Abstract
Many microbial exopolysaccharides (EPS) have been reported in the last decade, and their fermentation processes, functional properties and applications, structural characterization, and biological activities have been extensively studied. Despite the great diversity of biological activities already described for EPS, only a few have been exploited industrially. The main reason for this is that the structure–activity relationship of EPS has not been clearly defined. In this review, we collected EPS-related publications from two databases, the Web of Science and China National Knowledge Infrastructure, and reviewed the correlation between the structural characteristics of EPS and observed biological activity, as reported in studies over the last decade. This review focused on the antioxidant, antitumor, immunomodulatory, hypoglycemic, antibacterial, and gut microbial-modulating activities of EPS. This review aimed to lay a foundation for researching the structure–activity relationship of EPS and provide a theoretical basis for important scientific studies and applications of EPS.
Graphical Abstract
Similar content being viewed by others
Introduction
Microorganisms are the most abundant source of biological diversity on Earth. They exhibit novel functions and extensive biological characteristics, and are special sources of various metabolites [1, 2]. Exopolysaccharides (EPS) are extracellular carbohydrate polymers produced by microorganisms, including bacteria, fungi, and microalgae [3,4,5]. In the natural environment, EPS usually participate in protecting microorganisms; they resist adverse conditions (e.g., desiccation, cold, and hypertonic conditions), enhance resistance, and promote nutrient uptake [6,7,8].
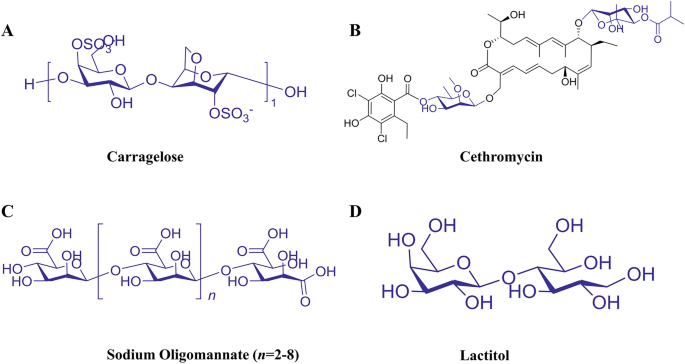
EPS have anticancer [9], antitumor [10], anti-inflammatory [11, 12], antidiabetic [13], antiviral [14], antioxidant [15], cholesterol-lowering [16], hypoglycemic/hypolipidemic [17, 18], immunomodulatory [19], and probiotic activities [20, 21]. Owing to their novel physiological functions and extensive biological activities [22], their formability is advantageous in terms of chemical composition and structure. EPS have been widely used in the fields of food, chemicals, and cosmetics [23, 24], and have also shown great potential for medical applications [25, 26]. Currently, the applications of microbial EPS in medicine include drug targeting [27], delivery [28, 29], vaccine preparation [30], tissue engineering [31], wound healing [32], anti-proliferation [33], cell carriers, and diagnostic tool manufacturing [3]. A number of carbohydrate-based drugs are also clinically used, including carragelose [34, 35] (Fig. 1A), cethromycin [36] (Fig. 1B), sodium oligomannate [37] (Fig. 1C), and lactitol [38] (Fig. 1D).
The biological activities of EPS are closely related to their structure [39], including monosaccharide composition, molecular weight, glycosidic linkage type and position, and chain conformation [40]. Therefore, in this review, we summarized the structural characteristics and biological activities of microbial EPS and explored their structure–activity relationship provide a reference and theoretical basis for the research and application of microbial EPS.
Research status of EPS
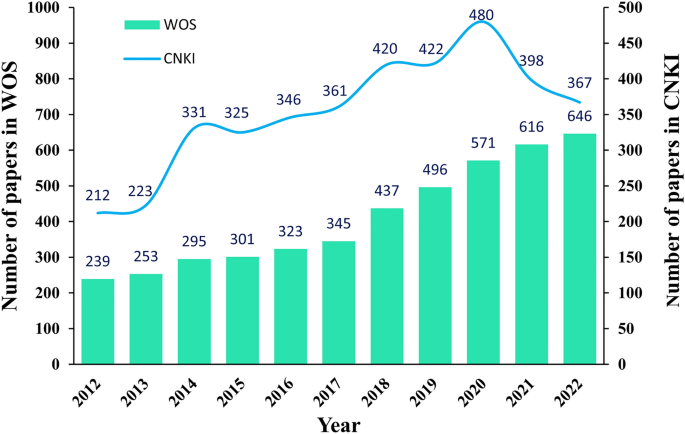
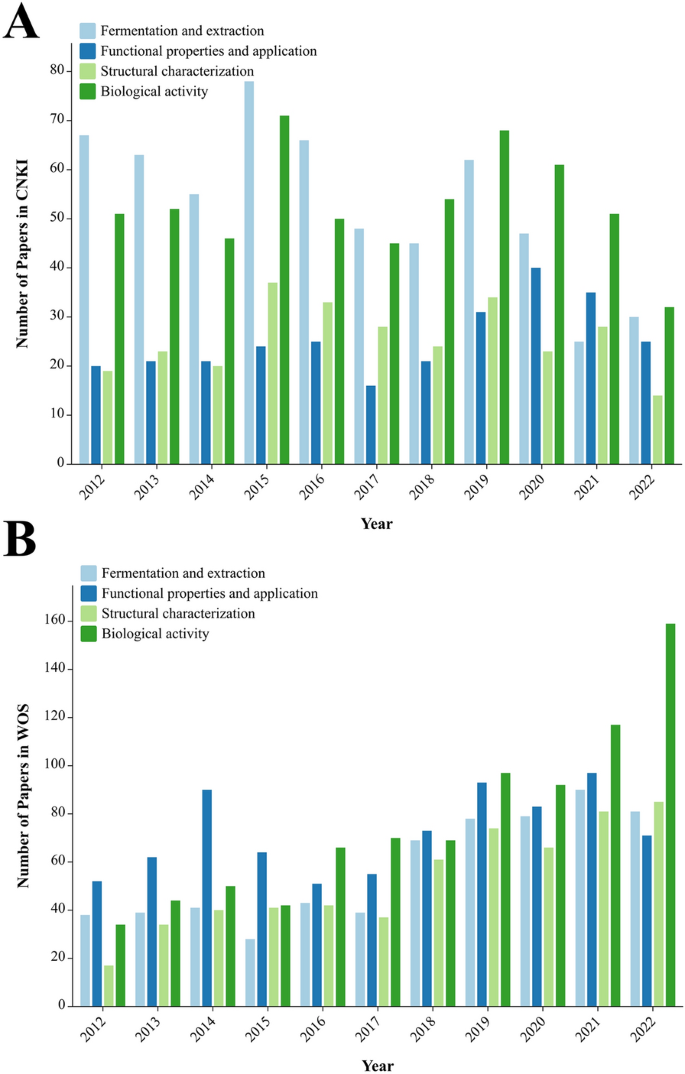
With the mining of EPS bioactivities and their wide range of applications in numerous research areas [41, 42], EPS research is gradually becoming an internationally cutting-edge topic with a large number of literature reports [3, 43, 44]. The EPS-related publications from 2012 to 2022 were statistically analyzed using the Web of Science (WOS) and China National Knowledge Infrastructure (CNKI) series databases with "exopolysaccharides" as the subject term (Fig. 2). The plot in Fig. 2 is similar to the global reasoning approach proposed by Chen et al. [45]. We projected the data of the two databases from the coordinate space to the nodes in an interaction space graph, which allowed us to directly analyze the information of the two databases from a global perspective.
The number of EPS-related publications on the WOS has been increasing annually since the past decade; the number of EPS-related publications was 239 in 2012, increasing to 646 in 2022—an increase of 170%. Over the past five years, the number of publications on EPS has increased, with an average annual increase of approximately 70 articles. As of March 9, 2023, the total number of EPS research publications reached 4,522 in the last decade. An annual analysis of the CNKI database shows that the number of EPS-related publications has increased annually over the past decade. As of March 9, 2023, the total number of EPS research papers published in the last decade was 3,885. An increase in the number of EPS-related studies in recent years has shown that EPS have gradually become a focus of attention and a research hotspot.
Currently, research on EPS focuses on four aspects: preparation process, functional properties and applications, structural characterization, and biological activities. The classification results of the studies on EPS in the two databases over the past decade are shown in Fig. 3, which shows that the focus of EPS research on CNKI differs significantly from that on the WOS.
Comparative statistical analysis of CNKI/WOS published literature from 2012 to 2022. Comparative general data of the number of CNKI/WOS published papers using different keywords/phrases to search: 1 fermentation and extraction; 2 functional properties and applications; 3 structural characterization; and 4 biological activity. WOS Web of Science, CNKI China National Knowledge Infrastructure
In recent years, many studies on the EPS preparation processes for CNKI have been reported and are increasing annually. Research on the biological activity of EPS has been steadily increasing annually, with a slower increase than that of the preparation process studies. However, the number of studies on the structural characterization of EPS is relatively small, with approximately 20 papers published each year (Fig. 3A). In contrast, the number of research publications on the structural characteristics of EPS in WOS remained above 60, but the overall number accounted for a small percentage of the publications (Fig. 3B). In summary, basic research on the structural characteristics of EPS is still very limited; therefore, conducting relevant research on these aspects is a key direction for researchers focusing on EPS.
Structural features of EPS
EPS have two different extracellular secretion states: capsular polysaccharides that adhere to the microbial cell wall to form a capsule, or slime polysaccharides that are loosely attached or even completely released into the surrounding environment to form slime [46, 47]. EPS can be homopolysaccharides composed of the same monosaccharide, such as curdlan, or heteropolysaccharides composed of different monosaccharides[48] (Fig. 4A). Heteropolysaccharides consist of different monosaccharides, including not only commonly observed sugars (such as glucose, galactose, and fructose), but also some rare monosaccharides (such as rhamnose, xylose, fucose, and mannose), uronic acids and amino sugars [20, 49] (such as xanthan) [50] (Fig. 4B). EPS with straight chains of monosaccharides, such as pullulan, are called linear polysaccharides [8, 51] (Fig. 4C). EPS with arms and bends, such as EPS-W1 extracted from Lactobacillus plantarum W1, are called branched polysaccharides [52,53,54] (Fig. 4D).
The structural description of EPS usually includes monosaccharide composition and conformation, molecular weight range, glycosidic bond conformation, repeating units, linkage sites, and spatial structures [55]. Table 1 summarizes the various EPS obtained from different microorganisms from 2018 to 2022. According to Table 1, we can speculate that EPS with a porous structure is more likely to have antioxidant activity, and that EPS with immunomodulatory activity are all triple-helical structures. Analyses of the monosaccharide composition, molecular weight, conformation, and biological activity of these EPS can provide useful information about their structure–activity relationships.
Structure–activity relationship of EPS
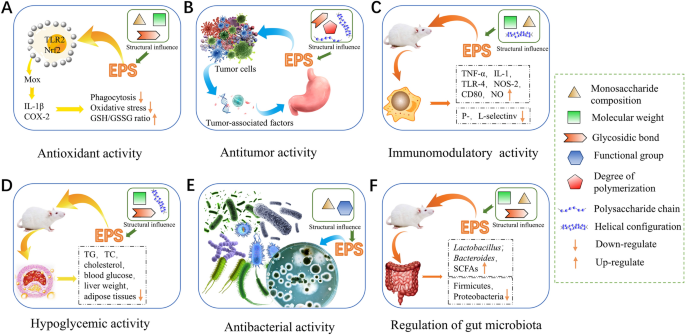
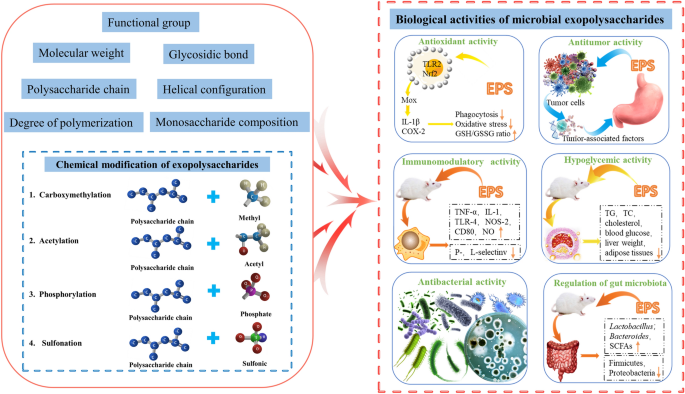
The composition and structure of EPS determine their microstructure and surface morphology, which affect their biological activity [77]. In this section, we focus on the antioxidant, antitumor, immunomodulatory, hypoglycemic, antibacterial, and gut microbial-modulating activities of microbial EPS (Fig. 5).
Antioxidant activity
Studies have shown that EPS have significant antioxidant activity. Similar to the mechanism of other sources of polysaccharides, the hydrogen-donating capacity of bacteria-derived EPS is considered the main property of its antioxidant function, but the underlying mechanism is not clear [80]. The antioxidant activity of EPS is affected by several factors, including monosaccharide composition, glycosidic bond type, and branching patterns.
The monosaccharide composition and composition ratio of EPS significantly a effected the antioxidant activity of EPS. As an example, EPS consisting of glucose-repeating units exhibit strong superoxide and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) scavenging activities and low hydroxyl radical scavenging activity, similar to that of the ascorbic acid standard [81]. The EPS obtained from Weissella cibaria SJ14, purified with a mannose content of 75.9%, exhibited excellent hydroxyl radical scavenging activity. This may be due to the higher mannose content [82]. In addition, high galactose-containing heteropolysaccharides obtained from W. confusa show strong scavenging ability and effective reduction of DPPH and hydrogen peroxide radicals [83]. Similarly, the two EPS components produced by Lactobacillus delbrueckii ssp. bulgaricus SRFM-1, r-EPS1 and r-EPS2, had higher proportions of galactose and exhibited stronger antioxidant activities [84]. Moreover, EPS, which consist of galactose, glucose, and rhamnose as the main monosaccharides exhibit antioxidant activity. This has been verified in EPS studies on Porphyridium cruentum and Bacillus sp. S-1, and Enterobacter ludwigeii [85, 86].
These results suggest that the glycosidic bond type and branching pattern can affect the antioxidant activity of EPS [84]. It has been suggested that α-1,2 and 1,6 glycosidic bonds are more flexible than β-1,3 and 1,4 glycosidic bonds [87,88,89]. The EPS produced by B. amyloliquefaciens is an α-glucan composed of glucose with two α-(1 → 3) and one α-(1 → 6) glycosidic bonds, which has a superoxide anion-scavenging ability [75]. However, the role of glycosidic bonds in antioxidant activity remains unclear and requires further investigation. It has been found that EPS with a high degree of branching also has good antioxidant activity. Yang et al. isolated and purified two fractions, THPS-1 and THPS-2, from the Tetragenococcus halophilus SNTH-8, both of which were highly branched polysaccharides with high antioxidant and emulsifying abilities [90].
Furthermore, changes in bacterial fermentation conditions (e.g., pH) can alter the structure of EPS, thereby affecting its antioxidant activity. For example, the EPS produced by Alteromonas australica QD under different pH conditions. The results revealed that acidic pH EPS (AC-EPS) and alkaline pH EPS (AL-EPS) contained similar types of monosaccharides with different proportions of Man, Gal, and GlcA. AL-EPS has been found to have high antioxidant activity [91]. Similar results have been reported by Ju [92].
Antitumor activity
According to a study on EPS antitumor activity regarding its structure, the high-order structure of EPS is more important than the primary structure for EPS antitumor activity [93, 94]. It includes the main chain composition, flexibility, molecular chain conformation, degree of branching, helical conformation, and spatial structure.
Antitumor EPS structural studies have shown that β-1,3 glycosidic bonds on glucose chains and β-1,6 glycosidic bonds on branched chains are required for their activities [95]. For instance, a variety of polysaccharide were isolated from Porphyra mushroom, whose antitumor active fraction is β-(1,3)-D-glucan with (1 → 6) branched chains [96]; the antitumor polysaccharide extracted from Auricularia auricula-judae was also composed of β-1,3-bound straight-chain glucan [97].
The flexibility of the polysaccharide backbone determines the antitumor activity of EPS to a certain extent [98]. Flexibility consists of a combination of hydrogen bonding and electrostatic repulsion of substituents within the polysaccharide molecule. High flexibility facilitates the interaction between the polysaccharide and the immune system, thus enhancing the antitumor activity of EPS [41, 99]. It has also been reported that polysaccharide branches can weaken intramolecular interactions and disrupt intermolecular binding, thus affecting antitumor activity [100, 101]. Bohn suggested that EPS with branching degrees of 0.2–0.33 have higher antitumor activity [102].
Morphological characteristics and chain conformation may also influence EPS antitumor activity [103]. Polysaccharides with a triple-helical conformation exhibit antitumor activity [104]. For instance, Misaki et al. found that lentinan and Auricularia auricula-judae polysaccharides with antitumor activity have β-triple helix conformation [97]. It has been found that chain conformation facilitates the interaction of polysaccharides with the immune system and enhances the antitumor activity of EPS [105]. The in vivo antitumor activity of different chain conformations of lentinan showed that the triple-helix conformation plays an important role in the antitumor activity of lentinan. Once the helical chain is disrupted, the antitumor activity decreases significantly or even disappears [106]. Poria polysaccharide is similar to lentinan; both have β-1,6 side chain glucan and no tumor activity, but through the oxidation of periodate and by Smith degradation after the removal of β-1,6 chain, the antitumor activity of polysaccharides was observed. X-ray diffraction analysis revealed that the polysaccharides formed a triple helical configuration [107]. Similarly, several other polysaccharides with antitumor activity extracted from mushrooms exhibit a triple-helical conformation in solution [108, 109]. Furthermore, characteristic viscosity is a key factor. An appropriate characteristic viscosity is conducive to the adhesion of polysaccharides to tumor cells [110].
The antitumor activity of sulfated polysaccharides was higher than that of non-sulfated polysaccharides. EPS from Lactobacillus plantarum 70810 (e.g., r-EPS1 and r-EPS2) inhibited tumor cell growth at a higher rate than the inhibition associated with r-EPS1; the authors hypothesized that the significant antitumor activity of r-EPS2 may be closely related to the composition of the sulfate group and β-glycosidic bond in r-EPS2 [111]. Sulfated galactans isolated from Halomonas aquamarina EG27S8QL also exhibit antitumor activity [112, 113]. However, EPSR3 from Bacillus cereus is a sulfate-free EPS, and its main component is uronic acid (28.7%). The results of this study showed that EPSR3 exhibited antitumor activity. The authors suggested that the antitumor activity of EPSR3 may be due to its uronic acid content [114]. Therefore, the relationship between acidic polysaccharides and their antitumor activities requires further investigation.
Immunomodulatory activity
Many studies have reported that EPS with certain compositions and molecular weights may be involved in immune responses [39, 115]. Results based on the structural features and immunomodulatory activity revealed that the presence of galactose is closely related to the immunomodulatory activity of EPS [115]. Reactions between polysaccharide antigens and antibodies produced in rabbits for galactose were reported as early as 1988 [116]. EPS from probiotic Enterococcus hirae WEHI01 are composed only of galactose, with a molecular weight of 2.59 × 103 Da, and it effectively improves macrophage-mediated immune responses [117].
Structure–activity relationship analysis showed that the molecular weight was significantly correlated with the immunomodulatory activity of EPS. EPS with higher molecular weights may inhibit immune responses [118]. In general, the degradation of higher-to lower-molecular-weight EPS significantly improves their biological activity [119]. Surayot et al. investigated the effect of EPS produced by Lactobacillus confusus TISTR 1498 on immunomodulatory activity, which consisted only of glucose with a high molecular weight of 65 000–506 000 kDa and was unable to stimulate the production of the pro-inflammatory factors nitric oxide and cytokines by RAW264.7 cells. After partial acid hydrolysis, its molecular weight was less than 70 kDa, and it was able to significantly stimulate macrophages and induce the production of nitric oxide as well as cytokines such as TNF-α, IL-1β, IL-6, and IL-10 [120]. The mechanism may be that lower-molecular-weight EPS and cell receptors bind more strongly and are more conducive to stimulating the production of pro-inflammatory factors in RAW264.7 cells. However, another study purified two homogeneous EPS, EPS53 (high molecular weight) and EPS53d (low molecular weight), from skimmed milk fermented by S. thermophilus XJ53; EPS53 showed stronger immune activity by promoting phagocytic ability and NO release from macrophages [121]. Therefore, the relationship between the molecular weight and immune activity of EPS needs to be further studied.
Acidic heteropolysaccharides are better at inducing immune responses [118]. For example, high-molecular-weight sulfated heteropolysaccharides from Lactobacillus paracasei VL8 are mainly composed of glucose and galactose, which have strong immunomodulatory activities [122]. Nishimura-Uemura studied a heteropolysaccharide produced by Lactobacillus delbrueckii subsp. Bulgaricus OLL1073R-1, consisting of both neutral and acidic polysaccharides in a 3:2 ratio of glucose to galactose. The acidic polysaccharides contained a small amount (0.1%) of phosphate, which was able to strongly induce the proliferation of various types of macrophages, whereas the neutral polysaccharides were unable to function, and dephosphorylation of this heteropolysaccharide caused a significant reduction in the stimulatory effect [123].
Furthermore, EPS with a triple-helical conformation may have immunomodulatory activity. For instance, EPS have been isolated from Aureobasidium pullulans CGMCC 23063, which has a triple-helical conformation linked to chains and round spheres. In an in vitro cellular assay, EPS showed immunoreactivity in RAW264.7 cells [56]. A novel crude EPS with a triple helical structure produced by Lactobacillus plantarum KX041 possesses prominent immune activity, promoting the proliferation and phagocytosis of Raw264.7 [73].
Hypoglycemic activity
Current studies on the hypoglycemic mechanism of EPS mainly focus on the regulation of related enzyme activities [124] and the improvement of insulin sensitivity [125]. EPS can reduce blood sugar by inhibiting digestive enzymes [126]. The hypoglycemic activity of EPS is closely related to its molecular weight, branched structure, and high-order structure [127, 128].
The hypoglycemic activity of EPS is closely related to its molecular weight [129,130,131]. The optimal activity of EPS can only be achieved at the appropriate molecular weight. Generally, EPS with low molecular weights exhibit better hypoglycemic activity [128]. A novel Codyceps polysaccharide with low molecular weight of 28 kDa was obtained by acid hydrolysis, and its inhibition rate on α-d-glucosidase was calculated as 40.01% [132]. Wang et al. used birch mushroom polysaccharides to simulate digestion in the intestine; the digested polysaccharide (UIOPS-1I) had a reduced molecular mass and significantly higher inhibitory activity against glucosidase [127].
Glycosidic bonds play an important role in the hypoglycemic activity of EPS [133]. It was found that most of the EPS with hypoglycemic activity have 1 → 3, 1 → 4, and 1 → 6 glycosidic bonds [128]. For example, the EPS backbone of Cordyceps militaris is dominated by a galactose 1 → 4 linkage, which effectively inhibits-glucosidase activity and restores glucose tolerance in mice [134].
It has also been suggested that EPS with a helical structure are more likely to have hypoglycemic activity, which has been verified in studies on Cordyceps militaris and Pleurotus citrinopileatus [17, 65]. Furthermore, sulfated EPS exhibited better hypoglycemic ability than that of natural EPS. For example, the EPS isolated from the fermentation broth of Lachnum sp. YM240 is sulfated, and sulfated EPS have a higher ability to inhibit glucosidase and amylase activities than that of unsulfated EPS [135].
Antibacterial activity
EPS contain various functional groups, such as carbonyl, phosphate, and hydroxyl groups. To some extent, these functional groups interact with bacterial cell membranes to exert antimicrobial activity [136, 137]. Novel Aspergillus spp. DHE6 produces EPS with the main functional groups -OH,–CH,–C = C, and C–O–C, which exhibit strong antibacterial activity against harmful human pathogens (Staphylococcus aureus, Bacillus subtilis, Bacillus pertussis, and Pseudomonas aeruginosa) [138].
EPS composed of glucose and rhamnose are more likely to exhibit antimicrobial activity, as verified in studies on EPS obtained from Lactobacillus gasseri FR4, Streptococcus thermophilus GST-6, and Lactococcus garvieae C47 [139,140,141]. Similarly, EPS are produced by Pediococcus pentosaceus SSC-12, with monosaccharide fractions of mainly glucose and rhamnose; they exhibit a strong antibacterial capacity [142]. However, an EPS with a molecular weight of 53,887 Da is produced by Lactobacillus crispatus, consisting mainly of mannose and glucose. It also has excellent antibacterial activity, which can effectively limit bacterial translocation and increase the abundance of Lactobacillus and Bifidobacterium [143]. Enterobacter sp. ACD2 EPS, with monosaccharide fractions of glucose, galactose, and fucose, containing 10% uronic acid and a small amount of fructose, showed high antibacterial activity against S. aureus and Eschericia coli [144]. In another study, dextran formed by Lactobacillus inhibited biofilm formation by C. albicans [145]. Moreover, negatively-charged EPS can interact better with pathogens through their sulfate groups, exhibiting antifungal activity [70].
Regulation of gut microbiota
EPS can also regulate the composition and function of the gut microbiota [20, 146]. Cordyceps sinensis polysaccharides (CSPs) are composed mainly of glucose, galactose, mannose, galacturonic acid, arabinose, trace proteins, and phenolic compounds. The backbone of CSPs consist of 1,4-glucose and 1,4-galactose, with a molecular weight of approximately 28 kDa [147]. CSPs increase the abundance of probiotics (Lactobacillus, Bifidobacterium, Bacteroides) and decrease that of pathogenic bacteria (Clostridium and Flexispira) [148]. E. coli EPS (EPS-m2) are composed of glucuronic acid, glucose, fucose, galactose/N-acetyl glucosamine, arabinose, xylose, and ribose in a molar ratio of approximately 77:44:29:28:2:1:1. EPS-m2 increases the abundance of Alistipes, Acinetobacter, Alloprevotella, Howardella, and Oxalobacter, and GC detection illustrates that EPS-m2 enhances the production of SCFAs [149]. The dextran (LM742) produced by Leuconostoc mesenteroides SPCL742, with a molecular weight of 1.3 × 106 Da, contains α-1,6 and α-1,3 glycosidic bonds in a ratio of 26.11:1. The LM742 glucan is resistant to digestive enzymes in the human gastrointestinal conditions [150]. Additionally, EPS from Paecilomyces cicadae TJJ1213 regulates the gut microbiota and metabolism and increases the abundance of probiotics [151].
However, few studies have reported on the role of EPS in the regulation of gut microbiota, and current studies have some shortcomings; thus, the mechanism of how EPS regulate gut microbiota is yet to be further elucidated. Therefore, researchers should strengthen the study of EPS structure and its relationship with the regulation of gut microbiota in the future, and reveal the intrinsic mechanism of EPS regulation of the gut microbiota.
Other biological activities
In addition to the aforementioned biological activities, EPS exhibit other biological activities. These include emulsifying, anti-inflammatory, and antimetastatic properties. EPS containing galactose generally have emulsifying properties, as demonstrated by the EPS generated by Lysinibacillus fusiformis and Streptococcus thermophilus [59, 76]. Sulfated heteropolysaccharides with branched and multichain structures may exhibit anti-inflammatory activities [152, 153]. Similarly, EPS isolated from Lactobacillus crispatus with a molecular weight of 53,887 Da, consisting mainly of mannose and glucose, possesses excellent anti-inflammatory activity [143]. Acidic EPS can degrade cholesterol more effectively than neutral polysaccharides can [154]. Moreover, EPS with irregularly curled conformations may have antimetastatic properties [61].
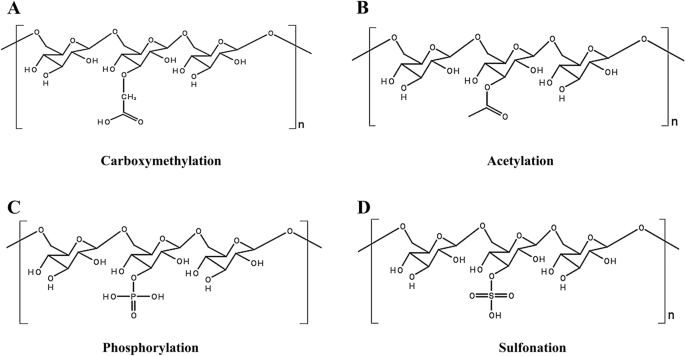
Chemical modification of EPS
The modification of EPS by group substitution to alter the structure of polysaccharides and enhance targeted biological activity has been reported as an emerging trend [138, 155, 156]. Current polysaccharide modification methods include carboxymethylation, acetylation, phosphorylation, and sulfonation. Lasiodiplodan, an exocellular fungal (1 → 6)-β-D-glucan, was used to illustrate the linkage of functional group to the glucan chain [157, 158] (Fig. 6).
Carboxymethylation modifications
Carboxymethylation involves the introduction of carboxymethyl groups to polysaccharide chains via etherification reactions of polysaccharides with acids or carboxylic acid derivatives to achieve changes in the spatial structure and water solubility of the polysaccharides; thus, affecting their biological activities [13, 159]. This modification has been shown to have a significant role in enhancing EPS bioactivity [160, 161]. For example, polysaccharides obtained from Lachnum YM240 fermentation broth were carboxymethyl-modified, and the results showed that diabetic mice fed carboxymethylated Lachnum polysaccharides had significantly lower fasting glucose and serum triglyceride levels and significantly higher insulin sensitivity [162]. Similarly, the EPS extracted from Lachnum YM281 were modified by carboxymethylation and exhibited enhanced biological activity [163].
Acetylation modifications
Acetylation alters the spatial structure of polysaccharides, thereby affecting their biological activities [159, 164]. EPS derived from Paenibacillus polymyxa EJS-3 have a higher reducing power than that of native EPS after various chemical modifications, including acetylation and phosphorylation [165]. Similarly, the EPS produced by Lactobacillus plantarum 70810 exhibited antioxidant activity after the introduction of a new acetylation moiety [166].
Phosphorylation modifications
Phosphorylation is a reliable method for enhancing the bioactivity of EPS [167]. EPS obtained from Lactococcus lactis subsp. lactis were phosphorylated and showed antioxidant effects in vivo and in vitro [168]. Similarly, EPS produced by Lachnum YM405 were subjected to sulfonation and phosphorylation treatments. The antioxidant activity of the modified derivatives was significantly enhanced [169].
Sulfonation modifications
EPS are sulfonated to achieve the desired chain length and water solubility of the polysaccharide, which affects its biological activity. The sulfonation EPS extracted from Enterobacter cloacae Z0206 protected RAW264.7 mouse macrophages from H2O2-induced oxidative damage and inhibited DNA breakage. These results suggest that sulfonation enhances antioxidant activity by modulating water solubility and chain length and protects cells by exchanging more hydrogen atoms [170]. Similarly, sulfonated EPS produced by Streptococcus thermophilus GST-6 and S. thermophilus ASCC1275 showed stronger antimicrobial efficacy against various Gram-positive and harmful pathogens than the efficacy of non-sulfonated EPS [141, 171].
Conclusion and future perspectives
EPS produced by microorganisms have attracted attention worldwide owing to their safety, diverse potent biological activities, and favorable advantages over other natural agents for industrial and therapeutic applications. However, the structure of EPS is complex and difficult to analyze, resulting in difficulties in investigating their structure–activity relationship, and its specific mechanism of action has not yet been revealed. In this review, we suggest that the structural characteristics of EPS, such as molecular weight, monosaccharide composition, glycosidic bond type, branching pattern, spatial structure, and chemical modifications may affect their biological activity. We believe that more advanced technology should to be used to analyze the structure of EPS. On this basis, the mechanism of action of the structure–activity relationship should be revealed from a new perspective to lay the foundation for the targeted synthesis and design of glycans. Moreover, many studies have shown that incubation conditions (e.g., time, temperature, and pH) can also affect the structure and biological activity of EPS. Therefore, changing the composition and structure of EPS through the influence of external factors can broaden its applications to some extent.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Demain AL. Importance of microbial natural products and the need to revitalize their discovery. J Ind Microbiol Biotechnol. 2014;41:185–201.
Poli A, Finore I, Romano I, Gioiello A, Lama L, Nicolaus B. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms. 2017;5:25.
Moscovici M. Present and future medical applications of microbial exopolysaccharides. Front Microbiol. 2015;6:1012.
Freitas F, Torres CAV, Reis MAM. Engineering aspects of microbial exopolysaccharide production. Bioresour Technol. 2017;245:1674–83.
Xiao M, Fu X, Wei X, Chi Y, Gao W, Yu Y, et al. Structural characterization of fucose-containing disaccharides prepared from exopolysaccharides of Enterobacter sakazakii. Carbohydr Polym. 2021;252:117139.
Singha T. Microbial extracellular polymeric substances: production, isolation and applications. IOSR J Pharm (IOSRPHR). 2012;2:271–81.
Flemming H-C. EPS—then and now. Microorganisms. 2016;4:41.
Decho AW, Gutierrez T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol. 2017. https://doi.org/10.3389/fmicb.2017.00922/full.
Gargouch N, Elleuch F, Karkouch I, Tabbene O, Pichon C, Gardarin C, et al. Potential of exopolysaccharide from Porphyridium marinum to contend with bacterial proliferation, biofilm formation, and breast cancer. Mar Drugs. 2021;19:66.
Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol. 2020;162:853–65.
Hou C, Chen L, Yang L, Ji X. An insight into anti-inflammatory effects of natural polysaccharides. Int J Biol Macromol. 2020;153:248–55.
Stellavato A, Dabous A, D’Ambrosio S, Cimini D, Schiraldi C. Anti-inflammatory effect of exopolysaccharides from Lactobacillus brevis on co-culture models of macrophage-Like and enterocyte. FEBS Open Bio. 2022;12:186–186.
Al-Nabulsi AA, Jaradat ZW, Al Qudsi FR, Elsalem L, Osaili TM, Olaimat AN, et al. Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT-Food Sci Technol. 2022;167:113817.
Chen L, Huang G. The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;115:77–82.
Kodali VP, Sen R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol J. 2008;3:245–51.
Zou Y, Xue W, Lin X, Hu T, Liu S-W, Sun C-H, et al. Taxonomic description and genome sequence of Christensenella intestinihominis sp. Nov., a novel cholesterol-lowering bacterium isolated from human gut. Front Microbiol. 2021;12:632361.
Sun H, Yu X, Li T, Zhu Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int J Biol Macromol. 2021;166:496–508.
Qi Y, Wang D, Fang L, Liu X, Liu C, Zhao F, et al. Hypoglycemic effect of exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on streptozotocin and high-fat diet-induced type 2 diabetic mice. Foods. 2022;11:3571.
Chen F, Huang G. Preparation and immunological activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;112:211–6.
Oerlemans MMP, Akkerman R, Ferrari M, Walvoort MTC, de Vos P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J Funct Food. 2021;76:104289.
Xu M, Li Z, Zhao X, Li W. Prebiotic properties of exopolysaccharides from Lactobacillus helveticus LZ-R-5 and L. pentosus LZ-R-17 evaluated by in vitro simulated digestion and fermentation. Foods. 2022;11:2501.
Wu J-Y, Siu K-C, Geng P. Bioactive ingredients and medicinal values of Grifola frondosa (Maitake). Foods. 2021;10:95.
Freitas F, Alves VD, Reis MAM. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–98.
Hao L, Liu W, Liu K, Shan K, Wang C, Xi C, et al. Isolation, optimization of fermentation conditions, and characterization of an exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Mar Drugs. 2019;17:703.
Wang C, Fan Q, Zhang X, Lu X, Xu Y, Zhu W, et al. Isolation, characterization, and pharmaceutical applications of an exopolysaccharide from AerococcusUriaeequi. Mar Drugs. 2018;16:337.
Xu L, Qiu Z, Gong H, Zhu C, Sang Q, Li Y, et al. Synergy of microbial polysaccharides and branched-preformed particle gel on thickening and enhanced oil recovery. Chem Eng Sci. 2019;208:115138.
Huang G, Huang H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J Control Release. 2018;278:122–6.
Huang G, Liu Y, Chen L. Chitosan and its derivatives as vehicles for drug delivery. Drug Delivery. 2017;24:108–13.
Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Delivery. 2018;25:766–72.
Tang Q, Huang G. Preparation and applications of glyconanoparticles. Int J Biol Macromol. 2018;116:927–30.
Di W, Zhang L, Yi H, Han X, Zhang Y, Xin L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol Lett. 2018;16:3577–86.
Gugliandolo C, Spano A, Maugeri TL, Poli A, Arena A, Nicolaus B. Role of bacterial exopolysaccharides as agents in counteracting immune disorders induced by herpes virus. Microorganisms. 2015;3:464–83.
Gao X, Li X, Mu J, Ho C-T, Su J, Zhang Y, et al. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporusumbellatus polysaccharide. Int J Biol Macromol. 2020;152:605–15.
Nagae M, Yamaguchi Y. Sugar recognition and protein–protein interaction of mammalian lectins conferring diverse functions. Curr Opin Struct Biol. 2015;34:108–15.
Cao X, Du X, Jiao H, An Q, Chen R, Fang P, et al. Carbohydrate-based drugs launched during 2000–2021. Acta Pharmaceutica Sinica B. 2022;12:3783–821.
He XM, Liu H. Formation of unusual sugars: mechanistic studies and biosynthetic applications. Annu Rev Biochem. 2002;71:701–54.
Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29:787–803.
Livesey G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev. 2003;16:163–91.
Chaisuwan W, Jantanasakulwong K, Wangtueai S, Phimolsiripol Y, Chaiyaso T, Techapun C, et al. Microbial exopolysaccharides for immune enhancement: fermentation, modifications and bioactivities. Food Biosci. 2020;35:100564.
Hou C, Yin M, Lan P, Wang H, Nie H, Ji X. Recent progress in the research of Angelica sinensis (Oliv.) diels polysaccharides: extraction, purification, structure and bioactivities. Chem Biol Technol Agric. 2021;8:13.
Ji X, Peng B, Ding H, Cui B, Nie H, Yan Y. Purification, structure and biological activity of pumpkin polysaccharides: a review. Food Rev Intl. 2023;39:307–19.
Ji X, Hou C, Shi M, Yan Y, Liu Y. an insight into the research concerning panax ginseng C. A. Meyer polysaccharides: a review. Food Rev Int. 2022;38:1149–65.
Shukla S. Secondary metabolites from marine microorganisms and therapeutic efficacy: a mini review. IJMS Vol45(10) [October 2016]. 2016. http://nopr.niscpr.res.in/handle/123456789/35709. Accessed 12 Apr 2023.
Singh R, Kumar M, Mittal A, Mehta PK. Microbial metabolites in nutrition, healthcare and agriculture. 3 Biotech. 2017;7:1–14.
Chen Y, Rohrbach M, Yan Z, Shuicheng Y, Feng J, Kalantidis Y. Graph-Based Global Reasoning Networks. 2019. p. 433–42. https://openaccess.thecvf.com/content_CVPR_2019/html/Chen_Graph-Based_Global_Reasoning_Networks_CVPR_2019_paper.html. Accessed 16 Aug 2023.
Lynch KM, Zannini E, Coffey A, Arendt EK. Lactic acid bacteria exopolysaccharides in foods and beverages: isolation, properties, characterization, and health benefits. Annu Rev Food Sci Technol. 2018;9:155–76.
Barcelos MCS, Vespermann KAC, Pelissari FM, Molina G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit Rev Food Sci Nutr. 2020;60:1475–95.
Zhang R, Edgar KJ. Properties, chemistry, and applications of the bioactive polysaccharide curdlan. Biomacromol. 2014;15:1079–96.
Jiang Y, Yang Z. A functional and genetic overview of exopolysaccharides produced by Lactobacillus plantarum. J Funct Food. 2018;47:229–40.
Sharma V, Harjai K, Shukla G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P-aeruginosa PAO1 biofilm. Folia Microbiol. 2018;63:181–90.
Cheng K-C, Demirci A, Catchmark JM. Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol. 2011;92:29–44.
Jaiswal P, Sharma R, Sanodiya BS, Bisen PS. Microbial exopolysaccharides: natural modulators of dairy products. J app pharm sci. 2014;4:105–9.
Schmid J, Farina J, Fiehm B, Sieber V. Editorial: microbial exopolysaccharides: from genes to applications. Front Microbiol. 2016;7:308.
Do TBT, Tran BK, Tran TVT, Le TH, Cnockaert M, Vandamme P, et al. Decoding the capability of Lactobacillus plantarum W1 isolated from soybean whey in producing an exopolysaccharide. ACS Omega. 2020;5:33387–94.
Wang Z. Analysis of monosaccharide composition of extracellular polysaccharides from Lactobacillus casei. Food Industry. 2017;10:88–92.
Liao Y, Gao M, Wang Y, Liu X, Zhong C, Jia S. Structural characterization and immunomodulatory activity of exopolysaccharide from Aureobasidium pullulans CGMCC 23063. Carbohydr Polym. 2022;288:119366.
Sun L, Cheng L, Ma Y, Lei P, Wang R, Gu Y, et al. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int J Biol Macromol. 2022;209:396–404.
Li J, Wu H, Liu Y, Nan J, Park HJ, Chen Y, et al. The chemical structure and immunomodulatory activity of an exopolysaccharide produced by Morchella esculenta under submerged fermentation. Food Funct. 2021;12:9327–38.
Mathivanan K, Chandirika JU, Vinothkanna A, Govindarajan RK, Meng D, Yin H. Characterization and biotechnological functional activities of exopolysaccharides produced by Lysinibacillus fusiformis KMNTT-10. J Polym Environ. 2021;29:1742–51.
Roger O, Kervarec N, Ratiskol J, Colliec-Jouault S, Chevolot L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonasinfernus. Carbohyd Res. 2004;339:2371–80.
Mazza M, Alliot C, Sinquin C, Colliec-Jouault S, Reiller PE, Huclier-Markai S. Marine exopolysaccharide complexed with scandium aimed as theranostic agents. Molecules. 2021;26:1143.
Qing Z, Jie W, Qing S, Shu-Ming Z, Xiang-Yang S, Chan-Yuan L, et al. Characterization and antioxidant activity of released exopolysaccharide from potential probiotic Leuconostocmesenteroides LM187. J Microbiol Biotechnol. 2021;31:1144–53.
Zhao D, Jiang J, Liu L, Wang S, Ping W, Ge J. Characterization of exopolysaccharides produced by Weissellaconfusa XG-3 and their potential biotechnological applications. Int J Biol Macromol. 2021;178:306–15.
Aburas H, Ispirli H, Taylan O, Yilmaz MT, Dertli E. Structural and physicochemical characterisation and antioxidant activity of an alpha-D-glucan produced by sourdough isolate Weissellacibaria MED17. Int J Biol Macromol. 2020;161:648–55.
Hao Y, Sun H, Zhang X, Wu L, Zhu Z. A novel acid polysaccharide from fermented broth of Pleurotus citrinopileatus: Hypoglycemic activity in vitro and chemical structure. J Mol Struct. 2020;1220:128717.
Chen L, Wang Z, Zhang B, Ge M, Ng H, Niu Y, et al. Production, structure and morphology of exopolysaccharides yielded by submerged fermentation of Antrodia cinnamomea. Carbohydr Polym. 2019;205:271–8.
Liu T, Zhou K, Yin S, Liu S, Zhu Y, Yang Y, et al. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int J Biol Macromol. 2019;134:516–26.
Long Z, Liu H, Li J, Sun J, Xue Y, Hu Z, et al. Preliminary characterization of exopolysaccharides produced by Abortiporusbiennis in submerged fermentation. Sains Malays. 2019;48:2633–40.
Min W-H, Fang X-B, Wu T, Fang L, Liu C-L, Wang J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J Biosci Bioeng. 2019;127:758–66.
Nehal F, Sahnoun M, Smaoui S, Jaouadi B, Bejar S, Mohammed S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb Pathog. 2019;132:10–9.
Sahana TG, Rekha PD. A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro. Int J Biol Macromol. 2019;131:10–8.
Wang K, Niu M, Yao D, Zhao J, Wu Y, Lu B, et al. Physicochemical characteristics and in vitro and in vivo antioxidant activity of a cell-bound exopolysaccharide produced by Lactobacillus fermentum S1. Int J Biol Macromol. 2019;139:252–61.
Xu Y, Cui Y, Wang X, Yue F, Shan Y, Liu B, et al. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int J Biol Macromol. 2019;128:480–92.
Jia X, Qu L, Panpan R, Liu S, Wu Y, Xu C. Characterization and antioxidant activity of an exopolysaccharide produced by Rigidoporusmicroporus (Agaricomycetes). Int J Med Mushrooms. 2018;20:311–20.
Zhao W, Zhang J, Jiang Y-Y, Zhao X, Hao X-N, Li L, et al. Characterization and antioxidant activity of the exopolysaccharide produced by Bacillus amyloliquefaciens GSBa-1. J Microbiol Biotechnol. 2018;28:1282–92.
Zhang H, Ren W, Guo Q, Xiong Z, Wang G, Xia Y, et al. Characterization of a yogurt-quality improving exopolysaccharide from Streptococcus thermophilus AR333. Food Hydrocolloids. 2018;81:220–8.
Kanamarlapudi SLRK, Muddada S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed Res Int. 2017;2017:e4201809.
Xiao M, Ren X, Yu Y, Gao W, Zhu C, Sun H, et al. Fucose-containing bacterial exopolysaccharides: sources, biological activities, and food applications. Food Chem X. 2022;13:100233.
Yang M, Zhou D, Xiao H, Fu X, Kong Q, Zhu C, et al. Marine-derived uronic acid-containing polysaccharides: structures, sources, production, and nutritional functions. Trends Food Sci Technol. 2022;122:1–12.
Andrew M, Jayaraman G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr Res. 2020;487:107881.
Abdhul K, Ganesh M, Shanmughapriya S, Kanagavel M, Anbarasu K, Natarajaseenivasan K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int J Biol Macromol. 2014;70:450–4.
Zhu Y, Wang C, Jia S, Wang B, Zhou K, Chen S, et al. Purification, characterization and antioxidant activity of the exopolysaccharide from Weissellacibaria SJ14 isolated from Sichuan paocai. Int J Biol Macromol. 2018;115:820–8.
Adebayo-Tayo B, Ishola R, Oyewunmi T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissellaconfusa strains. Biotechnol Rep. 2018;19:e00271.
Tang W, Dong M, Wang W, Han S, Rui X, Chen X, et al. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp bulgaricus SRFM-1. Carbohydr Polym. 2017;173:654–64.
Hu X, Pan X, Wang PG, Chen M. Isolation and characterization of an antioxidant exopolysaccharide produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr Polym. 2019;204:9–16.
Paikra SK, Panda J, Sahoo G, Mishra M. Characterization of exopolysaccharide derived from Enterobacter ludwigii and its possible role as an emulsifier. 3 Biotech. 2022;12:212.
Delbarre-Ladrat C, Sinquin C, Lebellenger L, Zykwinska A, Colliec-Jouault S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front Chem. 2014;2:85.
Plazinski W, Drach M, Plazinska A. Ring inversion properties of 1→2, 1→3 and 1→6-linked hexopyranoses and their correlation with the conformation of glycosidic linkages. Carbohyd Res. 2016;423:43–8.
Peesapati S, Sajeevan KA, Patel SK, Roy D. Relation between glycosidic linkage, structure and dynamics of α- and β-glucans in water. Biopolymers. 2021;112:e23423.
Yang X, Wu J, An F, Xu J, Bat-Ochir M, Wei L, et al. Structure characterization, antioxidant and emulsifying capacities of exopolysaccharide derived from Tetragenococcushalophilus SNTH-8. Int J Biol Macromol. 2022;208:288–98.
Li F, Hu X, Sun X, Li H, Lu J, Li Y, et al. Effect of fermentation pH on the structure, rheological properties, and antioxidant activities of exopolysaccharides produced by Alteromonasaustralica QD. Glycoconjugate J. 2022;39:773–87.
Ju Y, Shan K, Liu W, Xi C, Zhang Y, Wang W, et al. Effect of different initial fermentation pH on exopolysaccharides produced by Pseudoalteromonas agarivorans Hao 2018 and identification of key genes involved in exopolysaccharide synthesis via transcriptome analysis. Mar Drugs. 2022;20:89.
Ohno N, Miura T, Miura NN, Adachi Y, Yadomae T. Structure and biological activities of hypochlorite oxidized zymosan. Carbohyd Polym. 2001;44:339–49.
Zhang H, Wang Z, Wang X, Yao L, Wu Z, Yang X. Research progress in chemical modification methods and biological activities of polysaccharides. Food Ferment Ind. 2010;36:102–7.
Huang F, Meng Y. Research progress in active polysaccharides. Natural Product Research and Development. 1999;5:90–8.
Ramamurthy D, Nundalall T, Cingo S, Mungra N, Karaan M, Naran K, et al. Recent advances in immunotherapies against infectious diseases. Immunother Adv. 2021;1:Itaa007.
Misaki A, Kakuta M, Sasaki T, Tanaka M, Miyaji H. Studies on interrelation of structure and antitumor effects of polysaccharides: Antitumor action of periodate-modified, branched (1 → 3)-β-d-glucan of auricularia auricula-judae, and other polysaccharides containing (1 → 3)-glycosidic linkages. Carbohyd Res. 1981;92:115–29.
Demleitner S, Kraus J, Franz G. Synthesis and antitumour activity of derivatives of curdlan and lichenan branched at C-6. Carbohyd Res. 1992;226:239–46.
Öner ET, Hernández L, Combie J. Review of Levan polysaccharide: from a century of past experiences to future prospects. Biotechnol Adv. 2016;34:827–44.
Kasaai MR. A comparative study of molecular structure, solution properties and food application for three branched polysaccharides: amylopectin, glycogen, and dextran. Curr Trends Polym Sci. 2012;16:49–63.
Guo MQ, Hu X, Wang C, Ai L. Polysaccharides: structure and solubility. In: Zhenbo Xu, editor. Solubility of polysaccharides. Rijeka: IntechOpen; 2017.
Bohn JA, BeMiller JN. (1→3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohyd Polym. 1995;28:3–14.
Tang W, Han S, Zhou J, Xu Q, Dong M, Fan X, et al. Selective fermentation of Lactobacillus delbrueckii ssp. Bulgaricus SRFM-1 derived exopolysaccharide by Lactobacillus and Streptococcus strains revealed prebiotic properties. J Funct Foods. 2020;69:103952.
Linghong N, Ning Z. Relationship between structure and activity of active polysaccharides. Chem Ind For Prod. 2003;23:89–94.
Chen X, Xu X, Zhang L, Zeng F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohyd Polym. 2009;78:581–7.
Zhang L, Li X, Xu X, Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohyd Res. 2005;340:1515–21.
Maeda YY, Hamuro J, Chihara G. The mechanisms of action of anti-tumour polysaccharides. I. The effects of antilymphocyte serum on the anti-tumour activity of lentinan. Int J Cancer. 1971;8:41–6.
Saitô H, Yoshioka Y, Yokoi M, Yamada J. Distinct gelation mechanism between linear and branched (1 → 3)-β-D-glucans as revealed by high-resolution solid-state 13C NMR. Biopolymers. 1990;29:1689–98.
Yu Z, Ming G, Kaiping W, Zhixiang C, Liquan D, Jingyu L, et al. Structure, chain conformation and antitumor activity of a novel polysaccharide from Lentinus edodes. Fitoterapia. 2010;81:1163–70.
Zhu Z-Y, Dong F, Liu X, Lv Q, YingYang LF, et al. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr Polym. 2016;140:461–71.
Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014;67:71–8.
Kokoulin MS, Kuzmich AS, Romanenko LA, Chikalovets IV. Sulfated capsular polysaccharide from the marine bacterium Kangiella japonica inhibits T-47D cells growth in vitro. Carbohydr Polym. 2022;290:119477.
Kokoulin MS, Sigida EN, Kuzmich AS, Ibrahim IM, Fedonenko YP, Konnova SA. Structure and antiproliferative activity of the polysaccharide from Halomonasaquamarina related to Cobetia pacifica. Carbohydr Polym. 2022;298:120125.
Selim S, Almuhayawi MS, Alharbi MT, Nagshabandi MK, Alanazi A, Warrad M, et al. In vitro assessment of antistaphylococci, antitumor, immunological and structural characterization of acidic bioactive exopolysaccharides from marine Bacillus cereus isolated from Saudi Arabia. Metabolites. 2022;12:132.
Wang N, Shan Z, Jia X, Wang Y, Song S, Xiao D, et al. Galf-containing polysaccharides from medicinal molds: sources, structures and bioactive properties. Trends Food Sci Technol. 2023;131:244–63.
Notermans S, Veeneman GH, Van Zuylen CWEM, Hoogerhout P, Van Boom JH. (15)-linked β-D-galactofuranosides are immunodominant in extracellular polysaccharides of Penicillium and Aspergillus species. Mol immunol. 1988;25:975–9.
Jia K, Wei M, He Y, Wang Y, Wei H, Tao X. Characterization of novel exopolysaccharides from Enterococcus hirae WEHI01 and its immunomodulatory activity. Foods. 2022;11:3538.
Hidalgo-Cantabrana C, Lopez P, Gueimonde M, de Reyes-Gavilan CG, Suarez A, Margolles A, et al. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob Proteins. 2012;4:227–37.
Liu S, Zhou W, Ye W, Chen J, Wu C, Chen D, et al. Research advance on biological activity and structure-activity relationships of bioactive polysaccharide. Food Res Dev. 2017;38:211–8.
Surayot U, Wang J, Seesuriyachan P, Kuntiya A, Tabarsa M, Lee Y, et al. Exopolysaccharides from lactic acid bacteria: structural analysis, molecular weight effect on immunomodulation. Int J Biol Macromol. 2014;68:233–40.
Xia W, Han J, Zhu S, Wang Y, Zhang W, Wu Z. Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity. Int J Biol Macromol. 2023;230:123177.
Liu Y, Mao K, Zhang N, Chitrakar B, Huang P, Wang X, et al. Structural characterization and immunomodulatory effects of extracellular polysaccharide from Lactobacillus paracasei VL8 obtained by gradient ethanol precipitation. J Food Sci. 2022;87:2034–47.
Nishimura-Uemura J, Kitazawa H, Kawai Y, Itoh T, Oda M, Saito T. Functional alteration of murine macrophages stimulated with extracellular polysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Food Microbiol. 2003;20:267–73.
Ji X, Guo J, Cao T, Zhang T, Liu Y, Yan Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci Human Wellness. 2023;12:1969–80.
Yang S, Qu Y, Zhang H, Xue Z, Liu T, Yang L, et al. Hypoglycemic effects of polysaccharides from Gomphidiaceae rutilus fruiting bodies and their mechanisms. Food Funct. 2020;11:424–34.
Huang Z, Lin F, Zhu X, Zhang C, Jiang M, Lu Z. An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Int J Biol Macromol. 2020;143:775–84.
Wang C, Li W, Chen Z, Gao X, Yuan G, Pan Y, et al. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res Int. 2018;103:280–8.
Yang Y, Liu J, Tan Y, Wang S, Chen H, Zhou A. Progress in understanding the structure-activity relationship and hypoglycemic mechanism of polysaccharides. Food Science. 2021;42:355–63.
Ma Y, Mao D, Geng L, Wang Z, Xu C. Production, fractionation, characterization of extracellular polysaccharide from a newly isolated Trametesgibbosa and its hypoglycemic activity. Carbohydr Polym. 2013;96:460–5.
Ayyash M, Abu-Jdayil B, Itsaranuwat P, Galiwango E, Tamiello-Rosa C, Abdullah H, et al. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int J Biol Macromol. 2020;144:938–46.
Ahmad W, Boyajian JL, Abosalha A, Nasir A, Ashfaq I, Islam P, et al. High-molecular-weight dextran-type exopolysaccharide produced by the novel Apilactobacilluswaqarii improves metabolic syndrome: in vitro and in vivo analyses. Int J Mol Sci. 2022;23:12692.
Zhu Z-Y, Guo M-Z, Liu F, Luo Y, Chen L, Meng M, et al. Preparation and inhibition on α-d-glucosidase of low molecular weight polysaccharide from Cordyceps militaris. Int J Biol Macromol. 2016;93:27–33.
Mansel BW, Ryan TM, Chen H-L, Lundin L, Williams MAK. Polysaccharide conformations measured by solution state X-ray scattering. Chem Phys Lett. 2020;739:136951.
Yu X. Study on Structure, Synthesis Related Enymes and Hypoglycemic Activity of Exopolysaccharide from Cordyceps Militaris Mycelium. Tianjin University of Science & Technology. 2019. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkHr3ADhkADnVu66WViDP_3OSMGO5HucmElnGcdQNvlVqxIp8JuV0GwGQzlMaw8gao&uniplatform=NZKPT. Accessed 8 Apr 2023.
Wang Y, Hou G, Li J, Surhio MM, Ye M. Structure characterization, modification through carboxymethylation and sulfation, and in vitro antioxidant and hypoglycemic activities of a polysaccharide from Lachnum sp. Process Biochem. 2018;72:177–87.
Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: a review. Carbohydr Polym. 2019;207:317–32.
Rajoka MSR, Wu Y, Mehwish HM, Bansal M, Zhao L. Lactobacillus exopolysaccharides: new perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci Technol. 2020;103:36–48.
El-Ghonemy DH. Antioxidant and antimicrobial activities of exopolysaccharides produced by a novel Aspergillus sp. DHE6 under optimized submerged fermentation conditions. Biocatal Agric Biotechnol. 2021;36:102150.
Zhang J, Cao Y, Wang J, Guo X, Zheng Y, Zhao W, et al. Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6. Carbohyd Polym. 2016;146:368–75.
Rani RP, Anandharaj M, David RA. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int J Biol Macromol. 2018;109:772–83.
Ayyash M, Abu-Jdayil B, Itsaranuwat P, Almazrouei N, Galiwango E, Esposito G, et al. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020;333:127418.
Fan Y, Li X, Tian R, Tang R, Zhang J. Characterization and biological activity of a novel exopolysaccharide produced by Pediococcuspentosaceus SSC-12 from silage. Microorganisms. 2022;10:18.
Ding C, Wu H, Cao X, Gao Z, Tang Z, Fan W, et al. Lactobacillus crispatus-derived exopolysaccharides with antibacterial activity limit Salmonella typhimurium invasion by inhibiting inflammasome-mediated pyroptosis. Food Funct. 2022;13:10501–15.
Almutairi MH, Helal MM. Biological and microbiological activities of isolated Enterobacter sp. ACD2 exopolysaccharides from Tabuk region of Saudi Arabia. J King Saud Univ Sci. 2021;33:101328.
Matsubara VH, Wang Y, Bandara HMHN, Mayer MPA, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl Microbiol Biotechnol. 2016;100:6415–26.
Wan C, Qian W-W, Liu W, Pi X, Tang M-T, Wang X-L, et al. Exopolysaccharide from Lactobacillus rhamnosus ZFM231 alleviates DSS-induced colitis in mice by regulating gut microbiota. J Sci Food Agric. 2022. https://www.webofscience.com/wos/alldb/full-record/MEDLINE:35707876. Accessed 29 Jun 2022.
Wang J, Nie S, Kan L, Chen H, Cui SW, Phillips AO, et al. Comparison of structural features and antioxidant activity of polysaccharides from natural and cultured Cordyceps sinensis. Food Sci Biotechnol. 2017;26:55–62.
Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, et al. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohyd Polym. 2020;235:115957.
Li B, Chen H, Cao L, Hu Y, Chen D, Yin Y. Escherichia coli exopolysaccharides induced by ceftriaxone regulated human gut microbiota in vitro. Front Microbiol. 2021;12:634204.
Kim G, Bae J-H, Cheon S, Lee DH, Kim DH, Lee D, et al. Prebiotic activities of dextran from Leuconostocmesenteroides SPCL742 analyzed in the aspect of the human gut microbial ecosystem. Food Funct. 2022;13:1256–67.
Tian J, Zhao X, Tang C, Wang X, Zhang X, Xiao L, et al. Protective effect of Paecilomyces cicadae TJJ11213 exopolysaccharide on intestinal mucosa and regulation of gut microbiota in immunosuppressed mice. Food Res Int. 2023;165:112477.
Abdel-Wahab BA, Abd El-Kareem HF, Alzamami A, Fahmy CA, Elesawy BH, Mahmoud MM, et al. Novel exopolysaccharide from marine Bacillus subtilis with broad potential biological activities: insights into antioxidant, anti-inflammatory, cytotoxicity, and anti-alzheimer activity. Metabolites. 2022;12:715.
Su Y, Zhang Y, Fu H, Yao F, Liu P, Mo Q, et al. Physicochemical and anti-uvb-induced skin inflammatory properties of Lacticaseibacillusparacasei Subsp. paracasei SS-01 strain exopolysaccharide. Fermentation. 2022;8:198.
Dilna SV, Surya H, Aswathy RG, Varsha KK, Sakthikumar DN, Pandey A, et al. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF(4). LWT-Food Sci Technol. 2015;64:1179–86.
Morris G, Harding SE. Polysaccharides, microbial. In: Schaechter M, editor. Encyclopedia of microbiology (Third Edition). Amsterdam: Elsevier; 2009. p. 482–94.
Larsen FH, Engelsen SB. Insight into the functionality of microbial exopolysaccharides by NMR spectroscopy and molecular modeling. Front Microbiol. 2015;6:1374.
Kagimura FY, da Cunha MAA, Barbosa AM, Dekker RFH, Maneck Malfatti CR. Biological activities of derivatized D-glucans: a review. Int J Biol Macromol. 2015;72:588–98.
Wang Z, Xie J, Shen M, Nie S, Xie M. Sulfated modification of polysaccharides: synthesis, characterization and bioactivities. Trends Food Sci Technol. 2018;74:147–57.
Ren Y-Y, Sun P-P, Ji Y-P, Wang X-T, Dai S-H, Zhu Z-Y. Carboxymethylation and acetylation of the polysaccharide from Cordyceps militaris and their α-glucosidase inhibitory activities. Nat Prod Res. 2019. https://doi.org/10.1080/14786419.2018.1533830.
Kagimura FY, da Cunha MAA, Theis TV, Malfatti CRM, Dekker RFH, Barbosa AM, et al. Carboxymethylation of (1→6)-β-glucan (lasiodiplodan): preparation, characterization and antioxidant evaluation. Carbohyd Polym. 2015;127:390–9.
Wang X, Zhang Z, Zhao M. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int J Biol Macromol. 2015;72:526–30.
Wang Y, Su N, Hou G, Li J, Ye M. Hypoglycemic and hypolipidemic effects of a polysaccharide from Lachnum YM240 and its derivatives in mice, induced by a high fat diet and low dose STZ. Med Chem Commun. 2017;8:964–74.
Wu Y, Ye M, Du Z, Jing L, Surahio M, Yang L. Carboxymethylation of an exopolysaccharide from Lachnum and effect of its derivatives on experimental chronic renal failure. Carbohydr Polym. 2014;114:190–5.
Li S, Xiong Q, Lai X, Li X, Wan M, Zhang J, et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr Rev Food Sci Food Saf. 2016;15:237–50.
Liu J, Luo J, Ye H, Zeng X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem Toxicol. 2012;50:767–72.
Wang K, Li W, Rui X, Li T, Chen X, Jiang M, et al. Chemical modification, characterization and bioactivity of a released exopolysaccharide (r-EPS1) from Lactobacillus plantarum 70810. Glycoconj J. 2015;32:17–27.
Zhang M, Su N, Huang Q, Zhang Q, Wang Y, Li J, et al. Phosphorylation and antiaging activity of polysaccharide from Trichosanthes peel. J Food Drug Anal. 2017;25:976–83.
Guo Y, Pan D, Sun Y, Xin L, Li H, Zeng X. Antioxidant activity of phosphorylated exopolysaccharide produced by Lactococcus lactis subsp. lactis. Carbohydr Polym. 2013;97:849–54.
He Y, Ye M, Jing L, Du Z, Surhio MM, Xu H, et al. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohyd Polym. 2015;117:788–96.
Jin M, Wang Y, Huang M, Lu Z, Wang Y. Sulphation can enhance the antioxidant activity of polysaccharides produced by Enterobacter cloacae Z0206. Carbohyd Polym. 2014;99:624–9.
Li S, Shah NP. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014;165:262–70.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This research was supported by the Natural Science Foundation of Shandong Province (ZR2022MD097, ZR2012CM019), Education and Industry Integration Innovation Pilot Project of Qilu University of Technology (Shandong Academy of Sciences) (2022JBZ01-06), the Foundation of State Key Laboratory of Biobased Material and Green Papermaking (No. ZZ20190302), the Foundation of Shandong Provincial Key Laboratory of Biosensors (SWCG 2018–01), and the Foundation (No. 202002) of Qilu University of Technology of Cultivating Subject for Biology and Biochemistry, Science, Education and Industry Integration Innovation Pilot Project of Qilu University of Technology (Shandong Academy of Sciences) (2020KJC-ZD08).
Author information
Authors and Affiliations
Contributions
WW writing-review & editing. LH supervision, writing review & editing. YJ and SS writing-review & editing. YJ writing-review & editing, visualization. NL project administration, funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, W., Ju, Y., Liu, N. et al. Structural characteristics of microbial exopolysaccharides in association with their biological activities: a review. Chem. Biol. Technol. Agric. 10, 137 (2023). https://doi.org/10.1186/s40538-023-00515-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00515-3