Abstract
Background
Plant biostimulants constitute a promising environmentally friendly alternative for increasing crop yield and tolerance to unfavorable conditions. Among various types of such formulations, botanical extracts are gaining more recognition as products supporting plant performance. Moreover, novel tools such as cold-plasma or low-pressure microwave plasma discharge are being proposed as techniques that might improve their efficacy. Elucidation of the biostimulant’s mode of action requires complex research at a molecular level. Transcriptional changes occurring after biostimulant spraying might be investigated using RT-qPCR. However, this technique requires data normalization against stable endogenous controls.
Results
Here, we tested the expression stability of ten candidate genes in soybean plants exposed to various biostimulants treatment. Selection of the best-performing reference genes was conducted using four algorithms (geNorm, NormFinder, BestKeeper, and ΔCt method). According to the obtained results, Bic-C2 (RNA-binding protein Bicaudal-C) and CYP (cyclophilin type peptidyl-prolyl cis–trans isomerase) showed highest expression stability, while expression of EF1B (elongation factor 1-beta) fluctuated the most among a tested set of candidate genes.
Conclusions
Overall, we recommend using Bic-C2 together with CYP for the RT-qPCR data normalization in soybean biostimulation experiments. To our best knowledge, this is the first comprehensive study of reference genes stability in plants subjected to biostimulant treatment. The results of this study will aid in further biostimulant research in crop plants, facilitating analyses performed on the transcriptional level.
Graphical Abstract

Similar content being viewed by others
Background
Changing climate conditions and growing world population require novel solutions to meet the demand for food and feed production without negatively affecting the environment. Various strategies are being implemented to overcome these problems—one of them is the application of biostimulants, which constitute a range of formulations based on natural products used to promote the growth and stress tolerance of crop plants [1]. The main categories of biostimulants comprise humic substances, protein hydrolysates, beneficial fungi and bacteria, chitosan and other biopolymers, seaweed extracts, and botanical extracts [2]. Compared to others, botanical extracts’ mechanism of action is much less characterized. Plant-based biostimulants are rich in biologically active compounds, such as different phytohormones, antioxidants, vitamins, and other secondary metabolites, which improve overall plant performance by acting on various levels and through different pathways [3]. The characterization of these complex multilayer interactions requires more research employing omics tools (transcriptomics, proteomics, metabolomics, and phenomics) [4]. Yet, despite us not totally understanding how, botanical extracts are effective in stimulating crop growth and development under both optimal [5,6,7] and stress conditions [8,9,10].
Horsetail (Equisetum arvense L.), dog rose (Rosa canina L.), and common soapwort (Saponaria officinalis L.) are plants widely known for their therapeutic effects promoted by various bioactive compounds. Horsetail is distinguished by its high content of silicon within its aerial parts [11], which can be useful in two ways, including improved absorption of liquid biostimulant by treated plant and induce the metabolic response of treated plant by the microscopic disruption of its tissues by silicon particles. Moreover, horsetail extract can exhibit antifungal properties [12] and thus can serve as the contact fungicide in the biostimulant. Rosehip, as the fruit of dog rose, is a rich source of antioxidants, especially vitamin C and polyphenolic compounds [13]. Its role in biostimulants can lie in increasing the antioxidant status of treated plants and improving the stability of biostimulants. Soapwort is characterized by a high content of saponins and their glycosides [14]. These compounds are known for their potential toxicity, so their application in biostimulants should be well considered. However, they represent non-ionic biosurfactants with excellent performance [15]. In general, the presence of a surfactant agent as a detergent adjuvant is important for the optimal formulation of agrochemicals, leading to better adhesion on the surface of plants [16]. Except for this, biosurfactants may be applied in plant disease and pest control, boost plant growth through microbial interaction and enhance plant immunity [17]. Concisely, the specific properties of each of these plants predetermine their application within the complex biostimulant.
Since the use of biostimulants has been recognized as a viable method for enhancing crop resilience and yield, scientists are looking for novel ways of improving their mode of action [3, 18]. A recently proposed strategy involves cold-plasma activation of plant-based extracts, including gliding arc plasma discharge [19]. Thus far, non-thermal plasma technology has been applied in agriculture mainly for seed treatment in terms of microbial inactivation and germination improvement [20, 21]. Another reported strategy is using plasma-activated water to enhance plants’ growth and tolerance to abiotic and biotic stresses [22, 23]. This is attributed to the generation of a mixture of reactive oxygen and nitrogen species during plasma discharges, some of which act as signaling molecules in cells and activate plants’ defense systems [24]. Coupling plant-based biostimulants with cold-plasma activation is an innovative approach that has a high potential for improving crop yield in future [19]. Another alternative way that might influence the effectiveness of plant-derived biostimulants is the employment of microwave plasma discharge. This type of plasma treatment can operate under atmospheric pressure so that it can be simply used for the treatment of various biological materials. Along with decontamination [25] or degradation of hazardous compounds, including mycotoxins [26], microwave plasma discharge can be used to improve the extractability of bioactive compounds from plant materials [27]. The inhibition of adverse enzymatic degradation of plant materials was reported after their treatment by microwave plasma discharge [26]. The pretreatment of dried herbs by plasma discharge may potentially improve the chemical properties and stability of derived water extracts and biostimulants, respectively.
Nevertheless, it should be emphasized that the mechanisms underlying the plants’ response to such novel biostimulants have not yet been elucidated. Analysis of transcriptional changes occurring after biostimulant treatment might provide insights into its mode of action. The RT-qPCR technique allows the examination of the expression profiles of various genes related, for instance, to plant redox homeostasis and defense responses, which might be involved in the process. However, this technique is sensitive to various experimental inaccuracies occurring during analysis such as differences in sample quality and quantity, RNA integrity, reverse transcription efficiency, dilution preparation, and pipetting errors. To correct for such non-biological variations, proper data normalization using reference genes (RGs) showing stable expression in tested material are required [28, 29]. Since many studies report spatiotemporal variation in the expression of commonly used reference genes, identification of reliable endogenous controls should precede analyses of genes of interest in every RT-qPCR experiment [28].
Here, we tested the expression stability of ten candidate reference genes in soybean plants sprayed with three different variants of novel plant-based biostimulants. Two variants of biostimulant were formulated using either the gliding arc plasma or low-pressure microwave plasma discharge. To our best knowledge, this is the first report on the identification of reference genes in biostimulant-treated soybean. The results of this study will aid in further biostimulant research in crop plants, facilitating analyses performed on the transcriptional level.
Materials and methods
Preparation of biostimulants
First, three different biostimulants were prepared. The biostimulants were made from the mixture of dried and milled field horsetail (Equisetum arvense L.) stems and branches, dog rose (Rosa canina L.) fruits and soapwort (Saponaria officinalis L.) roots in the following ratio (w/w): 95.3%: 4.6%: 0.1%, respectively. The biostimulants differed according to plasma treatment used. Untreated biostimulant (without plasma application) was prepared as follows: 25 g of the herbal mixture was mixed with 250 ml of water and then extracted at 100 °C for 30 min. Second type of biostimulant was prepared in the same manner as the control biostimulant but was subsequently treated with gliding arc (GA) atmospheric plasma discharge for 30 s with air as a working gas at a flow rate of 30 standard cubic feet per hour. In the case of the third type of the biostimulant, microwave plasma discharge [30] was applied (500 W for 30 s) on the solid herbal mixture. After the MW plasma treatment, the liquid extract was obtained under the same extraction conditions as mentioned above. Pure water served as control. The biostimulants preparation procedure is depicted in Supplementary Figure S1.
Experimental design of biostimulant application on soybean plants
The experimental material consisted of soybean plants (Abaca variety) growing separately in pots in controlled phytotron conditions (25/18 °C, photoperiod 16/8 h day/night, with photosynthetic photon flux density (PPFD) at a plant level of 500–700 µmol m−2 s−1 and 75% relative humidity). The plants were divided into four groups based on the biostimulant used: control (water only), untreated biostimulant, GA biostimulant and MW biostimulant. Each group consisted of 18 plants. The soybean seeds were pregerminated for three days on a moist filter paper. Subsequently, the seeds were sown into a sterile sowing substrate and were grown for a total of 24 days. First application of biostimulants (or water) was applied on 14th day in the form of spraying. After three days (day 17), first nine plants were harvested to collect samples for the analyses. The second spraying of the biostimulants was performed on day 21. Again after three days (day 24), the remaining nine plants were harvested to obtain samples for the analyses. Roots and aerial parts of the plants were separated in both sampling points. The experimental design is shown in Fig. 1.
Every treatment was analyzed in three biological replicates, with each sample consisting of pooled material from three randomly chosen independent plants. Collected samples of leaves and roots were immediately frozen in liquid nitrogen and stored at -80°C until further analysis.
RNA extraction and cDNA synthesis
RNA extraction, reverse transcription, and RT-qPCR reactions were performed using standard protocols which were described in our previous studies [31]. In short, collected samples were immediately homogenized in liquid nitrogen using a sterile mortar and pestle. The isolation of total RNA was performed using TRIzol reagent (Invitrogen) according to the manufacturer’s recommendations. Integrity and quality of RNA samples were evaluated electrophoretically on 1.5% agarose gel and spectrophotometrically with NanoDrop2000 (Thermo Scientific™). The Maxima First Strand cDNA Synthesis Kit for RT-qPCR, with dsDNase (Thermo Scientific™) was used to remove the genomic DNA contamination and conduct the reaction of reverse transcription. The cDNA synthesis was carried out in a final volume of 20 µl using 3 µg of RNA. Obtained cDNA was used as a template in the following RT-qPCR reactions. The good quality of cDNA samples was confirmed via RT-qPCR reactions by analysis of amplification plots, mean Cq values, melt curves, and standard curves. Lack of genomic contamination in the samples was confirmed by NRT controls (no reverse transcriptase control).
RT-qPCR reactions and data analysis
Based on the literature review, five commonly used reference genes CYP (cyclophilin type peptidyl-prolyl cis–trans isomerase), EF1A (elongation factor 1-alpha), EF1B (elongation factor 1-beta), F-box (F-box protein), TUA (tubulin alpha) and five recently identified candidates showing stable expression in soybean Bic-C2 (RNA-binding protein Bicaudal-C), GPX (glutathione peroxidase), IGPS (indole-3-glycerol-phosphate synthase), TIA (apoptosis-promoting RNA-binding protein TIA-1/TIAR), ZnF (zinc finger) were chosen for the evaluation (Table 1) [28, 32,33,34]. Some of the traditionally used reference genes exhibited rather poor expression stability in several reports (e.g., GAPDH [32, 35], UBQ10 [36, 37]), therefore along testing the most promising conventional controls, candidates emerging from RNA-seq data [33, 34] were also included in the experimental setup.
Soybean CDS sequences and gene annotation data were retrieved from Phytozome (Phytozome genome ID: 275, annotation version: Glycine max Wm82.a2.v1) [30, 38]. Primers for RT-qPCR were designed with the PrimerBLAST tool (Supplementary Table S1) [39]. The study employed only primer pairs which showed specific amplification (confirmed with dissociation curve analysis—Supplementary Figure S2) and displayed amplification efficiency of 90–110% and correlation coefficient (R2) over 0.990 (determined via standard curve analysis). The RT-qPCR reactions were performed on the QuantStudio™ 3 apparatus (Applied Biosystems) using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems™). The reactions were conducted in three technical replicates on 20 ng of template cDNA and 400 nM of each primer in 20 µl total volume, using the cycling profile recommended by the supplier.
Gene expression stability was determined using geNorm [44], NormFinder [45], BestKeeper (46), and ΔCt method [47]. Obtained results were subsequently compiled into the overall ranking generated as described in Velada et al. [48]. In short, each gene was assigned a weight according to its stability as assessed by abovementioned algorithms (weight of 1 assigned to the best-performing gene, weight of 10 assigned to the worst-peforming gene). Next, the geometric means of these weights were calculated and the comprehensive ranking was obtained. Three datasets were used in the analysis—the roots samples dataset, the leaves samples dataset, and the full dataset comprising all roots and leaves samples analyzed together. For the validation of selected reference genes, the expression level of target gene SOD encoding superoxide dismutase [Cu/Zn] (NCBI Reference Sequence: NM_001249007.3) was analyzed under tested experimental conditions. The RT-qPCR reactions were conducted as described above using the following primers F: TCCTCTCACTGGACCAAACAA and R: TCATGACCACCTTTCCCAAGATCA. The transcript level of SOD was normalized against the best-performing and the worst-performing candidate reference genes according to the obtained results. The relative expression level of the target gene was calculated using the 2−∆∆Ct method with control samples being used as calibrator.
Results
Determination of candidate RGs expression stability
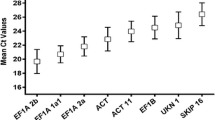
The average expression stability value (M) of reference genes was calculated by the geNorm algorithm. Candidate genes showing stable expression in the tested material have low M values, while those showing variable expression are characterized by high M values [44]. As shown in Fig. 2a, Bic-C2 and CYP were the best-performing pair of genes across all tested samples in this experiment, while EF1B was the worst-performing gene in the full dataset. In the leaves of the soybean plants treated with various biostimulants, the highest expression stability was exhibited by F-box and ZnF, while in the roots CYP and EF1A were considered to be the most stable (Supplementary Table S2).
Analysis performed by NormFinder includes intra- and intergroup variations in the calculation of the stability values (SV), with low SV indicating low expression variability [45]. According to the obtained results, Bic-C2 was identified as the best-scoring gene in full dataset (Fig. 2b) and ranked as second-best when roots and leaf samples were analyzed separately. In both of these datasets, the lowest variation of expression among all tested candidates was demonstrated by F-box (Supplementary Table S2).
The expression stability of tested genes was subsequently evaluated by the BestKeeper algorithm, which determines the correlation coefficient (r) of each candidate with the BestKeeper index (the geometric mean of all candidate genes). High values of correlation coefficient indicate high expression stability of the gene [46]. On the other hand, the ΔCt method ranks the genes based on the average standard deviation (mean SD). Both BestKeeper and ΔCt method produced identical results regarding the two most stably expressed genes in a given dataset. In full dataset, Bic-C2 and CYP were identified as most stable after the biostimulants treatment. Likewise, both calculation methods indicated EF1B as the worst reference gene among all candidates. In the leaves dataset and roots dataset, F-box together with Bic-C2 was designated as the two most stable reference genes. Nevertheless, the gene order in stability rankings generated by both approaches varied in further positions (Fig. 2c, Supplementary Table S2).
To determine how many reference genes should be used for reliable normalization of RT-qPCR data, the additional analysis of pairwise variation (Vn/n+1), was performed in the geNorm algorithm. The pairwise variation value below 0.15 indicates no need for the inclusion of an additional reference [44]. Results obtained in the present study show that a pair of best-performing reference genes is sufficient for accurate normalization of the expression data, regardless of the samples being analyzed in full or separate datasets (Supplementary Figure S3.).
As a final point, the obtained results were compiled into a comprehensive ranking (Table 2). In general, F-box and Bic-C2 were the most stable reference genes in the soybean leaves subjected to the biostimulants treatment. Out of all tested genes, the expression of TUA was the most affected by the experimental conditions used in this study. In roots, Bic-C2 was shown to display more stable expression than F-box, while expression of IGPS fluctuated the most among a tested set of candidate genes. Nevertheless, for the experiments involving both leaves and roots samples of soybean, Bic-C2 together with CYP is recommended as the best pair of controls for the normalization of RT-qPCR data.
Validation of candidate reference genes
The expression of SOD gene was estimated using the best- and the worst-performing reference genes identified in this study. When Bic-C2 and CYP were used as internal controls, the SOD expression in leaves of plants sprayed with different variants of biostimulant remained stable (Fig. 3a). However, when EF1B was used for data normalization, obtained results suggested downregulation of SOD transcription. Moreover, contradictory trends were shown in the roots (Fig. 3b) of the plants treated with biostimulant activated with cold plasma. Depending on the reference genes used in the calculation, SOD expression was either upregulated or downregulated by biostimulant application. This demonstrates the significance of proper reference gene selection and the influence it might have on the reliability of observed expression changes of genes of interest.
The expression profiles of SOD gene in soybean plants after biostimulant treatment. Relative expression level in leaves after first treatment (a) and in roots after second treatment (b). Normalization was performed using a pair of the most stable reference genes (Bic-C2 + CYP) in comparison to the most unstable reference gene (EF1B). Control: water (used as calibrator), Bio: untreated biostimulant, Bio MW: microwave plasma pre-treated biostimulant, Bio GA: gliding arc plasma pre-treated biostimulant. Data represents mean ± SD (n = 3), asterisk represents significant difference (P < 0.05, student’s t-test)
Discussion
Biostimulatory effects of plant extracts have been extensively studied in recent years as an alternative approach for promoting crop growth in sustainable agricultural production [3, 49,50,51,52]. Until now, no relevant studies have described the use of horsetail, dog rose, and soapwort in biostimulants to promote soybean plants growth and stress tolerance. Horsetail, as the main component of biostimulant in this study, is often used in organic farming for various claimed activities [53]. Its use in plant protection products was approved under Regulation of European Commission No. 1107/2009 as a basic substance since it has a preventive effect against fungal diseases due to its high silicon content. As a practical example, horsetail macerate was shown to be a promising Cu-free fungicide effective in protecting tomato plants against late blight [54]. Another study reported that horsetail extract increased the yield and improved the composition of basil essential oil, suggesting its positive effect on the quality of medicinal plants [55]. Polyphenol-rich rosehips, on the other hand, are neither commonly used nor approved for biostimulant production. However, polyphenol-based biostimulants can positively affect plant growth, especially at the root level [56], which is in coherence with using rosehip extracts as the biostimulant constituent in this study. Soapwort extract, as the material loaded with various saponins, is supposed to serve as an adjuvant in biostimulant. It occurs in this research-related biostimulant only to a minor extent, and its main purpose is to increase the efficiency of biostimulant. However, due to its properties, it may also partly act as a biocide. Application of common soapwort in biostimulants has not yet been reported in the scientific literature.
Better understanding of biostimulants’ mode of action requires complex studies conducted on a molecular level [57, 58]. Investigating transcriptional changes occurring after the exogenous application of biostimulatory substances might provide insight into the complex processes leading to the beneficial effects of improved growth, yield and increased resistance to adverse environmental factors [57, 58]. The RT-qPCR is a valuable and precise tool for evaluating changes in gene expression. However, in order to obtain accurate results proper data normalization is required. Thus, the step of reference genes selection is crucial in every experiment involving this technique [59, 60].
Previous studies conducted on soybean regarding reference gene selection focused on evaluating candidate genes’ stability under various abiotic and biotic stresses, in different organs, cultivars, or developmental stages [28, 32, 37, 40,41,42]. Although these results were obtained within the same species, they often are inconsistent or even contradictory, which might be attributed to a particular experimental setup. After testing soybean under different conditions, Wan et al. [28] reported that not a single gene displayed constant expression across all samples. Consequently, as ideal reference might not exist [61], it becomes crucial to precede each gene expression experiment with the identification of proper internal controls.
In this study, we evaluated the expression stability changes occurring in soybean plants subjected to foliar application of different variants of biostimulants. The experimental setup involved expression analysis of genes in both leaves and roots. We tested a set of ten potential reference genes—half of them represented commonly used internal controls (CYP, EF1A, EF1B, F-box, TUA), half comprised less-known but promising candidates (Bic-C2, GPX, IGPS, TIA, ZnF). Obtained data were analyzed via four different approaches (geNorm, NormFinder, BestKeeper, and ΔCt method), and the results were compiled into a comprehensive ranking of gene expression stability in leaves samples, roots samples and in the full dataset.
The results show that, regardless of the dataset, a pair of best-performing genes would be sufficient for gene expression normalization. Overall, Bic-C2 and CYP outperformed all other tested candidates in terms of expression stability in whole plants after biostimulants treatment. In fact, CYP was previously reported as being the most stable in different soybean organs [29], which corroborates our results. At the same time, when the leaves and roots samples were analyzed separately, other gene than CYP was classified as better candidate for data normalization. Along Bic-C2, high expression stability in sample subgroups was exhibited by F-box. In the study by Sharma et al. [43], F-box also showed stable expression in both root and shoot samples of soybean exposed to macronutrient stress (irrespective of the datasets being analyzed together or separately). Likewise, F-box was reported to display stable expression in soybean under other abiotic stresses, such as high salinity (shoots), low temperature (shoots) and dehydration (roots and shoots) [40].
To our best knowledge, this is the first comprehensive study of reference genes stability in plants subjected to biostimulants treatment. Even though gene expression changes in plants caused by biostimulants have been reported before, typically one [62,63,64] or at best three traditional reference genes [65,66,67] were used for data normalization without previous confirmation of their stability in the given experimental setup. Only few studies report testing a small set of three [68] or four [69] internal control candidates before analyzing genes of interest. Using unverified internal controls poses a risk of miscalculating the real expression changes of genes of interest and drawing false conclusions [70]. Therefore, the step of identification of accurate and reliable reference genes should not be omitted in gene expression studies. For instance, here F-box was shown to display the highest expression stability in the soybean leaves treated with biostimulants. Similarly, it was reported as the most stable gene in soybean subjected to viral stress [32]. Nevertheless, in the leaves of soybean exposed to Cd stress, it was ranked as the most unstable candidate [42]. Likewise, ACT was reported as highly stable in adzuki bean (Vigna angularis) under waterlogging stress and rust infection [71], yet it performed poorly when the plants of this species were growing under iron deficiency [72].
Many times some of the traditionally used reference genes, such as GAPDH, have been proven to show rather poor expression stability under given experimental conditions [32, 35, 73]. Therefore, there’s a need to find new candidates for reliable reference. Using RNA-seq datasets might significantly aid in this process. Transcriptome-based identification of novel reference genes has already been conducted in some plant species, e.g., Gossypium hirsutum [74], Allium tuberosum [75], Lactuca sativa [76] or Ardisia kteniophylla [77]. Yim et al. [33] also employed such strategy in order to find better internal controls for soybean studies. One of their newly identified candidates, Bic-C2, outperformed all of the genes tested in this experiment. Another one, GPX, also showed good overall performance as it ranked third in the full dataset. Likewise, Machado et al. [34] analyzed 1298 RNA-seq soybean samples and found 452 genes displaying uniform and constant expression, which might potentially serve as reference gene candidates. Six of them were also tested in this study. While CYP, EF1A and GPX remained stable after biostimulants exposure across both leaves and roots, ZnF, TIA and IGPS exhibited rather average expression stability. Therefore, the verification of candidates emerging from RNA-seq is still needed.
Since being identified as constitutively expressed in soybean [33], Bic-C2 has been used several times for RT-qPCR data normalization [78]. Yet, until now, its stability has not been confirmed by other authors. Based on the obtained results, we recommend Bic-C2 to be used in pair with CYP as reliable internal control in biostimulant-soybean research and to be considered as a worthy candidate for studies conducted in different species.
Conclusions
In summary, in this experiment, we tested the expression stability of ten candidate genes in soybean plants treated with three novel variants of plant biostimulants. The selected candidate genes included five commonly used reference genes: CYP, EF1A, EF1B, F-box, TUA; and five recently identified candidates showing stable expression in soybean: Bic-C2, GPX, IGPS, TIA, ZnF. Comprehensive analysis conducted with four algorithms points to Bic-C2 and CYP as the best-performing pair of reference genes in tested experimental material. The lowest expression stability was shown by one of the traditionally used reference genes, EF1B. Our results confirm that a pair of best-scoring genes will be sufficient for reliable RT-qPCR data normalization. Overall, we recommend Bic-C2 to be used together with CYP as a good internal control in the research of biostimulant applications on soybean plants. Moreover, these two candidate genes could be considered for biostimulation studies conducted in other plant species. The results of this study will aid in elucidating the biostimulant’s mode of action on the transcriptional level.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the first author (Magdalena Sozoniuk) on reasonable request.
Abbreviations
- Bic-C2:
-
RNA-binding protein Bicaudal-C
- cDNA:
-
Complementary deoxyribonucleic acid
- CDS:
-
Coding DNA sequence
- CYP:
-
Cyclophilin type peptidyl-prolyl cis–trans isomerase
- DNA:
-
Deoxyribonucleic acid
- EF1A:
-
Elongation factor 1-alpha
- EF1B:
-
Elongation factor 1-beta
- F-box:
-
F-box protein
- GA:
-
Gliding arc atmospheric plasma discharge
- GPX:
-
Glutathione peroxidase
- IGPS:
-
Indole-3-glycerol-phosphate synthase
- MW:
-
Microwave plasma discharge
- PPFD:
-
Photosynthetic photon flux density
- RGs:
-
Reference genes
- RNA:
-
Ribonucleic acid
- RT-qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- SOD:
-
Superoxide dismutase
- SV:
-
Stability value
- TIA:
-
Apoptosis-promoting RNA-binding protein TIA-1/TIAR
- TUA:
-
Tubulin alpha
- ZnF:
-
Zinc finger
References
Hasanuzzaman M, Parvin K, Bardhan K, Nahar K, Anee TI, Masud AAC, et al. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells. 2021;10(10):2537.
du Jardin P. Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic. 2015;196:3–14.
Bisht A, Chhabra R. Biostimulants: paving way towards sustainable agriculture and food security. Theor Exp Plant Physiol. 2024;36(2):139–63.
Martínez-Lorente SE, Martí-Guillén JM, Pedreño MÁ, Almagro L, Sabater-Jara AB. Higher plant-derived biostimulants: mechanisms of action and their role in mitigating plant abiotic stress. Antioxidants. 2024;13(3):318.
Kocira S, Szparaga A, Krawczuk A, Bartoš P, Zaguła G, Plawgo M, et al. Plant material as a novel tool in designing and formulating modern biostimulants—analysis of botanical extract from Linum usitatissimum L. Materials. 2021;14(21):6661.
Szparaga A, Kocira S, Findura P, Kapusta I, Zaguła G, Świeca M. Uncovering the multi-level response of Glycine max L. to the application of allelopathic biostimulant from Levisticum officinale Koch. Sci Rep. 2021;11(1):15360.
Godlewska K, Biesiada A, Michalak I, Pacyga P. The effect of plant-derived biostimulants on white head cabbage seedlings grown under controlled conditions. Sustainability. 2019;11(19):5317.
Francesca S, Raimondi G, Cirillo V, Maggio A, Barone A, Rigano MM. A novel plant-based biostimulant improves plant performances under drought stress in tomato. Biol Life Sci Forum. 2021;4(1):52.
Naboulsi I, Ben Mrid R, Ennoury A, Zouaoui Z, Nhiri M, Ben Bakrim W, et al. Crataegus oxyacantha extract as a biostimulant to enhance tolerance to salinity in tomato plants. Plants. 2022;11(10):1283.
Pacyga K, Pacyga P, Boba A, Kozak B, Wolko Ł, Kochneva Y, et al. Potential of plant-based extracts to alleviate sorbitol-induced osmotic stress in cabbage seedlings. Plants. 2024;13(6):843.
Peyghambarzadeh S, Babaeinejad T, Hadian J, Fallah A, Ghanavati N. Growth and phytochemical properties of horsetail plant affected by organic and mineral fertilization. SILICON. 2023;15(11):4751–9.
Garcia D, Ramos AJ, Sanchis V, Marín S. Equisetum arvense hydro-alcoholic extract: phenolic composition and antifungal and antimycotoxigenic effect against Aspergillus flavus and Fusarium verticillioides in stored maize. J Sci Food Agric. 2013;93(9):2248–53.
Koczka N, Stefanovits-Bányai É, Ombódi A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines. 2018;5(3):84.
Smułek W, Zdarta A, Pacholak A, Zgoła-Grześkowiak A, Marczak Ł, Jarzębski M, et al. Saponaria officinalis L. extract: surface active properties and impact on environmental bacterial strains. Colloids Surfaces B Biointerfaces. 2017;150:209–15.
Liu Z, Li Z, Zhong H, Zeng G, Liang Y, Chen M, et al. Recent advances in the environmental applications of biosurfactant saponins: a review. J Environ Chem Eng. 2017;5(6):6030–8.
Silva MGC, Medeiros AO, Converti A, Almeida FCG, Sarubbo LA. Biosurfactants: promising biomolecules for agricultural applications. Sustainability. 2024;16(1):449.
Ramesh M, Abinaya S. Chapter 12—Synergistic effect of biosurfactant with bioherbicides and their effectiveness in the management of weeds. In: Inamuddin D, Adetunji CO, editors. Applications of Biosurfactant in Agriculture: Academic Press; 2022. p. 227–44.
Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A. Biostimulants and crop responses: a review. Biol Agric Hortic. 2015;31(1):1–17.
Kocira S, Pérez-Pizá MC, Bohata A, Bartos P, Szparaga A. Cold plasma as a potential activator of plant biostimulants. Sustainability. 2022;14(1):495.
Pańka D, Jeske M, Łukanowski A, Baturo-Cieśniewska A, Prus P, Maitah M, et al. Can cold plasma be used for boosting plant growth and plant protection in sustainable plant production? Agronomy. 2022;12(4):841.
Lin S-P, Khumsupan D, Chou Y-J, Hsieh K-C, Hsu H-Y, Ting Y, et al. Applications of atmospheric cold plasma in agricultural, medical, and bioprocessing industries. Appl Microbiol Biotechnol. 2022;106(23):7737–50.
Gao Y, Francis K, Zhang X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res Int. 2022;157: 111246.
Konchekov EM, Gusein-zade N, Burmistrov DE, Kolik LV, Dorokhov AS, Izmailov AY, et al. Advancements in plasma agriculture: a review of recent studies. Int J Mol Sci. 2023;24(20):15093.
Adhikari B, Adhikari M, Ghimire B, Park G, Choi EH. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci Rep. 2019;9(1):16080.
Shimizu T, Steffes B, Pompl R, Jamitzky F, Bunk W, Ramrath K, et al. Characterization of microwave plasma torch for decontamination. Plasma Processes Polym. 2008;5(6):577–82.
Park BJ, Takatori K, Sugita-Konishi Y, Kim I-H, Lee M-H, Han D-W, et al. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf Coat Technol. 2007;201(9):5733–7.
Wiktor A, Hrycak B, Jasiński M, Rybak K, Kieliszek M, Kraśniewska K, et al. Impact of atmospheric pressure microwave plasma treatment on quality of selected spices. Appl Sci. 2020;10(19):6815.
Wan Q, Chen S, Shan Z, Yang Z, Chen L, Zhang C, et al. Stability evaluation of reference genes for gene expression analysis by RT-qPCR in soybean under different conditions. PLoS ONE. 2017;12(12): e0189405.
Miranda VJ, Coelho RR, Viana AAB, de Oliveira Neto OB, Carneiro RMDG, Rocha TL, et al. Validation of reference genes aiming accurate normalization of qPCR data in soybean upon nematode parasitism and insect attack. BMC Res Notes. 2013;6(1):196.
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–83.
Sozoniuk M, Jamioł M, Kankofer M, Kowalczyk K. Reference gene selection in bovine caruncular epithelial cells under pregnancy-associated hormones exposure. Sci Rep. 2022;12(1):12742.
Bansal R, Mittapelly P, Cassone BJ, Mamidala P, Redinbaugh MG, Michel A. Recommended reference genes for quantitative PCR analysis in soybean have variable stabilities during diverse biotic stresses. PLoS ONE. 2015;10(8): e0134890.
Yim AK-Y, Wong JW-H, Ku Y-S, Qin H, Chan T-F, Lam H-M. Using RNA-seq data to evaluate reference genes suitable for gene expression studies in soybean. PLoS ONE. 2015;10(9):e0136343.
Machado FB, Moharana KC, Almeida-Silva F, Gazara RK, Pedrosa-Silva F, Coelho FS, et al. Systematic analysis of 1298 RNA-Seq samples and construction of a comprehensive soybean (Glycine max) expression atlas. Plant J. 2020;103(5):1894–909.
Sowa S, Sozoniuk M, Toporowska J, Kowalczyk K, Paczos-Grzęda E. Validation of reference genes as an internal control for studying Avena sativa–Puccinia coronata interaction by RT-qPCR. Sci Rep. 2022;12(1):14601.
Jian B, Liu B, Bi Y, Hou W, Wu C, Han T. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Biol. 2008;9(1):59.
Hu R, Fan C, Li H, Zhang Q, Fu Y-F. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol. 2009;10(1):93.
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2011;40(D1):D1178–86.
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012;13(1):134.
Le DT, Aldrich DL, Valliyodan B, Watanabe Y, Ha CV, Nishiyama R, et al. Evaluation of candidate reference genes for normalization of quantitative RT-PCR in soybean tissues under various abiotic stress conditions. PLoS ONE. 2012;7(9): e46487.
Ma S, Niu H, Liu C, Zhang J, Hou C, Wang D. Expression stabilities of candidate reference Genes for RT-qPCR under different stress conditions in soybean. PLoS ONE. 2013;8(10): e75271.
Gao M, Liu Y, Ma X, Shuai Q, Gai J, Li Y. Evaluation of reference genes for normalization of gene expression using quantitative RT-PCR under aluminum, cadmium, and heat stresses in soybean. PLoS ONE. 2017;12(1): e0168965.
Sharma S, Vengavasi K, Kumar MN, Yadav SK, Pandey R. Expression of potential reference genes in response to macronutrient stress in rice and soybean. Gene. 2021;792: 145742.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):research0034.1.
Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Can Res. 2004;64(15):5245–50.
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—excel-based tool using pair-wise correlations. Biotech Lett. 2004;26(6):509–15.
Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7(1):33.
Velada I, Ragonezi C, Arnholdt-Schmitt B, Cardoso H. Reference genes selection and normalization of oxidative stress responsive genes upon different temperature stress conditions in Hypericum perforatum L. PLoS ONE. 2014;9(12): e115206.
Szparaga A, Kocira S, Kapusta I, Zaguła G. Prototyping extracts from Artemisia absinthium L. for their biostimulating properties yield-enhancing, and farmer income-increasing properties. Ind Crops Prod. 2021;160:113125.
Szparaga A, Kocira S, Kapusta I, Zaguła G. Solid–liquid extraction of bioactive compounds as a green alternative for developing novel biostimulant from Linum usitatissimum L. Chem Biol Technol Agric. 2023;10(1):108.
Hafeez MB, Hanif A, Shahzad S, Zahra N, Ahmad B, Kausar A, et al. Chapter 11 - Use of plant water extracts as biostimulants to improve the plant tolerance against abiotic stresses. In: Husen A, editor. Biostimulants in Plant Protection and Performance: Elsevier; 2024. p. 165–84.
Ali Q, Shehzad F, Waseem M, Shahid S, Hussain AI, Haider MZ, et al. Plant-based biostimulants and plant stress responses. In: Hasanuzzaman M, editor., et al., Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I: general consequences and plant responses. Singapore: Springer Singapore; 2020. p. 625–61.
Bertrand C, Gonzalez-Coloma A, Prigent-Combaret C. Chapter Four—Plant metabolomics to the benefit of crop protection and growth stimulation. In: Pétriacq P, Bouchereau A, editors. Advances in Botanical Research. 98: Academic Press; 2021. p. 107–32.
Trebbi G, Negri L, Bosi S, Dinelli G, Cozzo R, Marotti I. Evaluation of Equisetum arvense (Horsetail Macerate) as a copper substitute for pathogen management in field-grown organic tomato and durum wheat cultivations. Agriculture. 2021;11(1):5.
Eghlima G, Chegini KG, Farzaneh M, Aghamir F. Effect of common horsetail extract on growth characteristics, essential oil yield and chemical compositions of basil (Ocimum basilicum L.). Sci Rep. 2024;14(1):11082.
Zuzunaga-Rosas J, González-Orenga S, Tofei AM, Boscaiu M, Moreno-Ramón H, Ibáñez-Asensio S, et al. Effect of a biostimulant based on polyphenols and glycine betaine on tomato plants’ responses to salt stress. Agronomy. 2022;12(9):2142.
Xu L, Trinh HK, Geelen D. Biostimulant Mode of Action. The Chemical Biology of Plant Biostimulants. 2020. p. 245–59.
González-Morales S, Solís-Gaona S, Valdés-Caballero MV, Juárez-Maldonado A, Loredo-Treviño A, Benavides-Mendoza A. Transcriptomics of biostimulation of plants under abiotic stress. Front Genet. 2021;12: 583888.
Chapman JR, Waldenström J. With reference to reference genes: a systematic review of endogenous controls in gene expression studies. PLoS ONE. 2015;10(11): e0141853.
Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends Biotechnol. 2019;37(7):761–74.
Cieslak J, Mackowski M, Czyzak-Runowska G, Wojtowski J, Puppel K, Kuczynska B, et al. Screening for the most suitable reference genes for gene expression studies in equine milk somatic cells. PLoS ONE. 2015;10(10): e0139688.
Mostafa AA, El-Rahman SNA, Shehata S, Abdallah NA, Omar HS. Assessing the effects of a novel biostimulant to enhance leafminer resistance and plant growth on common bean. Sci Rep. 2021;11(1):20020.
Shukla PS, Nivetha N, Nori SS, Bose D, Kumar S, Khandelwal S, et al. Understanding the mode of action of AgroGain®, a biostimulant derived from the red seaweed Kappaphycus alvarezii in the stimulation of cotyledon expansion and growth of Cucumis sativa (cucumber). Front Plant Sci. 2023;14.
Ali O, Farrell AD, Ramsubhag A, Jayaraman J. Beneficial effects of an Ascophyllum nodosum extract on tomato (Solanum lycopersicum L.) during water stress. J Appl Phycol. 2024;36(1):385–97.
Della Lucia MC, Baghdadi A, Mangione F, Borella M, Zegada-Lizarazu W, Ravi S, et al. Transcriptional and physiological analyses to assess the effects of a novel biostimulant in tomato. Front Plant Sci. 2022;12.
Vaseva II, Simova-Stoilova L, Kostadinova A, Yuperlieva-Mateeva B, Karakicheva T, Vassileva V. Heat-stress-mitigating effects of a protein-hydrolysate-based biostimulant are linked to changes in protease, DHN, and HSP gene expression in Maize. Agronomy. 2022;12(5):1127.
Bertoldo G, Chiodi C, Della Lucia MC, Borella M, Ravi S, Baglieri A, et al. Brown seaweed extract (BSE) application influences Auxin- and ABA-related gene expression, root development, and sugar yield in Beta vulgaris L. Plants. 2023;12(4):843.
Łangowski Ł, Goñi O, Quille P, Stephenson P, Carmody N, Feeney E, et al. A plant biostimulant from the seaweed Ascophyllum nodosum (Sealicit) reduces podshatter and yield loss in oilseed rape through modulation of IND expression. Sci Rep. 2019;9(1):16644.
Campobenedetto C, Mannino G, Beekwilder J, Contartese V, Karlova R, Bertea CM. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci Rep. 2021;11(1):354.
Zheng T, Chen Z, Ju Y, Zhang H, Cai M, Pan H, et al. Reference gene selection for qRT-PCR analysis of flower development in Lagerstroemia indica and L. speciosa. PLoS ONE. 2018;13(3):e0195004.
Chi C, Shen Y, Yin L, Ke X, Han D, Zuo Y. Selection and validation of reference genes for gene expression analysis in Vigna angularis using quantitative real-time RT-PCR. PLoS ONE. 2016;11(12): e0168479.
Wang J, Yang K, Zhao B, Li Y, Wan P. Identification and validation of reference genes in the Adzuki Bean (Vigna angularis) under iron deficiency using quantitative real-time PCR. Plant Mol Biol Report. 2020;38(2):250–61.
Pombo MA, Zheng Y, Fei Z, Martin GB, Rosli HG. Use of RNA-seq data to identify and validate RT-qPCR reference genes for studying the tomato-Pseudomonas pathosystem. Sci Rep. 2017;7(1):44905.
Smitha PK, Vishnupriyan K, Kar AS, Anil Kumar M, Bathula C, Chandrashekara KN, et al. Genome wide search to identify reference genes candidates for gene expression analysis in Gossypium hirsutum. BMC Plant Biol. 2019;19(1):405.
Tong J, Hu M, Han B, Ji Y, Wang B, Liang H, et al. Determination of reliable reference genes for gene expression studies in Chinese chive (Allium tuberosum) based on the transcriptome profiling. Sci Rep. 2021;11(1):16558.
Medina-Lozano I, Arnedo MS, Grimplet J, Díaz A. Selection of novel reference genes by RNA-Seq and their evaluation for normalising real-time qPCR expression data of anthocyanin-related genes in lettuce and wild relatives. Int J Mol Sci. 2023;24(3):3052.
Wang W, Zhang X, Xu X, Xu X, Fu L, Chen H. Systematic identification of reference genes for qRT-PCR of Ardisia kteniophylla A. DC under different experimental conditions and for anthocyanin-related genes studies. Front Plant Sci. 2023;14.
Yung W-S, Wang Q, Huang M, Wong F-L, Liu A, Ng M-S, et al. Priming-induced alterations in histone modifications modulate transcriptional responses in soybean under salt stress. Plant J. 2022;109(6):1575–90.
Acknowledgements
This study was supported by Polish National Agency for Academic Exchange in frame of the project “Crucial, long-term collaborations for the development of an innovative, ecological approach in biostimulants production” (BPI/PST/2021/1/00034/U/00001).
Funding
This research was solely funded by the Polish National Agency for Academic Exchange, project No. BPI/PST/2021/1/00034/U/00001 “Crucial, long-term collaborations for the development of an innovative, ecological approach in biostimulants production”.
Author information
Authors and Affiliations
Contributions
MS: conceptualization, methodology, formal analysis, investigation, writing—original draft; MŚ: writing—review and editing; AB: funding acquisition, project administration, supervision; PB: funding acquisition, project administration; JB (Jan Bedrníček): investigation, writing—review and editing, visualization; FL: writing—review and editing, investigation; MJ: investigation; KP: investigation; AS (Adéla Stupková): investigation; JL: investigation; PO: investigation; JB (Jan Bárta): project administration; AS (Agnieszka Szparaga): investigation; methodology; MCPP: investigation; SK: funding acquisition, project administration, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sozoniuk, M., Świeca, M., Bohatá, A. et al. Selection of reference genes for expression profiling in biostimulation research of soybean. Chem. Biol. Technol. Agric. 11, 130 (2024). https://doi.org/10.1186/s40538-024-00660-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-024-00660-3