p = 0.004, 0.002, 0.002, 0.005, and 0.001 respectively). Mean VAS scores for the DCIVNPP group were 5.36 (SD 2.7), 5.11 (SD 2.4), 5.40 (SD 2.4), 4.31 (SD 2.3) and 3.54 (SD 2.3) respectively, while the mean scores for the control group were 5.93 (SD 2.7), 5.63 (SD 2.4), 5.93 (SD 2.6), 4.84 (SD 2.4) and 4.10 (SD 2.3), respectively (p < 0.05) (Table 2).
The maximum difference in the VAS between the groups was 0.57 on day 0 [0.16—0.98] and 0.56 [0.21—0.91] on day 3. The DCIVNPP group consistently demonstrated higher percentages of patients crossing the PASS threshold for pain (< 0.05). On day 3, a significantly greater proportion of patients in the DCIVNPP group (70.1%) crossed the PASS threshold compared to the control group (56.2%) (Table 4).
Side effects (Table 5)
Overall, the side effect profile of the DCIVNPP treatment were comparable to that of the control group, with some exceptions: the DCIVNPP group had higher rates of dizziness on day 0 and the first night (78 [24%] and 54 [15%], respectively) compared to 54 (17%) and 33 (9%) for the control group. They had also a higher rate of malaise on the third day (7 [3%]) compared to 18 (1%) for the control group. However, the control group had higher rate of abdominal pain on the first night and day 1 (4 [1%], and 7 [2%] respectively) compared to 15 (5%) and 18 (5%) respectively for the DCIVNPP group. Furthermore, the incidence of side effects decreased over time in both the DCIVNPP and control groups, from 39 and 32%, respectively, on Day 0 (p-value = 0.12), to 22% and 16%, respectively, on Day 3 (p-value = 0.06).
Discussion
This cohort study evaluated postoperative pain management and the secondary side effects of DCIVNPP following ACLR. The main finding of this study is that DCIVNPP led to a faster pain recovery than the control group.
Our study consistently showed that a higher percentage of patients in the DCIVNPP group crossed the threshold for PASS at each day post-operative assessment. Specifically, by day 3, more than 70% of patients in the DCIVNPP group reached the acceptable symptom state. These findings suggest that the DCIVNPP protocol is effective in facilitating a faster recovery and achieving satisfactory symptom control in the early post-operative period. This faster recovery may have important clinical implications because it suggests that the DCIVNPP protocol may be more effective in reducing pain and improving patient satisfaction in the early post-operative period which can influence patient recovery, rehabilitation, and overall satisfaction [2]. The use of PASS in our assessment is important because the results in the literature suggest that PASS can serve as a benchmark to evaluate treatment efficacy [23, 33]. Recent research has shown that PASS could be used to accurately predict the level of sports activity that can be resumed after shoulder surgery [23, 33].
On the other hand, the clinical significance of a 0.57 decrease in VAS, found in our study, is limited because it does not meet the MCID threshold for this score, which is typically set at a decrease of between 1 and 1.4 points [10,11,12,13]. Nevertheless, although the statistically significant difference may not be clinically significant, it still indicates a reduction in pain. This modest reduction in pain is likely responsible for the observed faster recovery in the DCIVNPP group.
The early postoperative VAS scores in our study (3.54 to 5.4) are slightly higher than those reported in the literature, which typically range from 2.5 to 5.4 [4, 5, 32]. This is probably because there was no morphine or any step 3 analgesics for pain management in our protocol, and that our VAS data was collected online, potentially allowing for less external influence on the patient's reported pain levels.
In the current study, we hypothesize that the most important element to the DCIVNPP is the DICC component. There are very few studies in literature comparing DICC to SCC. This include one experimental study by Holwerda et al. [21] and one therapeutic study by Murgier et al. [22]. In 2014, Murgier et al. performed a preliminary study in 39 patients, comparing DICC (using GameReady®) to SCC (using IceBand®). They concluded that DICC decreases the need for analgesic drugs following ACLR and improves postoperative recovery of knee range of motion [22]. However, the superiority of DICC group in their study was only based on lower VAS scores, and the p-values were not statistically significant [22]. Our study, however, shows a significant optimization of pain management, thus providing more solid evidence for the effectiveness of the DICC in reducing postoperative pain, resulting in faster recovery. Holwerda et al. performed an experimental study that compared the changes in tissue temperature (on the skin surface and in the quadriceps muscle) and cardiovascular response (mean arterial pressure, heart rate, and forearm blood flow) between the DICC using the GameReady® device and the SCC using elastic ice wrapping [21]. The study found no acute pathological cardiovascular strain with either technique, with a physiological response of a 5-min increase in mean arterial pressure (reaching a maximum of 6 mmHg). Also, there was no significant intramuscular temperature difference between the two modalities. SCC resulted in a temperature change of -14 ± 2 °C, while DICC was associated with a change of -7 ± 3 °C to -11 ± 6 °C, depending on the programmed compression pressure. This study provides further insight into the safety profile and the underlying changes associated with the DICC (GameReady®) [21]. In 2016, Song et al. performed a meta-analysis to compare the effects of compressive cryotherapy with NCC alone and found that compressive cryotherapy resulted in significantly better outcomes [20]. Similar results were reported in more recent studies [19, 34]. The metanalysis by Davey et al. in 2021 only identified 11 RCTs studying the effect of compression cryotherapy following ALCR, and none of them compared the different types of compression [35].
Our results show that the adverse effect profile of DCIVNPP treatment was comparable to that of the control group, with slightly higher rates of malaise and dizziness and lower rates of abdominal pain. We hypothesize that these changes are attributed to the mode of administration of Nefopam, which may influence these types of nonspecific adverse effects. Although the exact mechanism of action of Nefopam is unclear, it is thought to act centrally by inhibiting the reuptake of serotonin, norepinephrine, and dopamine [23]. In addition, nefopam may have some anti-inflammatory effect [25]. By means of these mechanisms of action, nefopam possess analgesic properties [36].
Although there is very little literature comparing the oral and intravenous forms of Nefopam, certain authors have suggested that there may be decreased tolerance associated with intravenous administration [24, 26]. Common adverse effects of Nefopam include dry mouth, drowsiness, dizziness, headache, nausea, and constipation. These adverse effects are more likely to occur with high dosages or prolonged use. More severe adverse effects of Nefopam, such as flushing, hypertension, tachycardia, and even seizures, may occur with rapid administration [24, 26]. However, none of these severe adverse effects were reported in our study.
The main limitation of the study is that it is non-randomized, which may create a selection bias. To control this potential bias, we used a matching strategy based on propensity scores. Lack of randomization can also create performance bias. The DCIVNPP group receives more attention from a nurse, which may make them feel more comfortable and have better outcomes, leading to an overestimation of the effectiveness of the tested protocol. Additionally, the surgical technique varied among patients, introducing potential bias into the study. For example, lateral extraarticular tenodesis was less frequently performed in the control group, which could potentially result in either reduced pain due to a lesser extent of the procedure, or conversely, increased pain as a consequence of potential residual instability. Another limitation of this study is the absence of specific validation studies for the MICD and PASS values in the French population. However, we addressed this limitation by utilizing a range of values from multiple references and selecting the highest reported MCID threshold and lowest reported PASS threshold for our analysis. Finally, a detection bias may have occurred as patients in the DCIVNPP group may have reported less pain due to their belief that they are receiving a more advanced treatment.
These findings should be extrapolated with caution. Further studies are needed, in particular a randomized controlled trial comparing the safety and effectiveness of this protocol to other cryotherapy and pain management techniques. Moreover, more studies are needed to determine if the 1-day-faster early recovery can be extended to longer-term faster recovery, such as returning to play after ACLR.
Conclusion
In conclusion, this study provides preliminary evidence for the effectiveness of DICC with IV nefopam in reducing early post-operative pain following ACLR. The main finding of this study is that patients in the DCIVNPP group cross the PASS threshold for pain faster. The route of nefopam administration may be associated with different side effect profiles: intravenous nefopam may increase the risk of dizziness and malaise while oral nefopam may increase the risk of abdominal pain.
References
Majewski M, Susanne H, Klaus S (2006) Epidemiology of athletic knee injuries: a 10-year study. Knee 13(3):184–188
Abdallah FW, Brull R, Joshi GP (2019) Pain management for ambulatory arthroscopic anterior cruciate ligament reconstruction: evidence-based recommendations from the society for ambulatory anesthesia. Anesth Analg 128(4):631–640
Filbay SR, Ackerman IN, Russell TG, Macri EM, Crossley KM (2014) Health-related quality of life after anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med 42(5):1247–1255
Chuaychoosakoon C, Parinyakhup W, Wiwatboworn A, Purngpiputtrakul P, Wanasitchaiwat P, Boonriong T (2021) Comparing the efficacy of postoperative pain control between intravenous parecoxib and oral diclofenac in ACL reconstruction. Orthop J Sports Med 9(10):232596712110416
Lefevre N, Klouche S, de Pamphilis O, Devaux C, Herman S, Bohu Y (2015) Postoperative discomfort after outpatient anterior cruciate ligament reconstruction: a prospective comparative study. Orthop Traumatol Surg Res 101(2):163–166
Sonnery-Cottet B, Saithna A, Quelard B, Daggett M, Borade A, Ouanezar H, Thaunat M, Blakeney WG (2019) Arthrogenic muscle inhibition after ACL reconstruction: a scoping review of the efficacy of interventions. Br J Sports Med 53(5):289–298
Tiao J, Wang K, Carbone AD, Herrera M, Zubizarreta N, Gladstone JN, Colvin AC, Anthony SG (2023) Ambulatory surgery centers significantly decrease total health care expenditures in primary anterior cruciate ligament reconstruction. Am J Sports Med 51(1):97–106
Secrist ES, Freedman KB, Ciccotti MG, Mazur DW, Hammoud S (2016) Pain management after outpatient anterior cruciate ligament reconstruction: a systematic review of randomized controlled trials. Am J Sports Med 44(9):2435–2447
Connelly JW, Galea VP, Rojanasopondist P, Matuszak SJ, Ingelsrud LH, Nielsen CS, Bragdon CR, Huddleston JI, Malchau H, Troelsen A (2019) Patient acceptable symptom state at 1 and 3 years after total knee arthroplasty: thresholds for the Knee Injury and Osteoarthritis Outcome Score (KOOS). J Bone Joint Surg 101(11):995–1003
Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC (2007) Understanding the minimum clinically important difference: a review of concepts and methods. The Spine Journal 7(5):541–546
Kelly A-M (2001) The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 18(3):205–207
Lee JS (2003) Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med 10(10):1128–1130
Tashjian RZ, Deloach J, Porucznik CA, Powell AP (2009) Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 18(6):927–932
Toomey CM, Whittaker JL, Palacios-Derflingher L, Emery CA (2021) Using the patient acceptable symptom state (pass) to evaluate KOOS outcomes 3–12 years following a youth sport-related knee injury. Osteoarthritis Cartil 29:S282–S283
Dougados M, Moore A, Yu S, Gitton X (2007) Evaluation of the Patient Acceptable Symptom State in a pooled analysis of two multicentre, randomised, double-blind, placebo-controlled studies evaluating lumiracoxib and celecoxib in patients with osteoarthritis. Arthritis Res Ther 9(1):R11
Crystal NJ, Townson DH, Cook SB, LaRoche DP (2013) Effect of cryotherapy on muscle recovery and inflammation following a bout of damaging exercise. Eur J Appl Physiol 113(10):2577–2586
Lefevre N, Bohu Y, Naouri JF, Klouche S, Herman S (2014) Validity of GNRB® arthrometer compared to Telos™ in the assessment of partial anterior cruciate ligament tears. Knee Surg Sports Traumatol Arthrosc 22(2):285–290
Raynor M, Pietrobon R, Guller U, Higgins L (2005) Cryotherapy after ACL Reconstruction - a meta-analysis. J Knee Surg 18(02):123–129
Mendes IE, Ribeiro Filho JC, Lourini LC, Salvador MD, de Carvalho AR, Buzanello MR, Bertolini GRF (2022) Cryotherapy in anterior cruciate ligamentoplasty pain: a scoping review. Therapeut Hypother Temp Manage 12(4):183–190
Song M, Sun X, Tian X, Zhang X, Shi T, Sun R, Dai W (2016) Compressive cryotherapy versus cryotherapy alone in patients undergoing knee surgery: a meta-analysis. Springerplus 5(1):1074
Holwerda SW, Trowbridge CA, Womochel KS, Keller DM (2013) Effects of cold modality application with static and intermittent pneumatic compression on tissue temperature and systemic cardiovascular responses. Sports Health 5(1):27–33
Murgier J, Cassard X (2014) Cryotherapy with dynamic intermittent compression for analgesia after anterior cruciate ligament reconstruction Preliminary study. Orthop Traumatol Surg Res 100(3):309–312
Kim KH, Abdi S (2014) Rediscovery of Nefopam for the treatment of neuropathic pain. Korean J Pain 27(2):103–111
Aymard G, Warot D, Démolis P, Giudicelli JF, Lechat P, Le Guern ME, Alquier C, Diquet B (2003) Comparative pharmacokinetics and pharmacodynamics of intravenous and oral nefopam in healthy volunteers*: PHARMACOKINETICS AND -DYNAMICS OF NEFOPAM. Pharmacol Toxicol 92(6):279–286
Chae JW, Kang DH, Li Y, Kim SH, Lee HG, Choi JI, Yoon MH, Kim WM (2020) Antinociceptive effects of nefopam modulating serotonergic, adrenergic, and glutamatergic neurotransmission in the spinal cord. Neurosci Lett 731:135057
Revol B, Delorme J, Jouanjus É, Spadari M, Djezzar S, Lepelley M, Khouri C, Fouilhé Sam-Laï N, Mallaret M (2021) Trente ans d’abus de néfopam en France. Therapies 76(6):527–537
Lysholm J, Tegner Y (2007) Knee injury rating scales. Acta Orthop 78(4):445–453
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49
Lang E, Hardy A, Tufis O, Grimaud O, Gerometta A, Bohu Y, Lefevre N, Meyer A (2022) Surgical technique of anterior cruciate ligament ligamentoplasty with pedicular hamstrings via an inside-out approach: BIOFAST hamstring tendons graft. Orthop Traumatol Surg Res 108(3):103192
Dos Santos A, Gerometta A, Bohu Y, Lefevre N, Van Hille W, Khiami F, Hardy A (2022) Anterior cruciate ligament arthroscopic reconstruction and lateral tenodesis with iliotibial band and gracilis tendon: Technical note. Orthop Traumatol Surg Res 108(8):103412
Lefevre N, Klouche S, de Pamphilis O, Herman S, Gerometta A, Bohu Y (2016) Peri-articular local infiltration analgesia versus femoral nerve block for postoperative pain control following anterior cruciate ligament reconstruction: Prospective, comparative, non-inferiority study. Orthop Traumatol Surg Res 102(7):873–877
Stein AM, Bouché P-A, Grimaud O, Vedrenne P, Hardy A (2023) La prégabaline ne diminue pas la douleur postopératoire après une chirurgie du LCA en ambulatoire : étude cas-témoin. Orthop Traumatol Surg Res 14:103596
Lutz C, Baverel L, Colombet P, Cournapeau J, Dalmay F, Lefevre N, Letartre R, Potel J-F, Roussignol X, Cucurulo T, Servien E (2016) Pain after out-patient vs. in-patient ACL reconstruction: French prospective study of 1076 patients. Orthop Traumatol Surg Res. 102(8):S265–S270
Glattke KE, Tummala SV, Chhabra A (2022) Anterior cruciate ligament reconstruction recovery and rehabilitation: a systematic review. J Bone Joint Surg 104(8):739–754
Davey MS, Hurley ET, Anil U, Moses A, Thompson K, Alaia M, Strauss EJ, Campbell KA (2021) Pain management strategies after anterior cruciate ligament reconstruction: a systematic review with network meta-analysis. Arthroscopy 37(4):1290-1300.e6
Schulz T, Lalande L, Aubrun F, Dziadzko M (2022) Nefopam prescribing preferences in French hospitals: results of a survey. Pan Afr Med J 41:213
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
MKM, NL, AM, AH contributed to the conception, design of the study, and interpretation of results. MKM, NL, YB, AH contributed to the acquisition of patients and their data. MKM, NL, FK, AM, OG, AH contributed to review of the database, critical review of data. NL, YB, AG, FK, YB, AH contributed to critical review of the manuscript. EV performed the statistical analysis and drafting of tables and figures. MKM, NL contributed to original creation and implementation of clinical database. MKM acts as the guarantor of the study and performed the drafting of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study involves human participants and was approved by ethics committee (CPP IDF VI). Participants gave informed consent to participate in the study/ collection of data before taking part. ClinicalTrials.gov ID: NCT02511158.
Competing interests
NL: Consultant at Websurvey Society, Paris France.
AH: Consultant for Arthrex and Depuy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moussa, M.K., Lefevre, N., Valentin, E. et al. Dynamic intermittent compression cryotherapy with intravenous nefopam results in faster pain recovery than static compression cryotherapy with oral nefopam: post-anterior cruciate ligament reconstruction. J EXP ORTOP 10, 72 (2023). https://doi.org/10.1186/s40634-023-00639-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-023-00639-3