Abstract
Background
Our previous study in 2011 concluded that permissive underfeeding may improve outcomes in patients receiving parenteral nutrition therapy. This conclusion was tentative, given the small sample size. We conducted the present systematic review and trial sequential meta-analysis to update the status of permissive underfeeding in patients who were admitted to the intensive care unit (ICU).
Methods
Seven databases were searched: PubMed, Embase, Web of Science, China National Knowledge Infrastructure, Wanfang, Chinese Biomedical Literature Database, and Cochrane Library. Randomized controlled trials (RCTs) were included. The Revised Cochrane risk-of-bias tool (ROB 2) was used to assess the risk of bias in the enrolled trials. RevMan software was used for data synthesis. Trial sequential analyses (TSA) of overall and ICU mortalities were performed.
Results
Twenty-three RCTs involving 11,444 critically ill patients were included. There were no significant differences in overall mortality, hospital mortality, length of hospital stays, and incidence of overall infection. Compared with the control group, permissive underfeeding significantly reduced ICU mortality (risk ratio [RR] = 0.90; 95% confidence interval [CI], [0.81, 0.99]; P = 0.02; I2 = 0%), and the incidence of gastrointestinal adverse events decreased (RR = 0.79; 95% CI, [0.69, 0.90]; P = 0.0003; I2 = 56%). Furthermore, mechanical ventilation duration was reduced (mean difference (MD) = − 1.85 days; 95% CI, [− 3.44, − 0.27]; P = 0.02; I2 = 0%).
Conclusions
Permissive underfeeding may reduce ICU mortality in critically ill patients and help to shorten mechanical ventilation duration, but the overall mortality is not improved. Owing to the sample size and patient heterogeneity, the conclusions still need to be verified by well-designed, large-scale RCTs.
Trial Registration The protocol for our meta-analysis and systematic review was registered and recorded in PROSPERO (registration no. CRD42023451308). Registered 14 August 2023
Similar content being viewed by others
Background
Nutritional therapy plays a pivotal role in critical care [1, 2]. Malnutrition in critically ill patients is related to prolonged stay in the intensive care unit (ICU), increased complications, and even associated with elevated risk of death [2, 3]. Appropriate nutritional intake helps critically ill patients maintain immune functions. It may also improve the hypercatabolic status and reduce occurrence of malnutrition, resulting in better clinical outcomes [1, 4].
According to the latest guidelines for nutrition in the ICU by the European Society for Clinical Nutrition and Metabolism (ESPEN), hypocaloric nutrition (not exceeding 70% of energy expenditure) in the early phase of acute illness limits the occurrence of overfeeding and other adverse outcomes [5]. Overfeeding is associated with complications such as hepatic steatosis and increased respiratory efforts, which have adverse effects on clinical outcomes [1, 6]. A recent study of 1,206 patients in 26 ICUs found that early high-energy feeding was detrimental in critically ill patients [7]. After decades of exploration, permissive underfeeding is a solution that balances the benefits of nutritional support with the adverse effects of overfeeding during the early stage of ICU admission [7,8,9,10,11]. In 2011, our team conducted a systematic review of randomized controlled trials (RCTs) to evaluate the clinical efficacy of hypocaloric nutrition in patients who received parenteral nutrition. Although we concluded that hypocaloric parenteral nutrition may shorten the length of hospital stay (LOS) and reduce the incidence of infection, the study was limited by its relatively small number of patients, with only 359 participants [12]. Subsequently, multiple well-designed studies that provided more data on permissive underfeeding in critically ill patients were published [9, 13]. Therefore, it is necessary to conduct a new systematic review and meta-analysis to update this evidence. In addition, trial-sequential meta-analysis techniques have emerged that can help researchers evaluate the power of evidence. Therefore, we conducted a systematic review and a trial sequential meta-analysis to evaluate the efficacy of permissive underfeeding in critically ill patients.
Methods
Protocol and registration
We designed and conducted this systematic review and meta-analysis following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. The protocol for our meta-analysis and systematic review was registered and recorded in PROSPERO (registration no. CRD42023451308, registered 14 August 2023).
Inclusion criteria
The inclusion criteria were established following the PICOS method, as outlined below:
-
P (Participants): Adult patients (age ≥ 18 years) admitted to the ICU with APACHE II scores ≥ 10 points
-
I (Intervention): Permissive underfeeding (actual calorie intake < 70% of target calorie or < 20 kcal/kg. d)
-
C (Comparison): Administration of isocaloric feeding (actual calorie intake ≥ 70% of target calorie or ≥ 20 kcal/kg. d)
-
O (Outcomes): Primary outcomes: overall mortality; secondary outcomes: duration of mechanical ventilation (days), ICU mortality, in-hospital mortality, length of hospital stay (days), incidence of overall infection, incidence of gastrointestinal adverse events
-
S (Study design): Randomized controlled trials
Exclusion criteria
The exclusion criteria encompassed the following: (a) post hoc analysis of a randomized controlled trial, (b) crossover randomized trial, (c) studies that did not address any primary or secondary outcomes, (d) pregnant or lactating women, (e) patients receiving previous nutritional support during the same hospitalization period, (f) studies involving transplantation programs, and (g) those specifically focused on cancer patients.
Literature sources and retrieval strategy
To achieve a thorough search of studies, two reviewers (YHY and WP) independently searched seven databases: PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang, Chinese Biomedical Literature Database (SinoMed), and Cochrane Library. RCTs published before October 31, 2023, that met the inclusion criteria were included for further analysis. The retrieval process is summarized in Additional file 1: Table S1.
Literature screening and data extraction
To avoid errors and missing data, two reviewers (YHY and WP) independently conducted literature screening and data extraction following the PRISMA guidelines. Controversies were initially managed using guidelines and discussed by the research team. If an initial resolution was not achieved, a third senior reviewer (JH) was consulted. The data extracted included study design, baseline patient information, statistics on ICU mortality, overall mortality, duration of mechanical ventilation (days), in-hospital mortality, length of hospital stay (days), incidence of overall infection, incidence of gastrointestinal adverse events.
Assessment of risk of bias
To guarantee the reliability of this study, two reviewers (YHY and JZ) independently assessed the risk of bias in the enrolled studies. The Revised Cochrane risk-of-bias tool (ROB 2) was used to assess the risk of bias in the RCTs. RCTs cover five dimensions of bias that can affect quality: bias arising from the randomization process, deviations from intended interventions, missing outcome data, outcome measurements, and selection of the reported result. Controversies were initially managed through discussion within the research team. If an initial resolution was not achieved, a third senior reviewer (JH) was consulted.
Statistical analyses
The Cochrane Collaboration’s Review Manager (RevMan) version 5.4 was used to pool the effects of interventions. Dichotomous variables were pooled and presented as risk ratios (RR) and 95% confidence intervals (CI) using the Mantel–Haenszel method. Continuous variables were pooled and presented as mean difference (MD) and 95% CI using the inverse variance method. The statistically significant level α was set at 0.05. Statistical differences were considered significant at P < 0.05. Statistical heterogeneity existed if I2 ≠ 0, and heterogeneity of pooled results was considered high if I2 > 50%. Random-effects models were used when heterogeneity was observed (I2 > 0) [14]; however, in cases where I2 was equal to 0, a fixed-effects model was used instead. Subgroup analysis was performed if the types of patients and interventions included in the studies were not identical. Publication bias was assessed only when the number of enrolled studies exceeded 10 because a limited number of studies undermined the power of the tests. Finally, sensitivity analysis was conducted to evaluate the reliability and authenticity of the results.
Trial sequential analysis
An updated meta-analysis with new RCTs may lead to false-positive results because sparse data increase the risk of random error. Trial sequential analyses (TSA) reduced the risk of random errors arising from inadequate sample sizes or repetitive tests and helped in estimating the required information size (RIS) for meta-analysis. We performed TSA for outcomes using TSA version 0.9.5.10 Beta software. Type 1 error and power were set to 5% and 80%, respectively.
Certainty and importance of evidence
The online tool GRADEpro, developed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, was used to evaluate the certainty and importance of the evidence [15]. The following items were individually rated: study design, risk of bias, imprecision, indirectness, and inconsistency. In accordance with the guidelines, the certainty for evidence was rated as “High, Moderate, Low, or Very low” by use of GRADEpro. And the importance of outcome was scored and categorized into one of three levels: “not important,” “important,” and “critical.”
Results
Literature retrieval results and characteristics
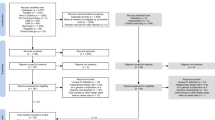
Initially 2708 records were retrieved. After removing duplicates, screening titles and abstracts, 52 studies were retained for full-text screening. Twenty-three RCTs involving 11,444 critically ill patients were included [9, 11, 13, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. The sample sizes of the included RCTs ranged from 16 to 3957. The mean Body Mass Index (BMI) of 14 RCTs was higher than the normal range, 25 kg/m2. The basic characteristics of the included RCTs are presented in Additional file 2: Table S2. A flowchart of the literature search and screening process is shown in Fig. 1.
Assessment of risk of bias
According to the criteria of ROB 2, three RCTs were considered high quality. ROB 2 indicated high risk for nine RCTs because they did not report the measurement of the outcome. Plots of the risk of bias among the 23 RCTs are demonstrated in Fig. 2 and Additional file 3. Fig S1.
Primary outcomes
Overall mortality
Nineteen RCTs, including 11,181 patients, reported overall mortality [9, 11, 13, 17, 19,20,21,22, 24,25,26,27,28,29,30,31,32,33, 35]. The pooled data indicated that there was no statistically significant difference in overall mortality between the two groups (RR = 0.96; 95% CI, [0.91, 1.01]; P = 0.13) without heterogeneity (I2 = 0%). The certainty of the evidence was deemed moderate. A forest plot of overall mortality is shown in Fig. 3.
Secondary outcomes
ICU mortality
Five RCTs including 4,361 patients reported ICU mortality [9, 19, 25, 28, 32]. Pooled data indicated that permissive underfeeding in critically ill patients was associated with lower ICU mortality than in the control group (RR = 0.90; 95% CI, [0.81, 0.99]; P = 0.02) without heterogeneity (I2 = 0%). The certainty of the evidence was deemed moderate. A forest plot of ICU mortality is shown in Additional file 3. Fig S2.
Duration of mechanical ventilation (days)
Three RCTs, including 411 patients, reported the duration of mechanical ventilation [19, 23, 32]. Pooled data indicated that permissive underfeeding in critically ill patients was significantly associated with shorter mechanical ventilation durations (MD = − 1.85 days; 95% CI, [− 3.44, − 0.27]; P = 0.02) without heterogeneity (I2 = 0%). The certainty of the evidence was deemed moderate. A forest plot of the mechanical ventilation duration is shown in Additional file 3: Fig S3.
In-hospital mortality
Nine RCTs, including 9563 patients, reported in-hospital mortality [9, 13, 19, 20, 24, 25, 28, 31, 32]. The pooled data indicated no statistically significant difference in hospital mortality between the two groups (RR = 0.95; 95% CI, [0.89, 1.02]; P = 0.18; I2 = 1%). The certainty of the evidence was deemed moderate. A forest plot of the in-hospital mortality rates is shown in Additional file 3: Fig S4.
Length of hospital stay (days)
Six RCTs, including 637 patients, reported the length of hospital stay [11, 17, 19, 26, 31, 32]. The pooled data indicated no statistically significant difference in the length of hospital stay between the two groups (MD = 1.11; 95% CI [− 2.16, 4.38]; P = 0.51; I2 = 77%). The certainty of the evidence was deemed low. A forest plot of length of hospital stay is shown in Additional file 3: Fig S5.
Incidence of overall infection
Fourteen RCTs, including 9782 patients, reported the incidence of overall infection [9, 11, 13, 17, 18, 20, 21, 25,26,27,28, 30, 33, 34]. The pooled data indicated no statistically significant difference in the incidence of overall infection between the two groups (RR = 0.92; 95% CI, [0.79, 1.06]; P = 0.25; I2 = 49%). The certainty of the evidence was deemed moderate. A forest plot of the incidence of overall infection is shown in Additional file 3: Fig S6.
Incidence of gastrointestinal adverse events
Nine RCTs, including 8423 patients, reported the incidence of gastrointestinal adverse events [9, 13, 21, 25, 28, 29, 33,34,35]. Pooled data indicated that permissive underfeeding in critically ill patients was significantly associated with a lower incidence of adverse gastrointestinal events in both groups (RR = 0.79; 95% CI, [0.69, 0.90]; P = 0.0003; I2 = 56%). The certainty of the evidence was deemed high. A forest plot of the incidence of gastrointestinal adverse events is shown in Additional file 3: Fig S7.
Subgroup analysis
Based on the various intervention periods, we performed subgroup analyses of overall mortality, in-hospital mortality, and incidence of overall infection. After excluding studies that did not report intervention periods, pooled data showed that no significant differences in overall mortality (RR = 1.01; 95% CI, [0.88, 1.16]; P = 0.89; I2 = 0%), in-hospital mortality (RR = 1.06; 95% CI, [0.82, 1.37]; P = 0.66; I2 = 8%), and incidence of overall infection (RR = 0.83; 95% CI, [0.48, 1.43]; P = 0.50; I2 = 75%) between the two groups in RCTs with an intervention period of < 7 days. Likewise, pooled data showed no significant differences in overall mortality (RR = 0.96; 95% CI, [0.90, 1.02]; P = 0.16; I2 = 0%), in-hospital mortality (RR = 0.96; 95% CI, [0.89, 1.03]; P = 0.22; I2 = 0%), and incidence of overall infection (RR = 0.93; 95% CI, [0.81, 1.07]; P = 0.30; I2 = 41%) between the two groups in RCTs with an intervention period of ≥ 7 days. Details of the subgroup analysis are shown in Additional file 3: Fig S8-10.
Publication bias
Funnel plots used to evaluate publication bias were symmetrical, indicating that no publication bias was observed. Funnel plots of the outcomes reported in more than 10 studies are shown in Additional file 3: Fig S11-12.
Sensitivity analysis
Sensitivity analysis was conducted to assess the stability of outcomes. Regarding overall mortality, the exclusion of individual studies did not have an impact on the results when compared with the pooled result. Regarding ICU mortality, the exclusion of the study by Reignier et al. [9] changed the results compared with the pooled results. Regarding in-hospital mortality, the exclusion of individual studies did not have an impact on the results when compared with pooled results. The details are shown in Additional file 3: Fig S13-15.
Trial sequential analysis
The TSA of overall mortality showed that the cumulative Z-curve crossed the no-boundary line. The RIS was estimated to be 16,789 as determined through TSA, and the cumulative Z-curve did not reach the RIS. The details are shown in Fig. 4. The TSA of ICU mortality showed that the cumulative Z-curve crossed the traditional boundary and did not cross the other boundary lines. The RIS was estimated to be 6955 as determined through TSA, and the cumulative Z-curve failed to exceed the RIS, which meant that the association between permissive underfeeding and lower ICU mortality was a false positive and more studies are needed to verify this association. The details are shown in Fig. 5.
GRADE summary of evidence table for key outcomes
The certainty of incidence of gastrointestinal adverse events was deemed high. The certainty of evidence for ICU mortality and in-hospital mortality were rated as moderate because studies did not report whether outcome measurements were blinded. The certainty of evidence for overall mortality was rated as moderate because different studies provided inconsistent results caused by different sample sizes. The overall certainty of the evidence of this systematic review and meta-analysis was deemed moderate. The details are summarized in Table 1.
Discussion
The benefits of nutritional treatment in critically ill patients have been repeatedly demonstrated in various studies. However, the question of how to provide appropriate and personalized nutrition still presents challenges for clinical practitioners [36, 37]. The recent French-Speaking ICU Nutritional Survey (FRANS) study and revised ESPEN guidelines lean towards supporting a low-calorie approach [5, 7].
ICU mortality was lower in the underfed patients. However, overall mortality and in-hospital mortality were not significantly different between the two groups. The interpretation for the difference is that ICU mortality is a more representative outcome in the acute phase and overall/in-hospital mortality represents the long-term outcomes. In addition to the effects of nutritional interventions, there are a number of complicated factors that influence long-term outcomes. Besides, the sample size of our study was not sufficient (approximately 70% of the RIS) and may lead to a tentative result. It may change in the future by large scale trials. Our findings are not entirely consistent with those of previous studies. A meta-analysis conducted by Zhou et al. concluded that there were no benefits in terms of reducing short-term mortality or the duration of mechanical ventilation [38]. A meta-analysis conducted by Pertzov et al. concluded that isocaloric nutrition was associated with a lower 28-day mortality, and no significant difference was observed in ICU mortality between the two groups [39]. However, these two reports included fewer studies and enrolled fewer patients (1052–6986), which may cause bias in their results.
Our systematic review and meta-analysis revealed that permissive underfeeding was associated with lower ICU mortality and mechanical ventilation duration in critically ill patients. Considering the impact of the intervention period on the outcomes, we performed subgroup analyses depending on whether the intervention period was < 7 days. The results showed that regardless of whether the permissive underfeeding intervention period was < 7 days, there were no significant differences in overall mortality, in-hospital mortality, and incidence of overall infection. In our meta-analysis, early high-energy intake was associated with poor tolerance. This is consistent with the newest ESPEN guidelines [5]. Berger et al. indicated that critically ill patients who are intolerant to early full nutrition have endogenous productions of 100–300 g of glucose per day to maintain a continuous blood glucose supply to vital organs. Production is unrepressed for at least 9 days if inflammation persists. Excessive energy intake can lead to overfeeding. Intolerance to overfeeding, which results in higher mortality, is now well-demonstrated and should therefore be avoided [40]. Because of the production of endogenous glucose, the rationality of early (first 48–72 h) hypocaloric feeding in critically ill patients is sound. However, the ESPEN guidelines recommend that full nutrition (70–100%) should be prescribed progressively within 3–7 days. This recommendation was based on a meta-analysis in 2016 that compared the impact of different enteral-parenteral routes in critically ill patients [5, 41]. When taking endogenous glucose into account, we conjectured that critically ill patients may have better clinical outcomes by continuing hypocaloric feeding for the first 48–72 h rather than being given full nutrition (70–100% within 3–7 days). In our meta-analysis, continuing hypocaloric feeding during and after the first 48–72 h indeed resulted in better clinical outcomes than those in the control group. Given the limited data reported, we were unable to perform further subgroup analyses using a 3-day cutoff, and it remains unknown whether the better clinical outcomes stemmed from hypocaloric feeding during the first three days or from continued hypocaloric feeding after three days, which should be the focus of future studies. Overall hypocaloric feeding was more effective.
Limitations
This systematic review and meta-analysis included more RCTs and sample sizes; however, yet it is still not enough to draw conclusions from the entire population of critically ill patients because most RCTs were conducted in developed countries, with only few reports were from developing countries. In high-income countries, especially the United States, patients had higher basic BMI values and the findings are therefore not generalizable to Asian and African countries. The result may not be extrapolated to malnourished patients as few patients included had a low baseline BMI. Additionally, some RCTs did not report whether they were blinded to the outcome assessment, which could increase the potential risk of bias. Outcomes such as length of hospital stay, and length of mechanical ventilation were reported by most studies while few studies reported “free days,” which limited us to conduct analysis of these more comprehensive parameters. In the subgroup analyses, we were unable to analyze all outcomes after excluding studies that did not report intervention periods because the remaining data were limited. For the same reason, we failed to conduct subgroup analyses based on the three or ten-day intervention periods. Finally, we included any route of nutritional support that is more relevant to the real world, which may also serve as a source of potential heterogeneity.
Conclusions
Permissive underfeeding may reduce ICU mortality in critically ill patients and help to shorten mechanical ventilation duration, but the overall mortality is not improved. Owing to the sample size and patient heterogeneity, the conclusions should be further verified by well-designed, large-scale RCTs.
Availability of data and materials
All data and materials related to this systematic review and meta-analysis are available from the corresponding author.
Abbreviations
- LOS:
-
Length of hospital stay
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis
- ICU:
-
Intensive care unit
- RCTs:
-
Randomized controlled trials
- CNKI:
-
China national knowledge infrastructure
- SinoMed:
-
Chinese biomedical literature database
- ROB2:
-
Revised Cochrane risk-of-bias tool
- RevMan:
-
Review manager
- RR:
-
Risk ratios
- CI:
-
Confidence intervals
- MD:
-
Mean difference
- ESPEN:
-
European Society for Clinical Nutrition and Metabolism
- GRADE:
-
Grading of recommendations assessment, development, and evaluation
- RIS:
-
Required information size
- FRANS:
-
French-speaking ICU nutritional survey
- BMI:
-
Body mass index
References
Matejovic M, Huet O, Dams K, Elke G, Vaquerizo Alonso C, Csomos A, et al. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. 2022;26:143. https://doi.org/10.1186/s13054-022-03997-z.
Lv C, Jiang X, Long Y, Liu Z, Lin J, Wu C, et al. Association between caloric adequacy and short-term clinical outcomes in critically ill patients using a weight-based equation: secondary analysis of a cluster-randomized controlled trial. Front Nutr. 2022;9: 902986. https://doi.org/10.3389/fnut.2022.902986.
Yeh DD, Fuentes E, Quraishi SA, Cropano C, Kaafarani H, Lee J, et al. Adequate nutrition may get you home: effect of caloric/protein deficits on the discharge destination of critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2016;40:37–44. https://doi.org/10.1177/0148607115585142.
Lee JH, Kim M, Choi D, Kwon J, Park YK. Isocaloric nutritional support reduces ventilator duration time in major trauma patients. Nutr Diet. 2023;80:435–44. https://doi.org/10.1111/1747-0080.12818.
Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. 2023;42:1671–89. https://doi.org/10.1016/j.clnu.2023.07.011.
Floro GC, Schwenger KJP, Ghorbani Y, Zidar DJ, Allard JP. In critically ill adult patients with COVID-19, lower energy and higher protein intake are associated with fewer mechanical ventilation and antibiotic days but not with ICU length of stay. Nut in Clin Prac. 2023. https://doi.org/10.1002/ncp.11019.
Pardo E, Lescot T, Preiser JC, Massanet P, Pons A, Jaber S, et al. Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care. 2023;27:7. https://doi.org/10.1186/s13054-022-04298-1.
Singer P, Bendavid I, BenArie I, Stadlander L, Kagan I. Feasibility of achieving different protein targets using a hypocaloric high-protein enteral formula in critically ill patients. Crit Care. 2021;25:204. https://doi.org/10.1186/s13054-021-03625-2.
Reignier J, Plantefeve G, Mira JP, Argaud L, Asfar P, Aissaoui N, et al. Low versus standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet Respir Med. 2023;11:602–12. https://doi.org/10.1016/S2213-2600(23)00092-9.
Hartl WH, Bender A, Scheipl F, Kuppinger D, Day AG, Küchenhoff H. Calorie intake and short-term survival of critically ill patients. Clin Nutr. 2019;38:660–7. https://doi.org/10.1016/j.clnu.2018.04.005.
Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. Am J Clin Nutr. 2014;100:1337–43. https://doi.org/10.3945/ajcn.114.088609.
Jiang H, Sun MW, Hefright B, Chen W, Lu CD, Zeng J. Efficacy of hypocaloric parenteral nutrition for surgical patients: a systematic review and meta-analysis. Clin Nutr. 2011;30:730–7. https://doi.org/10.1016/j.clnu.2011.05.006.
TARGET Investigators, for the ANZICS Clinical Trials Group, Chapman M, Peake SL, Bellomo R, Davies A, Deane A, et al. Energy-dense versus routine enteral nutrition in the critically ill. NEJM. 2018;379:1823–34. https://doi.org/10.1056/NEJMoa1811687.
Li SJ, Jiang H, Yang H, Chen W, Peng J, Sun MW, et al. The dilemma of heterogeneity tests in meta-analysis: a challenge from a simulation study. PLoS ONE. 2015;10: e0127538. https://doi.org/10.1371/journal.pone.0127538.
Online Tool to Evaluate the Certainty and Importance of the Evidence-GRADE Pro [cited Aug 1, 2023]. Available from: https://www.gradepro.org/.
Frankenfield DC, Smith JS, Cooney RN. Accelerated nitrogen loss after traumatic injury is not attenuated by achievement of energy balance. JPEN J Parenter Enteral Nutr. 1997;21:324–9. https://doi.org/10.1177/0148607197021006324.
McCowen KC, Friel C, Sternberg J, Chan S, Forse RA, Burke PA, et al. Hypocaloric Total Parenteral Nutrition: effectiveness in prevention of hyperglycemia and infectious complications–a randomized clinical trial. Crit Care Med. 2000;28:3606–11. https://doi.org/10.1097/00003246-200011000-00007.
Ahrens CL, Barletta JF, Kanji S, Tyburski JG, Wilson RF, Janisse JJ, et al. Effect of low-calorie parenteral nutrition on the incidence and severity of hyperglycemia in surgical patients: a randomized, controlled trial. Crit Care Med. 2005;33:2507–12. https://doi.org/10.1097/01.ccm.0000186746.64572.8a.
Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr. 2011;93:569–77. https://doi.org/10.3945/ajcn.110.005074.
Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med. 2011;39:967–74. https://doi.org/10.1097/CCM.0b013e31820a905a.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. https://doi.org/10.1001/jama.2012.137.
Berg A, Rooyackers O, Bellander BM, Wernerman J. Whole body protein kinetics during hypocaloric and normocaloric feeding in critically ill patients. Crit Care. 2013;17:R158. https://doi.org/10.1186/cc12837.
Rugeles SJ, Rueda JD, Díaz CE, Rosselli D. Hyperproteic hypocaloric enteral nutrition in the critically ill patient: a randomized controlled clinical trial. Indian J Crit Care Med. 2013;17:343–9. https://doi.org/10.4103/0972-5229.123438.
Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013. https://doi.org/10.1136/bmj.f1532.
Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs normocaloric nutrition in critically ill patients: a prospective randomized pilot trial. JPEN J Parenter Enteral Nutr. 2016;40:242–9. https://doi.org/10.1177/0148607114528980.
Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, et al. Intensive nutrition in acute lung injury: a clinical trial (INTACT). JPEN J Parenter Enteral Nutr. 2015;39:13–20. https://doi.org/10.1177/0148607114528541.
Owais AE, Kabir SI, Mcnaught C, Gatt M, MacFie J. A single-blind randomised clinical trial of permissive underfeeding in patients requiring parenteral nutrition. Clin Nutr. 2014;33:997–1001. https://doi.org/10.1016/j.clnu.2014.01.005.
Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398–408. https://doi.org/10.1056/NEJMoa1502826.
Rugeles S, Villarraga-Angulo LG, Ariza-Gutiérrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D. High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. J Crit Care. 2016;35:110–4. https://doi.org/10.1016/j.jcrc.2016.05.004.
Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43:1637–47. https://doi.org/10.1007/s00134-017-4880-3.
Rice TW, Files DC, Morris PE, Bernard AC, Ziegler TR, Drover JW, et al. Dietary management of blood glucose in medical critically ill overweight and obese patients: an open-label randomized trial. JPEN J Parenter Enteral Nutr. 2019;43:471–80. https://doi.org/10.1002/jpen.1447.
Aljada A, AlGwaiz GF, AlAyadhi D, Masuadi E, Zahra M, Al-Matar SH, et al. Effect of permissive underfeeding with intensive insulin therapy on MCP-1, sICAM-1, and TF in critically ill patients. Nutrients. 2019;11:987. https://doi.org/10.3390/nu11050987.
Mousavian SZ, Pasdar Y, Ranjbar G, Jandari S, Akhlaghi S, Almasi A, et al. Randomized controlled trial of comparative hypocaloric vs full-energy enteral feeding during the first week of hospitalization in neurosurgical patients at the Intensive Care Unit. JPEN J Parenter Enteral Nutr. 2020;44:1475–83. https://doi.org/10.1002/jpen.1782.
Xiong W, Qian K. Low-protein, hypocaloric nutrition with glutamine versus full-feeding in the acute phase in ICU patients with severe traumatic brain injury. Neuropsychiatr Dis Treat. 2021;17:703–10. https://doi.org/10.2147/NDT.S296296.
Sun JK, Nie S, Chen YM, Zhou J, Wang X, Zhou SM, et al. Effects of permissive hypocaloric vs standard enteral feeding on gastrointestinal function and outcomes in sepsis. World J Gastroenterol. 2021;27:4900–12. https://doi.org/10.3748/wjg.v27.i29.4900.
Wang Y, Lu CD, Chen W, Wang Q, Jiang H. Digital twin enabled personalized nutrition. Precis Nutr. 2023;2: e00030. https://doi.org/10.1097/PN9.0000000000000030.
Sun M, Wang Y, Yue H-Y, Hou J, Li J, Wu Q, et al. Dynamical monitoring and causal inference for respiratory metabolic indicators of septic patients. Precis Nutr. 2023;2(3):e00042. https://doi.org/10.1097/PN9.0000000000000042.
Zhou X, Fang H, Hu C, Xu J, Wang H, Pan J, et al. Effect of hypocaloric versus standard enteral feeding on clinical outcomes in critically ill adults—a meta-analysis of randomized controlled trials with trial sequential analysis. Med Intensiva. 2021;45:211–25. https://doi.org/10.1016/j.medin.2019.10.003.
Pertzov B, Bar-Yoseph H, Menndel Y, Bendavid I, Kagan I, Glass YD, et al. The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients-a systematic review and meta-analysis. Eur J Clin Nutr. 2022;76:5–15. https://doi.org/10.1038/s41430-021-00919-0.
Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:117. https://doi.org/10.1186/s13054-016-1298-1.
Berger MM, Singer P. Comment: early nutrition prescription in critically ill patients-learnings from the FRANS study. Crit Care. 2023;27:64. https://doi.org/10.1186/s13054-023-04346-4.
Acknowledgements
Not applicable.
Funding
This work was supported by the Sichuan Science and Technology Support Program [Grant ID: 2021YFS0378 to Hua Jiang] and Sichuan Committee of Healthcare [Grant ID: ChuanGanYan ZH2022-202 to Ping Zhou].
Author information
Authors and Affiliations
Contributions
H-YY, WP, and HJ conceptualized and designed the study. H-YY drafted the manuscript. H-YY and WP retrieved relevant databases. H-YY and WP conducted literature screening and data extraction. H-YY and JZ assessed the risk of bias. H-YY, JZ, YZ, and YW prepared the tables and figures. HJ resolved any controversy and revised the manuscript accordingly. HJ proofread the manuscript and improved the English writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing financial interests or personal relationships that may have influenced the work reported in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1:
The retrieval process.
Additional file 2. Table S2:
Basic characteristics of included RCT.
Additional file 3. Fig S1:
Reviewing authors’ judgements for each risk of bias item presented as percentage across all included studies. Fig S2: Forest plot for the comparison of ICU mortality. Fig S3: Forest plot for the comparison of duration of mechanical ventilation. Fig S4: Forest plot for the comparison of in-hospital mortality. Fig S5: Forest plot for the comparison of length of hospital stay. Fig S6: Forest plot for the comparison of incidence of overall infection. Fig S7: Forest plot for the comparison of incidence of gastrointestinal adverse events. Fig S8: Subgroup analysis of overall mortality according to intervention period. Fig S9: Subgroup analysis of in-hospital mortality according to intervention period. Fig S10: Subgroup analysis of incidence of overall infection according to intervention period. Fig S11: Funnel plot of overall mortality. Fig S12: Funnel plot of incidence of overall infection. Fig S13: Sensitivity analysis of overall mortality. Fig S14: Sensitivity analysis of ICU mortality. Fig S15: Sensitivity analysis of in-hospital mortality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yue, Hy., Peng, W., Zeng, J. et al. Efficacy of permissive underfeeding for critically ill patients: an updated systematic review and trial sequential meta-analysis. j intensive care 12, 4 (2024). https://doi.org/10.1186/s40560-024-00717-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-024-00717-3