Abstract
Arbuscular mycorrhizal fungi (AMF) typically exhibit reduced diversity in nutrient-rich soils. However, whether the influence of host plants on AMF within the rhizosphere is direct or indirect through the alteration of soil nutrient levels has yet to be determined. This study explored the diversity and colonization of AMF in the National Tropical Fruit Tree Genebank, where chemical fertilizers are used to preserve minor tropical fruit germplasms. We aimed to understand the direct and indirect effects of host plants on AMF community dynamics, sporulation, and colonization. By analyzing fine roots and rhizosphere soil from 12 tropical fruit trees, we assessed mycorrhizal colonization indices, soil nutrients, AMF spore density, and community structure. Despite their low colonization density and arbuscular abundance, all the tree roots harbored AMF, with spore densities ranging from 24.00 to 204.80 spores/100 g of dry soil. High-throughput sequencing identified 207 AMF virtual taxa (VTs) from 60 soil samples, with dominant taxa, including early-evolved Paraglomus fungi and ruderal AMF, being minimally affected by soil nutrient levels. Interestingly, there was no correlation between α diversity and spore density. Structural equation modeling (SEM) indicated that host plant evolutionary divergence time (ET) directly influenced AMF α diversity indices and indirectly impacted spore density via soil nutrients. However, neither ETs nor soil nutrients significantly affected the AMF community structure, with only arbuscular abundance showing a negative correlation with ET. This research underscores the intricate relationship between host plants and AMF in genebanks, offering insights for effective AMF resource management and informed conservation practices.
Graphical Abstract

Similar content being viewed by others
Introduction
Field genebanks play a critical role in supporting future food security by preserving plant genetic resources over the long term [1]. However, these genebanks are often primarily seen as repositories for resource conservation [2], leading to a lack of attention on the intricate microbial ecosystem in the soil environment where plants in field genebanks thrive. The relationship between plant roots and microorganisms is a result of complex molecular interactions that develop through the long-term coevolution of plant hosts and microbial partners [3]. Among these beneficial root associations, arbuscular mycorrhizae (AM) represent mutualistic symbioses that develop between the roots of 72% of vascular plants and arbuscular mycorrhizal fungi (AMF) [4]. Tropical fruit trees are an important source of edible fruits and economic income, especially in tropical and subtropical regions of China [5]. While some staple tropical fruits, such as avocado (Persea americana), banana (Musa nana), litchi (Litchi chinensis), and mango (Mangifera indica), have been shown to form AM associations [6,7,8], the mycorrhizal relationships of many less prominent tropical fruit trees (those with smaller production volumes traded in regional markets [9]) remain poorly characterized.
The presence of AMF in rhizosphere soil serves as the basis for the establishment of AM symbiosis within host plants. Predictable variations in AMF colonization within host roots and the diversity of AMF populations in rhizosphere soil have been observed in relation to soil pH and nutrient levels [10,11,12]. Many tropical ecosystems are characterized by low soil pH and limited nutrient availability [13, 14], leading to a general assumption of low species diversity among AMF communities in such environments [15]. However, ongoing research into AMF diversity, coupled with advances in high-throughput sequencing technology, has revealed that the species diversity and genetic variability in AMF in tropical ecosystems may have been substantially underestimated [16,17,18,19]. Moreover, it is important to highlight that AMF colonization and the diversity of AMF communities are being studied in the context of field genebanks situated in ultra-acidic, infertile soil with substantial nutrient inputs from manure. These genebanks make substantial investments in the application of rapid-acting nutrients to safeguard germplasm resources [20].
AMF receive lipids [21] and sugars [22] from their photosynthetic hosts in exchange for essential mineral nutrients. Consequently, host plants have the potential to significantly influence microbial communities within rhizosphere soils [23]. Recent investigations in grassland ecosystems have suggested that alterations in host plant species can trigger shifts in AMF diversity and community composition within rhizosphere soils [24,25,26]. Conversely, a separate study reported that 31 different cultivars of durum wheat (Triticum turgidum L. var. durum) had similar AMF communities under field conditions in Eastern Canada [23]. However, the aforementioned studies did not quantify the precise impact of host plants on AMF colonization and community assembly. Fortunately, the R package V.PhyloMaker [27, 28] offers a valuable tool for obtaining phylogenetic information about various plant species directly, obviating the need for genetic analysis. Importantly, different plant species exhibit varying nutrient requirements [29], and nutrient levels within the rhizosphere soil frequently influence both AMF colonization of host plants and the composition of microbial communities [10,11,12]. As such, it remains unknown whether host plants have a direct effect on AMF colonization metrics and community structure or this effect is mediated through the nutrient conditions of the rhizosphere soil. This represents an area of research that deserves further investigation. The National Tropical Fruit Tree Field Genebank, located in Zhanjiang city, China, encompasses more than 1300 germplasm resources representing 35 minor tropical fruit species, all within an 8-hectare area that maintains consistent initial soil nutrient levels and manure input conditions. Consequently, this site offers a promising opportunity to explore both the direct and indirect effects of host plants on AMF colonization parameters and community structure.
Therefore, we conducted a comprehensive study involving the collection of fine root and rhizosphere soil samples from 12 different minor tropical fruit trees within the confines of the National Tropical Fruit Tree Field Genebank. Our investigation included quantifying mycorrhizal colonization rates in fine roots, analyzing physiochemical properties and AMF spore density in rhizosphere soil, and utilizing high-throughput sequencing techniques to assess AMF diversity and community composition. Subsequently, we employed V.PhyloMaker to quantitatively estimate the evolutionary divergence time of the phylogenetic relationships among the various plant species. This information enabled us to scrutinize disparities in AMF community structure among fruit trees with differing evolutionary divergence times. Furthermore, we employed structural equation modeling (SEM) [30, 31] to systematically evaluate both the direct and indirect influences of host plants on AMF colonization and community structure. This research was primarily undertaken to achieve two major objectives: (1) to characterize AMF colonization and community diversity in 12 minor tropical fruit trees under ultra-acidic and infertile soil conditions coupled with substantial nutrient inputs from manure and (2) to ascertain whether the impact of host plants on AMF colonization metrics and community structure is direct or mediated by the nutrient conditions present in the rhizosphere soil.
Materials and methods
Study site and plant materials
The National Tropical Fruit Tree Field Genebank (GPS coordinates: 21°9′58″N; 110°16′47″E), established in 2012, is located near the city of Zhanjiang, Guangdong Province, China. This region features a tropical monsoon climate characterized by a mean annual temperature of 23.1 °C and an annual precipitation of 1750 mm. Approximately 70% of the precipitation occurs during the rainy season, spanning from April to September. The predominant soil type in this zone is latosol. Prior to the establishment of the Field Genebank, the 8-hectare core area was dedicated to pineapple (Ananas comosus) cultivation. The soil physicochemical properties of this 8-hectare core area were assessed using a random sampling approach during the establishment of the field genebank, and no significant differences were observed among samples (Additional file 4: Table S1). Since 2012, compound fertilizer (N:P2O5:K2O = 15:15:15) has been applied to the core area at a rate of 750 kg/ha annually through integrated water and fertilizer management; additionally, weed control and irrigation have been applied as needed based on the actual conditions. Fine root and soil samples were collected from 12 taxa representing 10 species of the same age: Averrhoa carambola (classified under Oxalidaceae, Ac); Annona squamosa (classified under Annonaceae, As); Clausena excavata (Ce) and 3 C. lansium cultivars (referred to as ClaA, ClaB, and ClaC); Hylocereus undatus (classified under Cactaceae, Hu); Psidium guajava (Pg); Rhodomyrtus tomentosa (Rt); Syzygium samarangense (classified under Myrtaceae, Ss); Synsepalum dulcificum (classified under Sapotaceae, Sd); and Ziziphus mauritiana (classified under Rhamnaceae, Zm).
Sample collection
Fine root and rhizosphere soil samples were collected to characterize the mycorrhizal colonization rates and AMF communities associated with the 12 minor tropical fruit trees. The sampling was conducted on June 23, 2020. For each minor tropical fruit tree taxon, five replicates were randomly selected from within the resource conservation area. The fine plant roots were carefully excavated and subsequently rinsed with distilled water. The collected roots for each replicate were then preserved in phials filled with formaldehyde–acetic acid–ethanol (FAA) fixative [32]. These phials were stored at 4 °C in a laboratory refrigerator until further processing. Rhizosphere soil samples were collected from the soil within a 1 cm radius of the fine roots and subsequently sieved through a 0.5-mm mesh [24]. The sieved rhizosphere soil from each replicate was divided into two parts: one part was transferred to a 50-mL freezing tube and stored at − 80 °C, while the other part was air-dried for subsequent analysis of soil properties and determination of AMF spore density in the rhizosphere soil.
Root colonization, soil properties and AMF spore density analysis
Root samples were prepared using the 'ink and vinegar' staining technique outlined in Stefani et al.'s work [23]. In each root sample, 30 random root fragments, each approximately 1 cm in length, were selected and examined for the presence of arbuscules, vesicles, and hyphae. The mycorrhizal colonization rate was determined using Mycocale software, and the relevant grading standards described in Votta et al.'s publication [33] were applied for assessment; colonization frequency (F), colonization density (M) and arbuscular abundance (A) were calculated. Seven soil properties, including total nitrogen (TN, determined using the Kjeldahl nitrogen method), total phosphorus (TP, determined using the molybdenum antimony anti-colorimetric method), total potassium (TK, determined using the flame photometer atomic absorption method), total organic carbon (TOC, determined using the potassium dichromate heating method), available phosphorus (AP, determined using the sodium bicarbonate leaching-colorimetric method), available potassium (AK, determined using the flame photometer atomic absorption method), and soil pH (determined using the acidimetry method), were evaluated following the procedures outlined in Bao [34]. For the convenience of subsequent analysis, we conducted principal component analysis (PCA) [35] on the soil nutrient indicators of 60 samples. For the separation of AMF spores, 25 g of each air-dried soil sample was subjected to wet sieving and sucrose centrifugation. The isolated spores were then placed in a Petri dish filled with distilled water and observed under a stereomicroscope, after which the spore density was calculated and is expressed as the number of AMF spores per 100 g of air-dried soil [6].
DNA sequencing
Total soil DNA was extracted from 0.25 g of rhizosphere soil using a DNeasy PowerSoil Kit (QIAGEN China Co. Ltd., Shanghai, China) following the manufacturer's instructions. The DNA concentration and purity were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, MA, USA) and 1.2% agarose gel electrophoresis. To characterize the AMF communities, the primer pairs AMV4.5NF (5’-AAGCTCGTAGTTGAATTTCG-3’) and AMDGR (5′-CCCAACTATCCCTATTAATCAT-3′) [36] were used. PCR amplification was carried out in a 25-µL reaction mixture as follows: 2 µL of DNA template, 5 μL of 5 × reaction buffer, 5 μL of 5 × GC buffer, 2 μL of dNTPs (2.5 mmol/L), 1 μL of AMV4.5NF (10 µmol/L), 1 μL of AMDGR (10 µmol/L), 8.75 μL of ddH2O, and 0.25 μL of Q5 high-fidelity DNA polymerase (NEB China Co. Ltd., Beijing, China). PCR amplification was carried out using an Applied Biosystems 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) with the following conditions: initial denaturation at 98 °C for 2 min; 30 cycles of thermocycling (98 °C for 15 s denaturation, 55 °C for 30 s annealing, 72 °C for 30 s extension); final extension at 72 °C for 5 min; and holding at 10 °C. The PCR products were purified using Vazyme VAHTSTM DNA Clean Beads (Vazyme Biotech Co., Ltd., Nanjing, China), and fluorescence was quantified using an FLx800 microplate reader (BioTek Instruments, Inc., Vermont, USA) and a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). The sequencing libraries were constructed, and high-throughput sequencing was performed by Shanghai Oebiotech Co. Ltd. (Shanghai, China) following procedures consistent with those of previous studies [6, 23] utilizing the Illumina platform to study AMF communities. The library was subsequently sequenced on an Illumina NovaSeq 6000 platform (Illumina, Inc., Hayward, CA, USA), generating 250 bp paired-end reads (PE250).
Sequencing data processing
The initial processing of the raw sequencing reads was performed with Vsearch software (version 2.13.4 for Linux x86_64) [37]. This process involved multiple steps, including read merging (which included decompression of the sequencing data, batch processing according to the experimental design, and consolidation of all the samples into a single file) and quality control (primarily focused on removing amplification primer sequences). Subsequently, denoising was performed using the unoise3 algorithm, and the data were filtered to eliminate chimeras. The central sequences in the output represented amplicon sequence variants (ASVs). The assignment of ASVs to AMF virtual taxa (VT) was carried out using the MaarjAM databases (http://maarjam.botany.ut.ee/, released on June 5th, 2019) [38] and the method described in Jiang et al. [6]. Taxonomic information for all the AMF VTs was obtained from the taxonomic results of representative sequences in the National Center for Biotechnology Information (NCBI) Taxonomy database (downloaded from https://ftp.ncbi.nlm.nih.gov/pub/taxonomy/ on April 20th, 2022) and the taxonomic data available in the Glomeromycota PHYLOGENY (http://www.amf-phylogeny.com/, last updated in 2021).
Subsequently, a taxonomic tree was constructed using the ggtree package [39] in the R programming environment. Furthermore, to determine the composition of the AMF community in this study, PCA was conducted using AMF classification and abundance data from 60 samples at both the family and order levels of AMF taxonomy. To assess α diversity, four indices, namely, richness (observed VTs), the Hill–Shannon index, the Hill–Simpson index, and Pielou's evenness, were calculated using QIIME2 software [40]. The results were visualized in a boxplot using R. To ensure consistency, subsequent analysis (β diversity) was performed at the same sequencing depth [41]. Therefore, all the sequencing data were subsampled to 95% of the sequence amount in the minimum sample using the 'qiime feature-table rarefy' function in QIIME2 software [40].
Impact of host plants on the AMF community
To examine the impact of host plants on the AMF community within rhizosphere soil. Our study initially utilized the V.PhyloMaker package [27, 28] in the R programming environment, this analysis focused on the phylogeny of ten species of minor tropical fruit trees (including 3 distinct cultivars of C. lansium). Subsequently, we conducted linear discriminant analysis effect size (LEfSe) analysis [41] using the evolutionary divergence times and subsampled AMF community data from different samples. The thresholds for the Kruskal‒Wallis (KW) and Wilcoxon tests were both set at 0.05, while the linear discriminant analysis (LDA) threshold was set to 3.0, this step was undertaken to analyze whether plants with different ETs exhibit a preference for specific AMF associations. Finally, nonmetric multidimensional scaling (NMDS) ordination was performed at the cultivar, species, genus, and family levels for the host plants using the Vegan [42] and ggplot2 [43] packages in R. The ordination was based on the binary Jaccard dissimilarity [44] of distinct plant AMF communities.
Construction of the structural equation model
To elucidate the connections among host plants, as quantified by their evolutionary divergence times (ETs), rhizosphere soil nutrient indicators (represented by the first 2 axes resulting from PCA conducted on the 7 soil nutrient parameters), AMF spore density, AMF colonization (F, M, and A), α diversity metrics (including richness, Shannon index, and Pielou's evenness), and the community structure of rhizosphere AMF (as depicted by the first 2 axes of the PCA at the order level of AMF taxonomy), we constructed two structural equation models. These structural equation models were constructed using the "psem" function within the R package “piecewiseSEM” [45]. This analytical approach allowed us to dissect the network into individual linear regressions that corresponded to the evolutionary divergence times of distinct host plants and rhizosphere soil nutrient indicators. Additionally, a generalized linear mixed-effects model was constructed for spore density, colonization status, α diversity, and the community structure of rhizosphere AMF, with each aspect assessed independently. These individual components were subsequently amalgamated to generate insights into the entire structural equation model. Within these models, we categorized the relationships between various indicators as either associative (indicated by solid red arrows for paths with P values less than 0.05) or independent (represented by black dotted arrows for paths with P values exceeding 0.05). We then assessed the goodness of fit of the model using Fisher's C statistic, considering the model to adequately fit the data when the P value exceeded 0.05 [45].
Statistical analyses
The normality of the 7 soil nutrient parameters, AMF spore density, AMF colonization status, and α diversity were assessed using the Kolmogorov‒Smirnov test [46]. Subsequently, the homoscedasticity of the aforementioned data was evaluated through the Levene test [47]. When the data did not satisfy the assumptions of normality or homogeneity, data transformation was applied to enhance the distribution of the residuals (refer to Additional file 5: Table S2). The influence of the host plant on the aforementioned 15 parameters in Additional file 5: Table S2 was examined using one-way analysis of variance (ANOVA). The Kolmogorov‒Smirnov test, Levene test, and one-way ANOVA were conducted within the R programming environment following the methodology described in a study on Argania spinosa [48].
Results
Soil properties and AMF colonization
Under the established nutrient inputs, there was notable variation in rhizosphere nutrient levels among the 12 different plants (Additional file 1: Fig. S1). Notably, C. excavata plants exhibited the highest soil pH but also had the lowest soil nutrient content within their rhizosphere, apart from the levels of TK and AK. According to the classification criteria outlined in the second national soil survey of China, the soil present within the National Tropical Fruit Tree Field Genebank was classified as an ultra-highly acidic latosol, and all the rhizosphere soil samples exhibited pH values less than 4.5 (Additional file 1: Fig. S1). The TP content across all the samples was predominantly classified as grade II and grade III. Furthermore, other soil total nutrients, including TN, TK, and TOC, were mostly categorized as grade IV and grade V. Conversely, for the two available nutrients, AP and AK, the majority of the 60 samples were classified as grade I (Additional file 1: Fig. S1). Interestingly, the PCA conducted on the 7 soil nutrient parameters, as represented in Fig. 1, highlighted a distinctive pattern. Nutrients with lower grades were primarily associated with PC1, explaining 56.50% of the total variation; these nutrients are referred to as "barren soil factors" (abbreviated as BSF). Conversely, indices with higher grades were predominantly associated with PC2, which accounted for 19.78% of the total variation, and were referred to as "fertile soil factors" (abbreviated as FSF).
The principal component analysis (PCA) plot includes 7 soil properties, namely, total nitrogen (TN), total phosphorus (TP), total potassium (TK), total organic carbon (TOC), available phosphorus (AP), available potassium (AK), and soil pH, for the 60 samples considered in this study. The loadings of 4 nutrient factors—TN, TK, TOC, and soil pH—classified as having low values in accordance with the data from the second national soil survey in China, are denoted by the gray arrows. The loadings of 3 nutrient factors—AP, AK, and TP—categorized as having high values according to the data from the second national soil survey in China are depicted by the black arrows. The abbreviations corresponding to the 12 plants are documented in the Materials and methods section
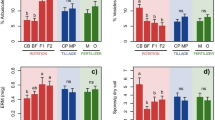
All 12 varieties of minor tropical fruit trees were found to be associated with AMF, with characteristic AM structures, including arbuscules, vesicles, and intraradical hyphae, clearly identified in their fine roots (Additional file 2: Fig. S2). Figure 2 presents the three principal indices of AM fungal colonization within the fine roots of all the minor tropical fruit trees studied in this investigation. Remarkably, C. excavata demonstrated the highest root mycorrhizal colonization density (M) and arbuscular abundance (A). The spore density ranged from 24.00 to 204.80 spores/100 g of air-dried rhizosphere soil across the 12 fruit trees, and the rhizosphere soil of C. excavata exhibited the highest density of AMF spores (Fig. 2).
The AM colonization frequency (F, black bar), colonization density (M, red bar), arbuscular abundance (A, green bar) and spore density (blue bar) for 12 kinds of minor tropical fruit trees in the National Tropical Fruit Tree Field Genebank. Data are shown as the means of 5 replicates with standard errors. For the same index, the same lowercase letters on the bar indicate that there are no significant differences between the two plant species (P < 0.05). The normality test and homoscedasticity test results of the 3 mycorrhizal colonization indices are listed in Additional file 4: Table S1. The abbreviations corresponding to the 12 plants are documented in the Materials and methods section
AMF communities in the rhizosphere soil of 12 minor tropical fruit trees
Following the processing of the initial raw reads, all the sequences were aligned against the MaarjAM database, yielding a collection of 207 AMF VTs (along with 4,681 AMF ASVs; for detailed information, refer to Additional file 6: Table S3). To visually represent the relative abundance of AMF communities among various plants, a taxonomic tree (depicted in Fig. 3) was constructed utilizing the recently identified AMF taxa and their respective abundance levels. A diverse AMF composition was observed across the 60 rhizosphere soil samples, encompassing a total of 4 orders, 11 families, and 21 genera. Notably, the predominant majority of the AMF were classified under the order Glomerales, which accounted for 57.118% of the total sequences. Additionally, a substantial portion of the sequences belonged to the order Paraglomerales, accounting for 31.622% of the sequences. At the family level, the prevailing groups were Glomeraceae (constituting 51.634% of the sequences) and Paraglomeraceae (making up 31.622% of the sequences). Upon further examination at the genus level, it was evident that the relative abundances of Paraglomus (31.619%), Rhizophagus (23.986%), Glomus (19.026%), and Claroideoglomus (5.484%) exceeded the threshold of 5%.
The taxonomic hierarchy tree is accompanied by abundance information. Each branch node within the taxonomic hierarchy tree is represented by a pie chart, which depicts the distribution of each AMF taxon associated with each plant variety. The size of the pie chart segments corresponds to the proportion of the respective taxon within the AMF community of the specific plants. The abbreviations corresponding to the 12 plants are documented in the Materials and methods section
The results of the PCA conducted at the family level are depicted in Fig. 4A. These results revealed that the foremost four loadings of PC1, which accounted for 19.53% of the total variation, corresponded to the families Glomeraceae, Paraglomeraceae, Claroideoglomeraceae, and Ambisporaceae. Notably, these 4 families also exhibited the highest relative abundances within the AMF communities across all 60 samples, as shown in Fig. 3. The primary loading for PC2, which explained 16.38% of the total variance, was attributed to Sacculosporaceae (as illustrated in Fig. 4A). Interestingly, Sacculosporaceae constituted only a marginal relative abundance of 0.002% within the entire community, as evident from Fig. 3. Consequently, in the context of PCA performed at the order level (as presented in Fig. 4B), PC1 (explaining 50.14% of the total variation) is representative of represent dominant species, whereas PC2 (accounting for 25.82% of the total variation) is representative of rare species.
The principal component analysis (PCA) plot shows the AMF communities across the 60 samples, differentiated at the family level (A) and order level (B). The red arrow signifies an AMF VT with substantial relative abundance across the entire community, whereas the green arrows denote AMF VTs with lower relative abundances
In relation to the α diversity observed within the AMF community, the richness, quantified by the number of observed VTs, varied across the 12 plants, ranging from 54.4 to 78 (Fig. 5). The Hill–Shannon and Hill–Simpson indices ranged from 4.86 to 14.38 and from 1.21 to 2.04, respectively, across the different plants. Additionally, the Pielou's evenness values ranged between 0.36 and 0.60 for the diverse plants. Remarkably, these 4 diversity indices were notably lower or higher for C. lansium ‘A’ than for the other plants (Fig. 5), as confirmed through the Kolmogorov‒Smirnov test and Levene test (as detailed in Additional file 5: Table S2). Conversely, the highest levels of α diversity were observed for the A. squamosa and Z. mauritiana plants (Fig. 5).
The boxplot illustrates the α diversity observed within the AMF communities of 12 fruit trees. The included α diversity indices encompass richness, the Shannon index, Simpson’s index, and Pielou's evenness. The presented data represent the means derived from 5 replicates, accompanied by their corresponding standard errors. In the case of the same α diversity index, identical lowercase letters displayed on the bars indicate the absence of significant differences between two plant species (P < 0.05). The results of the normality test and homoscedasticity test for the 4 α diversity indices are comprehensively outlined in Additional file 4: Table S1. The abbreviations corresponding to the 12 plants are documented in the Materials and methods section
Influence of host plant on AMF community composition
The phylogenetic analyses conducted using V.PhyloMaker are illustrated in Fig. 6A, revealing the initial divergence of the 10 minor tropical fruit tree species examined in this study. Specifically, the differentiation of A. squamosa plants commenced approximately 135.9 million years ago. The separation of the two Clausena species occurred approximately 12.3 million years ago, whereas the distinction among the three Myrtaceae species took place 29 million years ago (R. tomentosa and P. guajava plants) and 35.9 million years ago (Syzygium samarangense plants). Through the categorization of these 10 species based on their respective evolutionary separation times, the LEfSE analysis depicted in Fig. 6B demonstrated notable trends. These results revealed that the relative abundance of Paraglomeraceae was significantly elevated in R. tomentosa and P. guajava plants. Conversely, the abundance of Glomeraceae exhibited a significant increase in the three C. lansium cultivars and C. excavata. At the genus level, the relative abundance of Rhizophagus was notably greater in the Syzygium samarangense plants (see Fig. 6B). Interestingly, the NMDS analysis of the AMF communities in the rhizospheres of plants with varying evolutionary separation times (Additional file 3: Fig. S3) coincidentally revealed that host plants with either excessively short or exceptionally long evolutionary separation times did not influence the composition of the AMF community.
Cladograms illustrating the phylogenetic relationships generated by V.PhyloMaker for the 10 plant species investigated in the current study (A). In the context of the phylogeny for these 10 plant species, the length of the transverse segments signifies the evolutionary divergence time (measured in millions of years) among the various plants. The LEfSe analysis (B) was performed based on the relative abundance of different VT types within the AMF community of each sample. This diagram showcases the distinct AMF VTs across diverse plant groups. The 10 minor tropical fruit plant species were categorized according to their distinct evolutionary divergence times: As (135.9), Hu_Sd (119.9), Ac_Zm (115.8), Ss (35.9), Pg_Rt (29), and Ce_ClaA_ClaB_ClaC (12.3). The abbreviations corresponding to the 12 plants are documented in the Materials and methods section
The relationships among the evolutionary divergence times of host plants, soil factors and AMF parameters
After analyzing the rhizosphere nutrient content and various AMF parameters (including mycorrhizal colonization, spore density, α diversity, and community structure) among 12 distinct rare tropical fruit plants, it became evident that these factors exhibited variability. This variance occurred despite the presence of identical soil nutrient levels and consistent manual nutrient inputs. To further explore this phenomenon, we employed structural equation modeling (SEM) to explore the direct and indirect influences of host plants on AMF parameters. This quantification was based on the ET of different plant species.
The SEM proved to be an excellent fit for our data, as indicated by Fisher’s C statistic of 1.249 with a corresponding P value of 0.536, computed over 2 degrees of freedom. Specifically, we observed that the ET of host plants had a direct and positive significant impact on three α diversity indices of the AMF community. Notably, ET played a substantial role in enhancing richness, with a path coefficient of 0.6816 and a significance level of P < 0.001 (as illustrated in Fig. 7). Among the three mycorrhizal colonization indices, only the abundance of arbuscular structures (A) exhibited a direct correlation with ET, where the path coefficient was -0.3340 and the significance level was P < 0.05 (as depicted in Fig. 7). Interestingly, ET did not directly influence the AMF spore density; however, it did have a positive effect on both barren soil factors (BSFs) and fertile soil factors (FSFs). Notably, the density of AMF spores in the rhizosphere soil was directly affected by FSFs, with a path coefficient of -0.5890 and a significance level of P < 0.001 (as demonstrated in Fig. 7). Neither ET nor soil nutrients had a significant impact on the structure of the AMF community.
A structural equation model was constructed to depict the hypothesized causal relationships among 7 variables, including the evolutionary divergence times (ETs) of different plant species, 2 soil nutrient factors, and 4 AMF parameters. The 2 soil nutrient factors correspond to the first two principal components derived from a PCA of seven soil properties, namely, barren soil factors (BSFs) and fertile soil factors (FSFs), as depicted in Fig. 1. The AMF parameters encompass mycorrhizal colonization, spore density, α diversity, and community structure. In the structural equation model, the mycorrhizal colonization indices employed included colonization frequency (F), colonization density (M), and arbuscular abundance (A). Additionally, 3 α diversity indices, namely, richness, Shannon index, and Pielou's evenness, were included in the current SEM. Similar to the approach used for soil nutrient factors, the first two axes of the principle component analysis (PCA) at the order level (as shown in Fig. 4B) were utilized to quantify data on community structure. In the diagrams, the red solid arrows signify significant relationships between two indices in a given pathway, while the black dashed arrows denote nonsignificant relationships within that pathway. The numbers above the arrows represent path coefficients, with positive and negative values indicating positive and negative relationships between the two indicators, respectively. An asterisk (*) denotes a path with a P value less than 0.05, ** signifies P < 0.01, and *** indicates P < 0.001
Discussion
AMF colonization of minor tropical fruit trees in the National Tropical Fruit Tree Field Genebank
Tropical soils are generally considered highly acidic with relatively low nutrient levels [49]. According to the classification standards of the second national soil survey in China [50], before its establishment, the soil in the National Tropical Fruit Tree Field Genebank exhibited strong acidity, with all nutrient indicators being relatively low (Additional file 4: Table S1). With 9 consecutive years of chemical fertilizer application, the soil pH further decreased, but the contents of soil nutrients, especially available nutrients, significantly increased (the contents of AP and AK in the rhizosphere of most plants significantly exceeded the grade I standards, Additional file 1: Fig. S1). All the fine root samples in the present work were associated with AMF (Additional file 2: Fig. S2 and Fig. 2). However, the mycorrhizal colonization density (M) and arbuscular abundance (A) of all the samples were relatively low in this soil environment, and the highest M and A were found in C. excavata plants, at only 26.49% and 19.14%, respectively (Fig. 2). Similarly, compared with those in 12 other plants, the nutrient contents in C. excavata plants were consistently among the lowest (Additional file 1: Fig. S1). There are two pathways for the absorption of mineral elements by plants: the direct root cortical cell absorption pathway and the mycorrhizal pathway [51]. In cases where there is an ample supply of available nutrients in the soil, the plant's reliance on the mycorrhizal absorption pathway is significantly diminished [52]. Within the context of AM symbiosis, the host plant provides organic compounds to AMF with the prerequisite that AMF supplies mineral elements to the host plant [53]. As a result, in the National Tropical Fruit Tree Field Genebank, the extensive application of chemical fertilizers conspicuously suppressed the symbiotic relationship between fruit trees and AMF.
AMF community in 12 kinds of minor tropical fruit trees
The traditional morphological identification method has clear limitations in assessing the true distinctions among AMF communities because it relies heavily on the experience of the evaluator [54]. Therefore, through the utilization of high-throughput sequencing technology, we investigated AMF resources in the rhizosphere soils of 12 varieties of minor tropical fruit trees located within the National Tropical Fruit Tree Field Genebank. Similar to molecular identification-based research conducted on AMF communities in the rhizosphere soils of other plant species [6, 55, 56], this study identified numerous ASVs as Glomeromycota fungi. Among the studied plant species, the rhizosphere soil of C. excavata plants presented the highest number of identified ASVs, with an average of 70,540 ASVs per sample (Additional file 6: Table S3). This observation was consistent with the findings that C. excavata, the plant with the highest spore count in the rhizosphere soil, yielded consistent results (Fig. 2).
The National Tropical Fruit Tree Field Genebank, situated in southern China, has been documented as having Glomeraceae as the predominant AMF family in the region [6, 57]. Figure 3 further confirms Glomeraceae as the primary AMF family within the National Tropical Fruit Tree Field Genebank. In this study, sequencing data were utilized, and a comparative analysis was conducted using the MaarjAM database [38], which encompasses only 19 VTs of Paraglomus out of a total of 384 VTs. For the classification of AMF sequences, the NCBI Taxonomy database and the Glomeromycota PHYLOGENY database (collectively encompassing information on 341 AMF species) were used. However, these databases provide data on only 26 and 9 species of Paraglomus, respectively. Despite the identification of only 14 VTs of Paraglomus in the rhizosphere soils of 12 plants (Additional file 6: Table S3), Paraglomus constituted the largest proportion (31.622%) of the entire AMF community (Fig. 3). As an early diverging AMF [58], Paraglomus fungi were found to be widely distributed across the agricultural landscape, displaying a patchy distribution and low diversity. Notably, its distribution was found to be unrelated to soil physiochemical characteristics [59]. This observation may explain why Paraglomus was the dominant genus within the AMF community in the rhizosphere soil of the 12 examined plants.
Utilizing Grime's C (competitor)-S (stress tolerator)-R (ruderal) framework, AMF can be classified into C-AMF, S-AMF, and R-AMF [60]. Previous research has indicated that tilled agricultural soils exhibit an increase in the predominance of ruderal AMF species, particularly those belonging to the Glomeraceae family [60, 61]. Notably, studies have demonstrated that Claroideoglomus AMF also possesses ruderal traits [62,63,64,65]. At the VT level, the 20 most abundant VTs (Additional file 7: Table S4, accounting for 76.89% of the total sequence count) within the entire AMF community consisted of 12 affiliated with the Glomeraceae family and 3 affiliated with Claroideoglomeraceae, a subgroup within Claroideoglomus. This outcome suggested that the majority of the AMF species in the rhizosphere soil of the 12 plants in the National Tropical Fruit Tree Field Genebank adopted ruderal strategies.
Surprisingly, among the 12 varieties of minor tropical fruit trees examined, C. excavata exhibited the highest AMF spore density (Fig. 2). However, A. squamosa had significantly greater observed VTs than did the other trees (Fig. 5). These findings suggest the absence of a direct or intrinsic correlation between AMF spore density (determined via wet sieving and sucrose centrifugation) and the richness of AMF communities (determined through high-throughput sequencing). Two plausible explanations are proposed for this discrepancy. First, the AMF species identified by molecular methods originated from total soil DNA, while some AMF spores may have been lost during extraction using the wet sieving and sucrose centrifugation methods [66]. Additionally, distinct AMF species may exhibit varying sporulation periods [67]. In accordance with these findings, A. squamosa displayed the highest diversity and evenness (Fig. 5).
Notably, in a broader study encompassing 32 orchards across two Chinese provinces, a total of 96 samples were collected from mango and litchi plants, resulting in the identification of 104 distinct VTs [6]. Similarly, in a more localized study within the root rhizosphere soil of 31 wheat varieties in Lévis, Eastern Canada, 226 diverse VTs were identified from 93 samples [23]. In contrast, our analysis of 60 samples collected from 12 plants revealed 207 various VTs, a count significantly higher than that in previous studies in mango and litchi orchards [6] and only marginally lower than the number of AMF VTs found in durum wheat rhizosphere soil [23]. This finding suggested that the diversity of host plant species may have a more substantial impact on the increase in AMF species than the increase in sampling scale and sample quantity. In addition to the 10 species employed in the present work, there are an additional 25 minor tropical fruit tree species present in the Field Genebank. Our findings indicate the presence of high AMF species richness and diversity in the rhizosphere soils of minor tropical fruit trees within the National Tropical Fruit Tree Field Genebank.
Impact of host plants on the AMF community composition, α diversity, spore density, and colonization status in the rhizosphere
Understanding the spatial distribution of AMF within rhizosphere soils is pivotal for understanding the symbiotic relationships between AMF and their host plants. In contrast to plants with a random distribution, AMF exhibit specific patterns within the rhizosphere soil of host plants [68]. Nevertheless, varying conclusions have emerged from different studies regarding the influence of host plants on AMF community structure, irrespective of environmental conditions, ranging from minimal human disturbance to agricultural cultivation. For instance, in the temperate forests of Sasayama, Hyogo Prefecture, Japan, different plant species harbor similar compositions of AMF and ectomycorrhizal fungi [69]. However, three distinct studies focusing on grassland plant rhizosphere soil have reported a pronounced preference for AMF among specific hosts in these ecosystems [24,25,26]. Similarly, in southern Chinese orchards, litchi and mango plants have been found to significantly alter the composition of AMF communities in their surrounding rhizosphere soils [6]. In contrast, an examination of 31 varieties of durum wheat revealed a consistent composition of AMF communities in both the roots and surrounding rhizosphere soil [23]. Importantly, in the present work, variables such as tree age, fertilizer application, irrigation, and weed control were consistent, particularly among the diverse tropical fruits investigated. Moreover, we utilized the V.PhyloMaker tool to analyze the phylogenetic information of 12 distinct minor tropical fruit trees (Fig. 6A). This methodology allowed for the quantification of the influence of host plants on the structure of rhizosphere AMF communities while effectively controlling for potential confounding environmental factors.
In this investigation, advanced analytical methods, namely, LEfSe (Fig. 6B) and NMDS (Additional file 3: Fig. S3), were employed to scrutinize the AMF community structures associated with 5 plant species that exhibited later evolutionary development. These species included 3 plants from distinct genera within the same family and 2 from different species within a single genus. The results distinctly demonstrated that each of these plant species harbors a unique AMF community structure. In stark contrast, plants that underwent earlier evolutionary divergence displayed no significant variation in their AMF community compositions. Intriguingly, in cases of minimal evolutionary separation, such as among the 3 C. lansium cultivars, the differences in rhizosphere AMF communities were not substantial (Additional file 3: Fig. S3A). Consistent with these observations, the outcomes derived from SEM (Fig. 7) reinforced the notion that the evolutionary divergence time of the host plants does not exert a considerable influence on the configuration of their associated AMF communities.
Under natural conditions, plants engage in stringent reciprocal exchange with AMF, wherein AMF provide essential mineral nutrients to their host plants, which in turn supply AMF with organic matter [70]. This exchange has led to a prevailing assumption in traditional scientific discourse that AMF diversity is diminished in cultivated soils, particularly those enriched with heavy chemical fertilizers [15]. In contrast, extensive research has revealed the robust diversity of AMF in nutrient-rich soils [18, 55, 71]. Our study corroborates this finding by demonstrating the notable diversity of AMF within the rhizospheres of 12 distinct plants (Additional file 6: Table S3), despite the regular application of substantial chemical fertilizers.
Under consistent baseline nutrient conditions and annual nutrient inputs, the variation in rhizosphere nutrients among host plants may still reflect inherent differences in the host species. Consequently, we employed SEM to explore both the direct influence of host plants on the α diversity of their associated AMF communities and their indirect effects (mediated through alterations in rhizosphere nutrient profiles impacting AMF diversity). Ancient soils typically possess lower concentrations of mineral elements and organic matter than modern soils [72]. Plants that evolved earlier, and are adapted to resource-scarce environments, exhibit lower nutrient requirements [72]. Therefore, under uniform and sufficient chemical fertilization, the rhizospheres of phylogenetically older fruit trees tend to accumulate more nutrients. Consistent with this hypothesis, the evolutionary divergence time of host plants directly impacts both nutrient-poor and nutrient-rich soil factors (Fig. 7). In our study, the α diversity of the plant rhizosphere AMF communities, assessed by 3 distinct metrics, was directly correlated with the evolutionary divergence time of the host plants, with a particularly significant influence (P < 0.001) on the richness (Fig. 7). However, the impact of rhizosphere soil nutrient content on these metrics was not significant (Fig. 7). In the AM symbiotic relationship, AMF and host plants coevolve; early evolving plants typically form symbiotic associations with early evolving mycorrhizal fungi [73]. In this context, the dominant AMF in this study, Paraglomus fungi and ruderal AMF, both representative of early evolving AMF [58, 60], exhibited a distribution pattern that was minimally affected by soil nutrient content [59, 74]. This limited influence of soil nutrients on fungal distribution suggested that the evolutionary divergence time of host plants is a key factor shaping the α diversity of AMF communities across 12 fruit tree rhizospheres.
The observed negative influence of host plant evolutionary divergence time on rhizosphere AMF spore density and mycorrhizal colonization (Fig. 7) appears to be associated with the dominant ruderal strategy of these AMF. Compared with competitive and stress-tolerant AMF, ruderal AMF are characterized by higher growth rates, accelerated asexual spore reproduction, increased hyphal turnover, more effective hyphal repair, and more efficient spore dispersal [60]. Plants with recent evolutionary divergence exhibit a lower diversity of AMF in their rhizospheres (Fig. 5), resulting in a greater proportion and consequently greater density of ruderal AMF spores and arbuscules. Importantly, while ruderal AMF make a lesser contribution to nutrient uptake by host plants, they provide better protection to hosts against pathogens and herbivores [60], which are crucial challenges in tropical fruit cultivation [5]. Our study indicated that all 12 distinct fruit trees examined had relatively low AMF abundance within their roots and low AMF spore density in the rhizosphere (Fig. 2). Moreover, excessive application of readily available nutrients in the rhizosphere suppresses AMF sporulation (Fig. 7), highlighting the need to tailor chemical fertilizer application to the specific nutrient requirements of host plants to optimize the utility of ruderal AMF in the National Tropical Fruit Tree Field Genebank.
Conclusion
In the National Tropical Fruit Tree Field Genebank, 12 unique minor tropical fruit trees exhibited associations with AMF but displayed low mycorrhizal colonization density and arbuscular abundance. The highest AMF spore density in 100 g of air-dried rhizosphere soil was a mere 204.80 spores. High-throughput sequencing revealed diverse AMF communities, with 207 VTs and 4681 AMF ASVs in the soil samples. The dominant AMF were primarily early evolved Paraglomus fungi (31.619%) and ruderal AMF (56.848%). Despite the highly acidic and nutrient-rich conditions, a surprising diversity in the AMF communities was noted across the 12 fruit trees. Nonetheless, no correlation was found between α diversity and spore density. SEM indicated that the ET of host plants significantly influenced the three α diversity indices of the AMF community directly and spore density indirectly (mediated by fertile soil factors). However, neither ET nor soil nutrients significantly impacted the community structure. Among the mycorrhizal colonization indices, only the abundance of arbuscular structures showed a direct negative correlation with ET.
Availability of data and materials
Not applicable.
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- VT:
-
Virtual taxa
- ET:
-
Evolutionary divergence time
- SEM:
-
Structural equation modeling
- PCA:
-
Principal component analysis
- ASV:
-
Amplicon sequence variant
References
Mascher M, Schreiber M, Scholz U, Graner A, Reif JC, Stein N. Genebank genomics bridges the gap between the conservation of crop diversity and plant breeding. Nat Genet. 2019;51(7):1076–81. https://doi.org/10.1038/s41588-019-0443-6.
Olasupo FO, Adewale DB, Aikpokpodion PO, Muyiwa AA, Bhattacharjee R, Gutierrez OA, Motamayor JC, Schnell RJ, Ebai S, Zhang D. Genetic identity and diversity of Nigerian cacao genebank collections verified by single nucleotide polymorphisms (SNPs): a guide to field genebank management and utilization. Tree Geneti Genomes. 2018;14(2):32. https://doi.org/10.1007/s11295-018-1244-2.
Semchenko M, Barry KE, de Vries FT, Mommer L, Moora M, Maciá-Vicente JG. Deciphering the role of specialist and generalist plant–microbial interactions as drivers of plant–soil feedback. New Phytol. 2022;234(6):1929–44. https://doi.org/10.1111/nph.18118.
Genre A, Lanfranco L, Perotto S, Bonfante P. Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol. 2020;18(11):649–60. https://doi.org/10.1038/s41579-020-0402-3.
Yang X, Luo J, Li S, Liu C. Evaluation of nine pesticide residues in three minor tropical fruits from southern China. Food Control. 2016;60:677–82. https://doi.org/10.1016/j.foodcont.2015.08.036.
Jiang S, Hu X, Kang Y, Xie C, An X, Dong C, Xu Y, Shen Q. Arbuscular mycorrhizal fungal communities in the rhizospheric soil of litchi and mango orchards as affected by geographic distance, soil properties and manure input. Appl Soil Ecol. 2020;152:103593. https://doi.org/10.1016/j.apsoil.2020.103593.
Ortas İ, Rafique M, Akpinar C, Kacar YA. Growth media and mycorrhizal species effect on acclimatization and nutrient uptake of banana plantlets. Sci Hortic. 2017;217:55–60. https://doi.org/10.1016/j.scienta.2017.01.025.
Shu B, Liu L, Wei Y, Zhang D, Shi S. Differential selection pressure exerted by root rot disease on the microbial communities in the rhizosphere of avocado (Persea americana Mill.). Ann Appl Biol. 2019;175(3):376–87. https://doi.org/10.1111/aab.12547.
Arias RS, Borrone JW, Tondo CL, Kuhn DN, Irish BM, Schnell RJ. Genomics of tropical fruit tree crops. In: Schnell RJ, Priyadarshan PM, editors. Genomics of tree crops. New York: Springer; 2012. p. 209–39.
Sarr PS, Sugiyama A, Begoude ADB, Yazaki K, Araki S, Nawata E. Diversity and distribution of Arbuscular Mycorrhizal Fungi in cassava (Manihot esculenta Crantz) croplands in Cameroon as revealed by Illumina MiSeq. Rhizosphere. 2019;10:100147. https://doi.org/10.1016/j.rhisph.2019.100147.
Sheldrake M, Rosenstock NP, Mangan S, Revillini D, Sayer EJ, Olsson PA, Verbruggen E, Tanner EVJ, Turner BL, Wright SJ. Responses of arbuscular mycorrhizal fungi to long-term inorganic and organic nutrient addition in a lowland tropical forest. ISME J. 2018;12(10):2433–45. https://doi.org/10.1038/s41396-018-0189-7.
Wang H, Fang F-R, Chu H, Dou Q, Feng H, Yang C, Qing Q-Z, Tang M, Sung C-K, Wang C. Seasonal changes in Pinus tabuliformis root-associated fungal microbiota drive N and P cycling in terrestrial ecosystem. Front Microbiol. 2020;11:526898. https://doi.org/10.3389/fmicb.2020.526898.
Camenzind T, Hättenschwiler S, Treseder KK, Lehmann A, Rillig MC. Nutrient limitation of soil microbial processes in tropical forests. Eco Monogr. 2018;88(1):4–21. https://doi.org/10.1002/ecm.1279.
Turner BL, Brenes-Arguedas T, Condit R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature. 2018;555(7696):367–70. https://doi.org/10.1038/nature25789.
Ma X, Geng Q, Zhang H, Bian C, Chen HYH, Jiang D, Xu X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol. 2021;229:2957–69. https://doi.org/10.1111/nph.17077.
Prates Júnior P, Moreira BC. da Silva MdCS, Veloso TGR, Stürmer SL, Fernandes RBA, Mendonça EdS, Kasuya MCMJPo. Agroecological coffee management increases arbuscular mycorrhizal fungi diversity. PLoS ONE. 2019;14(1):e0209093. https://doi.org/10.1371/journal.pone.0209093.
Solís-Rodríguez URJ, Ramos-Zapata JA, Hernández-Cuevas L, Salinas-Peba L, Guadarrama P. Arbuscular mycorrhizal fungi diversity and distribution in tropical low flooding forest in Mexico. Mycol Prog. 2020;19(3):195–204. https://doi.org/10.1007/s11557-019-01550-x.
Marinho F, Oehl F, da Silva IR, Coyne D, Veras JSdN, Maia LC. High diversity of arbuscular mycorrhizal fungi in natural and anthropized sites of a Brazilian tropical dry forest (Caatinga). Fungal Ecol. 2019;40:82–91. https://doi.org/10.1016/j.funeco.2018.11.014.
Reyes HA, Ferreira PFA, Silva LC, da Costa MG, Nobre CP, Gehring C. Arbuscular mycorrhizal fungi along secondary forest succession at the eastern periphery of Amazonia: seasonal variability and impacts of soil fertility. Appl Soil Ecol. 2019;136:1–10. https://doi.org/10.1016/j.apsoil.2018.12.013.
Kameswara Rao N, Dulloo ME, Engels JMM. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet Resour Crop Ev. 2017;64(5):1061–74. https://doi.org/10.1007/s10722-016-0425-9.
Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D, et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356(6343):1172–5. https://doi.org/10.1126/science.aam9970.
Nadal M, Sawers R, Naseem S, Bassin B, Kulicke C, Sharman A, An G, An K, Ahern KR, Romag A, et al. An N-acetylglucosamine transporter required for arbuscular mycorrhizal symbioses in rice and maize. Nat Plants. 2017;3(6):17073. https://doi.org/10.1038/nplants.2017.73.
Stefani F, Dupont S, Laterrière M, Knox R, Ruan Y, Hamel C, Hijri M. Similar arbuscular mycorrhizal fungal communities in 31 durum wheat cultivars (Triticum turgidum L. var. durum) under field conditions in Eastern Canada. Front Plant Sci. 2020;11:1206. https://doi.org/10.3389/fpls.2020.01206.
Bi Y, Ma W, Xing F, Gao Y, Li Z, Chen C, Mu X, Li X, Zhu X. Diversity and specificity of arbuscular mycorrhizal fungi in the rhizosphere of six plants in the Songnen grassland China. Écoscience. 2020;27(1):11–21. https://doi.org/10.1080/11956860.2019.1662969.
Goldmann K, Boeddinghaus RS, Klemmer S, Regan KM, Heintz-Buschart A, Fischer M, Prati D, Piepho H-P, Berner D, Marhan S, et al. Unraveling spatiotemporal variability of arbuscular mycorrhizal fungi in a temperate grassland plot. Environ Microbiol. 2020;22(3):873–88. https://doi.org/10.1111/1462-2920.14653.
Šmilauer P, Košnar J, Kotilínek M, Šmilauerová M. Contrasting effects of host identity, plant community, and local species pool on the composition and colonization levels of arbuscular mycorrhizal fungal community in a temperate grassland. New Phytol. 2020;225(1):461–73. https://doi.org/10.1111/nph.16112.
Jin Y, Qian HV. PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography. 2019;42(8):1353–9. https://doi.org/10.1111/ecog.04434.
Jin Y, Qian HV. PhyloMaker2: an updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Diversity. 2022;44(4):335–9. https://doi.org/10.1016/j.pld.2022.05.005.
Brown PH, Zhao F-J, Dobermann A. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant Soil. 2022;476(1):11–23. https://doi.org/10.1007/s11104-021-05171-w.
Ouyang J-X, He Y-D, Yang B, Zhou J-Z, Li W, Cao Y. Elevation, but not phosphorus, shapes arbuscular mycorrhizal fungal colonization of plateau wetland plants: a case study of the Qinghai-Tibet Plateau. Glob Ecol Conserv. 2023;46:e02611. https://doi.org/10.1016/j.gecco.2023.e02611.
Sun D, Yang X, Wang Y, Fan Y, Ding P, Song XE, Yuan X, Yang X. Stronger mutualistic interactions with arbuscular mycorrhizal fungi help Asteraceae invaders outcompete the phylogenetically related natives. New Phytol. 2022;236(4):1487–96. https://doi.org/10.1111/nph.18435.
Hu W, Zhang H, Chen H, Tang M. Arbuscular mycorrhizas influence Lycium barbarum tolerance of water stress in a hot environment. Mycorrhiza. 2017;27(5):451–63. https://doi.org/10.1007/s00572-017-0765-0.
Votta C, Fiorilli V, Haider I, Wang JY, Balestrini R, Petřík I, Tarkowská D, Novák O, Serikbayeva A, Bonfante P, et al. Zaxinone synthase controls arbuscular mycorrhizal colonization level in rice. Plant J. 2022;111(6):1688–700. https://doi.org/10.1111/tpj.15917.
Bao S. Soil agrochemical analysis (in Chinese). Beijing: China Agricultural Press; 2000.
Greenacre M, Groenen PJF, Hastie T, D’Enza AI, Markos A, Tuzhilina E. Principal component analysis. Nat Rev Method Prime. 2022;2(1):100. https://doi.org/10.1038/s43586-022-00184-w.
Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol. 2010;12(8):2165–79. https://doi.org/10.1111/j.1462-2920.2009.02099.x.
Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. https://doi.org/10.7717/peerj.2584.
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier Ü, Zobel M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010;188(1):223–41. https://doi.org/10.1111/j.1469-8137.2010.03334.x.
Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. Ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8(1):28–36. https://doi.org/10.1111/2041-210X.12628.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. https://doi.org/10.1038/s41587-019-0209-9.
Kemp PF, Aller JY. Bacterial diversity in aquatic and other environments: what 16S rDNA libraries can tell us. FEMS Microbiol Ecol. 2004;47(2):161–77. https://doi.org/10.1016/S0168-6496(03)00257-5.
Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14(6):927–30. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x.
Wickham H. ggplot2. WIREs Comput Stat. 2011;3(2):180–5. https://doi.org/10.1002/wics.147.
Legendre P. Interpreting the replacement and richness difference components of beta diversity. Global Ecol Biogeogr. 2014;23(11):1324–34. https://doi.org/10.1111/geb.12207.
Lefcheck JS. piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol. 2016;7(5):573–9. https://doi.org/10.1111/2041-210X.12512.
Berger VW, Zhou Y. Kolmogorov-Smirnov test: overview. In: Kenett RS, Longford NT, Piegorsch WW, Ruggeri F, editors. Wiley StatsRef: statistics reference online. Hoboken: Wiley; 2014.
O’Neill ME, Mathews KL. Levene tests of homogeneity of variance for general block and treatment designs. Biometrics. 2002;58(1):216–24. https://doi.org/10.1111/j.0006-341X.2002.00216.x.
de la Fuente JL, Zunzunegui M, Barradas MCD. Physiological responses to water stress and stress memory in Argania spinosa. Plant Stress. 2023;7:100133. https://doi.org/10.1016/j.stress.2023.100133.
Page S, Mishra S, Agus F, Anshari G, Dargie G, Evers S, Jauhiainen J, Jaya A, Jovani-Sancho AJ, Laurén A, et al. Anthropogenic impacts on lowland tropical peatland biogeochemistry. Nat Rev Earth Environ. 2022;3(7):426–43. https://doi.org/10.1038/s43017-022-00289-6.
Chen S, Lin B, Li Y, Zhou S. Spatial and temporal changes of soil properties and soil fertility evaluation in a large grain-production area of subtropical plain China. Geoderma. 2020;357:113937. https://doi.org/10.1016/j.geoderma.2019.113937.
Zhang L, Zhou J, George TS, Limpens E, Feng G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022;27(4):402–11. https://doi.org/10.1016/j.tplants.2021.10.008.
Yan H, Freschet GT, Wang H, Hogan JA, Li S, Valverde-Barrantes OJ, Fu X, Wang R, Dai X, Jiang L, et al. Mycorrhizal symbiosis pathway and edaphic fertility frame root economics space among tree species. New Phytol. 2022;234(5):1639–53. https://doi.org/10.1111/nph.18066.
Smith SE, Smith FA. Roles of Arbuscular Mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2011;62(1):227–50. https://doi.org/10.1146/annurev-arplant-042110-103846.
Öpik M, Davison J, Moora M, Zobel M. DNA-based detection and identification of Glomeromycota: the virtual taxonomy of environmental sequences. Botany. 2013;92(2):135–47. https://doi.org/10.1139/cjb-2013-0110.
Dueñas JF, Camenzind T, Roy J, Hempel S, Homeier J, Suárez JP, Rillig MC. Moderate phosphorus additions consistently affect community composition of arbuscular mycorrhizal fungi in tropical montane forests in southern Ecuador. New Phytol. 2020;227(5):1505–18. https://doi.org/10.1111/nph.16641.
de Prado IGO, da Silva MCDS, de Prado DGO, Kemmelmeier K, Pedrosa BG, da Silva CC, Kasuya MCM. Revegetation process increases the diversity of total and arbuscular mycorrhizal fungi in areas affected by the Fundão dam failure in Mariana Brazil. Appl Soil Ecol. 2019;141:84–95. https://doi.org/10.1016/j.apsoil.2019.05.008.
Wang C, Gu Z, Cui H, Zhu H, Fu S, Yao Q. Differences in arbuscular mycorrhizal fungal community composition in soils of three land use types in subtropical hilly area of southern China. PLoS ONE. 2015;10(6):e0130983. https://doi.org/10.1371/journal.pone.0130983.
Malar CM, Wang Y, Stajich JE, Kokkoris V, Villeneuve-Laroche M, Yildirir G, Corradi N. Early branching arbuscular mycorrhizal fungus Paraglomus occultum carries a small and repeat-poor genome compared to relatives in the Glomeromycotina. Microbial Genomics. 2022;8(4):000810. https://doi.org/10.1099/mgen.0.000810.
Gosling P, Proctor M, Jones J, Bending GD. Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza. 2014;24(1):1–11. https://doi.org/10.1007/s00572-013-0505-z.
Chagnon P-L, Bradley RL, Maherali H, Klironomos JN. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013;18(9):484–91. https://doi.org/10.1016/j.tplants.2013.05.001.
Betancur-Agudelo M, Meyer E, Lovato PE. Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere. 2021;17:100307. https://doi.org/10.1016/j.rhisph.2021.100307.
Gao C, Courty P-E, Varoquaux N, Cole B, Montoya L, Xu L, Purdom E, Vogel J, Hutmacher Robert B, Dahlberg JA, et al. Successional adaptive strategies revealed by correlating arbuscular mycorrhizal fungal abundance with host plant gene expression. Mol Ecol. 2023;32(10):2674–87. https://doi.org/10.1111/mec.16343.
Horsch CCA, Antunes PM, Kallenbach CM. Arbuscular mycorrhizal fungal communities with contrasting life-history traits influence host nutrient acquisition. Mycorrhiza. 2023;33(1):1–14. https://doi.org/10.1007/s00572-022-01098-x.
Silva AMM, Feiler HP, Lacerda-Júnior GV, Fernandes-Júnior PI, de Tarso AS, de Araújo VAVP, Matteoli FP, de Araújo Pereira AP, de Melo IS, Cardoso EJBN. Arbuscular mycorrhizal fungi associated with the rhizosphere of an endemic terrestrial bromeliad and a grass in the Brazilian neotropical dry forest. Braz J Microbiol. 2023;54(3):1955–67. https://doi.org/10.1007/s42770-023-01058-3.
Ng A, Wilson BAL, Frew A. Belowground crop responses to root herbivory are associated with the community structure of native arbuscular mycorrhizal fungi. Appl Soil Ecol. 2023;185:104797. https://doi.org/10.1016/j.apsoil.2022.104797.
Boyno G, Demir S, Rezaee Danesh Y, Durak ED, Çevik R, Farda B, Djebaili R, Pellegrini M. A new technique for the extraction of arbuscular mycorrhizae fungal spores from rhizosphere. J Fungi. 2023. https://doi.org/10.3390/jof9080845.
Gai JP, Christie P, Feng G, Li XL. Twenty years of research on community composition and species distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza. 2006;16(4):229–39. https://doi.org/10.1007/s00572-005-0023-8.
Malicka M, Magurno F, Piotrowska-Seget Z. Phenol and polyaromatic hydrocarbons are stronger drivers than host plant species in shaping the arbuscular mycorrhizal fungal component of the mycorrhizosphere. Int J Mol Sci. 2022;23(20):12585. https://doi.org/10.3390/ijms232012585.
Toju H, Sato H. Root-associated fungi shared between arbuscular mycorrhizal and ectomycorrhizal conifers in a temperate forest. Front Microbio. 2018;9:433. https://doi.org/10.3389/fmicb.2018.00433.
Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Ann Rev Plant Biol. 2011;62(1):227–50. https://doi.org/10.1146/annurev-arplant-042110-103846.
Baltruschat H, Santos VM, da Silva DKA, Schellenberg I, Deubel A, Sieverding E, Oehl F. Unexpectedly high diversity of arbuscular mycorrhizal fungi in fertile Chernozem croplands in Central Europe. CATENA. 2019;182:104135. https://doi.org/10.1016/j.catena.2019.104135.
Lambers H, Raven JA, Shaver GR, Smith SE. Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol. 2008;23(2):95–103. https://doi.org/10.1016/j.tree.2007.10.008.
Hoysted GA, Kowal J, Jacob A, Rimington WR, Duckett JG, Pressel S, Orchard S, Ryan MH, Field KJ, Bidartondo MI. A mycorrhizal revolution. Curr Opin Plant Biol. 2018;44:1–6. https://doi.org/10.1016/j.pbi.2017.12.004.
Mansfield TM, Albornoz FE, Ryan MH, Bending GD, Standish RJ. Niche differentiation of Mucoromycotinian and Glomeromycotinian arbuscular mycorrhizal fungi along a 2-million-year soil chronosequence. Mycorrhiza. 2023;33(3):139–52. https://doi.org/10.1007/s00572-023-01111-x.
Acknowledgements
We thank Bin Zheng and Chengming Yan in the South Subtropical Crops Research Institute, Chinese Academy of Tropical Agricultural Science, for their help with sampling the roots and rhizosphere soil.
Funding
The Natural Science Foundation of China (No. 32260376), the Natural Science Foundation of Guizhou Province (No. QKHJC-ZK[2021]YB159), and the Doctoral Scientific Research Start-up Foundation from Tongren University (No. trxyDH1910) were provided in support of this work.
Author information
Authors and Affiliations
Contributions
All the authors contributed significantly to the activities described in the paper. In addition, JW and XG played major roles in the design of the experiment, the coordination of the activities, and the writing of the paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Seven soil properties, namely, total nitrogen (TN), total phosphorus (TP), total potassium (TK), total organic carbon (TOC), available phosphorus (AP), available potassium (AK), and soil pH, within the rhizosphere soils of 12 minor tropical fruit trees located in the National Tropical Fruit Tree Field Genebank. Each panel corresponds to a specific soil property index, with the abscissa denoting the plant labels and the ordinate representing the values of the corresponding soil properties. The upper and lower boundary lines of each box indicate the upper and lower interquartile ranges, respectively, while the central line within the box represents the average value. The gap between the two dashed lines signifies the range for a particular soil nutrient. Notably, when comparing the same soil property, identical capital letters associated with the bars signify the absence of significant differences between two plant species (with a significance level of P < 0.05). The outcomes of the normality test and the homoscedasticity test for these 7 soil properties are outlined in Table S1. The abbreviations corresponding to the 12 plants are documented in the Materials and methods section.
Additional file 2:
Figure S2. Representative arbuscular mycorrhizal (AM) structures, including arbuscules, vesicles, and intraradical hyphae, were visualized within the fine roots of 12 types of minor tropical fruit trees. Each image is specific to an individual plant, with the corresponding plant abbreviation provided in the upper right corner of the image. The magnification level employed for these images is 200×. The abbreviations corresponding to the 12 trees are documented in the Materials and methods section.
Additional file 3:
Figure S3. NMDS analysis was conducted on the rhizosphere AMF communities among various cultivars of the same species (A), distinct species within the same genus (B), separate genera within the same family (C), and diverse families (D) of 12 fruit trees. The evolutionary separation times among various species are depicted in Fig. 6A. The exact evolutionary divergence time among the 3 C. lansium cultivars cannot be definitively ascertained. Nevertheless, the intentional breeding of C. lansium cultivars began in the 1980s. Despite potential influences from human-driven selection and differentiation, the time of divergence for these cultivars remains relatively short, spanning approximately 50 years. The observations from the NMDS were corroborated by the results of the PERMANOVA, which yielded an R² value of 0.4059 and a significance level of P < 0.001
Additional file 4:
Table S1. The 7 soil physicochemical property indicators, namely, total nitrogen (TN), total phosphorus (TP), total potassium (TK), total organic carbon (TOC), available phosphorus (AP), available potassium (AK), and soil pH, of 5 randomly sampled points collected during the establishment of the National Tropical Fruit Tree Field Genebank in 2012.
Additional file 5:
Table S2. The normality test (P1, Kolmogorov‒Smirnov test) and homoscedasticity test (P2, Levene test) results of all the indices that were subjected to one-way ANOVA in the present work. The Kruskal‒Wallis test was carried out for the index that did not pass the normality test despite data transormation.
Additional file 6:
Table S3. Virtual taxa (VT) identified from all the AMF ASVs in the present work and their absolute abundances in the rhizospheres of the different plants.
Additional file 7:
Table S4. Twenty VT with the highest absolute abundances in the 60 samples analyzed in this study along with their corresponding taxonomic information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Gao, X., Wang, J. et al. Host plants directly determine the α diversity of rhizosphere arbuscular mycorrhizal fungal communities in the National Tropical Fruit Tree Field Genebank. Chem. Biol. Technol. Agric. 11, 20 (2024). https://doi.org/10.1186/s40538-024-00540-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-024-00540-w