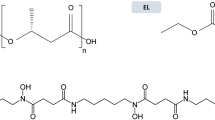
Abstract
Cellulose ethers, like methyl cellulose (MC) or hydroxypropyl cellulose (HPC), are widely used in conservation. They also occur as additives and rheology modifiers in various products like dispersions or gels. Do such products release harmful volatile organic compounds (VOC) during their accelerated aging? A mass testing series utilizing the Oddy test of 60 commercial cellulose ethers ranks the products in safe for permanent use (P, no corrosion), only for temporary use (T, slight corrosion), and unsuitable at all (F, heavy corrosion). Results show that 55% of the products passed the test whereas 33% are for temporary use as slight corrosion occurred on at least one metal coupon and only 11% failed the Oddy test. Raman measurements of the corrosion products identified oxides like massicot, litharge, cuprite, and tenorite among carbonates (hydrocerussite, plumbonacrite), and acetates like basic lead acetate, lead acetate trihydrate as well as lead formate as main phases. For example, commercial, industrial Klucel® G (HPC) scored a T rating through slight corrosion on the lead coupon. Basic lead acetate among other phases indicates the presence of acetic acid. Additional measurements of the sample with thermal desorption GC–MS utilizing the BEMMA scheme confirm the high acetic acid outgassing and reveal the presence of a small amount of formaldehyde.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Historical background
Cellulose nitrate was one of the first cellulose derivates and was already discovered in 1833 [1]. A first description of synthesis of acylated or alkylated cellulose derivates was given by W. Suida in 1905 [2]. Subsequent research on methyl- and ethyl derivates of cellulose was done in the 1910s [3]. Pioneering patents for nonionic cellulose ethers were submitted by Hubert in 1920 [4], Leuchs in 1912 [5] and Lilienfeld in 1916 [6]. Hubert [4] for example described the synthesis of hydroxyethyl cellulose, whereas Jansen [7] introduced the sodium carboxymethyl cellulose (Na-CMC). CMC was used in Germany for improving detergency in low grade synthetic detergents during the second world war [8]. For example, the product Tylose® HBR was recommended for use in fatty acid soap products [8]. Hydroxypropyl cellulose (HPC) was developed in the late 1940s by Eugene Klug and coworkers from Hercules Powder Company in the USA and was patented in 1951 [9]. HPC was introduced as Klucel® on the market. Methyl hydroxypropyl cellulose was developed even later by researchers from Dow Chemical Company and was patented in 1968 [10].
Industrial production of cellulose ethers started in Germany in the early 1920s and in 1936/37 in the USA [11]. Tylose®, one of the first commercial brands of cellulose ethers in Germany was registered in 1926 (1936 USA). It was developed by Chemische Fabrik Kalle & Co. that became part of I.G. Farben AG in 1925. The production palette included methyl cellulose (MC), and carboxymethyl cellulose [8, 12]. After the second world war the Tylose® business became part of Hoechst AG. In 1997 it was sold off to Shin-Etsu Chemical being manufactured by SE Tylose GmbH & Co. KG. Other familiar brands in conservation are for example Klucel®, Culminal®, Methocel® or Blanose®. Klucel® was patented in 1951 and became a registered trademark in the USA in 1963, and in Germany in 1968. Klucel® had been manufactured by Hercules Inc. (business division: Aqualon group) until it was sold off to Ashland Global Specialty Chemicals Inc. in 2008. Culminal® was registered as trademark by Hercules Inc in Germany in 1951 (1963 USA). Initially, it was a trademark for methyl cellulose (MC) [3]; however, in the following decades Culminal® product lines got extended and include genuine methyl cellulose (Culminal® MC types), hydroxypropyl methyl cellulose (Culminal® MHPC types), and hydroxyethyl methyl cellulose (Culminal® MHEC types) [13, 14]. Methocel® products have been manufactured by Dow Chemical Company and include genuine MC (Methocel A types) as well as HPMC (Methocel® E, F, J, K types). It was registered as trademark in the USA in 1938 (1959 in Germany). Blanose® is a trademark for sodium carboxymethylcellulose (Na-CMC) that was developed by Hercules Inc. (Aqualon group). It was introduced in the USA in 1946 and in Germany in 1947 [15].
Cellulose ethers in conservation
Methyl cellulose (MC) was used already in the 1920s as a consolidant for corroded leaden bullae [16]. In paper conservation water soluble MC has a long record of usage as adhesive, size and as consolidant [3, 17,18,19,20,21,22,23]. MC solutions have been tested on wall paintings [24, 25]. They have been used as a medium for pigments [26,27,28,29,30], as a consolidant for waterlogged wood [31] and for basketry [32], for relining of canvas [3], as an adhesive for textiles [33, 34] and wallpapers [35,36,37]. MC among other cellulose ethers have been added as additives (rheology modifiers, protective colloids) to starch adhesives and polymer dispersions [38,39,40,41,42,43] to improve working properties. Ethyl cellulose (EC) is hardly used in conservation [44] but is sold as film-forming agent for paints and for hot melt coating [45]. Hydroxy ethyl cellulose (HEC) has also a limited use in conservation [44] and mainly occurs as emulsifier, stabilizer, thickener or protective colloid in dispersions [39, 41, 46, 47]. Only few references document the single use of ethyl hydroxyethyl cellulose (EHEC), methyl hydroxyethyl cellulose (MHEC) and methyl hydroxypropyl cellulose (MHPC) in conservation. EHEC has been used for textile consolidation [44] and for formulations for jute consolidation [48]. HEMC was suggested for a consolidation treatment for canvas [49] and for canvas lining [50]. HPMC is mainly used as a thickening agent, stabilizer, emulsifier and film forming agent. HPMC and MC are the most common gelling agents used in aqueous conservation cleaning preparations [51]. Moreover, HPMC was used as consolidant of an ethnographical object [52]. HPC, especially Klucel® G have been used as leather consolidant [19, 53, 54], in textile [55, 56] and paper conservation [23, 57,58,59,60], for the consolidation of a wax sculpture [61], of archaeological cartonnage [62] and of herbarium specimen [63]. Klucel® J and G have been used in general for pigment consolidation where a non-aqueous treatment is required [19, 64, 65]. Na-CMC has many applications among conservators and include for example the use as adhesive, consolidant and detergent in paper conservation [44, 66], as relining agent for canvas [50], as paper or textile size, as cleaner for stones, murals, leather and textiles [3]. However, for wall paintings cellulose ethers with less impurity content (NaCl, a by-product from the etherification process) are recommended [25].
Oddy testing of cellulose derivates
Conservation materials like adhesives, consolidants or coatings stay on treated objects and enter with them display cases. Therefore, they need to be Oddy tested like materials for storage or display [67]. Due to its simplicity, the Oddy test is a suitable method for mass screening of materials for harmful emissions.
Oddy tests of cellulose derivates are scarcely found in the published literature. Comprehensive results are only available for cellulose nitrate [67,68,69]. All tested products of this type failed the Oddy test dramatically. Heavy corrosion occurred on all coupons and the analyzed phases include rouaite (Cu2(NO3)(OH)3), litharge (PbO), cerussite (PbCO3), hydrocerussite (Pb3(CO3)2(OH)2) and a silver cyanide–silver nitrate phase [67, 68]. The authors concluded that nitrous fumes were generated during the testing causing the massive corrosion. The results are particularly important as cellulose nitrate is still used as adhesive [44] and can occur as admixture in acrylic adhesives [70, 71]. Nel and Lau [71] showed that the manufacturer changed the composition of a ready-to-use adhesive based on the very stable Paraloid® B72 by adding small amounts of the unstable cellulose nitrate. It can be assumed that the aging characteristics and the corrosive gas activity of this new formulation is much worse compared to pure Paraloid® B72. This case demonstrates the importance of testing not only the primary polymers as commercial products often contain undeclared additives.
To the best of our knowledge Oddy test results of only three cellulose ethers are published. Korenberg et al. [72] tested Klucel® G (HPC) and Culminal® MC 2000 (MC) and both products passed the Oddy test. Recently, the Metropolitan Museum of Art (MMA) published also an Oddy test result of Klucel® G, rating it for temporary use as slight corrosion occurred on the silver and lead coupon [73]. Moreover, they reported the results for Methocel® A4C Methylcellulose, assigning it as suitable for use (P rating) [74].
Methods/experimental
Oddy test
The “3 in 1” Oddy tests were prepared according to the procedure of the British museum [75] that was revised in 2018 [72]. A detailed description of the work flow can be found in these publications, hence, only a quick summary will be given here. Two grams of the sample material are placed together with a small test tube with 0.5 ml ultra-pure water and stoppered with cotton wool in a 55 ml test tube (DURAN®, Carl Roth GmbH + Co. KG). Three high purity (> 99.9%) metal coupons (0.8 × 3.5 cm) made of silver (Carl Roth GmbH + Co. KG), copper (Carl Roth GmbH + Co. KG) and lead (Merck KGaA) are polished and cleaned in HPLC-grade acetone. They are fixed in a silicon stopper (Versilic™ Silicone Stopper, Th. Geyer GmbH & Co. KG) that seals the test tube. One blank test was included for every test series. They were placed in the oven at 60 °C for 28 days with a relative humidity of 100% inside the tubes. All materials were running in duplicate and were repeated if the results differ. The tests are evaluated visually by comparing them with the blank coupons and rated as P (permanent use, no corrosion), T (temporary use, slight corrosion) and F (fail, unsuitable, heavy corrosion). An overall rating is assigned to indicate a material’s general suitability. Please note that a P rating does not mean that the tested substance does not release any volatile components. There can be still VOC present, but they are not visibly corrosive to the three metals that are used in the Oddy test. According to the experience at the British Museum, the results for these metals can be generalized to all materials. ‘Since [the Oddy test] was implemented at the British Museum, there has been virtually no corrosion caused to objects by indoor pollutants’ [72].
The metal coupons are photo-documented using a camera (Canon EOS 7D Mark II) on a copy stand in diffuse light. Corroded coupons are further examined by optical microscopy and Raman spectroscopy.
Raman spectroscopy
Raman measurements of corroded Oddy test coupons allow to identify the corrosion products which may give hints to the corrosive gaseous agents. They were conducted with a Renishaw inVia™ Raman system equipped with two lasers (632 and 785 nm) and a Peltier-cooled CCD detector. The 632 nm laser (grating 1800 lines/mm) was focused through a 50× lens from a Leica DM 2550 M microscope. The spectral range was set to 100–3700 cm−1 using various measurement conditions (10–60 s; 2 accumulations, 1–50% laser power). The Origin(Pro), Version 9.0 (OriginLab Corporation, Northampton, MA, USA) software was used for spike removal and multi-point baseline subtraction.
BEMMA
Determination for the BEMMA (‘Bewertung von Emissionen aus Materialien für Museumsausstattungen’ = Evaluation of emissions from materials for museum equipments) evaluation is carried out in microchambers (µ-CTE, Markes company), six small cylinders with a volume of 45 ml each. The following parameters apply when loading the microchambers with the samples to be tested: Flow: 28 ml/min; 23 °C ± 2 K, ≈ 50% relative air humidity, synthetic air, surface-specific air-flow rate depends on the tested material. With these parameters the concentrations of VOCs in exhaust air of the microchambers can be calculated. Extract samples with Tenax® for VOCs (volatile organic compounds) and SVOCs (semi-volatile organic compounds) were analysed (in accordance with ISO 16000-6 [76]). Quantification is performed with a thermal desorption GC–MS combination. The evaluation of the individual peaks is carried out with the total ion chromatogram, which quantifies by toluene calibration. The summation is carried out for all components whose quantification through toluene equivalents equals or is greater than 5 µg/m3 (threshold for consideration). The resulting main components (≥ 5 µg/m3) are listed separately. Aldehydes and ketones were sampled with DNPH (dinitro-2,4-phenylhydrazine)-cartridges overnight (in accordance with ISO 16000-3 [77]). At least formaldehyde and acetaldehyde should be listed separately. Additionally, aldehydes beyond a concentration of 5 µg/m3 should be noted. Sample extraction for volatile, short-chain, organic acids, like formic and acetic acid was performed on silica gel cartridges with the second overnight sampling. The concentrations were quantified with ion-chromatography [78]. The sums of very volatile organic compounds (VVOC), a subset of the last two procedures, were also used for the evaluation.
Assessment criteria: Substances with high contamination potential, such as formic acid, acetic acid, formaldehyde and oximes must not be detectable. That means these compounds must be lower than the limit of detection. The limit of sum emissions for Σ VVOCs is 100 μg/m3, for Σ VOCs is 500 μg/m3 with the exception of sealing materials with Σ VOCs 2000 μg/m3 due to significantly smaller application surface and for Σ SVOCs is 100 μg/m3. All listed assessment criteria must be fulfilled, otherwise the product fails the BEMMA scheme.
Samples
Two types of samples are included in the study: (1) recent materials, that are currently used in conservation and that were freshly purchased and sampled; and (2) older materials from the 90 s and 00 s that had been used in the past and that may be part of art works nowadays. These samples are obtained from the materials’ collection of the Institute of Conservation Sciences, Stuttgart State Academy of Art and Design. Detailed information about the sample materials can be found in Table 1.
Results and discussion
From 60 samples 55% (i.e. 33 samples in total) passed the Oddy test and get a rating for permanent use. 33% (20 samples) are for temporary use as slight corrosion occurred on at least one metal coupon. Only 11% (7 samples) of the tested materials are unsuitable for use as they fail the Oddy test producing significant amount of corrosion due to a high concentration of harmful volatile components. Obviously, by checking the table of results (Table 1) no group of cellulose derivates entirely passes the Oddy test as there are always some products that release harmful volatile components.
Hydroxypropyl cellulose (HPC)
A total of 18 Klucel® HPC samples had been tested whereas 11 were freshly purchased and seven were historical or undated products from the materials’ collection. All historical samples failed the Oddy test with a T or and F rating (Table 1). Klucel® MF (Hercules, 2006, F rating) generated significant amount of corrosion on both copper and lead coupon. The phases were identified by means of Raman spectroscopy and include cuprite (Cu2O), massicot (PbO), hydrocerussite (Pb3(CO3)2(OH)2) and lead formate (Pb(HCOO)2). Cuprite yields Raman bands at 147, 215, 494 and 623 cm−1 which are often only visible as weak bands or shoulders (cf. Fig. 2a) [79, 80]. Massicot and its polymorph litharge are strong Raman scatterer. Both have their strongest band around 145 cm−1 and an additional main feature at 285 (massicot) and 340 cm−1 (litharge) [81, 82]. The main bands of hydrocerussite are located at 1048, 1051 cm−1 (shoulder) and c. 3540 cm−1 and can be used to differentiate it from plumbonacrite (Pb5O(OH)2(CO3)3) (see further discussion in this section and [83]). The Raman spectrum of lead formate is shown in Fig. 2c. Lead formate can be easily differentiated from lead acetates by its typical band pattern. The C–H stretching band doublet is located at 2843 and 2873 cm−1, the strong C–O stretching is centered at 1345 cm−1 and various spectral features occur at 760, 1075, 1375 and 1530 cm−1 [84, 85]. The identification of lead formate may not clearly proof the presence of formic acid as formaldehyde can be also oxidized during the test conditions, inducing the growth of metal formates [86, 87].
Regarding the fresh HPC products, Klucel® M and H passed the Oddy test with no visible corrosion on all metal coupons. The low viscosity type E generated little corrosion on the lead coupon leading to a T rating. Klucel® G was tested multiple times as ambivalent results occurred, causing at first a veritable confusion. A sample from 2017 (purchased from GMW) passed the Oddy test with no corrosion on any coupon (Fig. 1a). That was not surprising as Korenberg et al. [72] reported the same result for Klucel® G. However, the Oddy test from a freshly purchased product from 2021 (purchased from Deffner) shows some amount of corrosion on the lead coupon, yielding a T rating (Fig. 1c). The same result was achieved from Klucel® G purchased from Kremer (Fig. 1b). In both cases Raman spectroscopy revealed the presence of massicot/litharge (PbO), hydrocerussite and basic lead acetate (Pb3(CH3COO)2(OH)4). Basic lead acetate yields a plenty number of bands, with several bands (e.g. 370, 447, 612, 641, 648, 666, 912, 922, 929, 1345, 1410, 1428, 2923, 2970, 2996 cm−1) being diagnostic for this phase (cf. Fig. 2d). A detailed discussion and band assignment can be found elsewhere [84]. Especially the spectral feature at 370 cm−1 and the bands between 910 and 930 cm−1 can be used to differentiate it from lead acetate trihydrate (Pb(CH3COO)2·3H2O) [84]. Generally, lead acetates indicate the presence of acetic acid in the test tube. One gram of Klucel® G (Deffner) was more detailed tested utilizing the BEMMA scheme with a quantification of the outgassing VOC and VVOC. A high amount of acetic acid can be detected (about 4000 µg/m3). Furthermore, fragments of the hydroxypropyl cellulose can be detected with the VOC Tenax method and a small amount of formaldehyde was analyzed. This material does not fulfil the BEMMA scheme. A sum of VOC higher than 500 µg/m3 and the high acetic acid concentrations and the following high amounts of VVOCs were the reasons.
Lead coupons after the Oddy test of various Klucel® G samples. The lower part of the coupon was stuck into the stopper and may not be used for rating purpose. a Klucel® G 2017 (GMW), b Klucel® G 2021 (Kremer), c Klucel® G 2021 (Deffner), d Klucel® G IND (Ashland), e Klucel® GF (Ashland), f Klucel® GF Pharm (Ashland). Please note that the metallic reflections in f may lead to misleading interpretations regarding the metal corrosion
Raman spectra of common corrosion phases. Offset was applied for better comparison. a Cuprite and tenorite (Tn) (Cu coupon of the Hydroxyethylcelluose test); b anglesite (Pb coupon of the Blanose® 7H4Xf test); c lead formate (Pb coupon of the Klucel® MF test); d massicot (M), plumbonacrite (Pl), basic lead acetate (Pb coupon of the Hydroxyethylcelluose test); e plumbonacrite (Pl), lead acetate trihydrate (Pb coupon of the Hydroxyethylcelluose test)
In a next step, Klucel® G samples with different purity grades were obtained directly from the producer. The testing of the industrial quality (i.e. the same quality as for the Kremer and Deffner sample) shows unsurprisingly the same amount of corrosion as the two samples before (Fig. 1d). For Klucel® GF (food grade, i.e. higher purity) hardly any corrosion is visible, only some small white spots can be found on the edge of the coupon (Fig. 1e). Raman measurements show the presence of litharge and plumbonacrite. Plumbonacrite can be differentiated from hydrocerussite by means of Raman spectroscopy. It is characterized by a strong band at 1048 cm−1 with two additional sub-bands at 1052 and 1056 cm−1 that are visible as shoulders [83]. Additionally, among other band shifts in the fundamental modes the OH stretching results in two bands for plumbonacrite, whereas there is only one band for hydrocerussite [83]. Klucel® GF Pharm (pharmaceutical quality) passes the test with no corrosion on the lead coupon (Fig. 1f). The coupon even kept its metallic gloss after testing. The results for Klucel® G show that the amount of corrosion depends on the purity grade, hence, not the product itself releases the harmful volatile components but an impurity or a leftover from the production process causes the problems. The producer states upon request, that acetic acid is used in the production process for neutralization and that the acetate is removed during purification. Unfortunately, there is no data available how much acetate is left in the products after the purification. However, it is not surprising that the acetate amount is the highest in the industrial grade product. Moreover, according to the producer, there was an expand in production capacity for Klucel® in 2016 that may explain the different results for the older and new Klucel® G of industrial quality.
Hydroxyethyl cellulose (HEC), ethyl cellulose (EC), methyl cellulose (MC)
Only few HEC and only one EC sample were included in this study. EC (Ethylcellulose ET 200 purchased in 17.11.2015) failed the Oddy test, generating significant amount of corrosion on the lead coupon. The corrosion phases include massicot, litharge, hydrocerussite and basic lead acetate indicating the presence of acetic acid. For HEC, 2 of 5 samples passed the test, whereas one got a T rating (Tylose® H 100000 YP2, purchased in 11.2016) and two failed the test (Natrosol 250 MHBR purchased in 05.03.2004, Hydroxyethylcelluose (unknown date of purchase) (Table 1). The corrosion phases of the lead coupon from Hydroxyethylcelluose (Fluka) are particularly interesting as they include not only massicot and plumbonacrite but also basic lead acetate and lead acetate trihydrate. The Raman spectra of these phase associations are shown in Fig. 2d, e. Both lead acetates are good Raman scatterers and a direct comparison of their spectra reveal the differences. Lead acetate trihydrate yields its typical bands at 464, 617, 657, 930, 1345, 1429, 2930 and c. 2980 cm−1 [84]. The 370 cm−1 band is completely missing and there are significant differences in the 600–660 cm−1 and the 910–950 cm−1 ranges respectively. Both lead acetates indicate the generation of acetic acid during the Oddy test. Most of the MC products passed the Oddy test. Four freshly purchased samples were tested, three of them passed the test with no sign of corrosion. The only fresh sample that got a T rating was Methyl cellulose (Sigma) as little corrosion occurred on the lead coupon. Among the fresh samples, special emphasis was laid on Methocel® A4M as it is the most common cellulose ether in conservation practice. It was tested as industrial grade and passed the test. The same result was obtained from historical equivalents from 1997 and 2003. Generally, 9 historical or undated samples were included in this study of which 7 passed the Oddy test. Two low viscosity grade products got a different rating. Metolose® SM-15 (Shin Etsu) generated slight corrosion on the copper and lead coupon, yielding a T rating. Raman measurements reveal the presence of cuprite; litharge and hydrocerussite as main phases. Culminal® MC 15 S is the only tested MC that truly failed the Oddy test as heavy corrosion occurred on the lead coupon. Cuprite, massicot, hydrocerussite, plumbonacrite and lead formate could be identified by means of Raman spectroscopy.
Methyl hydroxyethyl cellulose (MHEC), Methyl hydroxypropyl cellulose (MHPC), sodium carboxymethyl cellulose (Na-CMC)
Nine MHEC products were included in this study of which five were freshly purchased. The results show that MHEC products mainly pass the Oddy test. Only two of the nine samples did not pass the test with a T rating for Tylose® MH 50 (Hoechst) and Tylose® MH 30000 YP4 (Shin Etsu). Raman measurements on the copper and lead coupon of the historical Tylose® MH 50 sample reveal the presence of cuprite, litharge, hydrocerussite and basic lead acetate. The corrosion phases of the high viscosity MHEC Tylose® MH 30000 YP4 include cuprite; massicot, hydrocerussite and lead formate. Most of the MHPC products got a P rating as no corrosion occurred during the tests. Culminal® MHPC 20000 P was tested as fresh and historical (2005) sample and both passed the Oddy test. A temporary rating was assigned to two historical products. The Oddy test of Methocel® K4MFG from 2003 yielded slight corrosion on copper (cuprite) and lead (hydrocerussite). Methocel® E5 Premium LV from 2000 created slight corrosion on the lead coupon with hydrocerussite as main phase.
Eight Na-CMC products were tested in this study and most of them passed the test. The two freshly purchased samples (Cekol® 700, Blanose® ref CMC 7M65) passed the Oddy test with no sign of corrosion. Slight corrosion on the lead coupon was observed from three historical or undated products. Raman measurements reveal the presence of hydrocerussite (Blanose® 7LF), anglesite (PbSO4) (Blanose® 7H4Xf; cf. Fig. 2b) or massicot, hydrocerussite and anglesite (Tylose® C6000) as main phases. Anglesite yields a characteristic Raman spectrum with a main band at 976 cm−1 and some weak to medium intensity bands at 439, 451 and 609 cm−1 [88].
Conclusion
60 commercial cellulose ether products have been Oddy tested for their VOC activity. Raman measurements of the corrosion products identified oxides like massicot, litharge, cuprite and tenorite among carbonates (hydrocerussite, plumbonacrite) and acetates like basic lead acetate, lead acetate trihydrate as well as lead formate as main phases. The results show the strong need of testing conservation materials for their ability of emitting harmful volatile organic compounds. In all tested cellulose ether classes, we found products that do not pass the Oddy test, generating a T or even a F rating. From the overall point of view, methyl cellulose, Na-CMC, MHEC, and MHPC gave the best results, whereas HEC and HPC yielded some bad surprises. Hence, there is no clear answer for the raised title question as there are many “good” examples that passed the test but there are also several products which failed. The example of Klucel® G shows, that even though the composition of a product did not change in recent time, some changes in the manufacture process can increase a leftover from the process in the product that can release VOC during aging. The Klucel® G sample from 2017 is rated for permanent use, whereas a freshly purchased sample from 2021 got a T rating, as slight corrosion occurred on the lead coupon. A regular retesting of such commonly used materials is absolutely mandatory. On the other hand, the popular methyl cellulose Methocel® A4M proved to be a product for permanent use. Both samples, the fresh one from 2021 and the older one from 1997 passed the Oddy test with no sign of corrosion. A further combination of Oddy mass screening and direct emission analysis of selected products utilizing the BEMMA scheme may also yield fruitful results in other product classes relevant for conservation.
Future work will expand the testing procedure to other product classes relevant for conservation (e.g. acrylics, polyvinyl acetate, etc.). Moreover, more extensive comparisons between Oddy tests and BEMMA results will be performed.
Availability of data and materials
The datasets used and/or analysed during the current study are available from CK on reasonable request.
Abbreviations
- BEMMA:
-
Bewertung von Emissionen aus Materialien für Museumsausstattungen
- DNPH:
-
Dinitro-2,4-phenylhydrazine
- HPC:
-
Hydroxypropyl cellulose
- EC:
-
Ethyl cellulose
- HEC:
-
Hydroxy ethyl cellulose
- MC:
-
Methyl cellulose
- MHEC:
-
Methyl hydroxyethyl cellulose
- MHPC:
-
Methyl hydroxypropyl cellulose
- Na-CMC:
-
Sodium carboxymethyl cellulose
- SVOC:
-
Semi-volatile organic compounds
- VOC:
-
Volatile organic compounds
- VVOC:
-
Very volatile organic compounds
References
Braconnot H. De la transformation de plusieurs substances végétales en un principe nouveau. Ann Chim Phys. 1833;52:290–4.
Suida W. Über den Einfluss der aktiven Atomgruppen in den Textilfasern auf das Zustandekommen von Färbungen. Monatsh Chem. 1905;26:413–27.
Feller R, Wilt M. Evaluation of cellulose ethers for conservation. Los Angeles: Getty Conservation Institute; 1990.
Hubert E. Verfahren zur Darstellung von Cellulosederivaten. DE 363,192; 1920.
Leuchs O. Verfahren zur Darstellung von Cellulosederivaten. DE 322,586; 1912.
Lilienfeld L. Alkyl ethers of cellulose and process of making the same. US 1,188,376; 1916.
Jansen E. Verfahren zur Herstellung von Celluloseverbindungen. DE 332,203; 1918.
Hader RN, Waldeck WF, Smith FW. Carboxymethylcellulose. Ind Eng Chem Res. 1952;44(12):2803–12.
Klug ED, Tennent HG. Manufacture of cellulose ethers. US 2,572,039; 1951.
Rodgers Jr SM, Wakeman BF, Savage AS. Novel hydroxypropyl methyl cellulose ethers, a process for preparing the same, and compositions utilizing such ethers. US 3,388,082; 1968.
Bülichen D. Wirkmechanismus verschiedener Celluloseether als Wasserretentionsmittel in der Tiefbohrzementierung und in Trockenmörtelsystemen. Dissertation, TU Munich. 2013.
Hopff H, Lüssi H, Hammer E. Zur Kenntnis der Perlpolymerisation. 5. Mitteilung: Einfluß der chemischen Struktur des Schutzkolloids. Makromol Chem. 1965;84:274–81.
Hercules Inc. CULMINAL® methylcellulose methylhydroxyethylcellulose methylhydroxypropylcellulose physical and chemical properties. Wilmington: Hercules Incorporated Aqualon Division; 1995.
Ashland Product data CulminalTM methylcellulose derivatives. https://www.brenntag.com/media/documents/bsi/product-data-sheets/material-science/ashland-cellulose-rheology-modifiers/culminal_mc_mhec_pds.pdf. Accessed 19 Jan 2022.
Hercules Inc. AQUALON® sodium carboxymethylcellulose physical and chemical properties. Wilmington: Hercules Incorporated Aqualon Division; 1999.
Jenkinson H. Some notes on the preservation, moulding and casting of seals. Antiq J. 1924;4:388–403.
Waechter O. Die Restaurierung einer armenischen Evangelien-Handschrift (cod 242) aus der Bibliothek der Mechitaristen-Congregation in Wien. Österreichische Zeitschrift für Kunst- und Denkmalpflege. 1968;22:43–7.
Asher CG. The conservation of a large collection of architectural drawings: the Howard Ship Yards & Dock Company Mss. In: Ninth annual meeting, Philadelphia, 27–31 May 1981, Preprints. American Institute for Conservation; 1981. p. 20–7.
Hofenk-de Graaff J. Hydroxy propyl cellulose, a multipurpose conservation material. In: ICOM Committee for conservation 6th triennial meeting, Ottawa, 21–25 September 1981, Preprints. Paris: ICOM; 1981. p. 81/4/9:1–7.
Ravines P, Faurie A. The impregnation and absorption behaviour of methyl cellulose of two modern papers. In: Bridgland J, editor. 10th triennial meeting, Washington, Preprints. Paris: ICOM-CC; 1993. p. 462–8.
Brückle I. Update: remoistenable lining with methyl cellulose adhesive preparation. Top Photogr Preserv. 1997;7:88–90.
Laroque C. Transparent papers: a technological outline and conservation review. Stud Conserv. 2000;45(1):21–31.
Hofmann C, Hartl A, Ahn K, Faerber I, Henniges U, Potthast A. Studies on the conservation of verdigris on paper. Restaurator. 2015;36(2):147–82.
Kottulinsky L. Bericht über die Restaurierung eines romanischen Deckenfreskos in Enns. Österreich Maltechnik Restauro. 1982;88:91–7.
Redman C. Cellulose sorbents: an evaluation of their working properties for use in wall painting conservation. Conservator. 1999;23(1):68–76.
Belen’kaia NG, Gorsenina WF, Kuzenetsova EN. The use of methylcellulose for the restoration of archival and library material. In: Fliate DM, editor. Starenie bumagi. Moscow: Akademiia nauk SSSR, Laboratoriia konservatskii i restavratsii dokumentov; 1965. p. 94–111.
Ranacher M. Painted lenten veils and wall coverings in Austria: technique and conservation. Stud Conserv. 1980;25(sup1):142–8.
Dignard C, Douglas R, Guild S. Ultrasonic misting. Part 2, treatment applications. J Am Inst Conserv. 1997;36:127–41.
O‘Donoghue E, Johnson AM, Mazurek J, Preusser F, Schilling M, Walton MS. Dictated by media: conservation and technical analysis of a 1938 Joan Miró canvas painting. Stud Conserv. 2006;51(sup2):62–8.
Sindlinger-Maushart K, Petersen K. Methylcellulose als Klebemittel für die Malschichtbefestigung auf Leinwandbildern: Untersuchung zur Klebkraft und zur mikrobiellen Resistenz. Zeitschrift für Kunsttechnologie und Konservierung. 2007;21(2):371–82.
Rosenqvist AM. The Stabilizing of Wood found in the Viking Ship of Oseberg—Part II. Stud Conserv. 1959;4(2):62–72.
Thomsen FG. Repair of a Tlingit basket using molded cotton fibers. In: ICOM Committee for conservation 7th triennial meeting, Ottawa, Canada 21–25 September 1981 preprints. Paris: ICOM; 1981. p. 81/3/2:1–3.
Masschelein-Kleiner L, Bergiers F. Influence of adhesives on the conservation of textiles. Stud Conserv. 1984;29(sup1):70–3.
Hillyer L, Tinker Z, Singer P. Evaluating the use of adhesives in textile conservation: Part I: an overview and surveys of current use. Conservator. 1997;21(1):37–47.
Schulte EK. Wallpaper conservation at the Longfellow national historic site: parlor and dining room. J Am Inst Conserv. 1981;20(2):100–10.
Karnes C, Ream J, Wendelin E. Wallpapers at Winterthur: seeing them in a “new light”. In: BPG annual. 2000;19.
Thomson R. Paper leather wallpapers: a contradiction in terms. Beiträge zur Erhaltung von Kunst- und Kulturgut. 2004;2:16–9.
Koller M, Hammer I, Paschinger H, Ranacher M. The abbey church at Melk: examination and conservation. Stud Conserv. 1980;25(sup1):101–12.
Howells R, Burnstock A, Hedley G, Hackney S. Polymer dispersions artificially aged. Stud Conserv. 1984;29(sup1):36–43.
Learner T. A review of synthetic binding media in twentieth-century paints. Conservator. 2000;24(1):96–103.
Jablonski E, Learner T, Hayes J, Golden M. The conservation of acrylic emulsion paintings: a literature review. Tate Papers. 2003. https://www.tate.org.uk/research/publications/tate-papers/02/conservation-concerns-for-acrylic-emulsion-paints-literature-review. Accessed 19 Jan 2022.
Doménech Carbo MT, Silva MF, Aura-Castro E, Fuster-López L, Kröner S, Martinez-Bazán ML, Más-Barberá X, Mecklenburg MF, Osete-Cortina L, Domenéch A, Gimeno-Adelatando JV, Yusá-Marco DJ. Study of behaviour on simulated daylight ageing of artists’ acrylic and poly(vinyl acetate) paint films. Anal Bioanal Chem. 2011;399(9):2921–37.
Melchiorre Di Crescenzo M, Zendri E, Sánchez-Pons M, Fuster-López L, Yusá-Marco DJ. The use of waterborne paints in contemporary murals: comparing the stability of vinyl, acrylic and styrene-acrylic formulations to outdoor weathering conditions. Polym Degrad Stabil. 2014;107:285–93.
Horie CV. Materials for conservation. 2nd ed. Oxford: Butterworth-Heinemann; 2010.
Kremer Pigmente. Produktdatenblatt 63720 Ethylcellulose ET 200. https://www.kremer-pigmente.com/elements/resources/products/files/63720.pdf. Accessed 19 Jan 2022.
De Witte E, Florquin S, Goessens-Landrie M. Influence of the modification of dispersions on film properties. Stud Conserv. 1984;29(sup1):32–5.
Mehra VR. Dispersion as lining adhesive and its scope. Stud Conserv. 1984;29(sup1):44–5.
Godfrey IM, King SN. Conservation of degraded rope from marine archaeological sites. AICCM Bull. 1990;16(3):93–107.
Böhme N, Anders M, Reichelt T, Schuhmann K, Bridarolli A, Chevalier A. New treatments for canvas consolidation and conservation. Herit Sci. 2020;8:16. https://doi.org/10.1186/s40494-020-0362-y.
Butkeviciute R, Lukseniene J, Senvaitiene J, Vaineikis A, Zickuviene G. Application of cellulose ethers for structure consolidation. In: Weyer A, editor. Konsolidieren und Kommunizieren: Materialien und Methoden zur Konsolidierung von Kunst- und Kulturgut im interdisziplinären Dialog. Schriften des Hornemann Instituts. Petersberg: Michael Imhof Verlag; 2018. p. 156–61.
Wolbers R, Stavroudis C. Aqueous methods for the cleaning of paintings. In: Stoner JH, Rushfield R, editors. Conservation of Easel paintings. Routledge series in conservation and museology. New York: Routledge; 2012. p. 500–23.
McDavis-Conway A, Godfrey J, Pouliot BP, Wolbers R. Hair consolidation and treatment of an insect-damaged dancing hat from Sierra Leone. Objects Spec Group Postprints. 2006;13:184–94.
Kite M, Thompson R. Conservation of leather and related materials. Oxford: Butterworth-Heinemann; 2006.
Johnson A. Evaluation of the use of SC6000 in conjunction with Klucel G as a conservation treatment for bookbinding leather: notes on a preliminary study. J Inst Conserv. 2013;36(2):125–44.
Gill K, Boersma F. Solvent reactivation of hydroxypropyl cellulose (Klucel G®) in textile conservation: recent developments. Conservator. 1997;21(1):12–20.
Lennard F, Ewer P. Textile conservation. 1st ed. Oxford: Butterworth-Heinemann; 2010.
Anderson P, Reidell S. Adhesive pre-coated repair materials. In: BPG annual. 2009;28:112.
Pataki A. Remoistenable tissue preparation and its practical aspects. Restaurator. 2009;30(1):51–69.
Dreyfuss-Deseigne R. A new mending material: nanocellulose film. J Paper Conserv. 2017;18(1):36–7.
Powell W. Creative problem solving in paper conservation: 4 case studies of complex treatments. In: 8th AICCM book, paper and photographic materials symposium. AICCM conference proceedings. 2014. p. 91–6.
Sàrries ZL. New technologies applied to restore a nineteenth-century Wax Medardo Rosso sculpture. Objects Spec Group Postprints. 2017;24:337–50.
Ali MF, El Sheikha AM, Ali AF. Analytical study and conservation of gilded mummy form cartonnage from the Greco-Roman period in Cairo museum. Mediterr Archaeol Archaeom. 2016;16(2):127–37.
Berli J, Belhadj O. Consolidating herbarium specimens using two-sided hydroxylpropylcellulose pre-coated paper. J Pap Conserv. 2020;21(1):31–4.
Berger GA. Formulating adhesives for the conservation of paintings. In: Brommelle N, Smith P, editors. Conservation and restoration of pictorial art. Oxford: Butterworth-Heinemann; 1976. p. 169–81.
Pataki-Hundt A. Conservation treatment and stabilization of the ninth-century Stuttgart Psalter. J Inst Conserv. 2012;35(2):152–64.
Baker C. Methylcellulose & sodium carboxy-methylcellulose: uses in paper conservation. In: BPG annual. 1982;1:16–9.
Eggert G, Kuiter R, Korenberg C, Ziegler J, Bette S, Stelzner J. Metal conservation, cellulose nitrate and the Oddy test. In: Chemello C, Brambilla L, Joseph E, editors. Metal 2019—proceedings of the interim meeting of the ICOM-CC Metals Working Group. September 2–6, 2019, Neuchâtel, Switzerland. Paris: ICOM-CC; 2019. p. 125–31.
Ziegler J, Kuhn-Wawrzinek C, Eska M, Eggert G. Popping stoppers, crumbling coupons—Oddy testing of common cellulose nitrate ceramic adhesives. In: Bridgland J, editor. ICOM-CC 17th triennial conference preprints, Melbourne, 15–19 September 2014. Paris: ICOM; 2014. p. 8.
Samide MJ, Liggett MC, Mill J, Smith GD. Relating volatiles analysis by GC–MS to Oddy test performance for determining the suitability of museum construction materials. Herit Sci. 2018;6:47.
Nel P. A preliminary investigation into the identification of adhesives on archaeological pottery. AICCM Bull. 2006;30(1):27–37.
Nel P, Lau D. Identification of a formulation change in a conservation-grade adhesive. In: Ambers J, Higgitt C, Harrison L, Saunders D, editors. Holding it all together: ancient and modern approaches to joining, repair and consolidation. London: Archetype; 2009. p. 99–106.
Korenberg C, Keable M, Phippard J, Doyle A. Refinements introduced in the Oddy test methodology. Stud Conserv. 2018;63(1):2–12.
Oddy test of Klucel® G, Metropolitan Museum of Art (MMA). https://www.conservation-wiki.com/w/images/e/ed/3003_Si.jpg. Accessed 19 Jan 2022.
Oddy test of Methocel® A4C methylcellulose, Metropolitan Museum of Art (MMA). https://www.conservation-wiki.com/w/images/4/42/3067_GA.jpg. Accessed 19 Jan 2022.
Thickett D, Lee LR. Selection of materials for the storage or display of museum objects. 2nd ed. British museum occasional papers 111. London: British Museum Press; 2004.
ISO 16000-6, Indoor air—Part 6: determination of organic compounds (VVOC, VOC, SVOC) in indoor and test chamber air by active sampling on sorbent tubes, thermal desorption and gas chromatography using MS or MS FID. Berlin: Beuth-Verlag; 2021.
ISO 16000-3, Indoor air—Part 3: determination of formaldehyde and other carbonyl compounds in indoor air and test chamber air—active sampling method. Berlin: Beuth-Verlag; 2011.
VDI 4301 Part 7—measurement of indoor air pollution–measurement of carboxylic acids. Berlin: Beuth-Verlag; 2018.
Welter N, Schüssler U, Kiefer W. Characterisation of inorganic pigments in ancient glass beads by means of Raman microspectroscopy, microprobe analysis and X-ray diffractometry. J Raman Spectrosc. 2007;38:113–21.
Ciupiński Ł, Fortuna-Zaleśna E, Garbacz H, Koss A, Kurzydłowski KJ, Marczak J, Mróz J, Onyszczuk T, Rycyk A, Sarzyński A, Skrzeczanowski W, Strzelec M, Zatorska A, Żukowska GZ. Comparative laser spectroscopy diagnostics for ancient metallic artefacts exposed to environmental pollution. Sensors. 2010;10:4926–49.
Bell IM, Clark RJH, Gibbs PJ. Raman spectroscopic library of natural and synthetic pigments (Pre- ≈ 1850 AD). Spectrochim Acta Part A. 1997;53:2159–79.
Burgio L, Clark RJH, Firth S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analyst. 2001;126:222–7.
Angelin EM, Babo S, Ferreira JL, Melo MJ. Raman microscopy for the identification of pearlescent pigments in acrylic works of art. J Raman Spectrosc. 2019;50:232–41.
Bernard M-C, Costa V, Joiret S. Assessing indoor lead corrosion using Raman spectroscopy during electrochemical reduction. e-PS. 2009;6:101–6.
Ghiara G, Campodonico S, Piccardo P, Martini C, Storme P, Maddalena M. Micro Raman investigation on corrosion of Pb-based alloy replicas of letters from the museum Plantin-Moretus, Antwerp. J Raman Spec. 2014;45:1093–102.
Raychaudhuri MR, Brimblecombe P. Formaldehyde oxidation and lead corrosion. Stud Conserv. 2000;45:226–32.
Eggert G, Fischer A. The formation of formates: a review of metal formates on heritage objects. Herit Sci. 2021;9:26.
Jehlicka J, Vitek P, Edwards HGM, Hargreaves D, Capounc T. Fast detection of sulphate minerals (gypsum, anglesite, baryte) by a portable Raman spectrometer. J Raman Spectrosc. 2009;40:1082–6.
Acknowledgements
The authors are grateful to S. Dietz for her support in the lab. We thank M. Paysan (Landesmuseum Württemberg) and our institutional colleagues U. Henniges, I. Brückle and A. Fischer for fruitful discussions and N. Nuechter (Ashland) for providing sample material.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project “Das Oddy-torium - Test von Restaurierungsmaterialien auf atmosphärische Korrosivität zum Schutz wertvoller Kulturgüter vor anthropogenen Luftschadstoffen” is funded by the Deutschen Bundesstiftung Umwelt (DBU), Osnabrück (Az. 35831/01).
Author information
Authors and Affiliations
Contributions
GE and CK designed the research project. SS performed the tests and Raman measurements, WH the BEMMA measurements and their evaluation. SS, GE, WH and CK interpreted the data and contributed to the draft of this paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Steger, S., Eggert, G., Horn, W. et al. Are cellulose ethers safe for the conservation of artwork? New insights in their VOC activity by means of Oddy testing. Herit Sci 10, 53 (2022). https://doi.org/10.1186/s40494-022-00688-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-022-00688-4