Abstract
Purpose
Running, jumping/landing and cutting/change of direction (CoD) are critical components of return to sport (RTS) following anterior cruciate ligament reconstruction (ACLR), however the electromyographic (EMG) activity patterns of the operated leg during the execution of these tasks are not clear.
Methods
A systematic review was conducted to retrieve EMG studies during running, jumping/landing and cutting/(CoD) in ACLR patients. MEDLINE, PubMed, SPORTDiscus and Web of Science databases were searched from 2000 to May, 2022 using a combination of keywords and their variations: “anterior cruciate ligament reconstruction” OR “ACLR”, “electromyography” OR “EMG”, “running”, “jumping” OR “landing”, “cutting” OR “change-of-direction” OR “CoD”. The search identified studies comparing EMG data during running, landing and cutting/(CoD) between the involved limb and contralateral or control limbs. Risk of bias was assessed and quantitative analyses using effect sizes were performed.
Results
Thirty two studies met the inclusion criteria. Seventy five percent (24/32) of the studies reported altered EMG activity pattern of the ACLR leg during running, jumping/landing and cutting/(CoD) when compared with either the healthy control leg or the contra-lateral leg. Twelve studies showed decreased, delayed or earlier onset and delayed peak in quadriceps EMG activity with small to large effect sizes and 9 studies showed increased, delayed or earlier onset and delayed peak in hamstrings EMG activity with small to large effect sizes. Four studies showed a “hamstrings-dominant” strategy i.e. decreased quadriceps coupled with increased hamstrings EMG activity in both running and jumping/landing irrespective of graft type. One study reported that on the grounds of decreased quadriceps activity, lower hamstrings EMG activity was predictive of ipsilateral re-injury in ACLR patients.
Conclusion
This systematic review of Level III evidence showed that the ACLR leg displays decreased quadriceps or increased hamstrings EMG activity or both despite RTS. Simultaneous decreased quadriceps and increased hamstrings EMG activity was shown for both running and jumping/landing. From a clinical perspective this “hamstrings dominant” strategy can serve as a protective mechanism against graft re-injury.
Level of evidence
III.
Similar content being viewed by others
Background
Anterior cruciate ligament (ACL) injuries most frequently occur during athletic activities/sports participation that involves some combination of running, jumping and cutting or changing direction (CoD) [59, 29, 76, 97, 104] and affect both amateur and professional athletes with injury rates ranging 3–15% per year [1, 50, 71]; though increasing trends in the average annual cases have been reported for both genders after adjusting for exposures [1, 95]. ACL reconstruction (ACLR) aims at reducing (though not eliminating) the risk for early-onset and accelerated progression of osteoarthritis (OA) [2, 65, 12,13,14, 20, 24, 63, 64, 77], propagate return to sport (RTS) to pre-injury levels [58] and eventually sustaining the same level of performance for the subsequent year [106].
Individuals who do RTS following ACLR, have an increased risk of re-injury [11, 75, 93, 108], with failure rates ranging ~ 3–5% in general population [37, 98] and ~ 5–17% in athletic populations [52, 56]. RTS is a complex, biopsychosocial process [5, 13, 17] that transcends choice of graft/graft-related functional outcomes [37, 75, 98], focuses on optimization of the functional recovery process [30, 31] and the restoration of “quality” of movement during running, jumping and cutting/ (CoD) [13, 31]. However, despite being cleared for RTS, ACLR athletes frequently display biomechanical alterations that are thought to predispose for either subsequent re-injury/graft failure or contra-lateral ACL injury [74, 35, 38, 60, 87, 103]. Whilst there is a host of factors impacting on these alterations [13, 17], neuromuscular activity patterns is a pivotal parameter because can be modified via training [68, 111].
Altered neuromuscular activity may be indicative of the ability to produce or accept force or identify potential areas of tissue overload [19, 41, 84]. Side to side differences in neuromuscular activity will result in altered movement quality, which in turn will induce further movement compensations and inappropriate patterns [13, 14, 19]. Finally it is not clear whether these differences in neuromuscular activity, are a function of time after surgery (thus reflecting tissue healing) [25, 47], functional criteria (highlighting restoration of motor control) [15, 16] or reflect pre-injury movement patterns [100, 110].
Thus, the objective of this systematic review was to synthesize the scientific literature regarding neuromuscular activity of the lower extremity muscles in adult, physically active ACL reconstructed patients during running, jumping and cutting/CoD tasks. The second aim was to examine whether EMG analyses of running, jumping and cutting/CoD could identify deficits with implications for either graft re-injury or contra-lateral ACL injury.
Methods
The present systematic review was designed, conducted and analyzed according to the guidelines of Preferred Reporting of Items for Systematic Reviews and Meta-Analyses (PRISMA) [73] and followed the recommendations of the Cochrane group [44].
Eligibility criteria
The following eligibility criteria had to be met in order for a study to be considered relevant for the purposes of the present systematic review
-
1.
Study participants: male, female or both
-
2.
Age ≥ 18 years
-
3.
ACL injury that was treated surgically
Furthermore studies had to have used running or jumping or cutting/CoD as testing modality and apply EMG recordings of at least one lower extremity muscle. We narrowed our search to original, peer-reviewed article published in English. We did not set a limit to time since surgery to be used as an eligibility criterion. Participants’ activity level was not restricted to a particular level. Studies were eligible for inclusion if they used surface EMG to assess magnitude of activity (peak/mean muscle activity) or timing (onset/offset and duration) in the injured side of ACLR patients and compared it with a control group and/or the intact contra-lateral leg. Thus, we excluded studies on ACL deficient patients, studies where EMG recordings were used to derive model-driven muscle forces, studies comparing post- to pre-surgery neuromuscular activity or assessing muscle activity during walking, jogging, downhill or uphill running. Finally we excluded editorials, theses, book chapters and conference abstracts.
Data sources
Our search was conducted from 2000 until May 2022 in the electronic databases MEDLINE/PubMed, SPORTDiscus and in the Web of Science. Furthermore, a manual search was done using the reference lists of included articles to identify additional and potentially relevant articles that had not been identified in the electronic searches.
Search strategy and study selection
The literature search was undertaken to locate articles that evaluated lower extremity muscle activity during running, jumping/landing and cutting/CoD tasks in individuals having undergone ACL reconstruction. The keywords were: “anterior cruciate ligament”/ “ACL”, “reconstruction”/”ACL reconstruction”/”ACLR”, “EMG/ “electromyography”/neuromuscular activity”/”muscle activity”/”EMG amplitude”/”EMG timing”/”muscle on-set”/”pre-activity”/”re-activity”, “running”, “jumping”, “landing”, “change-of-direction”. Keywords were used individually and in various combinations with OR/AND operators as follows: (anterior cruciate ligament OR ACL) AND (reconstruction) OR (ACLR) AND (EMG OR Electromyography OR neuromuscular activity OR muscle activity OR EMG amplitude OR EMG timing OR muscle on-set OR pre-activity OR re-activity) AND (Running OR Jumping OR Landing OR cutting OR change-of-direction OR CoD). The search was performed by two authors and was further supplemented by manual search of the reference lists of papers selected from the initial database search. All titles and abstracts were independently screened by the two authors performing the search to identify potentially relevant papers based on eligibility criteria. The full manuscripts were subsequently retrieved and each paper independently assessed for inclusion/exclusion criteria by the same two authors. If their decision was not unanimous, a third reviewer assessed the eligibility of the article.
Data collection process and data extraction
After final decision of all studies, data extraction for each included study was performed by two authors using a simple spreadsheet. The first author extracted study design, sample size and age of the ACLR and control group, graft type, time since surgery (in months) and activity level of groups. Furthermore the specific task per activity (running, jumping, cutting/CoD) was recorded along with EMG dependent variable and the muscle(s) studied. The EMG outcome of interest was registered as the reported comparison of the depended variable between ACLR leg and contra-lateral and/or control leg. Effect sizes (± 95%CI) were derived using sample size, mean and standard deviation of the reported values. When necessary data were unavailable, authors were contacted by email. The effect sizes were calculated according to the formula: Cohen d = mean (operated side) − mean (control or contra-lateral side)/SD (pooled) and were interpreted as small (≤ 0.4), moderate (≥ 0.5 up to 0.79) and large (≥ 0.8) [84].
Risk of bias assessment
A modified version of the Downs and Black checklist [84, 94] was used to determine the risk of bias of all the included articles [32]. The checklist examines features related to i) reporting (objectives/hypotheses, main outcomes, characteristics of the participants, interventions, main confounders/findings, estimates of random variability, reporting of p-values), ii) external validity (subjects/staff/places/facilities), iii) internal validity (blinding subjects/assessors, data dredging, follow-up lengths/same time period between intervention and outcome for cases and controls, appropriateness of statistical tests/main outcome measures), iv) selection (patients and controls from same population and over same period of time, randomization, allocation concealed, adjustments for confounding, loss to follow-up) and v) power analysis. For the purposes of this systematic review, studies with a total score ≥ 17 were rated as being of a low risk of bias (thus of “high” methodological quality) [94]. Studies between 13–16 points were rated as being of “medium” quality, and studies ≤ 13 were rated as being of high risk of bias (thus of “low” methodological quality). No study was excluded due to low methodological quality; our aim was to synthesize all available data regarding neuromuscular activity. Two of the authors independently reviewed and scored all included studies based on the checklist (Supplementary Tables S1-S3). Any discrepancy was resolved in a consensus meeting, and a third reviewer was available if needed, but that was not required.
Results
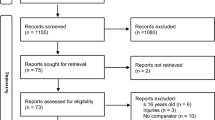
Returning hits from the electronic database search and manual search were screened for duplicates. After applying inclusion and exclusion criteria according to PRISMA flowchart [73], a total of 32 studies (6 running studies, 22 jumping studies and 5 cutting/CoD studies, one study contributed to both jumping and cutting/CoD), involving 884 subjects -482 participants with ACLR and 402 healthy controls could be used for analysis. There were 49 ACLR participants and 47 controls in studies dealing with running, 356 ACLR participants and 251 controls in studies dealing with jumping and 77 ACLR participants and 65 controls in studies dealing with cutting/CoD. Studies were excluded mainly because EMG recordings were used to derive model-based muscular forces rather than muscle activity, participants did not receive reconstruction surgery or muscle activity of the reconstructed leg was compared to muscle activity at the pre-injury state. The flowchart describing the steps of the search is depicted in Fig. 1.
Risk of bias assessment
Medium risk of bias was found for over 2/3 of the studies (22/32, 68.7%) [3, 4, 6, 7, 12, 21, 26,27,28, 34, 41, 46, 49, 61, 69, 80, 86, 90,91,92, 96, 101], 4/32 (12.5%) were of high methodological quality [10, 62, 78, 79] and 6/32 studies (18.8%) were of low quality [39, 72, 81, 82, 89, 109] (Supplementary Tables S1-S3). The main reasons for a medium to low methodological quality were due to unclear description of participants and/or prior interventions.
Study design
Running studies were case–control [34, 46, 89, 91] or case series [90, 92] (Table 1). Jumping studies were case–control [3, 4, 7, 12, 21, 26, 28, 39, 49, 61, 69, 72, 80,81,82, 86, 96, 101] and case series [27, 41, 62, 78, 79] (Table 2). Cuttting/CoD studies were case–control [6, 10, 26, 82] or case-study [109] (Table 3). The case–control studies compared the ACLR participants with at least one control group (e.g. healthy controls), whilst the case series studies made a comparison between the ACLR and the intact contra-lateral leg.
Participants
The sample size for the ACLR participants, ranged from n = 1 [109] to a maximum of n = 65 [78]. Running studies recruited exclusively males [34, 46, 89,90,91,92] receiving the median or medial 1/3 of the bone-patella tendon-bone (BPTB) [89,90,91,92], hamstrings HS [46] or a mixed BPTB and (HS) [34] grafts and their activity level was mainly amateur soccer players [89,90,91,92] (Table 1). Jumping studies recruited exclusively males [12, 27, 28, 41, 72, 96, 101], females [80,81,82] or both males and females [3, 4, 7, 21, 39, 49, 61, 62, 69, 78, 79, 86] receiving BPTB [39, 86, 101], hamstrings HS [41, 69, 80] or mixed BPTB and (HS) [3, 4, 7, 12, 21, 27, 28, 49, 61, 62, 78, 79, 81, 82, 96] grafts and included mostly some form of active sport-participants (Table 2). Cutting/CoD studies recruited exclusively females [10, 82, 109] or both males and females and their activity level was registered mainly as national level team-sport athletes (Table 3). Time since surgery ranged from as low as 4–8 months [34, 39, 46, 72, 86, 96, 101] to ≥ 60 [3, 4, 7, 26, 78, 79, 81, 82].
Interventions
The number of muscles assessed ranged 1–9 and investigators mainly assessed muscle activity around the thigh and recorded EMG signal from rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), biceps femoris (BF) and semitendinosus/semimebranosus (ST/SM) (Tables 4, 5 and 6). Less often EMG signal from gluteus maximus/medius (GMAX/GMED) and calf muscles such medial and lateral gastrocnemius (GM/GL) and soleus (SO) was also recorded (Tables 4, 5 and 6).
Running tasks involved conventional typically treadmill [34, 89,90,91,92] but also over-ground [46] running was used (Table 4). Jumping tasks included hops (single- and double- leg) [7, 21, 39, 41, 69, 79, 82, 86, 101], jumps [3, 4], drop jumps (single- and double- leg) [27, 28, 61, 62, 72, 80, 81], countermovement jumps (single-leg) [78] and box jumps (single-leg) [81] (Table 5). Finally cutting/CoD tasks involved a combination of hops interspersed with a change in direction such as cross-over hops [10, 82], or forward hop followed by diagonal hop [6, 26] (Table 6). In addition, some studies even investigated the influence of fatigue on neuromuscular activity during either running [89,90,91,92] or jumping tasks [4, 49, 61, 62].
Outcomes
All included studies assessed muscle activity using surface EMG according to standardized procedures and guidelines provided by the SENIAM project (Surface Electromyography for the Non-Invasive Assessment of Muscles) [42]. The EMG-related dependent variables included peak and/or mean amplitude, timing of peak muscle activity, preparatory and reactive muscle activity and onset of muscle activation. The outcome variables were expressed either as a percentage of maximum voluntary (isometric) contraction (%MVC), as a percentage of peak muscle activity during the task or as microvolts/milliseconds. Seventy five percent of the included studies reported statistically significant differences in muscle activity patterns during running, jumping and cutting/CoD tasks when the ACLR leg was compared to the contra-lateral intact and/or control leg [3, 7, 10, 21, 26, 28, 34, 39, 41, 46, 49, 62, 69, 72, 78,79,80, 86, 89,90,91,92, 96, 109] (Tables 4, 5 and 6).
Quadriceps muscles showed decreased EMG amplitude during running [46, 89,90,91] (Table 4) and jumping [21, 49, 62, 80, 86] (Table 5), increased EMG amplitude during jumping [80, 81, 96] (Table 5), earlier [39] or delayed [41] EMG onset during jumping (Table 5), delayed [26] EMG peak during cutting or no difference in EMG amplitude or onset during jumping [3, 4, 7, 12, 61, 69, 82] or cutting [6, 82, 109] (Tables 5 and 6]. Hamstrings muscles showed increased [46, 89] or decreased [34] EMG amplitude during running (Table 4), increased [49, 69] or decreased [80] EMG amplitude, earlier onset or delayed peak [39, 69, 96], or no difference [41, 61, 78, 79, 81, 82] during jumping (Table 5) and increased [10, 109] or decreased [10] EMG amplitude, delayed peak [26] or no difference [6, 82] during cutting (Table 6). Additionally GM/GL showed decreased EMG amplitude during running [46] (Table 4), increased [72, 78, 79], decreased [3] or no difference [4, 7, 86, 101] in EMG amplitude during jumping (Table 5) and delayed peak in EMG amplitude during cutting/CoD [26] (Table 6). Finally, GMAX/GMED showed increased [39, 78, 79, 81] or decreased [28] EMG amplitude during jumping and no difference in EMG amplitude during running [46] (Table 4) and jumping [46, 61, 62, 82, 101] (Table 5).
Research exclusively on males showed decreased or delayed quadriceps EMG activity in four studies involving running [34, 46, 89,90,91] (Table 4) and decreased [41], increased [96] or no difference [12, 101] during various hops (Table 5). Studies on males report increased BF [46, 89] or decreased [34] ST/SM EMG activity (specifically in individuals operated with hamstrings graft) during running or no difference during various jumps [12, 41, 96, 101] (Table 5). Decreased EMG activity during jumping in males has been reported for GM [41], and GMED [28]. In females increased [80, 81], decreased [80] or no difference [82] in quadriceps activity has been reported during various jump protocols (Table 5). In addition females have decreased BF EMG activity during jumping [80] and cutting/CoD [10]; others however report no difference [81, 82] (Table 5). Studies with both male and female participants report decreased quadriceps activity, delayed peak or earlier onset [21, 26, 39, 49, 62, 86] but others report no difference [4, 7, 61, 69, 78, 79] during jumping. Hamstrings display increased activity, delayed peak or earlier onset [7, 26, 39, 49, 69], whilst no difference is reported in others [4, 61, 78, 79] during jumping. Those who report no differences in either quadriceps or hamstrings EMG activity, do report increased GMAX activity [78, 79].
Decreased quadriceps activity or earlier onset following ACLR with BPTB graft is reported in all [39, 86, 89,90,91,92] but one [101] studies. Increased hamstrings activity is reported during running [89]. Regarding ACLR with hamstrings graft decreased quadriceps activity or onset has been reported [41, 46, 80] but no difference has also been reported [69, 109]. Regarding hamstrings muscle activity in these patients, increased [46, 49, 80] and decreased BF [10] has been reported. In addition ST/SM activity showed no difference [46] or decreased activity [109] in patients receiving hamstrings graft. Studies with mixed sample of grafts report decrease in quadriceps activity [49, 62], increase in quadriceps activity [81] or duration [96] or no difference [3, 4, 7, 12, 61, 78, 79, 82]. Regarding hamstrings, activity is decreased and there is also shorter BF onset [7, 34, 49] but other report no difference in hamstrings activity [3, 4, 12, 61, 82].
Discussion
The aim of the present review was to synthesize the scientific literature regarding EMG activity of the lower extremity muscles in adult, physically active ACLR individuals during running, jumping/landing and cutting/CoD tasks. All three tasks are important elements in the rehabilitation process of ACLR participants aiming to RTS [15, 16, 84] and thus any EMG alterations are deemed high relevant.
Results on running show a decrease in muscle activity of the VM (early stance) and VL, VM (late stance) with moderate to large effect sizes [46] as well as reduced progressive recruitment of the VL during late stance with mainly moderate effect sizes [89,90,91]. In addition, increased BF EMG activity (large effect size) [46] as well as increased progressive recruitment of the BF during the stance phase (small effect size) have been reported [89]. The coupled reduction in VM/VL and increase in BF activity muscle activity has been reported for both HS [46] and BPTD graft [89,90,91]. In non-injured subjects the preferential increase in agonist EMG activity, which characterizes the “quadriceps-dominant” strategy, is considered to reflect the physiological response to the accumulation of metabolic fatigue [22, 48, 66, 89, 113] as well as a biomechanical consequence that is associated with better neuromuscular control of the joint during fatigue [53, 54, 83]. Thus, following reconstruction these studies suggest a replacement of the typical “quadriceps-dominant” strategy by a “hamstrings-dominant” strategy for the ACLR leg during running, aiming to dynamically stabilize the reconstructed limb and decrease the anterior stress applied to the ACL graft [46, 89]. Importantly the lack of the anticipated increase in agonist EMG activity has also been reported for the VL muscle during single-leg drop-jump before and after a fatigue protocol [62]. Therefore the “hamstrings-dominant” strategy may reflect either an alteration of the local physiological response to accumulating fatigue or a biomechanical adaptation to stabilize the joint under fatigue. This hypothesis is further supported by evidence showing that aerobic endurance is more strongly correlated to the relative increase in VL EMG activity on the intact contra-lateral leg compared to the corresponding increase in the EMG activity of the ACLR leg during high-intensity fatiguing running [91]. From a clinical perspective the establishment of a “hamstrings-dominant” strategy during high-intensity running offers a potential protective mechanism after a unilateral ACLR. In addition it has been shown that BPTB ACLR individuals who experienced a secondary ipsilateral ACL injury have lower hamstrings activity compared to BPTB ACLR individuals who did not [86]. Thus, the lack of high BF activity following ACLR may increase the risk for ipsilateral ACL injuries at least in BPTD grafts [86] or increase the chances for a hamstring injury [105] irrespective of graft type [34].
Jumping studies are the most abundant, possible because of the clinical relevance of the hopping tests [56]. Results regarding timing of muscle activity are mixed with some studies reporting earlier onset (large effect sizes for all tested muscles) [7, 39] or longer duration during pre-impact (large effect sizes for both quadriceps and hamstrings) [96], but others have reported no significant difference in muscle onset [12] or even a delayed muscle onset (moderate effect size for VM only) [41]. Thus, three studies with large effect sizes show earlier onset or the longer duration at pre-impact for both quadriceps and hamstrings which may indicate increased pre-tension that serves as a protective mechanism by stiffening the joint for the subsequent impact [33]. A line of criticism is that the patients in these two studies were examined ~ 4–6 months following surgery; the corresponding time since surgery for the other studies was ~ 15 months [12] and ~ 60 months [41]; thus it is possible that the early EMG onset is observed only in the initial rehabilitation period. In addition the apparently opposite in direction trends regarding the onset of VM activity [39, 41] may be attributed to the different methodology. Indeed Gokeler et al., (2010) [39] defined the muscle onset as the first muscle burst in EMG activity before landing, whereas He et al., (2022) [41] defined the onset as the rising of linear envelopes representing muscle burst.
EMG amplitude has been examined both pre-impact (preparatory muscle activity) [28, 49, 86] and post-impact following initial contact (reactive muscle activity) [3, 21, 28, 49, 62, 69, 72, 78,79,80,81, 86]. Five studies indicate decreased post-impact activity for quadriceps (small to large effect sizes) [21, 49, 62, 80, 86], whilst three studies suggest increased post-impact activity for the quadriceps (small effect sizes) [72, 80, 81]. Regarding hamstrings, two studies report increased activity post-impact (large effect sizes) [49, 69] and one study reported decreased hamstrings activity (small effect size) [80]. Furthermore data for muscles above or below the knee show increased activity for GMAX (small to moderate effect sizes) [78, 79, 81] or decreased GMED activity (moderate effect size) [28] and either increased (moderate effect sizes) [72, 78, 79] or decreased (large effect size) [3] GM activity. The decreased quadriceps post-impact activity may bear resemblance to the “quadriceps-avoidance” gait that has been observed during hop landing in ACL-deficient subjects [8, 36]; however others indicate increased quadriceps post-impact activity [72, 80, 81] or no change in quadriceps activity [4, 12, 61, 82, 101]. In fact the same subjects show decreased quadriceps activity during double-leg drop jump and increased quadriceps activity during single-leg drop jump [80], therefore depending on the task the decreased quadriceps activity may represent merely a variation in landing strategy with subjects having increased use of the non-operate leg to control landing. Thus, from a clinical perspective regarding landing tasks most studies point towards reduced quadriceps activity which may be potentially dangerous, since decreased quadriceps activity is thought to lead to re-injury and may contribute to the development of post-traumatic OA [107, 85] and increased hamstrings activity that may act as a protector against anterior tibial shear [83]; however increased hamstring activity may also be present in ACLR patients with high degree of kinesiophobia [69]. The simultaneous decreased quadriceps activation coupled with increased hamstrings activation that characterizes the “hamstring-dominant” strategy reported in running [46, 89] appears to be also present in landing tasks [49, 86]. In fact in the latter study it was reported that lower hamstrings activity in ACLR subjects was predictive of subsequent re-injury [86]. Furthermore a recurring pattern that deserves further notice is the increased activation of the GMAX post-impact, which has been described as “hip-bias” compensation [78, 79, 81] and has been observed at the involved lower extremity among most subjects who report high perceived sports capability compared to pre-injury status [78, 79]. The authors hypothesized that these compensations may be related to a neuro-sensory deficit and subsequent CNS sensorimotor re-organization [79].
Regarding cutting/CoD in a limited number of studies, cross-over hops have been associated with either decreased or no change in BF [10, 82], whilst hop followed by unanticipated diagonal hop ort cut showed either no difference or delayed EMG activity of the quadriceps and hamstrings activity (with small to large effect sizes) [6, 26]. The delayed EMG activity seen in the ACLR leg during an unanticipated cut was considered to reflect sensory deficit in the operated knee [26], whilst the increased medial hamstring activity coupled with decreased lateral hamstring activity was viewed as a potential injury mechanism for the contra-lateral leg [10]. These limited studies exhibit inherent limitations that do not allow application as a monitoring tool during rehabilitation. CoD in chaotic sports environments is influenced by a multitude of factors and cannot be simply simulated as a series of hops [70].
Collectively we examined neuromuscular activity patterns regarding lower extremity muscles in individuals with primary ACLR during common athletic tasks such as running, landing and CoD/cutting. We observed reduced quadriceps coupled with increased hamstrings activity (i.e. a “hamstrings-dominant” strategy) for the operated leg during running and landing irrespective of graft [46, 49, 89] which may offer a plausible explanation for contra-lateral ACL injuries. In addition, BPTB ACLR individuals who experienced a secondary ipsilateral ACL injury had lower hamstrings activity during a landing task compared to BPTB ACLR patients who did not [86]; thus further supporting the importance of high hamstrings activity following ACLR given that reduced quadriceps activity of the operated leg has been established for running [90, 91] and jumping/landing [21, 49, 62, 80, 86]. Increased hamstrings activity may also indicate kinesiophobia especially if coupled with reduced functional performance [69]; on the contrary high performing sub-groups of patients may demonstrate higher GMAX activity [78, 79, 81]. A possibility for lower (medial) hamstrings activity may appear in ACLR with a hamstrings graft [34]. The reported neuromuscular alterations were observed despite that ACLR participants had completed all clinical criteria (deficit on isokinetic and functional field testing, pain-free, no swelling on swipe test and full ROM allowing resumption of high speed running) and had even RTS which may underscore the need for prolonged movement re-training [13, 15,16,17], interventions to modulate neuromuscular activity as soon as possible with minimal burden on joint/graft loading [40, 102] as well as targeting other strength-related qualities such as rate of force development [18].
Neuromuscular activity during athletic maneuvers in healthy subjects showed some potential in identifying individuals at increased risk for suffering ACL injury [100, 110]. Following RTS after primary ACLR there is the risk for ipsilateral re-injury or contra-lateral ACL injury [51, 55, 57, 67, 88, 93, 98]. Therefore examining EMG activity patterns during athletic tasks following primary ACLR appears to be an important factor to consider in establishing a potential connection between time since surgery, RTS and risk for re-injury. The risk for contra-lateral ACL injury following primary BPTB ACLR is higher compared to the risk of ipsilateral re-injury [51, 67, 88, 112], whilst primary HS ACLR is associated with higher rate of graft failure compared to contra-lateral ACL injury [51, 55, 57, 67, 88, 93, 98]. In addition at 15 years of follow up contra-lateral ACL tears are significantly more likely than graft failures [67], but graft failure is higher in hamstrings ACLR compared to BPTB ACLR [112]. Our results indicate that decreased quadriceps activity following ACLR is rather graft independent and has been reported for either BPTB [39, 86, 89,90,91,92] or HS [41, 46, 80] grafts. Whilst the down-regulation of quadriceps activity may be straightforward in the case of BPTB ACLR [19], the reported hamstrings neuromuscular facilitation that occurs even in the case low/medium hamstrings strength deficit, although “protective” in nature (by theoretically reducing shear forces at the knee joint), may be responsible for this down-regulation of quadriceps activation through reciprocal inhibition [85] regardless of graft selection. Thus optimization of quadriceps muscle function is always a high priority [19]; therefore we consider pivotal the role of hamstrings in the RTS following ACLR [14, 99]. Given that graft failure may occur when the hamstrings are actively lengthening to resist anterior tibial translation [9] and that hamstrings muscle tears mainly occur when the hamstrings act eccentrically to brake the knee extension at the end of the swing phase during running ([23], eccentric strengthening of the hamstrings following ACLR seems highly relevant; in fact persistent flexor strength deficits may not be revealed by “gold standard” isokinetic concentric testing, but with more functional eccentric strength testing [45]. In addition given that hamstrings act as both knee flexor and hip extensor, balancing “knee-dominant” and “hip-dominant” exercises may result in optimal functioning of the hamstrings especially during high-intensity running when their hip moment arm is double their knee moment arm [43]. Contra-lateral secondary ACL injuries following ACLR are more common compared to graft re-injuries [51, 67, 88, 112], thus the contra-lateral “healthy” leg should also be considered as training target. Instead of considering each leg separately, a more holistic approach regarding overall movement quality has been proposed [15, 16].
There are some limitations in this systematic review that need to be considered. (1) We included studies published only in English, (2) most of the studies had limited sample size and thus were underpowered to adjust for gender, or graft type, which may influence the reported outcomes. Thus, future investigations should assess the role of different graft types on muscle activation pattern during running, jumping and cutting/CoD tasks, in male and female ACLR participants separately, (3) there was high variation in time since surgery, ranging from ~ 4–6 months to 180 months years, and the rehabilitation protocols were not specified in most of the studies, (4) finally, the included studies investigated different running tasks such over-ground and treadmill running as well as landing tasks, such as single- and double- drop jump, vertical jump, or hop landing. Because of the heterogeneity in the methodologies and the absence of a gold standard execution protocol as mentioned above, caution is warranted regarding the interpretation of the results, (5) some studies used pooled quadriceps and hamstring muscle activity although the lateral and medial components of these muscle groups have differential actions.
Conclusion
Patients with ACLR displayed an altered muscle activity pattern during running, jumping and cutting/CoD tasks, even though they were considered to be capable for sport return. Although there was great heterogeneity in the subject selection and study methods, the ACLR leg displayed decreased quadriceps or increased hamstrings EMG activity or both despite RTS. Simultaneous decreased quadriceps and increased hamstrings EMG activity was shown for both running and jumping/landing irrespective of graft.
The clinical relevance is that this combination, i.e. “hamstrings dominant” strategy, can serve as a protective mechanism against graft re-injury by reducing anterior shear forces at the knee. More studies are needed to establish whether there is indeed a link between the “hamstrings dominant” strategy and reduced re-injury risk.
References
Agel J, Rockwood T, Klossner D (2016) Collegiate ACL injury rates across 15 sports: national collegiate athletic association injury surveillance system data update (2004–2005 through 2012–2013). Clin J Sport Med 26:518–523
Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D et al (2014) Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis. Am J Sports Med 42(9):2242–2252
Alanazi A, Mitchell K, Roddey T, Alenazi A, Alzhrani M, Ortiz A (2020) Landing evaluation in soccer players with or without anterior cruciate ligament reconstruction. Int J Sports Med 41(13):962–971
Alanazi AD, Mitchell K, Roddey T, Alenazi AM, Alzhrani MM, Almansour AM et al (2021) The effects of a high-intensity exercise bout on landing biomechanics post anterior cruciate ligament reconstruction: a quasi-experimental study. BMC Sports Sci Med Rehabil 13(1):36
Ardern CL, Kvist J, Webster KE (2016) Psychological aspects of anterior cruciate ligament injuries. Oper Tech Sports Med 24(1):77–83
Arumugam A, Hager CK (2022) Thigh muscle co-contraction patterns in individuals with anterior cruciate ligament reconstruction, athletes and controls during a novel double-hop test. Sci Rep 12(1):8431
Behnke AL, Parola LR, Karamchedu NP, Badger GJ, Fleming BC, Beveridge JE (2021) Neuromuscular function in anterior cruciate ligament reconstructed patients at long-term follow-up. Clin Biomech (Bristol, Avon) 81:105231
Berchuck M, Andriacchi TP, Bach BR, Reider B (1990) Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg 72:871–877
Boden BP, Dean GS, Feagin JA, Garrett WE (2000) Mechanisms of anterior cruciate ligament injury. Orthopedics 23:573–578
Briem K, Ragnarsdóttir AM, Árnason SI, Sveinsson T (2016) Altered medial versus lateral hamstring muscle activity during hop testing in female athletes 1–6 years after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 24(1):12–17
Brophy RH, Schmitz L, Wright RW (2012) Return to play and future ACL injury risk following ACL reconstruction in soccer athletes from the moon group. Am J Sports Med 40:2517–2522
Bryant AL, Newton RU, Steele J (2009) Successful feed-forward strategies following ACL injury and reconstruction. J Electromyogr Kinesiol 19(5):988–997
Buckthorpe M (2019) Optimising the late-stage rehabilitation and return-to-sport training and testing process after ACL reconstruction. Sports Med 49(7):1043–1058
Buckthorpe M, Danelon F, La Rosa G, Nanni G, Stride M, Della Villa F (2021) Recommendations for hamstring function recovery after ACL reconstruction. Sports Med 51(4):607–624
Buckthorpe M, Della Villa F, Della Villa S, Roi GS (2019) On-field rehabilitation part 1: 4 pillars of high-quality on-field rehabilitation are restoring movement quality, physical conditioning, restoring sport-specific skills, and progressively developing chronic training load. J Orthop Sports Phys Ther 49(8):565–569
Buckthorpe M, Della Villa F, Della Villa S, Roi GS (2019) On-field rehabilitation part 2: a 5-stage program for the soccer player focused on linear movements, multidirectional movements, soccer-specific skills, soccer-specific movements, and modified practice. J Orthop Sports Phys Ther 49(8):570–575
Buckthorpe M, Della VF (2020) Optimising the “mid-stage” training and testing process after ACL reconstruction. Sports Med 50(4):657–678
Buckthorpe M, Roi GS (2018) The time has come to incorporate a greater focus on rate of force development training in the sports injury rehabilitation process. Muscles Ligaments Tendons J 7(3):435–441
Buckthorpe M, La Rosa G, Villa FD (2019) Restoring knee extensor strength after anterior cruciate ligament reconstruction: a clinical commentary. Int J Sports Phys Ther 14(1):159–172
Buller LT, Best MB, Baraga MG, Kaplan LD (2015) Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med 3(1):1–8
Burland JP, Lepley AS, Frechette L, Lepley LK (2020) Protracted alterations in muscle activation strategies and knee mechanics in patients after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 28(12):3766–3772
Camic CL, Kovacs AJ, Enquist EA, McLain TA, Hill EC (2015) Muscle activation of the quadriceps and hamstrings during incremental running. Muscle Nerve 52(6):1023–1029
Chumanov ES, Schache AG, Heiderscheit BC, Thelen DG (2012) Hamstrings are most susceptible to injury during the late swing phase of sprinting. Br J Sports Med 46(2):90
Claes S, Hermie L, Verdonk R, Bellemans J, Verdonk P (2013) Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sports Traumatol Arthrosc 21(9):1967–1976
Claes S, Verdonk P, Forsyth R, Bellemans J (2011) The ‘“ligamentization”’ process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med 39(11):2476–2483
Coats-Thomas MS, Miranda DL, Badger GJ, Fleming BC (2013) Effects of ACL reconstruction surgery on muscle activity of the lower limb during a jump-cut maneuver in males and females. J Orthop Res 31(12):1890–1896
DashtiRostami K, Alizadeh M, Minoonejad H, Thomas A, Yazdi H (2020) Relationship between electromyographic activity of knee joint muscles with vertical and posterior ground reaction forces in anterior cruciate ligament reconstructed patients during a single leg vertical drop landing task. Res Sports Med 28(1):1–14
DashtiRostami K, Naderi A, Thomas A (2019) Hip abductor and adductor muscles activity patterns during landing after anterior cruciate ligament injury. J Sport Rehabil 28(8):871–876
Della Villa F, Buckthorpe M, Grassi A, Nabiuzzi A, Tosarelli F, Zaffagnini S et al (2020) Systematic video analysis of ACL injuries in professional male football (soccer): injury mechanisms, situational patterns and biomechanics study on 134 consecutive cases. Br J Sports Med 54(23):1423–1432
Diermeier T, Rothrauff BB, Engebretsen L, Lynch AD, Ayeni OR, Paterno MV et al (2020) Treatment after anterior cruciate ligament injury: panther symposium ACL treatment consensus group. Knee Surg Sports Traumatol Arthrosc 28(8):2390–2402
Dingenen B, Gokeler A (2017) Optimization of the return-to-sport paradigm after anterior cruciate ligament reconstruction: a critical step back to move forward. Sports Med 47(8):1487–1500
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and nonrandomized studies of health care interventions. J Epidemiol Community Health 52(6):377–384
Dyhre-Poulsen P, Simonsen EB, Voigt M (1991) Dynamic control of muscle stiffness and H reflex modulation during hopping and jumping in man. J Physiol 437(1):287–304
Einarsson E, Thomson A, Sas B, Hansen C, Gislason M, Whiteley R (2021) Lower medial hamstring activity after ACL reconstruction during running: a cross-sectional study. BMJ Open Sport Exerc Med 7(1):e000875
de Fontenay BP, Argaud S, Blache Y, Monteil K (2014) Motion alterations after anterior cruciate ligament reconstruction: comparison of the injured and uninjured lower limbs during a single-legged jump. J Athl Train 49(3):311–316
Gauffin H, Tropp H (1992) Altered movement and muscular-activation patterns during the one-legged jump in patients with an old anterior cruciate ligament rupture. Am J Sports Med 20(2):182–192
Gifstad T, Foss OA, Engebretsen L, Lind M, Forssblad m, Albrektsen G, et al (2014) Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med 42(10):2319–2328
Goerger BM, Marshall SW, Beutler AI, Blackburn JT, Wilckens JH, Padua DA (2015) Anterior cruciate ligament injury alters preinjury lower extremity biomechanics in the injured and uninjured leg: the JUMP-ACL study. Br J Sports Med 49(3):188–195
Gokeler A, Hof AL, Arnold MP, Dijkstra PU, Postema K, Otten E (2010) Abnormal landing strategies after ACL reconstruction. Scand J Med Sci Sports 20(1):e12–e19
Hansen C, Einarson E, Thomson A, Whiteley R, Witvrouw E (2017) Hamstring and calf muscle activation as a function of bodyweight support during treadmill running in ACL reconstructed athletes. Gait Posture 58:154–158
He X, Qiu J, Cao M, Ho YC, Leong HT, Fu SC et al (2022) Effects of deficits in the neuromuscular and mechanical properties of the quadriceps and hamstrings on single-leg hop performance and dynamic knee stability in patients after anterior cruciate ligament reconstruction. Orthop J Sports Med 10(1):1–9
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374
Higashihara A, Nagano Y, Ono T, Fukubayashi T (2018) Differences in hamstring activation characteristics between the acceleration and maximum-speed phases of sprinting. J Sports Sci 36:1313–1328
Higgins JPT, Green S (eds) (2008). Wiley, Chichester
Hogberg J, Bergentoft E, Piussi R, Wernbom M, Beischer S, Simonson R et al (2022) Persistent knee flexor strength deficits identified through the NordBord eccentric test not seen with “gold standard” isokinetic concentric testing during the first year after anterior cruciate ligament reconstruction with a hamstring tendon autograft. Phys Ther Sport 55:119–124
Jafarnezhadgero AA, Pourrahimghoroghchi A, Darvishani MA, Aali S, Dionisio VC (2021) Analysis of ground reaction forces and muscle activity in individuals with anterior cruciate ligament reconstruction during different running strike patterns. Gait Posture 90:204–209
Janssen RPA, Scheffler SU (2014) Intra-Articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22(9):2102–2108
Jones AM, Pringle JSM, Carter H (2005) Influence of muscle fiber type and motor unit recruitment on VO2 kinetics. In: Jones AM, Poole DC (eds) Oxygen uptake kinetics in sport, exercise and medicine. Routledge, London
Jordan MJ, Aagaard P, Herzog W (2017) Asymmetry and thigh muscle coactivity in fatigued anterior cruciate ligament-reconstructed elite skiers. Med Sci Sports Exerc 49(1):11–20
Kaeding CC, Leger-St-Jean B, Magnussen RA (2017) Epidemiology and diagnosis of anterior cruciate ligament injuries. Clin Sports Med 36(1):1–8
Kaeding CC, Pedroza AD, Reinke EK, Huston LJ, Spindler KP (2015) Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med 43(7):1583–1590
Kamath GV, Murphy T, Creighton RA, Viradia N, Taft TN, Spang JT (2014) Anterior cruciate ligament injury, return to play, and reinjury in the elite collegiate athlete: analysis of an NCAA division I cohort. Am J Sports Med 42(7):1638–1643
Kellis E, Liassou C (2009) The effect of selective muscle fatigue on sagittal lower limb kinematics and muscle activity during level running. J Orthop Sports Phys Ther 39:210–220
Kellis E, Zafeiridis A, Amiridis IG (2011) Muscle coactivation before and after the impact phase of running following isokinetic fatigue. J Athl Train 46:11–19
King E, Richter C, Jackson M, Franklyn-Miller A, Falvey E, Myer GD et al (2020) Factors Influencing return to play and second anterior cruciate ligament injury rates in level 1 athletes after primary anterior cruciate ligament reconstruction: 2-year follow-up on 1432 reconstructions at a single center. Am J Sports Med 48(4):812–824
Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E (2016) Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med 50(15):946–951
Laboute E, James-Belin E, Puig PL, Trouve P, Verhaeghe E (2018) Graft failure is more frequent after hamstring than patellar tendon autograft. Knee Surg Sports Traumatol Arthrosc 26(12):3537–3546
Lai CCH, Ardern CL, Feller JA, Webster KE (2018) Eighty-three per cent of elite athletes return to pre-injury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med 52(2):128–138
Landry SC, McKean KA, Hubley-Kozey CL, Stanish WD, Deluzio KJ (2007) Neuromuscular and lower limb biomechanical differences exist between male and female elite adolescent soccer players during an unanticipated side-cut maneuver. Am J Sports Med 35(11):1888–1900
Lee SP, Chow JW, Tillman MD (2014) Persons with reconstructed ACL exhibit altered knee mechanics during high speed maneuvers. J Sports Med 35(6):528–533
Lessi GC, Serrão FV (2017) Effects of fatigue on lower limb, pelvis and trunk kinematics and lower limb muscle activity during single-leg landing after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 25(8):2550–2558
Lessi GC, Silva RS, Serrao FV (2018) Comparison of the effects of fatigue on kinematics and muscle activation between men and women after anterior cruciate ligament reconstruction. Phys Ther Sport 31:29–34
Li RT, Lorenz S, Xu Y, Harner CD, Fu FH, Irrgang JJ (2011) Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. Am J Sports Med 39(12):2595–2603
Lie MM, Risberg MA, Storheim K, Engebretsen L, Øiestad BE (2019) What’s the rate of knee osteoarthritis 10 years after anterior cruciate ligament injury? An updated systematic review. Br J Sports Med 53(18):1162–1167
Luc B, Gribble PA, Pietrosimone BG (2014) Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed- to- treat analysis. J Athl Train 49:806–819
Macdonald JH, Farina D, Marcora SM (2008) Response of electromyographic variables during incremental and fatiguing cycling. Med Sci Sports Exerc 40(2):335–344
Magnussen RA, Meschbach NT, Kaeding CC, Wright RW, Spindler KP (2015) ACL graft and contralateral ACL Tear risk within ten years following reconstruction: a systematic review. JBJS Rev 3(1):e3
Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE (2001) A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech 16(5):438–445
Markstrom JL, Grinberg A, Hager CK (2022) Fear of reinjury following anterior cruciate ligament reconstruction is manifested in muscle activation patterns of single-leg side-hop landings. Phys The 102(2):1–10
Marques JB, Paul DJ, Graham-Smith P, Read PJ (2020) Change of direction assessment following anterior cruciate ligament reconstruction: a review of current practice and considerations to enhance practical application. Sports Med 50(1):55–72
Mayer SW, Queen RM, Taylor D, Moorman CT, Toth AP, Garrett WE et al (2015) Functional testing differences in anterior cruciate ligament reconstruction patients released versus not released to return to sport. Am J Sports Med 43:1648–1655
Melinska A, Czamara A, Szuba L, Bedzinski R (2015) Biomechanical characteristics of the jump down of healthy subjects and patients with knee injuries. Acta Bioeng Biomech 17(2):111–120
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y (2013) Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg 95(14):1271–1277
Mohtadi NG, Chan DS, Dainty KN, Whelan DB (2011) Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev 9:CD005960
Montalvo AM, Schneider DK, Webster KE, Yut L, Galloway MT, Heidt RS et al (2019) Anterior cruciate ligament injury risk in sport: a systematic review and meta-analysis of injury incidence by sex and sport classification. J Athl Train 54(5):472–482
Moses B, Orchard J, Orchard J (2012) Systematic review: annual incidence of ACL injury and surgery in various populations. Res Sports Med 20:157–179
Nyland J, Klein S, Caborn DN (2010) Lower extremity compensatory neuromuscular and biomechanical adaptations 2 to 11 years after anterior cruciate ligament reconstruction. Arthroscopy 26(9):1212–1225
Nyland J, Wera J, Klein S, Caborn DN (2014) Lower extremity neuromuscular compensations during instrumented single leg hop testing 2–10 years following ACL reconstruction. Knee 21(6):1191–1197
Ortiz A, Capo-Lugo CE, Venegas-Rios HL (2014) Biomechanical deficiencies in women with semitendinosus-gracilis anterior cruciate ligament reconstruction during drop jumps. PM R 6(12):1097–1106
Ortiz A, Olson S, Libby CL, Trudelle-Jackson E, Kwon YH, Etnyre B et al (2008) Landing mechanics between noninjured women and women with anterior cruciate ligament reconstruction during 2 jump tasks. Am J Sports Med 36(1):149–157
Ortiz A, Olson S, Trudelle-Jackson E, Rosario M, Venegas HL (2011) Landing mechanics during side hopping and crossover hopping maneuvers in noninjured women and women with anterior cruciate ligament reconstruction. PM R 3(1):13–20
Padua DA, Arnold BL, Perrin DH, Gansneder BM, Carcia CR, Granata KP (2006) Fatigue, vertical leg stiffness, and stiffness control strategies in males and females. J Athl Train 41:294–304
Pairot-de-Fontenay B, Willy RW, Elias ARC, Mizner RL, Dube MO, Roy JS (2019) Running biomechanics in individuals with anterior cruciate ligament reconstruction: a systematic review. Sports Med 49(9):1411–1424
Palmieri-Smith RM, McLean SG, Ashton-Miller JA, Wojtys EM (2009) Association of quadriceps and hamstrings cocontraction patterns with knee joint loading. J Athl Train 44:256–263
Palmieri-Smith RM, Strickland M, Lepley LK (2019) Hamstring muscle activity after primary anterior cruciate ligament reconstruction-a protective mechanism in those who do not sustain a secondary injury? A Preliminary Study Sports Health 11(4):316–323
Paterno MV, Kiefer AW, Bonnette S, Riley MA, Schmitt LC, Ford KR et al (2015) Prospectively identified deficits in sagittal plane hip-ankle coordination in female athletes who sustain a second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Clin Biomech 30(10):1094–1104
Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE (2012) Incidence of contra-lateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med 22(2):116–121
Patras K, Zampeli F, Ristanis S, Tsepis E, Ziogas G, Stergiou N et al (2012) Hamstring-dominant strategy of the bone-patellar tendon-bone graft anterior cruciate ligament-reconstructed leg versus quadriceps-dominant strategy of the contralateral intact leg during high-intensity exercise in male athletes. Arthroscopy 28(9):1262–1270
Patras K, Ziogas G, Ristanis S, Tsepis E, Stergiou N, Georgoulis AD (2009) High intensity running results in an impaired neuromuscular response in ACL reconstructed individuals. Knee Surg Sports Traumatol Arthrosc 17(8):977–984
Patras K, Ziogas G, Ristanis S, Tsepis E, Stergiou N, Georgoulis AD (2010) ACL reconstructed patients with a BPTB graft present an impaired vastus lateralis neuromuscular response during high intensity running. J Sci Med Sport 13(6):573–577
Patras K, Ziogas G, Ristanis S, Tsepis E, Tsiaras V, Stergiou N et al (2011) Endurance markers are related with local neuromuscular response for the intact but not for the ACL reconstructed leg during high intensity running. J Sports Med Phys Fitness 51(4):708–714
Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW (2020) Effect of graft choice on revision and contralateral anterior cruciate ligament reconstruction: results from the New Zealand ACL registry. Am J Sports Med 48(1):63–69
Ramsey CA, Lamb P, Kaur M, Baxter GD, Ribeiro DC (2019) How are running shoes assessed? A systematic review of characteristics and measurement tools used to describe running footwear. J Sports Sci 37(14):1617–1629
Renstrom P, Ljungqvist A, Arendt E, Beynnon B, Fukubayashi T, Garrett WE et al (2008) Non-contact ACL injuries in female athletes: an international Olympic committee current concepts statement. Br J Sports Med 42:394–412
Rocchi JE, Labanca L, Laudani L, Minganti C, Mariani PP, Macaluso A (2020) Timing of muscle activation is altered during single-leg landing tasks after anterior cruciate ligament reconstruction at the time of return to sport. Clin J Sport Med 30(6):e186–e193
Roos KG, Wasserman EB, Dalton SL, Gray A, Djoko A, Dompier TP et al (2017) Epidemiology of 3825 injuries sustained in six seasons of National Collegiate Athletic Association men’s and women’s soccer (2009/2010–2014/2015). Br J Sports Med 51(13):1029–1034
Samuelsen BT, Webster KE, Johnson NR, Hewett TE, Krych A (2017) Hamstring autograft versus patellar tendon autograft for ACL reconstruction: is there a difference in graft failure rate? A meta-analysis of 47,613 patients. Clin Orthop Relat Res 475(10):2459–2468
Sherman DA, Glaviano NR, Norte GE (2021) Hamstrings neuromuscular function after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Sports Med 51(8):1751–1769
Smeets A, Malfait B, Dingenen B, Robinson MA, Vanrenterghem J, Peers K et al (2019) Is knee neuromuscular activity related to anterior cruciate ligament injury risk? A pilot study Knee 26(1):40–51
Smeets A, Vanrenterghem J, Staes F, Vandenneucker H, Claes S, Verschueren S (2020) Are anterior cruciate ligament-reconstructed athletes more vulnerable to fatigue than uninjured athletes? Med Sci Sports Exerc 52(2):345–353
So BC, Kwok MY, Chan YL, Lam HK, Chang HH, Chan TK et al (2022) Lower-limb muscle activity during aquatic treadmill running in individuals with anterior cruciate ligament reconstruction. J Sport Rehabil 31(7):894–903
Sterns KM, Pollard CD (2013) Abnormal frontal plane knee mechanics during sidestep cutting in female soccer athletes after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med 41(4):918–923
Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD (2013) Epidemiology of knee injuries among US high school athletes, 2005/06-2010/11. Med Sci Sports Exerc 45(3):462–469
de Visser HM, Reijman M, Heijboer MP, Bos PK (2012) Risk factors of recurrent hamstring injuries: a systematic review. Br J Sports Med 46:124–130
Walden M, Hagglund M, Magnusson H, Ekstrand J (2016) ACL injuries in men’s professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med 50(12):744–750
Wang LJ, Zeng N, Yan ZP et al (2020) Post-traumatic osteoarthritis following ACL injury. Arthritis Res Ther 22:57
Wiggins AJ, Grandhi RK, Schneider DK, Stanfiled D, Webster KE, Myer GD (2016) Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med 44(7):1861–1876
Zebis MK, Andersen CH, Bencke J, Orntoft C, Linnebjerg C, Holmich P et al (2017) Neuromuscular coordination deficit persists 12 months after ACL reconstruction but can be modulated by 6 weeks of kettlebell training: a case study in women’s elite soccer. Case Rep Orthop 2017:4269575
Zebis MK, Andersen LL, Bencke J, Kjaer M, Aagaard P (2009) Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am J Sports Med 37(10):1967–1973
Zebis MK, Bencke J, Andersen LL, Dossing S, Alkjaer T, Magnusson SP et al (2008) The effects of neuromuscular training on knee joint motor control during side-cutting in female elite soccer and handball players. Clin J Sport Med 18(4):329–337
Zhao L, Lu M, Deng M, Xing J, He L, Wang C (2020) Outcome of bone-patellar tendon-bone vs hamstring tendon autograft for anterior cruciate ligament reconstruction: A meta-analysis of randomized controlled trials with a 5-year minimum follow-up. Medicine (Baltimore) 99(48):e23476
Zuniga JM, Malek MH (2013) Electromyographic responses of the superficial quadriceps femoris muscles during incremental treadmill running. Muscle Nerve 48:938–944
Acknowledgements
The author(s) declare that they have no acknowledgements
Funding
The present study received no funding.
Author information
Authors and Affiliations
Contributions
JDG assisted in designing the search strategy, conducted the search, screened the papers, extracted the data and wrote the manuscript. DM assisted in designing the search strategy, reviewed the findings of the search, verified scientific merit, and contributed to the discussion. KP assisted in designing the search strategy, conducted the search, screened the papers, extracted the data, performed the statistical analysis and assisted in writing the manuscript. PDM, IT, ODS assisted in designing the search strategy, reviewed the findings of the analysis, verified scientific merit, and contributed to the discussion. PJP assisted in designing the search strategy, reviewed the findings of the analysis, verified scientific merit, and contributed to the discussion. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received ethical approval by the local Institutional Review Board.
The study did not involved participation of subject and informed consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Risk of bias assessment for electromyographic running studies. Table S2. Risk of bias assessment for electromyographic jumping/landing studies. Table S3. Risk of bias assessment for electromyographic cutting/CoD studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Georgoulis, J.D., Melissaridou, D., Patras, K. et al. Neuromuscular activity of the lower-extremities during running, landing and changing-of-direction movements in individuals with anterior cruciate ligament reconstruction: a review of electromyographic studies. J EXP ORTOP 10, 43 (2023). https://doi.org/10.1186/s40634-023-00603-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-023-00603-1