Abstract
Background
To reduce pharmacy-related medical expenses, it is necessary to cut drug costs, potentially by increasing generic drug usage. This study analyzes the correlation between generic drug usage and monthly personal income by examining prescriptions for individual drugs.
Methods
We conducted a cross-sectional study based on the data set from the National Database of Health Insurance Claims and Specific Health Checkups of Japan Open Data Japan and the Basic Survey on Wage Structure. We calculated the correlation coefficient between the usage rate of generic drugs in each prefecture of Japan and monthly personal incomes. We then analyzed the correlation coefficients based on the therapeutic categories of medicinal drugs; the contingency table was visualized as a mosaic plot. To compare the proportions between multiple categories, the chi-squared test was applied as a statistical significance test that was used in the analysis of n × m contingency tables. We worked with the null hypothesis that there were no differences between classes in the population.
Results
Regarding the correlation coefficient between the usage rate of generic drugs and monthly personal incomes, the proportion of negative correlation coefficients for outpatient out-of-hospital and outpatient in-hospital prescriptions was over 70%, while that for inpatient prescriptions was 46.9%. The proportion of medicinal drugs exhibiting a negative correlation between the rates of generic drug usage and monthly personal incomes for outpatient out-of-hospital prescriptions and outpatient in-hospital prescriptions was higher than that of inpatient prescriptions. The proportion of statistically correlated medicinal drugs among inpatient prescriptions was lower than that among outpatient out-of-hospital and outpatient in-hospital prescriptions. The proportions of significant negative correlations for outpatient out-of-hospital, outpatient in-hospital, and inpatient prescriptions were 30.6%, 22.7%, and 3.5%, respectively. It was also observed that the rate of generic prescription usage for outpatient out-of-hospital and in-hospital prescriptions increased as monthly personal incomes decreased. In outpatients, the therapeutic categories with strong negative correlations were vasodilators and hyperlipidemia drugs.
Conclusions
Our results may help to increase the usage rate of generic drugs in different prefectures by providing useful information for promoting them throughout Japan.
Similar content being viewed by others
Background
The Japanese health insurance system guarantees public medical insurance to all citizens. Citizens can freely choose medical institutions. They can also receive advanced medical care at low costs. In principle, the share of medical expenses for Japanese citizens is 10% for those aged 75 and over, 20% for those aged 70 to 74, 30% for those aged 6 to 69, and 20% for those under 5 [1]. Rising medical costs have become a concern in Japan. Medical costs in fiscal year 2019 were 43.6 trillion yen [2], which represents an increase of 2.1 trillion yen from the 41.5 trillion yen spent in fiscal year 2015. By fiscal year 2019, approximately 16,000 items [3] were listed on the official bulletin as medical drugs to be used for medical treatment and were covered by insurance at medical institutions. Medical costs related to pharmacies were 7.7 trillion yen, of which 5.7 trillion yen was attributed to the cost of drugs [4]. In 2020, the usage rate of generic drugs in the United States reached 95%, but remained 70% in Japan [5, 6]. Increasing the usage rate of generic drugs can contribute to reducing drug costs in Japan. To reduce medical costs, the “Basic Policy on Economic and Fiscal Management and Reform 2021” [7] stipulates that the rate of generic drug usage should reach more than 80% by fiscal year 2023 in each prefecture. In Japan, dispensing generic drugs and generic substitution is governed by the Rules for Health Insurance-covered Medical Institutions and Physicians and Rules for Health Insurance-covered Dispensing Pharmacies and Pharmacists [8]. In Japan, nonproprietary name prescriptions are recommended to promote the use of generic drugs [9]. It is a system in which patients can choose between brand-name drugs and generic drugs at pharmacies. A pharmacist can dispense a generic drug unless the doctor has indicated a brand-name drug in the prescription [10].
For those aged 75 and over, those belonging to high-income groups who pay 30% of their costs under Japan’s public universal insurance system are more likely to choose brand-name drugs than those in general-income groups who pay 10% of their costs [8]. In a systematic review and meta-analysis, patients with lower income (i.e., < 200% federal poverty level) were more likely to use generic drugs than those with a higher income (≥ 200% FPL; pooled OR = 1.32, 95% CI 1.15–1.52) [11]. In the United States, it has been reported that generic drug discount programs are an option to provide affordable prescription medication to low-income individuals [12]. Large hospitals use a higher percentage of generic drugs as compared to smaller medical institutions, such as clinics [13]. Yokoi and Tashiro identified a positive correlation between the prescription rate of generic drugs and the extent of separation between a medical practice and the sites of drug dispensation [14]. There are regional differences in the use of generic drugs as well [15, 16]. In addition, although the government incentivizes the use of generic drugs, antihypertensive drugs are not effective in relatively high-income areas [15]. As prescriptions comprise outpatient out-of-hospital, outpatient in-hospital, and inpatient prescriptions, it is conceivable that the correlation between the usage rate of generic drugs and monthly personal income varies according to the difference in prescriptions. However, the correlation between the usage rate of generic drugs, sorted by prescription and monthly personal income, has not been previously investigated.
There are few research reports on the overall usage of generic drugs based on comprehensive drug prescription data in Japan. To the best of our knowledge, correlations between the usage rate of generic drugs by prescription type and monthly personal income have not been investigated for each individual drug using the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) Open Data Japan (NODJ). Therefore, we analyzed this correlation for each individual drug using the NODJ and Basic Survey on Wage Structure (BSWS), and then evaluated the therapeutic category that demonstrates the correlation.
Methods
National Database of Health Insurance Claims and Specific Health Checkups of Japan Open Data Japan (NODJ)
In 2009, based on the Act on Assurance of Medical Care for Elderly People, the Ministry of Health, Labour and Welfare (MHLW) began operating the NDB. This database accumulates monthly data on health insurance claims, and annual data on specific health checkups, thus making it one of the most exhaustive national healthcare databases worldwide. Its insurance-related data are also useful in developing government policies for national healthcare insurance systems and for academic research. NDB reflects healthcare trends in Japan, because the national healthcare system covers most medical care in the country [17]. Compared to the data obtained through sampling surveys, the NDB consists of a more comprehensive data set of individuals who have received specific health checkups in Japan. On account of its national coverage, it is also suitable for understanding the healthcare conditions in each prefecture [18].
The MHLW recently released the “NODJ.” This database provides a variety of NDB summaries that are freely available to the public. As the NODJ contains open data, analyses entail less effort, cost, and ethical consideration, and can be conducted quickly. We obtained data from the MHLW website for the fourth NODJ in 2017 [19]. The NODJ contains data items, such as medical and dental fee schedules, dental injuries, drug data, specified insurance medical materials, specified medical examination test items, and a specified medical examination questionnaire. The medication usage records in the fourth NODJ were restricted to the top 100 medication codes for each therapeutic category. The NODJ contains information on prescription drug usage, including outpatient out-of-hospital prescriptions, outpatient in-hospital prescriptions, and inpatient prescriptions for internal use, external use, and injection, based on the standard unit of drug price listings. We evaluated the effects of drugs based on the therapeutic categories set by the Standard Commodity Classification Number of Japan [20]. We used medications stratified by 47 Japanese prefectures, therapeutic categories, and drug prices from data tables 000711952.xlsx, 000711957.xlsx, and 000711961.xlsx [19]. We then calculated the usage rates of generic drugs stratified by outpatient out-of-hospital prescriptions, outpatient in-hospital prescriptions, and inpatient prescriptions. Medicinal drugs were classified into brand-name and generic drugs by their commercial names, and the usage rate of generic drugs was calculated according to the method prescribed by Ono et al. (Fig. 1) [21].
Basic Survey on Wage Structure (BSWS)
In Japan, various large-scale medical information databases have been developed for clinical and epidemiological research purposes [22, 23]. Since 1948, the MHLW has annually conducted the “BSWS” [24, 25], based on the “Statistics Law.” The BSWS was conducted in 49,541 offices across the country in fiscal year 2017, aiming to present a clear picture of the employee/wage structure in major industries [25], defined as wage distribution stratified according to the types of employment and work, occupation, sex, age, school career, length of service, and occupational career. BSWS data are accessible to the public, and the data from 2017 are available from the MHLW and e-Stat websites (www.e-stat.go.jp) [26]. In this study, monthly personal income by prefecture was considered.
Data analysis
We conducted a cross-sectional study based on the data set from the NODJ and BSWS. We calculated the correlation coefficient between the usage rate of generic drugs in each prefecture and monthly personal incomes (Additional file 1: Table S1). We then analyzed correlation coefficients based on the therapeutic categories of medicinal drugs; the contingency table was visualized as a mosaic plot. To compare the proportions between multiple categories, the chi-squared test was applied as a statistical significance test used in the analysis of n × m contingency tables. We worked with the null hypothesis that there were no differences between classes in the population. A p value of less than 0.05 (typically ≤ 0.05) was considered statistically significant.
The data analysis was performed using the JMP Pro 11 software (SAS Institute, Cary, NC, United States).
Results
Numbers of drugs by prescription category
The numbers of medicinal drugs for outpatient out-of-hospital, outpatient in-hospital, and inpatient prescriptions were 4904, 4937, and 4907, respectively (Fig. 2). The numbers of brand-name drugs that exhibited a significant negative correlation between the usage rate of generic drugs and monthly personal incomes for outpatient out-of-hospital, outpatient in-hospital, and inpatient prescriptions were 195, 98, and 9, respectively (Fig. 2).
Correlation coefficients and prescription classifications (mosaic plot)
Using a mosaic plot, we summarized the proportion of positive/negative ratios of correlation coefficients and prescription classifications (outpatient out-of-hospital, outpatient in-hospital, and inpatient) (Fig. 3). The following significantly different categorical features according to prescription classification were detected using the chi-squared test. Regarding the correlation coefficient between the usage rate of generic drugs and monthly personal incomes, the proportion of negative correlation coefficients for outpatient out-of-hospital and outpatient in-hospital prescriptions was over 70%, while that for inpatient prescriptions was 46.9% (Fig. 3a). The proportion of inpatient prescriptions was significantly lower than that of outpatient out-of-hospital and outpatient in-hospital prescriptions (Fig. 3b).
Proportions of significant negative correlations by prescription category
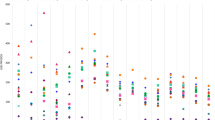
The proportions of significant negative correlations for outpatient out-of-hospital, outpatient in-hospital, and inpatient prescriptions were 30.6%, 22.7%, and 3.5%, respectively. For the therapeutic categories with a negative correlation for outpatient out-of-hospital prescriptions, the numbers of antihypertensives (code: 214), vasodilators (code: 217), agents for peptic ulcer (code: 232), and agents for hyperlipidemia (code: 218) were 16, 13, 12, and 12, respectively (Table 1 and Fig. 4). The correlation coefficients for Marzulene® S-blended granules (l-glutamine, agents for peptic ulcer, code: 232), Gaster® D tablets 20 mg (Famotidine, agents for peptic ulcer, code: 232), and Cerocral® tablets 20 mg (Ifenprodil tartrate, other cardiovascular agents, code: 219) were - 0.7058 (p value < 0.0001), - 0.6922 (p value < 0.0001), and - 0.656 (p value < 0.0001), respectively (Additional file 2: Table S2).
Regarding the therapeutic categories with negative correlations for outpatient in-hospital prescriptions, vasodilators (code: 217), agents for hyperlipidemias (code: 218), agents for peptic ulcer (code: 232), and hypnotics and sedatives, antianxietics (code: 112) accounted for 11, 9, 8, and 8 items, respectively (Table 1 and Fig. 5). The correlation coefficients of Mucosolvan® Tablets 15 mg (Ambroxol hydrochloride, expectorants, code: 223), Amlodin® Tablets 5 mg (Amlodipine besilate, vasodilators, code: 217), and Lendormin® Tablets 0.25 mg (Brotizolam, hypnotics and sedatives, antianxietics, code: 112) were - 0.6504 (p value < 0.0001), - 0.5744 (p value < 0.0001), and - 0.5675 (p value < 0.0001), respectively (Additional file 3: Table S3).
Regarding the therapeutic categories with negative correlations for inpatient prescriptions, hypnotics and sedatives, antianxietics (code: 112), antiparkinsonism agents (code: 116), antispasmodics (code: 124), diuretics (code: 213), other cardiovascular agents (code: 219), agents for peptic ulcer (code: 232), antacids (code: 234), adrenal hormone preparations (code: 245), and Chinese medicines (code: 520) accounted for 1, 1, 1, 1, 1, 1, 1, 1, and 1 items, respectively (Table 1 and Fig. 6). The correlation coefficients for Marzulene® S-blended granules (l-glutamine, agents for peptic ulcers, code: 232), Ternerin® tablets 1 mg (Tizanidine Hydrochloride, antispasmodics, code: 124), and Diart® tablets 60 mg (Azosemide, diuretics, code: 213) were - 0.4904 (p = 0.0006), - 0.3864 (p value = 0.0073), and - 0.3726 (p value = 0.0099), respectively (Additional file 4: Table S4).
The average drug prices (Japanese yen: mean ± standard deviation) associated with significant negative correlations between the usage rate of generic drugs and monthly personal incomes for outpatient out-of-hospital, outpatient in-hospital, and inpatient prescriptions were 91.4 ± 150.2, 71.1 ± 113.8, and 13.8 ± 9.2, respectively (Table 2).
Discussion
In Japan's medical system, additional insurance premiums are charged according to the usage rate of generic drugs for outpatient out-of-hospital prescriptions, outpatient in-hospital prescriptions, and inpatient prescriptions [27]. However, the usage rate of generic drugs differs among the three. The self-pay rate is essentially 10% for those aged 75 and over, 20% for those aged 70 to 74, 30% for those aged 6 to 69, and 20% for those under 5 [1]. For outpatient out-of-hospital prescriptions and outpatient in-hospital prescriptions, the proportion of medicinal drugs that exhibited a negative correlation between the usage rate of generic drugs and monthly personal incomes was higher than that of inpatient prescriptions (Fig. 3a). The proportion of statistically correlated medicinal drugs for inpatient prescriptions was lower than that for outpatient out-of-hospital and outpatient in-hospital prescriptions (Fig. 3b). This result could be due to the difference in the system of additional insurance premiums for the use of generic drugs among the three groups of patients. Another possible reason is that inpatients are more likely to be aged 70 or more, and have a lower self-pay rate than outpatients [28]. To promote the use of generic drugs, it may be necessary to review the premium system for the use of generic drugs in in-hospital prescriptions.
Moreover, the rate of generic prescription usage for outpatient out-of-hospital and outpatient in-hospital prescriptions increased as monthly personal incomes decreased (Fig. 3a). This result is consistent with previous research findings that show that incentivizing the use of generic antihypertensive drugs was not effective in areas with relatively high-income levels [15]; for the elderly aged 75 and over, those in high-income groups are more likely to choose brand-name drugs than those in general-income groups [8]. These results may contribute to policies targeted at reducing medical costs. For outpatients, the therapeutic categories related to negative correlations were vasodilators and hyperlipidemia drugs. The generic usage rates of these categories of drugs prescribed nationwide for the treatment of lifestyle-related diseases negatively correlated with monthly personal incomes. In addition, we investigated the correlation coefficient for each drug, with no clear trend identified (Figs. 4, 5, 6). Federman et al. reported that the elderly with low incomes or no prescription coverage were more likely to use generic cardiovascular drugs than high-income and insured seniors [29]. A possible reason for this is that people with lower incomes need to reduce their expenditure, and this is done by reducing the cost of drugs for treating lifestyle-related diseases that need to be purchased on a regular basis.
For medicinal drugs with significant negative correlations, the median price for outpatient out-of-hospital and outpatient in-hospital prescriptions was higher than that of inpatient prescriptions (Table 2). This indicates that a high-priced, brand-name drug may be prescribed as a generic drug outside of hospitals. This result, which is related to monthly personal income by region and the usage rate of generic drugs, suggests that the usage of generic drugs reduces medical costs. It is important to consider economic measures to promote the usage of generic drugs among high-income people to further increase the usage rate of generic drugs.
The average price of brand-name drugs that are significantly positive correlated with outpatient in-hospital care was the lowest among the prescription categories. Although this is an interesting discovery, an investigation of the reasons must be reserved for future studies.
Several limitations should be considered when interpreting the NODJ results. The use of medications in the NODJ database is restricted to the top 100 medication codes in each therapeutic category, and not all the NDB medication codes were included. Therefore, the drugs studied were not representative of all the drugs in their respective therapeutic categories. The ratio of the drugs surveyed to all drugs was assumed to vary according to their category.
This study investigated the correlations at the regional prefectural level. Regional correlation studies cannot evaluate the associations between individual-level exposure and outcomes, nor can they elucidate the causal relationships and risk assessment of the usage rate of generic drugs at the individual level. It is important to consider these factors when a prefecture develops measures to increase the usage rate of generic drugs.
Conclusion
Our results may help increase the usage rate of generic drugs in different prefectures by providing useful information for promoting their usage throughout Japan. Furthermore, our statistical analysis may serve as a valuable resource for policymakers attempting to reduce healthcare costs by encouraging the use of generic prescription drugs.
Availability of data and materials
Data used in this study are available from the following links: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00003.html, www.e-stat.go.jp, https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450091&tstat=000001011429&cycle=0&tclass1=000001098975&tclass2=000001098977&tclass3=000001098986&tclass4val=0.
Abbreviations
- MHLW:
-
Ministry of Health, Labour, and Welfare
- BSWS:
-
Basic Survey on Wage Structure
- NDB:
-
National Database of Health Insurance Claims and Specific Health Checkups of Japan
- NODJ:
-
National Database of Health Insurance Claims and Specific Health Checkups of Japan from Open Data Japan
References
Ministry of Health, Labour and Welfare. About medical insurance in Japan. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/iryouhoken01/index.html. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Trends in medical expenses in fiscal year 2019. https://www.mhlw.go.jp/content/12400000/000550869.pdf. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. List of items listed in National Health Insurance (NHI) drug price standards and information on generic drugs (until March 31, 2020). https://www.mhlw.go.jp/topics/2019/08/tp20190819-01.html. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Trends in dispensing medical expenses (computer processing) in fiscal year 2019. https://www.mhlw.go.jp/topics/medias/year/19/dl/gaiyo_data.pdf. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Fiscal year 2020 Generic drug use promotion roadmap verification study project report summary. https://www.mhlw.go.jp/content/10800000/000829151.pdf. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Announcement of the usage rate of generic drugs by insurer (for medical treatment in March, 2019). https://www.mhlw.go.jp/stf/newpage_14403.html. Accessed 10 Feb 2023.
Cabinet Office. Basic Policy on Economic and Financial Management and Reform 2021. https://www5.cao.go.jp/keizai-shimon/kaigi/cabinet/2021/2021_basicpolicies_ja.pdf. Accessed 10 Feb 2023.
Ito Y, Hara K, Yoo B, Tomio J, Kobayashi Y. Can income-based co-payment rates improve disparity? The case of the choice between brand-name and generic drugs. BMC Health Serv Res. 2019;19:780.
Central Social Insurance Medical Council Medical Fee Revision Results Verification Subcommittee. About the results of the special survey (fiscal year 2016 survey) pertaining to the verification of the results of the fiscal year 2016 medical fee revision. https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000152477.pdf. Accessed 10 Feb 2023.
Hamada Y, Uchida M, Arai S, Yamazaki K, Takeda M, Arai K, et al. Analysis of patients’ request to switch from a generic drug to the original drug in external prescriptions. J Pharm Health Care Sci. 2020;6:27.
Mishuk AU, Qian J, Howard JN, Harris I, Frank G, Kiptanui Z, et al. The association between patient sociodemographic characteristics and generic drug use: a systematic review and meta-analysis. J Manag Care Spec Pharm. 2018;24:252–64.
Omojasola A. Predictors of $4 generic prescription drug discount programs use in the low-income population. Res Social Adm Pharm. 2014; 10.
Ministry of Health, Labour and Welfare. Special survey on verification of results of revision of medical fees in 2016 (fiscal year 2017 survey) Impact of measures to promote the use of generic drugs and implementation status survey report. https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000192295.pdf. Accessed 10 Feb 2023.
Yokoi M, Tashiro T. Separating prescription from dispensation medicines: economic effect estimation in Japan. Glob J Health Sci. 2018;10:88–96.
Ohashi H, Nishikawa K. Diffusion of generic drugs and incentive policies: the effect of medical fee revision in FY 2012 on reducing drug costs. Jpn J Health Econ Policy. 2019;31:77–101.
Hellerstein JK. The importance of the physician in the generic versus trade-name prescription decision. RAND J Econ. 1998;29:108–36.
Ministry of Health, Labour and Welfare. 4th NDB open data commentary. https://www.mhlw.go.jp/content/12400000/000711802.pdf. Accessed 10 Feb 2023.
Tsushita K. PDCA of insurance business utilizing data of 20 million specific medical examinations. J Natl Inst Public Health. 2014;63:438–48.
Ministry of Health, Labour and Welfare. 4th NDB open data. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00003.html. Accessed 10 Feb 2023.
Mukai R, Shimada K, Suzuki T, Nakao S, Tanaka M, Matsumoto K, et al. Trends associated with hemorrhoids in Japan: data mining of medical information datasets and the national database of health insurance claims and specific health checkups of Japan (NDB) open data Japan. Biol Pharm Bull. 2020;43:1831–8.
Ono S, Komatsuzaki A, Iguchi A, Kikuchi H, Motoi S, Susuga M. Generic drug usage in dentistry across Japan: analysis using a Japanese national database. Int J Environ Res Public Health. 2021;18:11329.
Itoh H, Saito T, Nojiri S, Hiratsuka Y, Yokoyama K. National burden of the pharmaceutical cost of wet compresses and its cost predictors: nationwide cross-sectional study in Japan. Health Econ Rev. 2019;9:20.
Sakakibara Y, Ochibe T, Amari S, Tohkin M. Study on the risk factors for postoperative delirium using the national health insurance claims database in Japan. Jpn J Pharm Health Care Sci. 2019;45:195–207.
Statistics Bureau of Japan. (Reference table) Cash salary, regular salary, annual bonus and other special salaries paid by prefecture (list of 47 prefectures). https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450091&tstat=000001011429&cycle=0&tclass1=000001098975&tclass2=000001098977&tclass3=000001098986&tclass4val=0. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Results of the 2017 Basic Survey on Wage Structure. https://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2017/dl/12.pdf. Accessed 10 Feb 2023.
Statistics Bureau of Japan. Statistics of Japan. https://www.e-stat.go.jp. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Matters to be noted in implementing the partial revision of the method for calculating medical fees. https://www.mhlw.go.jp/content/12404000/000907837.pdf. Accessed 10 Feb 2023.
Ministry of Health, Labour and Welfare. Overview of the 2017 patient survey. https://www.mhlw.go.jp/toukei/saikin/hw/kanja/17/dl/kanja-01.pdf. Accessed 10 Feb 2023.
Federman AD, Halm EA, Zhu C, Hochman T, Siu AL. Association of income and prescription drug coverage with generic medication use among older adults with hypertension. Am J Manag Care. 2006;12:611–8.
Acknowledgements
Not applicable.
Funding
This research was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number, 20K10408, 21K06646, and 22K10446.
Author information
Authors and Affiliations
Contributions
TS and MN conceived of the study and participated in its design and coordination and drafted the manuscript. MI, MM, MI, and RS conceived of the study and conducted the statistical analysis and drafted the manuscript. WW, KO, HT, SH, KM, and FG collected and analyzed the data. SN and MM helped in the interpretation of the data. KI participated in the design of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Monthly personal income and the usage rate of generic drugs in prescriptions by each prefecture.
Additional file 2: Table S2.
Correlation coefficients between the usage rate of generic drugs and monthly personal income for outpatient out-of-hospital prescriptions in each prefecture.
Additional file 3: Table S3.
Correlation coefficients between the usage rate of generic drugs and monthly personal income for outpatient in-hospital prescriptions in each prefecture.
Additional file 4: Table S4.
Correlation coefficients between the usage rate of generic drugs and monthly personal income for inpatient prescriptions in each prefecture.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Suzuki, T., Iwata, M., Maezawa, M. et al. Promoting generic drug usage in Japan: correlation between generic drug usage and monthly personal income. J of Pharm Policy and Pract 16, 27 (2023). https://doi.org/10.1186/s40545-023-00532-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40545-023-00532-5