Abstract
Fungi and bacteria are important causes of damage to historical textiles. Many methods are used to resist innate damage in historical textiles. The study aim was to use an innovative method that loaded a mahogany plant extract onto natural chitosan and gelatin nanocomposite polymers to prepare chitosan / mahogany plant extract composite and gelatin mahogany plant extract nanocomposite and evaluate their potential for protecting historical textiles from biological damage. The fungi and bacteria found on historical textile samples were identified by biochemical methods. We performed an antifungal activity assessment of the mahogany—natural chitosan and mahogany—gelatin polymers to study the effect of these materials on the mechanical, chemical, and optical properties of dyed linen textiles. New linen fabrics dyed with madder, turmeric, and pomegranate were mordanted with alum, copper, and iron mordants. These materials were applied to dyed linen fabrics, and then the treated linen was artificially aged. The mechanical, chemical, and optical characteristics of the dyed linen fabric were examined by scanning electron microscopy, Fourier-transform infrared spectroscopy, CIELab, the tensile strength and elongation test, and the air permeability test. Mahogany – chitosan was more effective than mahogany – gelatin as an antifungal and antibacterial treatment of dyed linen and caused fewer changes in the mechanical, chemical, and optical characteristics. The mahogany – chitosan composite is recommended for preservation of historical linen textiles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Historical textiles collections in museums are an important part of our cultural heritage. However, these historical textiles in museums or excavation sites are exposed to microbiological damage caused by fungi—and bacteria, and humidity, that can depend on the chemical composition of the fibers, and the natural composition of the historical textiles [1,2,3]. For example, historical linen textiles consist mainly of vegetable fibers, which are primarily made of cellulose (cotton > 90%, flax 60–70%, and hemp up to 77%) [4, 5].
Various aspects of microbiological damage on historical textiles can be observed in several ways, such as their loss of durability, change in color gradients, and complete loss of historical textiles subjected to extreme degrees of biological damage. The fungi and bacteria found on textiles and historical organic materials include (Staphylococcus aureus, Candida albicans, Aspergillus niger, and Aspergillus fumigatus) [6, 7].
Chemical methods are used to protect historical textiles from bacterial fungal damage. It has been observed that chemical preservation methods show different degrees of toxicity to humans as well as changes in the characteristics of historical textiles. Several other preservation methods such as inert gases and radiation, are being studied [8, 9].
Chitosan, a cationic polymer derived from chitin in nature, is a promising antimicrobial agent because of its biocompatibility. Gelatin is an animal protein obtained by controlled hydrolysis of the fibrous insoluble collagen from the bones and skins of animals and fish. Gelatin comprises non-polar aliphatic amino acids, such as glycine (33.0%), proline (13.2%), alanine (11.2%), and hydroxyproline (9.1%) [10,11,12,13].
The main aim of this study was to use innovative method that loads a to that loads a mahogany plant extract onto either natural chitosan or gelatin nanocomposite polymers to prepare a chitosan–mahogany plant extract composite and a gelatin–mahogany plant extract composite and evaluate their potential for protecting historical textiles from biological damage.
The effects of these composites on the mechanical, chemical, and optical properties of dyed linen fabrics with natural dyes were also examined. Because early identification of fungi and bacteria would be necessary prior to any preservation treatments, a secondary goal was to identify the fungi and bacteria found on Egyptian textiles in the museum environment.
Materials and methods
Materials
-
Egyptian linen fabrics were supplied by Eglan Co., Egypt.
-
The number of threads per square centimeter of the linen fabric was 20 threads in the warp direction, and 14 threads in the weft direction and the weight per square meter was 85 g.
-
Alum, iron III chloride FeCl3 and copper sulfate CuSO4.5H2O were purchased from Fluka.
-
Madder dye and turmeric dye ware obtained from Wild Colours, Birmingham, UK. www.wildcolours.co.uk.
-
Pomegranate dye was obtained from a local market.
-
Chitosan with a powder acetylation degree of 14%, and an average molecular weight of 40.000 kDa was supplied by Fluka Chemical, Germany.
-
Gelatin type A from porcine skin (175 Bloom), was purchased from Alnasr Co. (Egypt).
-
Mahogny plant material from Mahogani plants grown in Egypt was collected from plants cultivated in Giza Zooogical Garden (Giza Zoo), Giza. The identities of the plant materials were authenticated by a plant taxonomist at El-Orman Botanical Garden, Giza, Egypt. According to the Linnaeus taxonomic classification system, the main formal names (Genus and species) are Swietenia macrophylla and Swietenia mahagoni.
Methods
Plant extraction
Five grams of dry powdered plant extracted materials were subjected to successive organic solvent extraction by refluxing in Soxhlet apparatus each for 10 h. In this study, the 80% aqueous methanol was used as solvent. All of the extracts were concentrated by oven drying. Each fraction was collected when no further elution of compounds was observed. The collected extracts were then distilled followed by drying in incubator. The dried extracts were stored in sterile containers in a refrigerator until further analysis [14].
Microbiological examination:
Historical textile samples were collected from different Egyptian museums to identify the type of fungi and bacteria to help establish the most common types of fungi and bacteria on Egyptian historical textiles and guide the experimental part of this study that evaluated the resistance of these types to potential preservation treatments. A sample was inoculated onto different microbiological broth media including brain heart infusion and Sabouraud dextrose broth. A loopful was streaked on blood agar, mannitol salt agar, MacConkey agar and Sabouraud dextrose agar (SDA). Broth and solid media were incubated at 37 °C for 24–48 h. The pure isolates were identified biochemically by conventional methods [15]. The samples are very small fibers dropped from the historical textiles object; these separate fiber samples cannot be returned and reattached to the historical textiles objects is and were about 0.5 cm in length.
Linen fabric preparation process
Dye extraction
The linen fabrics in the wet state were placed into the mordanting or dyeing bath. This action led to the liquor being taken up evenly. It is preferable not to expose the fiber to high temperature suddenly. The fibers were first placed in the cold solution slowly heated and then dyed with turmeric, madder, and pomegranate dyes according to the following steps.
-
1.
Grind the dry fibers to a fine powder.
-
2.
Prepare a 10% solution (w/v)
-
3.
Soak the dyes in distilled water for 12 to extract the color solution from the dyes powder.
-
4.
Heat the extract for 1.5 h at 70 °C with continuous stirring. Add water to compensate for the evaporated water during the heating process.
-
5.
Cool and filter the extract many times to obtain a clear colored solution.
Dyeing procedures
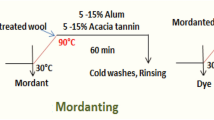
The dyeing process was performed using the exhaustion method and a liquor ratio (LR) of 1:20 (1 g of goods placed in a dye bath volume of 20 ml). The dyeing process was achieved in beakers in which linen fabric was used a cellulose fiber substrate and gently stirred at 70 °C for 1.5 h. Mordants, such as alum Al2(NH4)2(SO4)4.24H2O (Fluka), FeCl2, and CuSO4, were added as concentrated solution (50 g L) to give a final dyebath concentration of 2.5 g L or 5 g L of mordant. The mordanting bath continued for 30 min at temperature 60 °C. After dyeing, the unfixed dyestuff was removed by rinsing three times with cold water (5 min, room temperature, LR 1:20) [16, 17]. The characterizations of linen fabrics (color values, tensile strength and elongation) after dyeing are presented in Table 1.
Preparation of polymer—mahogany extracts composites
The desired amounts of extracted materials from mahogany were sonicated in a natural polymer solution (chitosan—alginate and chitosan—Alginate mixture) for 20 min using sonication dose 350 watts.
Then the solution was stirred at room temperature for 8 h and washed with ultra-pure water by ultracentrifugation to remove unbound chitosan and then collected and dried at room temperature to obtain chitosan—mahogny, gelatin—mahogny and chitosan / aliginate—mahogny nanocomposites.
Transmission electron microscopy (TEM) images of a gelatin—plant extract nanocomposite, and chitosan—mahogny nanocomposite are shown in Fig. 1.
Antimicrobial effect
The antimicrobial activity of the chitosan—mahogny, gelatin—mahogny, and chitosan/aliginate—mahogny nanocomposites prepared by a nano- technique were determined using an agar—disk diffusion assay and compared with that chitosan, gelatin, penicillin and miconazole 2% (w/v) against different microorganism isolates using the method described by Raeisi et al. [18].
One hundred microliters of bacterial suspensions (1.5 × 108 CFU/ mL) was spread on Muller-Hinton agar plates and then filtered through paper disks (6 mm in diameter). Then 10 μL of the collected material was studied. The plates were incubated at 37 °C for 24—48 h according to the tested bacteria. The diameters of the inhibition zones were measured in mm. All experiments were performed in triplicate.
Thermal ageing
The Thermal ageing experiment was comparable to that used by Feller (1994) in maturation tests for preservation materials. Accelerated thermal ageing is used to study the behavior of materials used in historical textile conservation, and to study the mechanical, chemical and optical properties of historical textiles in museums. Some previous studies have demonstrated that the quickened temperature restriction of 100 0C degrees for 72 h is equivalent the type of ageing of historical materials that occurs in a gallery over long periods [19, 20].
The treated linen fabrics samples were hang in a temperature-controlled oven (Herous – Germany). The treated linen fabrics samples were thermally aged separately at a temperature of 100 °C for 72 h and 144 h.
Examinations and analysis
Digital microscope
Many magnified images of the experimentally aged materials were taken under a digital microscope to examine the surface of the linen cloth and study the homogeneity of the treatment applications (Digital Microscope, VHX-6000 Series—KEYENCE, USA) [21].
Morphological study
Scanning Electron Microscopy (SEM) is very useful for studying the surface morphology of treated and untreated fibers. Different fibers were taken from different areas of the treated fabric and examined by a HITACHI-SU-1500 Scanning Electron Microscope [22]. The linen samples were placed and adhered directly without any preparation on the SEM samples holder, and then the holder was placed in the SEM chamber.
Color measurement
The CIE Lab values of the color change of the treated and untreated dyed linen fabrics were measured using a double beam Optimatch spectrophotometer (Datacolor International Spectraflash -SF450-UK). The literatures refers to the (L*) corresponding to the brightness (100 = white, 0 = black), and to the (a*) corresponding to the red – green coordinate (positive sign = red, negative sign = green). In addition, (b*) refers to the yellow – blue coordinate (positive sign = yellow, negative sign = blue) [23, 24].
Fourier—transform infrared spectroscopy (FTIR)
The change in chemical structure of the treated and untreated dyed linen fabrics were monitored by spectroscopy FTIR (BRUKER’S VERTEX 70 with attenuated total reflection (FTIR-ATR) at a resolution of 4 cm−1 [25, 26]. The textile samples were placed directly on the sample holder of FTIR- ATR devise, this type of investigation is consider as nan-destructive technique. Therefore, no preparation step was done on the sample before investigation.
Mechanical measurements
Mechanical properties (tensile strength and elongation) of the treated and untreated dyed linen fabrics were tested using a Shimadzu Universal Tester type S-500 (Japan). The measurements were performed according to the ASTM 2000, D 3822-96 standard test method for tensile properties of single textile fibers [27]. The spacing of initial jaw was 50 mm and the speed of the test was 25 mm/ min, at temperature 23 °C, and R.H. 65%.
Air permeability test
The air Permeability of the treated and untreated dyed linen fabrics was tested by using a FX3300 Air Permeability SDL tester at 65 ± 2% humidity and 20% ± 2 °C according to the EN ISO 9237 standard. First, the FX3300 was calibrated by using a calibration check plate [28].
Results and discussion
Microbial identification
Culture detection revealed small golden—yellow colonies on mannitol salt agar, different colony shape on SDA, including cream white colored raised colonies or an initial white growth becoming black later to give a “salt and pepper appearance”; and blue–green powdery colonies that were and pale yellow on reverse. Biochemical analysis revealed that Staphylococcus aureus, Candida albicans, Aspergillus niger, and Aspergillus fumigatus were the main microorganisms. These microorganisms are known to cause damage and decomposition of historical textiles, which threatens complete loss of these textiles. Therefore, we first identified the microorganisms, and then designed natural nanomaterials, and finally evaluated safety and efficacy for protection\preservation. The goal was to use suitable nanomaterials to protect real historical textiles.
Antimicrobial effect
The antimicrobial effects of the different 2% chitosan–mahogany, gelatin—mahogany, and chitosan/alginate—mahogany nanocomposites 2% against different microorganism isolates were compared (Table 2). The 2% chitosan—alginate –mahogany nanocomposite had the best antimicrobial activity against S. aureus, C. albicans, A. niger, and A. fumigatus.
Visual examination
Visual examination of the treated linen fabrics was the first important step in the examination process. Changes in the physical appearance can often be observed by the naked eye [29, 30]. It was clear by the visual examination that the linen fabric treated with mahogany—chitosan was softer than the linen fabric treated with mahogany—gelatin. In addition, the surface of the linen fabric treated with mahogany—chitosan was smoother than the surface treated with mahogany/gelatin.
Effect of mahogany—chitosan and mahogany—gelatin on the fiber morphology
Figure 2 shows digital microscope images of the surface morphology of the linen fabric that was treated with mahogany—chitosan (A and B) as well as of the surface morphology of the linen fabric treated with mahogany—gelatin (C and D). The images clearly show that the application to the fiber surface was more homogeneous with chitosan than with gelatin. The linen fabric surface appearance is clearly shown after treatment with mahogany – chitosan but treatment with mahogany—gelatin caused what appeared to be a layer that partially coved the actual surface of the linen fabric. In addition, the treatment with mahogany—gelatin caused bridging of the interfaces between the warp and the weft threads. These results of the treated linen fabrics were confirmed by SEM. Figure 3 shows (SEM) images of the surface of the dyed fabrics treated with mahogany plant extract applied onto the natural chitosan—gelatin. The images clearly show that the application of the mahogany plant extract applied to the natural chitosan was more homogeneous and the surface structure was smoother. The shapes of the linen fibers after treatment are shown in Fig. 3A–C.
It can be seen that treatment with the mahogany plant extract applied onto the natural gelatin has coated the surface of the treated linen fabrics as shown in Fig. 3D–F. The woven structure of the linen fabric treated with mahogany – gelatin is not clear after treatment.
Effect of treatment with mahogany – chitosan and mahogany—gelatin on color change
Figure 4 show the color change of the linen dyed fabrics treated with mahogany—chitosan and with mahogany—gelatin. It is clear that the application of mahogany led to a very slight change in the color grades of the dyed linen. For example, the brightness coordinate L of linen dyed with turmeric and modanted with iron was 61.90 and was 58.13 CIELab units after treatment. The red—green coordinate (a) of linen dyed with turmeric and modanted with iron was 4.18 and was 4.19 CIELab units after treatment. The yellow–blue coordinate (b) of linen dyed with turmeric and modanted with iron was 17.16 and was 16.79 CIELab units after treatment. On the other hand, slight changes in the linen fabric caused by treatment with mahogany—chitosan and with mahogany—gelatin were observed after thermal ageing for 72 h and 144 h. The brightness coordinate L in CIELab units of the treated linen dyed with turmeric dye and modanted with iron was 58.13 and was 57.41 after thermally ageing for 72 h and was 56.61 after thermally ageing for 144 h. In other words, the ∆L value of the linen treated with mahogany—chitosan after thermal ageing for 72 h and 144 h were −0.72 and −1.52 CIELab units respectively, whereas the ∆L values of the linen treated with mahogany—gelatin after thermal ageing for 72 h and 144 h were −0.95 and −1.31 CIELab units, respectively, which cannot be seen with the naked eye. The treated linen fabrics become slightly darker. On the other hand, the ∆a values of the dyed linen with turmeric and modanted with iron and treated with mahogany—chitosan after thermal ageing for 72 h and 144 h were 0.43 and 0.70 CIELab units, respectively, which cannot be seen it by the naked eye. The ∆a values of the linen treated with mahogany—gelatin after thermal ageing for 72 h and 144 h were 0.48 and 0.2 CIELab units, respectively. The treated linen fabrics dyed with turmeric become slightly red. The ∆b values of the linen dyed with turmeric and mordanted with iron and treated with mahogany—chitosan after thermal ageing for 72 h and 144 h were −1.0 and −0.75 CIELab units, respectively. The ∆a value of linen treated with mahogany—gelatin after thermal ageing for 72 h and 144 h were −1.28 and −0.76 CIELab units, respectively, but the changes cannot see it with the naked eye. The treated linen fabrics become a slightly blue.
Effect of treatment with mahogany – chitosan and with mahogany—gelatin on the brightness coordinate (L) of dyed linen fabric (A and B). Effect of treatment with mahogany – chitosan and with mahogany—gelatin on the red—green coordinate (a) of dyed linen fabric (C and D). Effect of treatment with mahogany – chitosan and with mahogany—gelatin on the yellow—blue coordinate (b) of dyed linen fabric (E and F)
Effect of treatment with mahogany – chitosan and with mahogany—gelatin on the on mechanical properties
Figure 5 show the obtained data of the effects of treatment Effect of treatment with mahogany – chitosan and with mahogany—gelatin on mechanical properties of treated linen fabric. It is clear that the application of mahogany led to a very slight change in the mechanical properties of the treated linen fabric. There is a slight increase in tensile strength and elongation of the linen fabric treated with mahogany—chitosan and with mahogany—gelatin. Application of treatment with mahogany—chitosan and with mahogany—gelatin forms a film on the surface of the linen fabric and lesser amounts can penetrate into the inter-fiber regions. The application with mahogany—chitosan and with mahogany—gelatin can affect load—bearing capability of the fabric and the symmetrical distribution of the load. There was also reduction in strength due to thermal ageing a finding is in agreement with Chattopadhayay [31].
Effect of treatment with mahogany – chitosan and with mahogany—gelatin on chemical properties
The FTIR spectra of linen fabrics treated by mahogany—chitosan nanocomposite are shown in Fig. 6A. New peaks at 803.19 cm−1, 1262.34 cm−1, 1315.08 cm−1, 1374.02 cm−1, and 2900.36 cm−1 appear in the FTIR spectra of the treated linen by mahogany—chitosan. The FTIR spectra of linen fabrics treated by mahogany—gelatin are shown in Fig. 6B. New peaks at 812.48 cm−1, 1522.91 cm−1, 1563.24 cm−1, 1544.63 cm−1, and 1653.2 cm−1 appear in the FTIR spectra of the treated linen by mahogany—gelatin. The new peaks were attributed to the amino group of chitosan and the amide group of gelatin, respectively. These findings are in agreement with those of kweon et al. and Yang et al. [32,33,34].
Effect of treatment by with mahogany – chitosan and with mahogany—gelatin on the air permeability of linen fabric
The rate of the air stream through a known zone of texture was balanced to secure an endorsed pressure differential between the two textile surfaces within the test region; from this stream rate, the air permeability of the texture was calculated. Air permeability was measured according to standard BS 5636. This system measures air permeability with to a precision of + 3%. The following testing parameters were used test area of 38 cm2; test pressure of 200 pa; unit of measurement of 1/m2/second) [35]. Table 3 shows the effects of mahogany—chitosan and mahogany—gelatin on the air permeability of dyed linen fabric before and after treatment. It can be seen that the air permeability was slightly decreased after treatment by mahogany—chitosan and mahogany – gelatin. The reason for the decreased permeability in the treated linen fabrics can be attributed to the lower and decreased interfaces and spacing in the fabric structure between the warp and weft threads. The decrease in the air permeability was less for the linen treated with mahogany—chitosan than for linen treated linen with mahogany—gelatin. This indicates that the amount of coverage of the interfaces and spacing in the fabric structure was lower using mahogany—chitosan than when using mahogany- gelatin.
Conclusion
This study identified the first time to identify the most important types of microorganisms that attack and cause damage and decomposition of historical LINEN textiles in Egyptian museums. We used a biological chemistry method to identify S. aureus, C. albicans, A. niger, and A. fumigatu as the primary microorganisms on historical Egyptian textiles. Mahogany– chitosan and mahogany–gelatin nanocomposites were prepared, and the effectiveness for preventing microbial growth in extracts of the nanocomposites was evaluated.
SEM images were showed that the homogeneity of the application on the fiber surface was better with mahogany – chitosan than with mahogany—gelatin. The surface appearance of the linen fabric treated was more clearly shown after treatment with mahogany—chitosan. A slight change in color of the linen fabrics treated with with mahogany–chitosan and with mahogany–gelatin of < 1 CIELab unit was measured, but this change is too small to be observed with the naked eye. Some new peaks appeared in the FTIR spectra after treatment which confirmed that the linen was grafted successfully.
There was also a slight increase in tensile strength and elongation of the linen fabric treated with mahogany—chitosan and with mahogany—gelatin. The air permeability was slightly decreased after treatment with mahogany—chitosan and with mahogany—gelatin. The antifungal and antibacterial effectiveness was greater for mahogany–chitosan treatment than for mahogany–gelatin treatment of the dyed linen fabrics.
Fewer changes in the mechanical, chemical, and optical properties of the dyed linen fabrics were observed after treatment with mahogany—chitosan than with mahogany—gelatin. The study results support the use mahogany—chitosan for preservative treatment of historical linen textiles in Egypt.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Tímár-Balázsy Á, Eastop D. Chemical Principles of Textile Conservation. 1st ed. Oxford: Butterworth-Heinemann; 1998.
Ahmed H. Strategy For Preservation Of Ptolemaic Wrapped Mummy’s Linen In Tuna El-Gebel Excavation, Egypt. A Case Study. Int J Con Sci. 2011;2(3):155–64.
Brzozowska I, Bogdanowicz A, Szczęsny P, Zielenkiewicz U, Laudy A. Evaluation of bacterial diversity on historical silk velvet textiles from the Museum of King John III’s Palace at Wilanów Poland. Int Biodet Biodeg. 2018;131:78–87. https://doi.org/10.1016/j.ibiod.2017.02.017.
Kavkler K, Demsar D. Application of FTIR and Raman spectroscopy to qualitative analysis of structural changes in cellulosic fibres. Tekstilec. 2012;55:19–31.
Gleba M, Harris S. First Plant Bast Fibre Technology: identifying splicing in archaeological textiles. Arch Anth Sci. 2019;11:2329–46.
Sülar V, Devrim G. Biodegradation behaviour of different textile fibres: visual, morphological, structural properties and soil analyses. Fib Tex Eas Eur. 2019;27(1):100–11. https://doi.org/10.5604/01.3001.0012.7751.
Unango FJ, Ramasamy KM. A review on the investigation of biologically active natural compounds on cotton fabrics as an antibacterial textile finishing. Int Res J Sci Tech. 2019;1(1):49–55. https://doi.org/10.46378/irjst.2019.010107.
Mansour M, Ahmed H. Occurrence of fungi on some deteriorated ancient Egyptian materials and their controlling by ecofriendly products. Eg J Arch Res St. 2012;2(2):91–101. https://doi.org/10.21608/EJARS.2012.7465.
Landi S. The Textile Conservator’s Manual. Oxford: Butterworth-Heinemann Series in Conservation and Museology; 1998.
Shu XZ, Zhu KJ. Controlled drug release properties of ionically cross-linked Chitosan beads: the influence of anion structure. Int J Pharm. 2002;233(1–2):217–25. https://doi.org/10.1016/S0378-5173(01)00943-7.
Ravi Kumar MNV. A review of chitin and chitosan applications. React Funct Polym. 2001;46(1):1–27. https://doi.org/10.1016/S1381-5148(00)00038-9.
Ma W, Tang CH, Yin SE, Yang XA, Wang Q, Liu F, Wei ZH. Characterization of gelatin-based edible films incorporated with olive oil. Fo Res Int. 2012;49(1):572–9. https://doi.org/10.1016/j.foodres.2012.07.037.
Jiang Y, Li Y, Chai Z, Leng XJ. Study of the physical properties of whey protein isolate and gelatin composite films. J Agr Fo Che. 2010. https://doi.org/10.1021/jf9040904.
Azhari H, Abdurahman H, Jessinta, Mashitah MY. Antibacterial Activity of Different Extract of Swietenia Macrophylla King. In 13th Medicinal and Aromatic Plants Seminar 2012. Industrial sciences and technology, University Malaysia Pahang, Malasia. 2012. http://umpir.ump.edu.my/id/eprint/3030/1/MAPS2012,_Antibacterial_activity_of_S._macrophylla.pdf
Quinn PJ, Carter ME, Markey B, Carter GR. Clinical Veterinary Microbiology. London: Wolf/Mosby; 1994.
Abdel-Hamied M, Hassan RRA, Salem MZM, Ashraf T, Mohammed M, Mahmoud N, El-Din YS, Ismail SH. Potential effects of nano-cellulose and nano-silica/polyvinyl alcohol nanocomposites in the strengthening of dyed paper manuscripts with madder: an experimental study. Sci Rep. 2022 Nov 15;12(1):19617. https://doi.org/10.1038/s41598-022-23907-1. PMID: 36380061; PMCID: PMC9666511./S0959-6526(02)00077-X.
Ahmed HE. History of natural dyes in North Africa ‘Egypt.’ In: Bechtold T, editor. Handbook of natural colorants. London: Welly; 2009. p. 27–36.
Mojtaba R, Hossein T, Arman Y, Sirvan S. Antimicrobial effect of cinnamon essential oil against Escherichia Coli and Staphylococcus aureus. Heal Sco. 2015;4(4):1–5. https://doi.org/10.17795/jhealthscope-21808.
Feller RL. Accelerated ageing: photochemical and thermal aspects. Los Angels: Getty Publications; 1995.
Feller RL. Accelerated ageing: photochemical and thermal aspects: Marina del Rey, The Getty conservation institute. Ann Arbor: Edwards Bros; 1994.
Ahmed HE, Kolisis FN. A study on using of protease for removal of animal glue adhesive in textile conservation. J App Pol Sci. 2012;124(5):3565–76. https://doi.org/10.1002/app.34053.
Batcheller JC. Optical and scanning electron microscopy techniques for the determination of hair fibres from Romano-Egyptian textiles. In: Scientific analysis of ancient and historic textiles: informing preservation, display and interpretation: postprints, first annual conference, 13–15 July 2004. London: Archetype Publications; 2005. p. 51–56.
Booth JE. Principles Of Textile Testing. India: CBS Publishers and Distributors Pvt LTD; 1969.
Wyszecki G, Stiles W. Color Science. New York: Wiley; 2000.
Fierascu, I., R.C. Fierascu, and P. Fotea. Application of Fourier-Transform Infrared Spectroscopy (FTIR) for the Study of Cultural Heritage Artifacts. in VR Technologies in Cultural Heritage: First International Conference. VRTCH 2018, Brasov, Romania, May 29–30, 2018, Revised Selected Papers. Springer. 2019.
Balta ZI, Demetrescu I, Lupu M. ATR/FTIR investigation into the nature of the metal threads from Romanian medieval textiles. U P B Sci Bull Series B. 2017;79(2):25–36.
Saha P, Manna S, Chowdhury S, Sen R, Roy D, Adhikari B. Enhancement of tensile strength of lignocellulosic jute fibers by alkali-steam treatment. Biore Tech. 2010;101(9):3182–7. https://doi.org/10.1016/j.biortech.2009.12.010.
Özdemir H. Air permability of worsted fabrics. Ind Text. 2018;69(4):322–7.
Ahmed HE. A new approach to the conservation of metallic embroidery threads in historic textile objects from private collections. Int J Con Sci. 2014;5(1):21–34.
Ahmed H, Yahia D, Zidan Y. Restoration and storage procedures of a rare historical textile in the museum of the faculty of applied arts of Helwan University Egypt. Eg J Arch Rest Stu. 2018;8(1):35–43.
Chattopadhyay D, Inamdar M. Improvement in properties of cotton fabric through synthesized nano-chitosan application. Ind J Fib Tex Res. 2013;38:14–21.
Yang Z, Zeng Z, Xiao Z, Ji H. Preparation and controllable release of chitosan/vanillin microcapsules and their application to cotton fabric. Fla Frag J. 2014;29(2):114–20. https://doi.org/10.1002/ffj.3186.
Scacchetti FA, Pinto E, Soares GM. Preparation and characterization of cotton fabrics with antimicrobial properties through the application of chitosan/silver-zeolite film. Pro Eng. 2017;200:276–82. https://doi.org/10.1016/j.proeng.2017.07.039.
Kweon H, Chul Ha H, Chul Um I, Hwan PY. Physical properties of silk fibroin/chitosan blend films. J App Poly Sci. 2001;80(7):928–34. https://doi.org/10.1002/app.1172.
Behera B. Comfort and handle behaviour of linen-blended fabrics. AUTEX Res J. 2007;7(1):33–47.
Acknowledgements
SEM, digital microscopy of treated samples was done at the Saga University-Japan during the JSPS invitation fellowship. Therefore, the author would like to thank JSPS, Saga University, and Dr. Mei Ishii. Furthermore, FTIR, air permeability test, mechanical properties measurement, and CIELab measurements of treated samples were done at National Institute of Standards, Egypt. So authors thank Prof. Mahmoud Morsy and Mrs. Rasha Sadik.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no funding from any governmental or non-governmental agencies. Any necessary funding was made by the authors themselves.
Author information
Authors and Affiliations
Contributions
HA collected historical samples, dyed and prepared the textile samples, applied the nanocomposites to the textile samples, aged the treated samples, test and analyzed the textile samples before and after treatment, and participated in the writing of the research. WM prepared the nanocomposites of mahogany plant extract with natural chitosan and with gelatin, performed TEM of nanocomposites, and participated in writing the manuscript. SM identified the microorganisms, evaluated the microbiological and antimicrobial effects, and participated in writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ahmed, H.E., Marouf, S. & Mohamed, W.S. Antifungal activity assessment of nanocomposites of natural chitosan and gelatin with a mahogany plant extract for conservation of historical textiles. Herit Sci 10, 198 (2022). https://doi.org/10.1186/s40494-022-00822-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-022-00822-2