Abstract
Purpose
Anterior cruciate ligament rupture is associated with characteristic bone contusions in approximately 80% of patients, and these have been correlated with higher pain scores. Bone bruising may indicate joint damage that increases inflammation and the likelihood of posttraumatic osteoarthritis. We sought to characterize the severity of bone bruising following acute anterior cruciate ligament injury and determine if it correlates with synovial fluid and serum levels of the proinflammatory chemokine monocyte chemoattractant protein-1 associated with posttraumatic osteoarthritis.
Methods
This was a retrospective analysis of data collected prospectively from January 2014 through December 2016. All patients who sustained an acute ligament rupture were evaluated within 15 days of injury, obtained a magnetic resonance imaging study, and underwent bone-patellar-tendon-bone autograft reconstruction were offered enrollment. The overall severity of bone bruising on magnetic resonance imaging was graded (sum of 0–3 grades in 13 sectors of the articular surfaces). Serum and synovial fluid levels of monocyte chemoattractant protein-1 were measured within 14 days of injury, and serum levels were again measured 6 and 12 months following surgery. Separate univariate linear regression models were constructed to determine the association between monocyte chemoattractant protein-1 and bone bruising severity at each time point.
Results
Forty-eight subjects were included in this study. They had a mean age of 21.4 years and were 48% female. Median overall bone bruising severity was 5 (range 0–14). Severity of bone bruising correlated with higher synovial fluid concentrations of monocyte chemoattractant protein-1 preoperatively (R2 = 0.18, p = 0.009) and with serum concentrations at 12 months post-reconstruction (R2 = 0.12, p = 0.04).
Conclusions
The severity of bone bruising following anterior cruciate ligament rupture is associated with higher levels of the proinflammatory cytokine monocyte chemoattractant protein-1 in synovial fluid acutely post-injury and in serum 12-months following anterior cruciate ligament reconstruction. This suggests that severe bone bruising on magnetic resonance imaging after ligament rupture may indicate increased risk for persistent joint inflammation and posttraumatic osteoarthritis.
Level of evidence
III ― retrospective cohort study.
Similar content being viewed by others
Background
Characteristic bone contusions occur in approximately 80% of patients who sustain an anterior cruciate ligament (ACL) injury [2, 11, 23, 27]. Bone bruising typically occurs when ligamentous insufficiency allows the posterolateral tibial plateau to abut the lateral femoral condyle as the mechanism of injury occurs [25]. The presence of bone bruising is associated with greater patient reported pain during the first four weeks post-injury as well as prolonged antalgic gait [15, 22]. However, bone bruising has not been shown to correlate with instability or functional outcomes [1, 3, 8, 12, 19]. Even in the absence of identifiable articular cartilage injury, subchondral bone bruising may indicate damage to the joint that increases acute inflammation and may hasten progression to posttraumatic osteoarthritis (PTOA) [7, 21]. PTOA prevention and treatment remain limited by an inability to identify patients at highest risk. Establishing an association between severe bone bruising, which is readily identified on magnetic resonance imaging (MRI), and biomarkers known to be associated with PTOA would allow for early identification of patients at increased risk for PTOA. This would facilitate further research and may eventually allow patient-specific delivery of treatments to minimize risk of PTOA following ACL rupture.
Separately, several synovial fluid biomarkers indicative of cartilage injury have been associated with development of arthritis, including the proinflammatory cytokine monocyte chemoattractant protein 1 (MCP-1), also known as chemokine C–C motif ligand 2 (CCL2) [6, 10, 13]. Elevated levels of MCP-1 have been correlated with radiographic and symptomatic PTOA after injury [4, 5, 9, 17, 20, 26, 28]. Bone bruising following ACL rupture has not yet been linked to increased levels of proinflammatory cytokines.
The purpose of our study was to determine if the severity of bone bruising on MRI following acute ACL injury correlates with biomarkers associated with PTOA. We hypothesized that the severity of bruising as an indicator of damage to the joint would correlate with increased concentrations of the proinflammatory chemokine MCP-1 (CCL2) associated with posttraumatic osteoarthritis (Fig. 1).
Methods
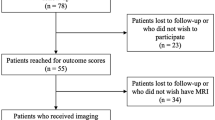
This was a retrospective analysis of prospectively collected data conducted as part of a study investigating clinical outcomes of ACL reconstruction (ACLR) at an academic medical center in the southeastern United States. It was approved by the local institutional review board. Informed consent was obtained from all subjects. Subjects were enrolled from January 2014 through December 2016. Enrollment was offered to all English-speaking patients aged 16 to 35 years with no prior diagnosis of inflammatory arthritis who sustained an acute ACL rupture, were evaluated within fifteen days of injury, obtained an MRI, and underwent bone-patellar-tendon-bone autograft ACLR.
Analysis of bone bruising
MRI studies including T2 sequence images were obtained at the time of initial ACL injury diagnosis. MRIs were reviewed, and the severity of bone bruising was graded on T2 sequences in 13 sectors of the femoral and tibial articular surfaces using the scheme developed for the Whole-Organ Magnetic Resonance Imaging Score (WORMS) [24]. In this system the femoral and tibial articular surface are divided into six sectors each (the anterior, central, and posterior regions of the medial and lateral joint surfaces), and the region beneath the tibial spines is graded as a separate sector. In each sector the severity of bone bruising was graded 0–3, with 0 corresponding to no contusion, 1 to increased signal intensity in < 25% of the sector, 2 to 25–50%, and 3 to > 50% of the sector [24].
Each MRI was graded 4 times, twice by 2 resident physicians (orthopaedics and radiology) at least 6 weeks apart. Grades from all 4 reads were collated, and a final grade for each sector was assigned by a fellowship-trained musculoskeletal radiologist. The prevalence and severity of bone bruising in each sector was recorded. Reliability of this grading system was evaluated using kappa for intra- and inter-rater agreement [16]. The overall severity of bone bruising on MRI (sum of grades 0–3 in 13 sectors, theoretical range 0–39) was selected as the primary radiographic outcome, as logically it may reflect the relative energy of the mechanism of injury.
Biomarker analysis
Serum and synovial fluid were collected via phlebotomy and sterile knee joint aspiration within 14 days of ACL injury, and serum was collected at 6 and 12 months following ACLR. Fluids were centrifuged and stored at -80 °C prior to batch analysis. Commercial enzyme-linked immunosorbent assays (ELISA) were used to evaluate concentrations of MCP-1 (ng/mL; R&D Systems, Minneapolis, MN, USA). The assay detection sensitivity for MCP-1 (mean minimal detectable dose) was reported as 1.7 pg/ml. All assays were performed in duplicate and exhibited < 10% inter- and intra-assay variability. These methods have been described in greater detail previously [18].
Statistical analysis
Separate univariate linear regression models were constructed to determine the variance in MCP-1 levels associated with overall severity of bone bruising on MRI at each time point. P-values ≤ 0.05 were considered statistically significant. Data were analysed using SPSS version 16.0.0.2 [14].
Results
Sixty-four patients were recruited for enrollment, of whom 14 were excluded due to history of prior ACLR. Two patients declined to have serum or synovial fluid collected for biomarker analysis and were also excluded. All 48 subjects who had an MRI obtained acutely post-injury and biomarker data at all time points were included. Subjects had a mean age of 21.4 years (SD 3.5) and were 48% female. Mean height was 175 cm (SD 11), and mean weight was 76 kg (SD 15).
Bone bruising
On all 4 reads by both resident graders, there was bruising > 50% of the time in the lateral femoral central (LFc), lateral tibial posterior (LTp), and medial tibial posterior (MTp) regions, and there was bruising < 50% of the time in all other sectors (Table 1). Intra-rater agreement was best in LFc, LTp, and MTp sectors where bruising was common, with agreement ranging from 87–92% and kappa ranging from 0.54 (moderate) to 0.81 (near perfect). Intra-rater agreement in all other sectors where bruising was less common ranged from 90–100%, though Kappa (quickly affected by numerous zero values) was more variable, ranging from 0.27 (fair) to 1.0 (perfect). Combined inter-rater agreement in LFc, LTp, and MTp sectors was 80–90% with kappa ranging from 0.38 (fair) to 0.53 (moderate). Overall bone bruising severity (sum of grades in all sectors) ranged from 0 to 14 with median 5 and IQR 3.
Correlation of bone bruising and biomarker data
The median concentration of MCP-1 in knee synovial fluid within 14 days of injury was 1261 ng/mL (IQR 1295), with higher concentrations observed among the top quartile of patients by severity of bone bruising on MRI and lower concentrations among the bottom quartile of patients by bone bruising severity on MRI. The trend towards higher MCP-1 concentrations among patients with more severe bone bruising was observed in serum concentrations also at all time points (Table 2). All MCP-1 concentrations exceeded the lower limit of detectability. Separate univariate linear regressions demonstrated that greater overall bone bruising severity correlated with higher synovial fluid concentrations of MCP-1 acutely post-injury (R2 = 0.18, p = 0.009) and with serum concentrations of MCP-1 at 12 months post-ACLR (R2 = 0.12, p = 0.04). There were no statistically significant associations between bone bruising severity and serum concentrations of MCP-1 acutely post-injury (R2 = 0.001; P = 0.799) or at 6 months post-ACLR (R2 = 0.07; P = 0.10).
Discussion
The findings of this study support our hypothesis that greater bone bruising severity post-ACL rupture is associated with elevated levels of the proinflammatory chemokine MCP-1. Notably, bone bruising was associated with higher MCP-1 levels both in synovial fluid preoperatively and in serum at 12-months post-injury. Bone bruising associated with ACL rupture has been studied extensively, but to date it has been correlated only with pain scores and not with instability or functional outcomes [1, 3, 8, 12, 15, 19, 22]. The current translational study provides a link between radiographic and biomarker data suggesting that greater bone bruising severity may be an indicator of increased risk of PTOA after ACL rupture.
The pattern and prevalence of bone bruising we observed is consistent with previous studies, with bruising occurring > 50% of the time in the lateral femoral condyle and posterior tibial plateau [7, 22]. We found that the severity of bone bruising on MRI can be reliably graded by orthopaedic surgeons and radiologists alike with good agreement between them. This suggests that clinicians could realistically note and quantify severe bone bruising in patients with acute ACL rupture and be able to better prognosticate their individual risks for persistent joint inflammation and PTOA.
This study has a number of limitations. It is non-randomized, but it assesses the natural history of ACL rupture rather than the effects of an intervention, making a prospective cohort study the ideal study design. Additionally, we offered enrollment consecutively to all eligible patients of all orthopaedic sports surgeons at our institution, minimizing selection bias. Retrospective studies are often susceptible to recall bias, but all data in the current study including biomarkers and MRI images were collected prospectively, mitigating this as a potential source of bias. Graded outcomes such as bone bruising severity are subject to experimenter’s bias (the tendency of values to drift towards the value expected by the rater), but the rating system we used with two raters making two passes each and a third rater to adjudicate discrepancies minimizes this as well. Finally, the distribution of bone bruising grades with many zero values is inherently non-normal, but the linear regressions used are tolerant of non-normal distributions, eliminating this as a source of error.
To our knowledge, this is the first study to demonstrate and association between higher synovial fluid concentrations of MCP-1 and bone bruising severity acutely post-ACL rupture. This suggests that MRI may be able to indirectly detect injuries likely to cause higher concentrations of pro-inflammatory cytokines, which could theoretically lead to early identification of those patients at greatest risk of developing post-traumatic osteoarthritis. Additionally, the severity of bone bruising at the time of ACL injury may be linked to higher serum concentrations of pro-inflammatory cytokines that persist 12 months post-ACLR. The lack of statistically significant correlations between bone bruising severity and serum concentrations of MCP-1 acutely and 6 months post-injury is of unclear significance, though the consistent trends observed in Table 2 at all time points suggest that a study with greater power might detect similar correlations at these time points as well. These data suggest that the severity of bone bruising at the time of ACL injury is linked to the inflammatory process acutely post-injury and 12 months post-ACLR that may be associated with the development of PTOA.
Conclusions
The severity of bone bruising following ACL rupture is associated with higher levels of the proinflammatory chemokine MCP-1 (CCL2) in synovial fluid preoperatively and in serum 12-months post-ACLR. This suggests that severe bone bruising on MRI acutely post-ACL rupture may indicate greater joint inflammation, which may increase the risk for future posttraumatic osteoarthritis.
Availability of data and materials
The data used in this study can be provided upon reasonable request. They are not otherwise publicly available.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ACLR:
-
Anterior cruciate ligament reconstruction
- CCL2:
-
Chemokine C–C motif ligand 2 (also known as MCP-1)
- ELISA:
-
Enzyme-linked immunosorbent assay
- LFc:
-
Lateral femoral central
- LTp:
-
Lateral tibial posterior
- MCP-1:
-
Monocyte chemoattractant protein 1 (also known as CCL2)
- MRI:
-
Magnetic resonance imaging
- MTp:
-
Medial tibial posterior
- PTOA:
-
Posttraumatic osteoarthritis
- WORMS:
-
Whole-Organ Magnetic Resonance Imaging Score
References
Bastos R, Andrade R, Vasta S, Pereira R, Papalia R, van der Merwe W et al (2019) Tibiofemoral bone bruise volume is not associated with meniscal injury and knee laxity in patients with anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc 27(10):3318–3326
Bordoni V, di Laura FG, Previtali D, Tamborini S, Candrian C, Cristallo Lacalamita M et al (2019) Bone Bruise and Anterior Cruciate Ligament Tears: Presence, Distribution Pattern, and Associated Lesions in the Pediatric Population. Am J Sports Med 47(13):3181–3186
Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L (2001) Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy 17(5):445–449
Cuéllar VG, Cuéllar JM, Golish SR, Yeomans DC, Scuderi GJ (2010) Cytokine profiling in acute anterior cruciate ligament injury. Arthroscopy 26(10):1296–1301
Cuéllar VG, Cuéllar JM, Kirsch T, Strauss EJ (2016) Correlation of Synovial Fluid Biomarkers With Cartilage Pathology and Associated Outcomes in Knee Arthroscopy. Arthroscopy 32(3):475–485
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6):313–326
Filardo G, Andriolo L, di Laura FG, Napoli F, Zaffagnini S, Candrian C (2019) Bone bruise in anterior cruciate ligament rupture entails a more severe joint damage affecting joint degenerative progression. Knee Surg Sports Traumatol Arthrosc 27(1):44–59
Frobell RB, Le Graverand MP, Buck R, Roos EM, Roos HP, Tamez-Pena J et al (2009) The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage 17(2):161–167
Furman BD, Kimmerling KA, Zura RD, Reilly RM, Zlowodzki MP, Huebner JL et al (2015) Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheumatol 67(5):1234–1239
Gong JH, Ratkay LG, Waterfield JD, Clark-Lewis I (1997) An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med 186(1):131–137
Graf BK, Cook DA, De Smet AA, Keene JS (1993) “Bone bruises” on magnetic resonance imaging evaluation of anterior cruciate ligament injuries. Am J Sports Med 21(2):220–223
Hanypsiak BT, Spindler KP, Rothrock CR, Calabrese GJ, Richmond B, Herrenbruck TM et al (2008) Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med 36(4):671–677
Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lipsky PE (2001) Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res 3(2):118–126
IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 16.0.0.2. Armonk, NY: IBM Corp.
Johnson DL, Bealle DP, Brand JC Jr, Nyland J, Caborn DN (2000) The effect of a geographic lateral bone bruise on knee inflammation after acute anterior cruciate ligament rupture. Am J Sports Med 28(2):152–155
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Li L, Jiang BE (2015) Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem 52(Pt 2):276–282
Lisee C, Spang JT, Loeser R, Longobardi L, Lalush D, Nissman D, Schwartz T, Hu D, Pietrosimone B (2021) Tibiofemoral articular cartilage composition differs based on serum biochemical profiles following anterior cruciate ligament reconstruction. Osteoarthritis Cartilage 29(12):1732–1740
Marot V, Corin B, Reina N, Murgier J, Berard E, Cavaignac E (2021) Femoral and tibial bone bruise volume is not correlated with ALL injury or rotational instability in patients with ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc. 29(3):900–6
Monibi F, Roller BL, Stoker A, Garner B, Bal S, Cook JL (2016) Identification of Synovial Fluid Biomarkers for Knee Osteoarthritis and Correlation with Radiographic Assessment. J Knee Surg 29(3):242–247
Nakamae A, Engebretsen L, Bahr R, Krosshaug T, Ochi M (2006) Natural history of bone bruises after acute knee injury: clinical outcome and histopathological findings. Knee Surg Sports Traumatol Arthrosc 14(12):1252–1258
Papalia R, Torre G, Vasta S, Zampogna B, Pedersen DR, Denaro V et al (2015) Bone bruises in anterior cruciate ligament injured knee and long-term outcomes. A review of the evidence. Open Access J Sports Med 6:37–48
Patel SA, Hageman J, Quatman CE, Wordeman SC, Hewett TE (2014) Prevalence and location of bone bruises associated with anterior cruciate ligament injury and implications for mechanism of injury: a systematic review. Sports Med 44(2):281–293
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D et al (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12(3):177–190
Sanders TG, Medynski MA, Feller JF, Lawhorn KW (2000) Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury. Radiographics 20:S135-51
Scanzello CR (2017) Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J Orthop Res 35(4):735–739
Vellet AD, Marks PH, Fowler PJ, Munro TG (1991) Occult posttraumatic osteochondral lesions of the knee: prevalence, classification, and short-term sequelae evaluated with MR imaging. Radiology 178(1):271–276
Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J et al (2016) Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical Outcomes. Arthritis Rheumatol 68(9):2129–2140
Acknowledgements
None.
Informed consent
Informed consent was obtained from all participants of this study.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
LK designed the study and data collection instruments, contributed to data acquisition and analysis, and drafted the manuscript. DO contributed to data acquisition. LP contributed to literature review. GK, RC, and JS enrolled subjects in the parent study. DN helped design data collection instruments and contributed to data acquistion. BP and JS conceived the study and contributed to study design, analysis, and drafting the manuscript. All authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The local institutional review board approved this study.
Consent for publication
All authors consent to submission of this manuscript and to its publication if accepted.
Competing interest
All authors have no potential conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keil, L.G., Onuscheck, D.S., Pratson, L.F. et al. Bone bruising severity after anterior cruciate ligament rupture predicts elevation of chemokine MCP-1 associated with osteoarthritis. J EXP ORTOP 9, 37 (2022). https://doi.org/10.1186/s40634-022-00478-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-022-00478-8