Abstract
Background
Up to 11% of critically ill patients with sepsis have an unknown source, where the pathogen and site of infection are unclear. The aim of this scoping review is to document currently reported diagnostic criteria of sepsis of unknown origin (SUO) and identify the types and breadth of existing evidence supporting diagnostic processes to identify the infection source in critically ill patients with suspected SUO.
Methods
A literature search of Embase, MEDLINE and PubMed for published studies from 1910 to August 19, 2021 addressing the topic of SUO was performed. Study type, country of origin according to World Bank classification, diagnostic criteria of sepsis of unknown origin, and investigative approaches were extracted from the studies.
Results
From an initial 722 studies, 89 unique publications fulfilled the inclusion and exclusion criteria and were included for full text review. The most common publication type was case report/series 45/89 (51%). Only 10/89 (11%) of studies provided a diagnostic criteria of SUO, but a universally accepted diagnostic criterion was not identified. The included studies discussed 30/89 (34%) history, 23/89 (26%) examination, 57/89 (64%) imaging, microbiology 39/89 (44%), and special tests 32/89 (36%) as part of the diagnostic processes in patients with SUO.
Conclusions
Universally accepted diagnostic criteria for SUO was not found. Prospective studies on investigative processes in critically ill patients managed as SUO across different healthcare settings are needed to understand the epidemiology and inform the diagnostic criteria required to diagnose SUO.
Similar content being viewed by others
Background
Sepsis is life threatening organ dysfunction due to abnormal host response to an infection [1]. Treatment of underlying infection with appropriate early antimicrobials and source control are cornerstones of sepsis management [2]. The use of antimicrobial agents is guided by identification of causative organisms whilst timely source control relies on locating a surgical source of infection. However, culture negative sepsis is found in up to one half of septic patients [3,4,5,6,7,8]. Lack of confirmatory microbiology may be caused by the use of antimicrobials prior to cultures, inadequate sampling or fastidious organisms [9,10,11]. Furthermore, it has been reported that 2–11% of critically ill patients have sepsis of unknown origin (SUO) [4, 12,13,14,15,16,17]. In these patients, not only are microbiological investigations negative, the site of infection is also unclear. Failure to identify the source of sepsis, or identify the offending microorganism is associated with higher risk of severe organ dysfunction and mortality [8, 18]. To complicate matters further, patients may instead have non-infective cause of organ dysfunction rather than sepsis. The key question to determine in patients with suspected SUO is whether an infection is the cause of the manifested systemic inflammation and organ dysfunction.
For these reasons a systematic and comprehensive workup to identify the underlying source of infection or non-infective pathology is often recommended in patients with suspected SUO [19]. However, there is lack of empirical data to guide the optimal diagnostic approach to identify the underlying infection source in these patients. Observational data suggests that this is a common problem as only 74% of patients with suspected septic shock have an infection source identified within 24 h of ICU admission [16]. Additional, albeit un-protocolized workup, will result in a further 7% who will have the source of infection identified after the initial 24 h of admission for shock. Nevertheless, this study showed that further diagnostic workup over time will lead to a definitive diagnosis in a significant portion of patients initially thought to have SUO [16]. Yet, if identification of infective cause of sepsis is dependent on time and extent of investigation, then when should patients be considered to have SUO?
The primary objective of this scoping review is to document currently reported diagnostic criteria for SUO. The secondary objective was to characterize the types and breadth of existing evidence supporting diagnostic processes to identify the cause of infection in critically ill patients with suspected SUO.
Methods
Study design and search strategy
This scoping review was designed using the PRISMA Extension for Scoping Reviews and the research protocol is published online (https://doi.org/10.6084/m9.figshare.20493444.v1) [20]. The key elements of the research question were as follows: the “population” was adult patients with SUO, “concept” was diagnostic criteria and investigative approach and “context” was intensive care unit. We performed a literature search of Embase, MEDLINE and PubMed for published studies since 1910 on August 19, 2021 using the search strategy shown in Additional file 1. Studies published prior to the first consensus definition of sepsis in 1992 was included in this review. Our rationale was that the need of a diagnostic approach to identify a suspected infective cause in patients presenting with severe inflammation or organ dysfunction was recognized much earlier [21]. Furthermore, although the definition of sepsis has changed over the years, the association between infection and life-threatening organ dysfunction and inflammation has remained consistent. Thus, this scoping review aimed to document the diagnostic criteria used to define “suspected infection” in patients with SUO [1]. An iterative search strategy was utilized, and the final search strategy was based on content extracted from included studies. The term “adult” was not used in the search strategy as it resulted in very limited studies for review. We included all studies published in English, Chinese and German.
Eligibility criteria and selection process
Studies were included if they included: (1) diagnostic criteria for SUO or (2) described quantitative or qualitative diagnostic methods to establish an infective cause of sepsis which was initially unknown. Studies must include critically ill patients managed in the ICU. Articles that were limited to describing patients with primary bacteraemia or fungaemia of unknown source were excluded. This exclusion was based on the rationale that these patients had a confirmed infection, despite an unclear physical origin of the pathogen detected in blood cultures. This group of patients is fundamentally different to the group of patients who have suspected SUO, where both the infection site and pathogen are unknown, or who may not have any infection. It is evident that the latter group require a substantially different diagnostic approach, which is the focus of this scoping review. Studies focused on pyrexia of unknown cause in non-critically ill patients were also not included. Although the methods of investigation may overlap to some degree, our rationale was that critically ill ICU patients with SUO represent a different population, with different urgency relating to decision making. Compared to stable patients with pyrexia of unknown origin, diagnosis of the presence of infection in ICU patients with suspected SUO presents a constrained opportunity for lengthy and first-person history, often demonstrates clouded clinical signs and a restricted time-frame for implementing progressively more invasive, often serial, and time-consuming investigations to make a diagnosis and initiate appropriate treatment.
Two independent researchers (LL and OOYM) used the eligibility criteria described and screened the title and abstracts of studies gathered from the search. Full texts of initially selected studies were reviewed to confirm eligibility. Disagreements were resolved by discussion, and if remained unresolved an adjudicator made the final decision.
Data extraction
A data extraction chart was developed by two reviewers (LL and OOYM) to extract information from included studies. This chart was constructed in the following steps. First, a template to extract data on characteristics of the article including author, publication year, country/territory of origin, language, title, and type of study was constructed. Country/territory of origin was defined as the location of the study population or address of the corresponding author. Second, a free text entry box was created to document the following: the presence of diagnostic criteria for SUO, clinical findings, extent of investigations, and the timing and duration of diagnostic method required prior to diagnosis of SUO. Diagnostic criteria for SUO was recorded when there was direct reference to a stated set of criteria for individual classification, or study inclusion criteria for patients. Third, all studies were reviewed for diagnostic thematic processes used to identify the infection source or an alternative non-infective pathology. We observed that the main processes described included history, examination, imaging, microbiological sampling and special tests. These themes were then added to the data extraction template and the corresponding data extracted. Critical appraisal of potential bias and heterogeneity was not performed.
Statistics
Descriptive statistics using percentages were used to summarize extracted information. Studies were grouped by World Bank income classification to show the distribution of results by different resource settings. Inter-rater reliability between reviewers on inclusion and exclusion of studies was assessed using Kappa statistic [22].
Results
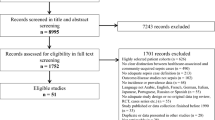
We found a total of 722 potentially relevant studies, with 603 from PubMed, 90 from Embase and 29 from MEDLINE (Fig. 1). After screening the titles and abstract, 133 met the inclusion and exclusion criteria. Subsequently, 39 duplicated studies were excluded, and therefore, 94 studies were included for full-text procurement. Of these 94 studies, only 63 studies had full accessible text, with the remaining 31 studies reviewed in abstract or conference proceeding format. After full text review, 5 studies not related to the inclusion criteria were excluded, because they were not related to diagnostic criteria or diagnosis of SUO, focused on diagnostic workup on patients with primary bacteraemia and described case of pancreatitis rather than sepsis. Therefore, a final collection of 89 studies was included in the scoping review (Additional file 2). The inter-rater agreement for inclusion and exclusion of studies prior to discussion was good between the two reviewers (κ = 0.83, 682/722 agreement). After discussion, the two reviewers agreed on all study inclusions and a third reviewer was not required for adjudication.
Characteristics of studies
An overview of the included studies is shown in Table 1. All included papers were published from 1982 to 2021, with 33/89 (37%) published between 2015 and 2019. The most common publication type was case reports or case series 45/89 (51%). Included studies originated from 23 different countries/territories, with the United States contributing to the greatest number of studies at 28/89 (31%) (Table 2). The majority of the studies 82/89 (92%) were from high income countries/territories, and none were from low-income countries/territories. Studies included in the final review were either in English 85/89 (96%) or German 4/89 (4%).
Diagnostic Criteria of SUO
Criteria used to define SUO were not provided in 79/89 (89%) of included studies. Even in the remaining 10/89 (11%), there was no consensus on the diagnostic criteria of SUO, or reference to known consensus diagnostic criteria (Table 3) [23]. The criteria, when present, often described as failure to identify source of sepsis despite “radiological—including CT—and microbiological technology, and systematic diagnostic workups” or after “extensive diagnostics” [24, 25]. However, the breadth and depth of examination and investigations that constitute a comprehensive workup was rarely described explicitly. Most diagnostic criteria focused on laboratory and radiological investigations, whilst the extent and thoroughness of medical history was never mentioned as a requirement. Clinical signs or examination was stated as necessary in 6/89 (7%) of publications but none explained the scope of negative findings required to fulfill the diagnostic criteria for SUO [26,27,28,29,30,31]. Even for microbiological investigations, the requirement for all of routine blood, urine and respiratory bacterial cultures was mentioned in only 3/89 (3%) of studies [28, 32, 33]. In addition, 3/89 (3%) of studies classified patients as SUO if investigative work up was negative after a cutoff time of 24 to 48 h [16, 28]. None of the diagnostic criteria mentioned whether non-infective differential diagnoses, if any, should be investigated for before attributing a patient’s clinical condition to SUO.
History
Despite the universal absence of history as a component of the diagnostic criteria for SUO (when provided), 30/89 (34%) of studies discussed the merits of specific, focused history taking. Most of these studies describe how a particular aspect of history helped direct further investigations to confirm the infective source or to suggest alternative diagnoses [34,35,36,37,38,39,40]. Important aspects of history taking included travel history which may reveal exposure to endemic pathogens, such as malaria and rickettsia [34, 37]. Other studies highlighted the importance of drug history which may reveal use of drugs which may cause symptoms and signs that mimic sepsis [35, 36, 38]. Non-specific symptoms of prolonged fever, myalgia and weight loss were described as suggesting malignancy, rheumatological disease or hematological disorders [41,42,43,44,45,46]. None of the studies described what a thorough and comprehensive history should encompass to best investigate SUO.
Examination
Although 23/89 (26%) of studies described specific signs on examination that provided clues to infective source, none of them outlined what would be considered a systematic examination. Isolated and specific recommendations were, however, made. Examination of the oral cavity and soft tissues in the neck may suggest localized infection or even Lemierre’s syndrome [40, 47]. Furthermore, examination based on context such as finding soft issue infection in patients with burns may be helpful [47, 48]. Repeated examinations were helpful to identify source of infection in patients who did not initially have localizing signs [49]. Nonspecific signs such as lymphadenopathy or maculopapular rash may suggest lymphoma or rheumatological conditions, such as Adult Onset Still’s Disease, respectively [50, 51]. Seven studies noted the significance of hepato- and/or splenomegaly which suggests underlying hematological disorders [41, 44,45,46, 52,53,54]. None of the studies reported inter-observer variability in identification of clinical signs.
Imaging
Utility of imaging was discussed in 57/89 (64%) of studies, specifically to identify the source of sepsis. Point of care ultrasound was demonstrated to be useful to assess many body compartments including pulmonary, urinary, biliary and musculoskeletal [55,56,57]. Computed tomography (CT) was often reported as a helpful screening tool to increase diagnostic yield when source of sepsis is unknown [26, 31, 39, 49, 51, 57,58,59,60,61,62,63]. Along with plain radiographs, ultrasound and echocardiography, studies have generally recommended that it is essential to perform a screening CT scan before a diagnosis of SUO is made [25, 28,29,30, 32, 33]. Furthermore, the addition of positron emission tomography–CT (PET–CT) has been shown to be a key investigation resulting in a positive diagnosis in critically ill patients with suspected SUO [25, 28, 32, 64,65,66]. One study showed that 99 m Tc labeled white cell has high sensitivity (95%) and specificity (91%) for identifying site of infection in trauma and surgical ICU patients with SUO [29].
Microbiological Culture Sampling
Microbiological cultures were described in 39/89 (44%) of studies. Blood culture was considered mandatory in only 6 studies [23, 28, 32, 33, 66, 67]. Overall, none of the studies specified the criteria for properly performed cultures but one study mentioned that a minimum of two peripheral blood cultures was needed [23]. Beyond cultures from blood, respiratory and urinary tract, cultures from catheter tips and hubs, sinus lavage and bronchoalveolar lavage were often described as part of the workup to establish SUO [16, 23, 28, 29, 32, 35, 37, 58, 60, 61, 64, 67,68,69]. Culture from bone marrow may also be helpful [70]. Lumbar puncture may be undertaken in patients with neurological symptoms [36, 71].
Special Tests
Special investigations were described in 32/89 (36%) studies and included advanced microbiological tests, special techniques to obtain microbiological samples and other tests performed to look for alternative diagnoses. Streptococcus pneumoniae and legionella pneumophila urine antigens, thyroid hormones, cortisol and toxicology screening were also part of the diagnostic workup described by some authors [35, 36, 72]. Serology for rickettsial infections, screening for schistosomiasis, leishmaniasis were used in areas, where the pathogens are endemic [37, 42]. Interestingly, malaria screening may be warranted even when patients have not travelled to endemic areas as rare events may allow vector borne diseases to transmit in non-endemic areas [73]. Skin biopsy of rashes/lesions may help rule out septic emboli whilst histology may facilitate diagnosis of rheumatological or drug sensitivity disorders [35].
Monospot test and Epstein–Barr Virus polymerase chain reaction (PCR) may be useful as part of the diagnostic workup for hemophagocytic lymphohistiocytosis (HLH) [44, 62, 74]. QuantiFERON-TB Gold to screen for mycobacterium tuberculosis was mentioned in one study [74]. Five studies mentioned the need for human immunodeficiency virus testing [25, 37, 39, 42, 53]. Procalcitonin was tested in 10/89 (11%) studies as a marker suggestive of bacterial sepsis, although it cannot help to localize the site of infection [18, 30, 34, 38, 41, 52, 62, 65, 66, 75]. Bone marrow examination and biopsy of lesions or lymph nodes were used to rule out malignancy or HLH [41, 44,45,46, 50,51,52,53, 62, 74, 76]. Extremely high concentrations of ferritin may be helpful to screen for HLH [33, 41, 44,45,46, 52,53,54, 62]. Utilization of percutaneous cholecystostomy or diagnostic laparoscopy was found to be helpful in patients with SUO in certain instances when other clinical, laboratory and imaging tests were negative [30, 33, 77, 78]. A diagnostic autoimmune panel has been recommended to screen for alternative diagnoses, such as granulomatous polyangiitis or other rheumatological disorders [71].
Discussion
To our knowledge this is the first comprehensive scoping review seeking to document the diagnostic criteria of SUO and characterize the existing evidence supporting diagnostic processes to identify the infection source in critically ill patients with suspected SUO. The systematic literature search identified 89 relevant studies of which the majority were case reports that almost exclusively originated from high income countries/regions. Universal diagnostic criteria for SUO were not found. There is also currently no standardized diagnostic approach for patients with suspected SUO, although common investigative processes included history, physical examination, imaging, microbiological investigations, and special tests.
Surprisingly, universally accepted diagnostic criteria for SUO were not found despite reported data suggesting 2–11% of septic patients have an unknown infectious source [4, 12,13,14,15,16,17]. We found that common elements described in different criteria for SUO included negative clinical, microbiological, imaging and special tests (Table 3). Yet, what constitutes a thorough clinical history and examination to localize the infective source was not standardized. Similarly, only 3% of studies listed sputum, blood and urine bacterial cultures as essential microbiological investigations, yet it would seem reasonable that they should be. Furthermore, the need for fungal and parasitic workup was not described or mentioned in any of the diagnostic criteria reviewed. The use of serological tests was mentioned, but the types of serology to be tested were not specified. Thus, using these current loose criteria may result in the premature conclusion that patients have SUO if non-bacterial microbiological tests were not performed, especially since 21% of infections in ICU are viral, parasitic or fungal in origin [79]. In addition, CT imaging was frequently listed as a criterion, but which body part(s) should be scanned is often not specified. Overall, the published diagnostic criteria of SUO included in this review (Table 3) are focused on the prerequisite infection workup in SUO without an explicit mention on what constitutes sepsis. This suggests most diagnostic criteria of SUO are concerned with how best to define “suspected infection” with the assumption that infection is responsible for the life threatening organ dysfunction and inflammation in sepsis.
Investigations such as microbiological culture require time for results to become available, and delay in diagnosis may be incurred for patients in smaller centers who require transfer for imaging, such as CT [80]. Indeed, an allowable time delay of 24 to 48 h was part of the criteria in 2 of the included studies [16, 28]. Thus, patients initially managed as suspected SUO may have a source identified or an alternative diagnosis established as time progresses [16]. The diagnosis in patients suspected to have SUO should be treated as an evolving, working diagnosis which is time dependent.
There is currently no consensus on the type, extent and sequence of investigative processes, or the minimal time interval required before a patient should be considered to have SUO. To better define this common clinical problem, empiric data from prospective studies are required to inform the drafting of evidenced-based diagnostic criteria for SUO in critically ill patients. The current information gap includes three key areas. First, the proportion of patients who on ICU admission have suspected sepsis but the infection site and pathogen are unknown. Second, the time interval from suspicion of sepsis to confirmation of infection. Third, the time sequence and breadth of diagnostic workup commonly taken to locate the infection or establish an alternative diagnosis. With these data it may be possible to construct a criteria to epidemiologically define patients with SUO as a diagnosis, likely by exclusion after protocolized workup has failed to identify a source of infection or establish a non-infective alternative diagnosis of life threatening organ dysfunction.
Development of a standard, protocolized diagnostic approach in patients with suspected SUO may reduce lapses in workup efficiency and minimize missed opportunities to identify the infection source or an alternative diagnosis. Our search found that core components of common investigative processes include: history, clinical examination, imaging, microbiological tests and special tests. Apart from localizing symptoms, one study highlighted the importance of social history, where a woman living in Frankfurt without travel history was suspected to have contracted malaria by living near the airport [73]. Although systematic examination is advocated, we were unable to find a set of examination components for this task. Over 50% of the included studies mentioned imaging tests in their diagnostic approach. Comparative studies have generally found CT to be superior to USG in detecting intra-abdominal sepsis, particularly in patients with recent surgery [81, 82]. Interestingly, at least 6 studies discussed the merits of PET–CT in identifying infection source in critically ill patients with SUO [25, 28, 32, 64,65,66]. Whilst microbiological culture is currently the gold standard to confirm infection, polymerase chain reaction and next generation sequencing pathogen detection may offer superior sensitivity for fastidious bacterial organisms, prior antimicrobial therapy or non-bacterial pathogens [83,84,85].
Finally, 92% of the available literature on diagnostic criteria of sepsis of unknown cause and investigative approach is from high income countries/regions alone with none from low-income countries. Advanced diagnostic tests and procedures may not always be available to all clinicians. Furthermore, variation in case-mix and endemic pathogens will likely determine the specific investigations required for different healthcare settings [86, 87]. An example would be the need to routinely screen for malaria in many parts of sub-Saharan Africa, whereas malaria screen would be of much lower priority in northern European countries unless there is a travel history. Therefore, it may be necessary to tailor the protocolized investigate workup in patients suspected of SUO based on the healthcare setting, country income, population structure, comorbidities, resource and local pathogens.
Our scoping review has a few important limitations. First, we restricted our search strategy to SUO in ICU patients which may have reduced the volume of available evidence. However, we decided not to include ward patients with mild organ dysfunction, because conceptually, they represent a different population with less clinical urgency and more time for investigations for suspected SUO. Second, because of limited relevant literature identified, we included case reports, abstracts and conference proceedings in this review. Review articles were also included to report expert opinion as there was lack of empirical data to guide the diagnostic approach in SUO. Although we were unable to systematically assess heterogeneity, the limited range of high level of published evidence showed that there is likely significant bias. Third, we used ICU admission to indicate the presence of critical illness and organ dysfunction as a study inclusion criteria, because a substantial number of studies did not provide sufficient information about organ dysfunction to allow a precise definition of sepsis or sepsis severity. Fourth, we were unable to separately summarize the diagnostic approach used for SUO patients presenting from the community or hospital setting, because the number of studies was limited.
Conclusions
A universally accepted diagnostic criteria of SUO was not found. Prospective studies on investigative processes in critically ill patients managed as SUO across different healthcare settings are needed to understand the epidemiology and inform the diagnostic criteria of SUO.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- CT:
-
Computed tomography
- HLH:
-
Hemophagocytic lymphohistiocytosis
- ICU:
-
Intensive care unit
- USG:
-
Ultrasonography
- PCR:
-
Polymerase chain reaction
- PET–CT:
-
Positron emission tomography–computed tomography
- SUO:
-
Sepsis of unknown origin
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143.
Phua J, Ngerng W, See K, Tay C, Kiong T, Lim H, Chew M, Yip H, Tan A, Khalizah H, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17(5):R202.
Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N, et al. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med. 2009;37(1):81–8.
Brun-Buisson C, Meshaka P, Pinton P, Vallet B, Group ES. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30(4):580–8.
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53.
Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Namendys-Silva SA, Martin-Loeches I, Leone M, Lupu MN, Vincent JL, et al. Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit. Open Forum Infect Dis. 2018;5(12):ofy313.
Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture-negative severe sepsis: nationwide trends and outcomes. Chest. 2016;150(6):1251–9.
Cheng MP, Stenstrom R, Paquette K, Stabler SN, Akhter M, Davidson AC, Gavric M, Lawandi A, Jinah R, Saeed Z, et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: a diagnostic study. Ann Intern Med. 2019;171(8):547–54.
Bouza E, Sousa D, Rodriguez-Creixems M, Lechuz JG, Munoz P. Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections? J Clin Microbiol. 2007;45(9):2765–9.
Doern GV. Detection of selected fastidious bacteria. Clin Infect Dis. 2000;30(1):166–73.
Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, Gomersall CD, Faruq MO, Shrestha BR, Gia Binh N, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. 2011;342: d3245.
Abe T, Yamakawa K, Ogura H, Kushimoto S, Saitoh D, Fujishima S, Otomo Y, Kotani J, Umemura Y, Sakamoto Y, et al. Epidemiology of sepsis and septic shock in intensive care units between sepsis-2 and sepsis-3 populations: sepsis prognostication in intensive care unit and emergency room (SPICE-ICU). J Intensive Care. 2020;8:44.
Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, Ma X, Cao X, Chen D, Lu W, et al. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit Care Med. 2020;48(3):e209–18.
Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth. 2017;119(4):626–36.
Contou D, Roux D, Jochmans S, Coudroy R, Guerot E, Grimaldi D, Ricome S, Maury E, Plantefeve G, Mayaux J, et al. Septic shock with no diagnosis at 24 hours: a pragmatic multicenter prospective cohort study. Crit Care. 2016;20(1):360.
Alberti C, Brun-Buisson C, Goodman SV, Guidici D, Granton J, Moreno R, Smithies M, Thomas O, Artigas A, Le Gall JR, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168(1):77–84.
Klein Klouwenberg PM, Cremer OL, van Vught LA, Ong DS, Frencken JF, Schultz MJ, Bonten MJ, van der Poll T. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319.
De Waele JJ, Sakr Y. How I search for a sepsis source. Crit Care. 2019;23(1):386.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101(6):1644–1655.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82.
Agarwal R, Gupta D, Ray P, Aggarwal AN, Jindal SK. Epidemiology, risk factors and outcome of nosocomial infections in a Respiratory Intensive Care Unit in North India. J Infect. 2006;53(2):98–105.
Birndt S, Fetscher S, Frimmel M, Hochhaus A, La Rosée P: Cytokine adsorption in a patient with severe coagulation abnormalities due to hemophagocytic lymphohistiocytosis (HLH). In: 23rd European Hematology Association Congress: 2018; 2018.
Fort R, Ledochowski S, Friggeri A. PET-CT in critically ill patients: diagnosing the unsuspected. Crit Care Med. 2018;46(2):e166–9.
Barkhausen J, Stoblen F, Dominguez-Fernandez E, Henseke P, Muller RD. Impact of CT in patients with sepsis of unknown origin. Acta Radiol. 1999;40(5):552–5.
Kelly JJ, Puyana JC, Callery MP, Yood SM, Sandor A, Litwin DE. The feasibility and accuracy of diagnostic laparoscopy in the septic ICU patient. Surg Endosc. 2000;14(7):617–21.
Mandry D, Tatopoulos A, Chevalier-Mathias E, Lemarie J, Bollaert PE, Roch V, Olivier P, Marie PY, Gibot S. (1)(8)F-fluorodeoxyglucose positron emission tomography combined with whole-body computed tomographic angiography in critically ill patients with suspected severe sepsis with no definite diagnosis. Eur J Nucl Med Mol Imaging. 2014;41(10):1924–30.
Minoja G, Chiaranda M, Fachinetti A, Raso M, Dominioni L, Torre D, De Palma D. The clinical use of 99m-Tc-labeled WBC scintigraphy in critically ill surgical and trauma patients with occult sepsis. Intensive Care Med. 1996;22(9):867–71.
Peris A, Matano S, Manca G, Zagli G, Bonizzoli M, Cianchi G, Pasquini A, Batacchi S, Di Filippo A, Anichini V, et al. Bedside diagnostic laparoscopy to diagnose intraabdominal pathology in the intensive care unit. Crit Care. 2009;13(1):R25.
Velmahos GC, Kamel E, Berne TV, Yassa N, Ramicone E, Song Z, Demetriades D. Abdominal computed tomography for the diagnosis of intra-abdominal sepsis in critically injured patients: fishing in murky waters. Arch Surg. 1999;134(8):831–6 (discussion 836-838).
Kluge S, Braune S, Nierhaus A, Wichmann D, Derlin T, Mester J, Klutmann S. Diagnostic value of positron emission tomography combined with computed tomography for evaluating patients with septic shock of unknown origin. J Crit Care. 2012;27(3):316.e311-317.
Lee MJ, Saini S, Brink JA, Hahn PF, Simeone JF, Morrison MC, Rattner D, Mueller PR. Treatment of critically ill patients with sepsis of unknown cause: value of percutaneous cholecystostomy. Am J Roentgenol. 1991;156(6):1163–6.
Panjwani R, Davis S. Malaria with Acute Respiratory Distress Syndrome: Developing Country Complications Seen in America. Am J Respir Crit Care Med. 2018;197:A5329.
van den Hoven D, Veldhuijzen N, Röckmann H, de Jong B. Azathioprine hypersensitivity syndrome, a drug reaction mimicking sepsis. Netherlands Journal of Critical Care. 2020;28(6):257–62.
Kansagra A, Patel S, Wilcox SR. Prolonged hypothermia due to olanzapine in the setting of renal failure: a case report and review of the literature. Ther Adv Psychopharmacol. 2013;3(6):335–9.
Chueng TA, Koch KR, Anstead GM, Agarwal AN, Dayton CL. Case report: early doxycycline therapy for potential rickettsiosis in critically ill patients in flea-borne typhus-endemic areas. Am J Trop Med Hyg. 2019;101(4):863–9.
Sexe J, Mayes C, Tofts P. Euglycemic diabetic ketoacidosis in a lung cancer patient using empagliflozin. Case Rep Crit Care. 2020;2020:7437892.
Takano Y, Fukuda K, Takayasu H, Shinmura K, Koizumi G, Sasai M, Nagayama Y, Kawamo M, Yasuda T, Watanabe K, et al. Liver abscessation and multiple septic pulmonary emboli associated with Lemierre’s syndrome: a case report. BMC Res Notes. 2015;8:65.
Jane R, Johnson P. Critical care nurses be aware: Lemierre’s syndrome is on the rise. Aust Crit Care. 2003;16(4):126–32.
Padhi S, Varghese RG, Ramdas A, Phansalkar MD, Sarangi R. Hemophagocytic lymphohistiocytosis: critical reappraisal of a potentially under-recognized condition. Front Med. 2013;7(4):492–8.
Lin N, Hippensteel J, Neumeier A: Secondary hemophagocytic lymphohistiocytosis in AIDS associated EBV+ diffuse large B-cell lymphoma. In: Critical care case reports: hematology, oncology, rheumatology, and immunology: 2018; San Diego; 2018: A3392-A3392.
Ngo C, Nguyen O: Certainly sirs, but sepsis misses the sweet spot. In: 38th Annual Meeting of the Society of General Internal Medicine: 2015; Toronto; 2015: S375-S376.
Kapoor S, Naydenov S, Poddar N, Madmud G, Dawson J, Colin H, Mayer J, Martin T: A rare case of EBV induced hemophagocytic lymphohistocytosis. In: Critical Care Congress 2015: 2015; Phoenix; 2015: A1633.
Khaliq A, Zumberg M, Xiong G, Lo M: Walks like sepsis, talks like sepsis, but still not sepsis: Hematophagocytic lymphohistiocytosis. In: 35th Annual Meeting of the Society of General Internal Medicine: 2012; Orlando; 2012: S500.
Jafar N, Orenstein A: Similar but not the same. In: Chest Annual Meeting 2019: 2019; New Orleans; 2019: 1802A.
Czupryna P, Garkowski A, Moniuszko A, Pancewicz S, Ciemerych A, Zajkowska J. Patients with sepsis in infectious diseases department in years 1997–2010—epidemiology and clinical features. Przegl Epidemiol. 2013;67(3):429–34.
Penuelas O, Cerda E, Espino J, Garcia-Dominguez J, Hierro PG, de la Cal MA, Lorente JA. Limb intracompartmental sepsis in burn patients associated with occult infection. Burns. 2010;36(4):558–64.
Heidelberg LS, Pettke EN, Wagner T, Angotti L. An atypical case of necrotizing fasciitis secondary to perforated cecal cancer. J Surg Case Rep. 2020;2020(11):rjaa371.
Neel A, Wahbi A, Tessoulin B, Boileau J, Carpentier D, Decaux O, Fardet L, Geri G, Godmer P, Goujard C, et al. Diagnostic and management of life-threatening Adult-Onset Still Disease: a French nationwide multicenter study and systematic literature review. Crit Care. 2018;22(1):88.
Haqiqi A, Patel R, Mathew L, Myers A, Cliff S, G I: Purpura fulminans as a medical emergency, in a patient with angioimmunoblastic lymphoma. In: 15th Medical Dermatology Meeting: 2020; London: British Journal of Dermatology; 2020: pp. e118–e137.
Hindi Z, Khaled AA, Abushahin A. Hemophagocytic syndrome masquerading as septic shock: an approach to such dilemma. SAGE Open Med Case Rep. 2017. https://doi.org/10.1177/2050313X17746309.
Maheshwari N, Mandal AK, Sahni N. Sepsis of unknown origin with multiorgan failure syndrome: think of hemophagocytic lymphohistiocytosis. Indian J Crit Care Med. 2015;19(7):419–21.
Saevels K, Robert D, Van den Broeck S, Malfait R, Gadisseur A, Jorens P, Verlinden A. EBV-associated hemophagocytic lymphohistiocytosis complicated by severe coagulation disorders and opportunistic infections: case report of a survivor. Clin Case Rep. 2018;6(1):115–8.
Balik M. Importance of ultrasound examination in diagnosing acute conditions. Vnitr Lek. 2019;65(3):177–86.
Lichtenstein DA. Point-of-care ultrasound: Infection control in the intensive care unit. Crit Care Med. 2007;35(5 Suppl):S262-267.
Cengiz M, Celikbilek G, Andic C, Dosemeci L, Yilmaz M, Karaali K, Ramazanoglu A. Maxillary sinusitis in patients ventilated for a severe head injury and with nostrils free of any foreign body. Injury. 2011;42(1):33–7.
Stelter L, Steffen I, Pinkernelle JG, von Dossow-Hanfstingl V, Kastrup M, Denecke T, Grieser C. Computed tomography findings in septic patients with acute respiratory distress syndrome: correlation with survival and pulmonary versus extrapulmonary septic focus. J Comput Assist Tomogr. 2013;37(4):602–9.
Tonolini M, Ippolito S. Cross-sectional imaging of complicated urinary infections affecting the lower tract and male genital organs. Insights Imaging. 2016;7(5):689–711.
Samek M, Iversen K, Belmar Campos C, Berneking L, Langebrake C, Wolschke C, Ayuk F, Kroger N, Christopeit M. Monocenter study on epidemiology, outcomes, and risk factors of infections in recipients of 166 allogeneic stem cell transplantations during 1 year. Eur J Haematol. 2020;105(2):126–37.
Seiden AM. Sinusitis in the critical care patient. New Horiz. 1993;1(2):261–70.
Mascia G, Argiolas D, Carta E, Ibba S, Piredda GB. Hemophagocytic lymphohistiocytosis in renal transplant recipients: a 2-case report. Transplant Proc. 2020;52(5):1566–9.
Adam EJ, Page JE. Intra-abdominal sepsis: the role of radiology. Baillieres Clin Gastroenterol. 1991;5(3 Pt 1):587–609.
Huang CK, Huang JY, Ruan SY, Chien KL. Diagnostic performance of FDG PET/CT in critically ill patients with suspected infection: a systematic review and meta-analysis. J Formos Med Assoc. 2020;119(5):941–9.
Kampe KK, Rotermund R, Tienken M, Thomalla G, Regier M, Klutmann S, Kluge S. Diagnostic value of positron emission tomography combined with computed tomography for evaluating critically Ill neurological patients. Front Neurol. 2017;8:33.
Muckart D, Hardcastle T, Peer F. Positron emission tomography/computed tomography scanning for the diagnosis of occult sepsis in the critically injured. S Afr J Surg. 2016;54(1):43–8.
Darwich M, Gleeson K. Fever of unknown origin in the ICU: Key components of the work-up. Journal of Critical Illness. 1998;13(5):287–97.
Tanguy M, Seguin P, Laviolle B, Desbordes L, Malledant Y. Hub qualitative blood culture is useful for diagnosis of catheter-related infections in critically ill patients. Intensive Care Med. 2005;31(5):645–8.
Urli T, Perone G, Acquarolo A, Zappa S, Antonini B, Ciani A. Surveillance of infections acquired in intensive care: usefulness in clinical practice. J Hosp Infect. 2002;52(2):130–5.
Ivory J, Ashai K, Schmidt J: Hide and go seek: A case of vancomycin-resistant enterococcus in the bone marrow. In: 2019 Annual Meeting of the Society of General Internal Medicine: 2019; Washington; 2019: S548.
Hofmann T, Kainz J, Koc C, Smolle KH, Brunner G. Isolated unilateral otitis with facial nerve paralysis as initial symptom of Wegener granulomatosis. An unusual clinical course. Laryngorhinootologie. 1998;77(6):352–4.
Karanikolas M, Velissaris D, Karamouzos V, Filos KS. Thyroid storm presenting as intra-abdominal sepsis with multi-organ failure requiring intensive care. Anaesth Intensive Care. 2009;37(6):1005–7.
Praetorius F, Altrock G, Blees N, Schuh N, Faulde M. Imported Anopheles: in the luggage or from the airplane? A case of severe autochthonous malaria tropica near an airport. Dtsch Med Wochenschr. 1999;124(34–35):998–1002.
Bajaj P, Clement J, Bayerl MG, Kalra N, Craig TJ, Ishmael FT. High-grade fever and pancytopenia in an adult patient with common variable immune deficiency. Allergy Asthma Proc. 2014;35(1):78–82.
Scawn N, Saul D, Pathak D, Matata B, Kemp I, Stables R, Lane S, Haycox A, Houten R. A pilot randomised controlled trial in intensive care patients comparing 7 days’ treatment with empirical antibiotics with 2 days’ treatment for hospital-acquired infection of unknown origin. Health Technol Assess. 2012;16(36):1–70.
Kozlova A, Gleason J, Maroz N: Multiple Organ Dysfunction Syndrome in Setting of Sweet's Syndrome Without Evidence of Underlying Infectious, Rheumatologic, or Oncologic Etiology. In: ATS International Conference: 2019; 2019: A6544.
Grundmann RT, Petersen M, Lippert H, Meyer F. The acute (surgical) abdomen—epidemiology, diagnosis and general principles of management. Z Gastroenterol. 2010;48(6):696–706.
Rodrigues M: Cholecystostomy. In: Cardiovascular and Interventional Radiological Society of Europe 2009: 2009; Lisbon; 2009: 190.
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, Finfer S, Pelosi P, Brazzi L, Aditianingsih D, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–87.
Bergeron C, Fleet R, Tounkara FK, Lavallee-Bourget I, Turgeon-Pelchat C. Lack of CT scanner in a rural emergency department increases inter-facility transfers: a pilot study. BMC Res Notes. 2017;10(1):772.
Dobrin PB, Gully PH, Greenlee HB, Freeark RJ, Moncada R, Churchill R, Reynes C, Henkin R. Radiologic diagnosis of an intra-abdominal abscess. Do multiple tests help? Arch Surg. 1986;121(1):41–6.
Go HL, Baarslag HJ, Vermeulen H, Lameris JS, Legemate DA. A comparative study to validate the use of ultrasonography and computed tomography in patients with post-operative intra-abdominal sepsis. Eur J Radiol. 2005;54(3):383–7.
Grumaz S, Grumaz C, Vainshtein Y, Stevens P, Glanz K, Decker SO, Hofer S, Weigand MA, Brenner T, Sohn K. Enhanced performance of next-generation sequencing diagnostics compared with standard of care microbiological diagnostics in patients suffering from septic shock. Crit Care Med. 2019;47(5):e394–402.
Bloos F, Hinder F, Becker K, Sachse S, MekontsoDessap A, Straube E, Cattoir V, Brun-Buisson C, Reinhart K, Peters G, et al. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010;36(2):241–7.
Rossoff J, Chaudhury S, Soneji M, Patel SJ, Kwon S, Armstrong A, Muller WJ: Noninvasive diagnosis of infection using plasma next-generation sequencing: a single-center experience. Open Forum Infect Dis. 2019;6(8):ofz327.
Dunser MW, Festic E, Dondorp A, Kissoon N, Ganbat T, Kwizera A, Haniffa R, Baker T, Schultz MJ. Global Intensive Care Working Group of European Society of Intensive Care M: recommendations for sepsis management in resource-limited settings. Intensive Care Med. 2012;38(4):557–74.
Thwaites CL, Lundeg G, Dondorp AM. sepsis in resource-limited settings-expert consensus recommendations group of the European Society of Intensive Care M, the Mahidol-Oxford Research Unit in Bangkok T: recommendations for infection management in patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. 2016;42(12):2040–2.
Hulst AMV, Rijk MCV, Bavelaar-Croon CDL, Tjan DHT. The value of F-18-fluorodeoxyglucose positron emission tomography ( FDG-PET/CT ) in the intensive care unit: a review. Netherlands J Crit Care. 2019;27(3):108–14.
Acknowledgements
CaBoOM investigators: Tom Stelfox, Daniel Niven, Rameiya Paramalingam, Derek Vonderhaar, Ross Freebairn, Gavin M Joynt, Lowell Ling, Patricia Leung, Dean Gopalan, Jean Yves Lefrant, Sophie Lloret, Loubna Elotmani, Jason A. Roberts, Jeffrey Lipman, Kevin B. Laupland, Cheryl Fourie, Renee Saba, Dougal Carlisle, Felicity Edwards.
Funding
This was not a funded study.
Author information
Authors and Affiliations
Consortia
Contributions
LL designed the study. LL and OOYM performed the literature search, collected and analyzed the data. LL wrote the first draft of the manuscript. OOYM, KL, JYL, JR, PDG, JL, GMJ and other authors critiqued the manuscript and approved of the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search Strategy. Search strategy used for Embase, PubMed and MEDLINE in this scoping review.
Additional file 2.
Summary of Included Studies. Background information and extracted data from included studies.
Additional file 3.
Diagnostic Workup Extraction. Extraction of data on diagnostic thematic processes from articles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ling, L., Mui, O.O.Y., Laupland, K.B. et al. Scoping review on diagnostic criteria and investigative approach in sepsis of unknown origin in critically ill patients. j intensive care 10, 44 (2022). https://doi.org/10.1186/s40560-022-00633-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-022-00633-4