Abstract
Background
Increasing demand for renewable energy resources and the need to mitigate climate change have raised interest in short-rotation forestry with fast-growing deciduous trees like hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in northern Europe. Given that climate warming has already considerably extended the growing season in this region, northward transfer of genotypes could improve forest plantation productivity and enable more efficient mitigation of climate change. We studied the spring and autumn phenology of hybrid aspen genotypes of different geographic origin (European P. tremula parent from 51° to 60° N and North American P. tremuloides parent from 45° to 54° N) 3 and 6 years after planting in a progeny trial established in Estonia at 58° N.
Findings
The effect of geographic origin on spring and autumn phenology of hybrid aspen was evident at the age of 3 and 6 years. Geographic origin did not affect spring phenology. However, hybrids with P. tremula parents of northern origin, with bud-burst occurring some days later, were able to unfold and develop full-sized leaves faster than genotypes with early bud-burst. The main differences between different geographic origins appeared in the autumn of year 6, when genotypes of northern origin (60° N) started autumn defoliation significantly earlier than those of southern origin (51° to 57° N). The genotypes of southern origin (55° 53′ to 57° 31′ N) had a period from bud-burst to defoliation 27 days longer than that of genotypes of northern origin (60° 22′ N). The interval between spring and autumn phenological processes showed significant positive correlation with current annual height growth for both study years.
Conclusions
Hybrid aspen genotypes from 55° to 57° N responded well to northward transfer, having a longer leafy period and greater height increment than southward transferred genotypes. Northward-transferred genotypes were apparently better adapted to climate-change-induced extension of the growing season at higher latitudes.
Similar content being viewed by others
Findings
Introduction
Short-rotation plantation forestry (SRF) is a novel and promising land-use system for the intensive production of energy and pulp wood in northern Europe (Weih 2004; Tullus et al. 2013). This type of forestry has great potential for mitigating climate change by replacing fossil fuels and sequestrating atmospheric CO2 (Paquette and Messier 2010; Haus et al. 2014; Lutter et al. 2016). Hybrid aspen (Populus tremula L. × P. tremuloides Michx.) is considered to be one of the most promising tree species for SRF and intensive pulp and biomass production in northern Europe (Tullus et al. 2012) owing to a faster growth rate compared with local European aspen (Yu et al. 2001a), the existence of long-term breeding programmes (Yu et al. 2001a; b; Stener and Karlsson 2004; Rytter and Stener 2003) and good cold resistance (Tullus et al. 2012). So far, hybrid aspen plantations in hemiboreal Estonia have been established with clonal plants that have been transferred southward, i.e. hybrids with one or both parents of northern origin (Tullus et al. 2007).
In the higher latitudes of the northern hemisphere, it is predicted that, besides the positive impact of increasing CO2 and temperature on the productivity of forests (Cole et al. 2010; Lindner et al. 2010), climate change will also extend the growth period for plants (Keenan et al. 2014), primarily through the earlier onset of springs (Jaagus 2006; Menzel et al. 2006; Schwartz et al. 2006), i.e. the date when daily mean air temperature rises above +5 °C (Jaagus and Ahas 2000; Jaagus 2006). Moreover, several recent studies also show clear trends of growth season extension in autumn, resulting in delayed leaf senescence (Garonna et al. 2014; Gill et al. 2015). Length of growing season is strongly controlled by climatic conditions (Cleland et al. 2007; Rohde et al. 2011), and tree populations display latitudinal adaptation (Pellis et al. 2004; Elferjani et al. 2016) as demonstrated by differences in phenological processes like growth initiation in spring (bud-burst) and growth cessation in autumn (bud-set) (Rohde et al. 2011). For example, northern populations are adapted to temperature-controlled delayed bud-burst in spring (Luquez et al. 2008) and photoperiod-sensitive bud-set in autumn (Way and Montgomery 2015) to avoid cold damage (Howe et al. 2003). Therefore, the warming climate and longer growing periods in autumn in northern Europe might advantage southern-origin genotypes that have adapted to a longer growing season in their local environment (Pellis et al. 2004; Way and Montgomery 2015). Northward transfer and selection of southern genotypes could improve future forest plantation biomass production and mitigate climate change more efficiently (Marris 2009; Way and Montgomery 2015).
In northern Europe, hybrid aspen progeny trials have so far mainly focused on tree growth potential (e.g. Yu et al. 2001a; Yu and Pulkkinen 2003; Stener and Karlsson 2004) and wood properties (Yu et al. 2001b; Rytter and Stener 2003). The few studies conducted on the phenological traits of hybrid aspen clones have concluded that genotypes selected in local environmental conditions with earlier bud-burst are more productive (Yu et al. 2001a; b; Jansons et al. 2014). However, given the great variability of phenological traits of Populus spp. and of climate change, hybrid aspen phenology is still understudied in progeny trials, bearing in mind that phenological traits like bud-burst, duration of leafy period, bud-set, etc. are important factors determining tree adaptability to climatic conditions, with consequent effects on productivity and survival (Cooke et al. 2012; Elferjani et al. 2016).
In order to test the suitability of hybrid aspen genotypes of different geographic origin for Estonian conditions, a progeny trial with completely randomised design was established, whereby phenological observations in spring and autumn were carried out during the third and sixth growing season since planting. The hypotheses were as follows: (1) northern transfer of hybrid aspen genotypes is superior to southern transfer because of better adaptability with an extended growing season—a consequence of climate change in northern regions; (2) genotypes with a longer leafy period also have a greater annual height increment.
Methods
Study area
A genetic trial testing a number of clones and full-sib families of hybrid aspen was established in south-eastern Estonia in the village of Agali (58° 17′ 10″ N, 27° 17′ 18″ E). Estonia is situated in the hemiboreal vegetation zone of the northern hemisphere within a transition zone of maritime to continental climate (Ahti et al. 1968). During the study period (2009–2014), the mean annual temperature was 6.1 °C and the mean annual precipitation was 676 mm according to data from the nearest weather station to the trial (Estonian Environment Agency). The mean annual temperature of the 2 years when the phenological survey was carried out was similar (6.7 °C in 2011 and 6.6 °C in 2014). The 1966–2010 long-term average temperature was 5.1 °C in this region (Tarand et al. 2013).
The trial was established in spring 2009 on flat agricultural land in the territory of the Järvselja Training and Experimental Forest Centre. The soil type of the study site according to IUSS Working Group WRB classification (2014) was fertile Eutric Glossic Retisol with a sandy loam texture in the uppermost 0–30-cm soil layer. The progeny trial was fenced to prevent animal damage. Mechanical weed control was carried out annually and neither fertiliser nor irrigation was applied after establishment.
Background of the studied hybrid aspen genotypes
The geographic origin of the studied hybrid aspen genotypes was determined by the latitudinal origin of their native European aspen (P. tremula) parent (Table 1). The exact origin of the P. tremuloides parent of these genotypes was often less precisely known but represented various states and regions along the border of the USA with Canada.
The tested hybrid aspen progenies representing northward transfer were as follows: (1) seven clones with a P. tremula parent from Latvia (LV); (2) five clones with a P. tremula parent from Sweden (SE); (3) six different full-sib families with a P. tremula parent from Germany (DE). The trial includes also four hybrid aspen clones that represent southward transfer with a P. tremula parent from Finland (FI).
The comparison trial was established using 1-year-old container plants with 1 × 2 m spacing, planted in a completely randomised design. Each of the 22 treatments (clones or full-sib families) was represented by three replicate plots (16 trees per plot). After the fifth growing season, half of the trees (every second row) were systematically harvested to reduce within-stand competition, and the remaining trees were left growing with 2 × 2 m spacing.
Phenological observations
Phenological observations in the trial were carried out during the third (2011) and the sixth (2014) growing season by adapting the methodology described by Yu et al. (2001a) (Table 2). During both surveys, the onset (day-of-year) of spring phenological stages were recorded using a 3-day monitoring interval and that of autumn stages with a 4-day interval. Phenology was described for each replication separately and based on visual tree crown observations. For statistical analysis, the arithmetical mean day-of-year of onset of each stage was calculated for each treatment.
Tree growth measurement
The height (m) of all trees growing in the trial was measured before growth initiation and after growth cessation in both study years, using an extendable measuring rod (at age 3) and a Vertex IV (Haglöf Inc.) height metre (at age 6). Current annual increment of height (CAIH, m) was calculated as the difference between initial and final height in the given year.
Statistical analysis
The effect of geographic origin (based on the origin of P. tremula parents) on phenology for each study year was tested separately with one-way analysis of variance (ANOVA). The effect of geographic origin on CAIH in each study year was tested separately with a mixed model (Eq. 1), where replication and individual treatment (clone or family) within origin were treated as random factors. The Tukey LSD test was applied to distinguish groups within each study year if a significant origin effect was detected.
where μ is overall mean, α i is the fixed effect of geographic origin, β j is the random effect of treatment within origin and γ k(j) is random effect of replication within treatment and ε ijk is error term.
The relationships between time intervals of different phenological stages and CAIH in each study year were studied with Pearson’s linear correlation analysis.
The normality of the variables was tested with the Shapiro-Wilk test. Q-Q plots and residual distribution were applied to assess the normality of model residuals. A significance level of α = 0.05 was used to reject the null hypothesis after statistical tests. All statistical analyses were carried out using R Statistics software (R Core Team 2015).
Results and discussion
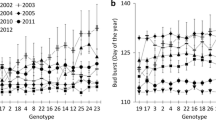
Clonal (or family-wise) mean bud-burst occurred between day 117 and day 131 in year 3 (Fig. 1) but no significant differences between geographic origins were found in the mean bud-burst date (Table 3). Also, geographic origin had no significant impact on the mean onset of other tree phenological stages or on intervals between spring and autumn phenological stages during the third growing season (Table 3).
Significant impact of geographic origin on the mean onset of autumn phenological stages was revealed during the sixth growing season (Table 3). In year 6, southward transferred FI genotypes (60° 22′ N) started leaf defoliation processes and bud-set significantly earlier than northward-transferred genotypes (51° 16′ to 57° 31′ N). This is in accordance with the study by Pellis et al. (2004) reporting that poplar genotypes originating from lower latitudes shed their foliage later than genotypes from higher latitudes. Earlier leaf-fall in the genotypes of more northerly origin can be explained by their adaptation to longer photoperiod (Luquez et al. 2008; Way and Montgomery 2015). Shorter autumn days in the case of southern translocation cause them to start preparation for the dormancy season (Luquez et al. 2008; Saikkonen et al. 2012). At the same time, delayed abscission of northward-transferred genotypes could be caused by their adaptation to shorter photoperiod. This means that critical day-length triggering of autumnal senescence processes arrives later for them under northern conditions with longer photoperiod (Way and Montgomery 2015). Moreover, climate-change-induced temperature rise enables them to grow longer in the autumn than northern-origin genotypes without major risk of frost damage in autumn compared with northern-origin genotypes (Garonna et al. 2014; Gill et al. 2015).
Mean bud-burst started 6–10 days later in the northern-origin genotypes than in southern ones in year 6 but the differences were not significant (Table 3). This result is contrary to what has been observed in hybrid aspen genotypes grown in more northern regions (Yu et al. 2001a). The earlier onset of spring because of climate warming (Jaagus 2006; Menzel et al. 2006; Schwartz et al. 2006) suggests that northward-transferred trees adapt to initiate a growth period earlier than northern populations (Pellis et al. 2004; Luquez et al. 2008) as spring bud-burst is more controlled by temperature than photoperiod (Laube et al. 2014). The timing of bud-burst is considered to be an important characteristic that determines the growth rate of different hybrid aspen genotypes (Yu et al. 2001a; Jansons et al. 2014) and other fast-growing Salicaceae family species (Lennartsson and Ögren 2004; Weih 2009; Müller et al. 2013). Genotypes with early bud-burst are able to develop a higher number of leaves by capturing priority resources (Bergh et al. 2003; Müller et al. 2013) and therefore grow much faster than late flushing genotypes (Yu et al. 2001a; Bergh et al. 2003; Müller et al. 2013; Jansons et al. 2014).
Also, the mean day-of-year when trees obtained full-sized leaves at year 6 varied only by 4 days among all the studied genotypes, and this was also not significant (Table 3). However, genotypes with later bud-burst developed full-sized leaves much faster compared with early bud-burst genotypes (Fig. 1). Our results for hybrid aspen are in correspondence with the study by Yu et al. (2001a) about hybrid aspen in Finland and other studies about willow (Weih 2009) and silver birch (Possen et al. 2014) in northern European regions. Leaf unfolding and leaf development are controlled by temperature (Davi et al. 2011; Laube et al. 2014), and later-flushing genotypes are able to develop leaves faster than early flushing ones because they are adapted to avoid late-frost damage in northern growing conditions (Howe et al. 2003).
Earlier abscission of FI genotypes shortened their leafy period (S1–A3) compared with southern genotypes (DE, SE, LV) at year 6. The period from bud-burst to defoliation ranged from 177 to 188 days for southern-origin genotypes and was 27 days shorter for northern FI genotypes compared with the longest growing SE genotypes (Table 3). The intervals between several spring (growth initiation) and autumn (growth cessation) phenological processes were significantly and positively correlated with CAIH in both study years: in year 3, the strongest relation was observed with the interval between S3 to A1 (Fig. 2a); in year 6, the strongest relation was observed with the interval between S2 to A1 (Fig. 2b). That means that faster height growth of northward-transferred genotypes (SE and LV but not DE; Fig. 3) at both 3 and 6 years is explained, at least in part, by their longer leafy period compared with southward transferred FI genotypes (Fig. 2). It has already been found that the fast growth of hybrid aspen compared with the European aspen is partly explained by a longer growth period (from bud-burst to half of the leaves yellowed) (Yu et al. 2001a). However, correlation between the length of leafy period and height growth among different hybrid aspen genotypes after latitudinal transfer has not been reported previously.
Linear relationship between the ‘interval between spring and autumn phenological processes’ and current annual height increment (CAIH), where a the relationship with the interval that showed the strongest correlation during year 3, i.e. S3–A1, and b the relationship with the interval that showed the strongest correlation during year 6, i.e. S2–A1, are presented (p < 0.01**, p < 0.001***)
At the same time, wide latitudinal transfer should be approached cautiously (Marris 2009). For example, CAIH of DE genotypes representing the southern-most origin in our trial (51° 16′ to 52° 16′ N), did not differ significantly from the northern-origin FI genotypes (60° 22′ N) during both phenological surveys (Fig. 3) based on the Tukey LSD test. However, it must be remembered that DE genotypes represent full-sib families, meaning that they are less intensively selected than the clones from the other countries. Given the results after 6 years, northward-transferred SE (55° 53′ to 57° 31′ N) and LV (56° 06′ to 57°10′ N) genotypes can be regarded as most suitable for Estonian conditions as they showed significantly higher growth potential than southward transferred FI genotypes (Fig. 3).
Conclusions
A warming climate has already caused extension of the growing season in the hemiboreal region (a trend predicted to continue in future). Consequently, hybrid aspen genotypes with a southern origin responded well to a northward transfer, whereby they had a longer leafy period and greater annual height growth than southward transferred genotypes. The main differences among genotypes of different geographic origin were revealed in autumn phenology, when northern genotypes started earlier defoliation and bud-set. The effects of geographic transfer on performance of hybrid aspen genotypes should be studied in various environmental conditions for a longer time to draw final conclusions in terms of selecting the most suitable genotypes for commercial propagation and large-scale plantation establishment.
Abbreviations
- A:
-
Autumn phenology
- A1:
-
Half of the leaves are yellowed, defoliation has not started
- A2:
-
Defoliation of half of the leaves
- A3:
-
Defoliation of all the leaves, bud-set
- CAIH :
-
Current annual increment of height
- DE:
-
Germany
- FI:
-
Finland
- LV:
-
Latvia
- S:
-
Spring phenology
- S1:
-
Bud-burst, leaves emerge from the bud about 5 mm
- S2:
-
All the leaves are unfolded
- S3:
-
All the leaves have attained full size
- SE:
-
Sweden
- SRF:
-
Short-rotation forestry
References
Ahti, T., Hamet-Ahti, L., & Jalas, J. (1968). Vegetation zones and their sections in northwest Europe. Annales Botanici Fennici, 5, 169–211.
Bergh, T., Freeman, M., Sigurdsson, B., Kellomäki, S., Laitinen, K., Niinistö, S., Peltola, H., & Linder, S. (2003). Modelling the short-term effects of climate change on the productivity of selected tree species in Nordic countries. Forest Ecology and Management, 183, 327–340.
Cleland, E. E., Chuine, I., Menzel, A., Mooney, H. A., & Schwartz, M. D. (2007). Shifting plant phenology in response to global change. Trends in Ecology Evolution, 22, 357–365.
Cole, C. T., Anderson, J. E., Lindroth, R. L., & Waller, D. M. (2010). Rising concentration of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremuloides). Global Change Biology, 16, 2186–2197.
Cooke, J., Eriksson, M., & Junttila, O. (2012). The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant, Cell & Environment, 35, 1707–1728.
R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL (http://www.R-project.org/).
Davi, H., Gillmann, M., Ibanez, T., Cailleret, M., Bontemps, A., Fady, B., & Lefevre, F. (2011). Diversity of leaf unfolding dynamics among tree species: New insights from a study along an altitudinal gradient. Agricultural and Forest Meteorology, 151, 1504–1513.
Elferjani, R., DesRochers, A., & Tremblay, F. (2016). Plasticity of bud phenology and photosynthetic capacity in hybrid poplar plantations along a latitudinal gradient in northeastern Canada. Environmental and Experimental Botany, 125, 67–76.
Garonna, I., de Jong, R., de Wit, A. J. W., Mücher, C. A., Schmid, B., & Schaepman, M. E. (2014). Strong contribution of autumn phenology to changes in satellite-derived growing season length estimates across Europe (1982–2011). Global Change Biology, 20, 3457–3470.
Gill, A. L., Gallinat, A. S., Sanders-DeMott, R., Rigden, A. J., Short Gianotti, D. J., Mantooth, J. A., & Templer, P. H. (2015). Changes in autumn senescence in northern hemisphere deciduous trees: a meta-analysis of autumn phenology studies. Annals of Botany, 116, 875–888.
Haus, S., Gustavsson, L., & Sathre, R. (2014). Climate mitigation comparison of woody biomass systems with the inclusion of land-use in the reference fossil system. Biomass and Bioenergy, 65, 136–144.
Howe, G. T., Aitken, S. N., Neale, D. B., Jermstad, K. D., Wheeler, N. C., & Chen, T. H. H. (2003). From genotype to phenotype: unravelling the complexities of cold adaptation in forest trees. Canadian Journal of Botany, 81, 1247–1266.
IUSS Working Group WRB. (2014). World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. Rome: FAO.
Jaagus, J. (2006). Climatic changes in Estonia during the second half of the 20th century in relationship with changes in large-scale atmospheric circulation. Theoretical and Applied Climatology, 83, 77–88.
Jaagus, J., & Ahas, R. (2000). Space-time variations of climatic seasons and their correlation with the phenological development of nature in Estonia. Climate Research, 15, 207–219.
Jansons, A., Zeps, M., Rieksts-Riekstins, J., Matisons, R., & Krisans, O. (2014). Height increment of hybrid aspen Populus tremuloides × P. tremula as a function of weather conditions in south-western part of Latvia. Silva Fennica, 48, 1–13.
Keenan, T. F., Gray, J., Friedl, M. A., Toomey, M., Bohrer, G., Hollinger, D. Y., Munger, J. W., OˈKeefe, J., Schmid, H. P., Wing, I. S., Yang, B., & Richardson, A. D. (2014). Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nature Climate Change, 4, 598–604.
Laube, J., Sparks, T. H., Estrella, N., Höfler, J., Ankerst, D. P., & Menzel, A. (2014). Chilling outweighs photoperiod in preventing precocious spring development. Global Change Biology, 20, 170–182.
Lennartsson, M., & Ögren, E. (2004). Clonal variation in temperature requirements for budburst and dehardening in Salix species used for biomass production. Scandinavian Journal of Forest Research, 19, 295–302.
Lindner, M., Maroschek, M., Netherer, S., Kremer, A., Barbati, A., Garcia-Gonzalo, J., Seidl, R., Delzon, S., Corona, P., Kolström, M., Lexer, M. J., & Marchetti, M. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management, 259, 698–709.
Luquez, V., Hall, D., Albrectsen, B. R., Karlsson, J., Ingvarsson, P., & Jansson, S. (2008). Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genetics and Genomes, 4, 279–292.
Lutter, R., Tullus, A., Kanal, A., Tullus, T., & Tullus, H. (2016). The impact of former land-use type to above- and below-ground C and N pools in short-rotation hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations in hemiboreal conditions. Forest Ecology and Management, 378, 79–90.
Marris, E. (2009). Forestry: planting the forest of the future. Nature, 459, 906–908.
Menzel, A., Sparks, T. H., Estrella, N., Koch, E., Aasa, A., Ahas, R., Alm-Kübler, K., Bissolli, P., Braslavska, O., Briede, A., Chmielewski, F. M., Crepinsek, Z., Curnel, Y., Dahl, A., Defila, C., Donnelly, A., Filella, Y., Jatczak, K., Mage, F., Mestre, A., Nordli, Ø., Penuelas, J., Pirinen, P., Remisova, V., Scheifinger, H., Striz, M., Susnik, A., Van Vliet, A. J. H., Wielgolaski, F. E., Zach, S., & Zust, A. (2006). European phenological response to climatic change matches the warming pattern. Global Change Biology, 12, 1969–1976.
Müller, A., Horna, V., Kleemann, F., Vornam, B., & Leuschner, C. (2013). Physiological vs. morphological traits controlling the productivity of six aspen full-sib families. Biomass and Bioenergy, 56, 274–283.
Paquette, A., & Messier, C. (2010). The role of plantations in managing the world’s forests in the Anthropocene. Frontiers in Ecology and the Environment, 8, 27–34.
Pellis, A., Laureysens, I., & Ceulemans, R. (2004). Genetic variation of the bud and leaf phenology of seventeen poplar clones in a short rotation coppice culture. Plant Biology, 6, 38–46.
Possen, B. J. H., Rousi, M., Silfver, T., Anttonen, M. J., Ruotsalainen, S., Oksanen, E., & Vapaavuori, E. (2014). Within-stand variation in silver birch (Betula pendula Roth) phenology. Trees, 28, 1801–1812.
Rohde, A., Bastien, C., & Boerjan, W. (2011). Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiology, 31, 472–482.
Rytter, L., & Stener, L. G. (2003). Clonal variation in nutrient content in woody biomass of hybrid aspen (Populus tremula L. × P. tremuloides Michx.). Silva Fennica, 37, 313–324.
Saikkonen, K., Taulavuori, K., Hyvönen, T., Gundel, P. E., Hamilton, C. E., Vänninen, I., Nissinen, A., & Helander, M. (2012). Climate change-driven species’ range shifts filtered by photoperiodism. Nature Climate Change, 2, 239–242.
Schwartz, M. D., Ahas, R., & Aasa, A. (2006). Onset of spring starting earlier across the Northern Hemisphere. Global Change Biology, 12, 343–351.
Stener, L. G., & Karlsson, B. (2004). Improvement of Populus tremula × P. tremuloides by phenotypic selection and clonal testing. Forest Genetics, 11, 13–27.
Tarand, A., Jaagus, J., & Kallis, A. (2013). Eesti kliima minevikus ja tänapäeval (p. 631). Tartu: Tartu Ülikooli Kirjastus (in Estonian).
Tullus, A., Tullus, H., Vares, A., & Kanal, A. (2007). Early growth of hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations on former agricultural lands in Estonia. Forest Ecology and Management, 245, 118–129.
Tullus, A., Rytter, L., Tullus, T., Weih, M., & Tullus, H. (2012). Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scandinavian Journal of Forest Research, 27, 10–29.
Tullus, H., Tullus, A., & Rytter, L. (2013). Short-rotation forestry for supplying biomass for energy production. In S. Kellomäki, A. Kilpeläinen, & A. Ashraful (Eds.), Forest bioenergy production: management, carbon sequestration and adaptation (pp. 39–56). New York: Springer.
Way, D. A., & Montgomery, R. A. (2015). Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant, Cell & Environment, 38, 1725–1736.
Weih, M. (2004). Intensive short rotation forestry in boreal climates: present and future perspectives. Canadian Journal of Forest Research, 34, 1369–1378.
Weih, M. (2009). Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): effects on shoot growth and nitrogen economy. Tree Physiology, 29, 1479–1490.
Yu, Q., & Pulkkinen, P. (2003). Genotype-environment interaction and stability in growth of aspen clones. Forest Ecology and Management, 173, 25–35.
Yu, Q., Tigerstedt, P. M. A., & Haapanen, M. (2001). Growth and phenology of hybrid aspen clones (Populus tremula L. × P. tremuloides Michx.). Silva Fennica, 35, 15–25.
Yu, Q., Pulkkinen, M., Rautio, M., Haapanen, M., Alen, R., Stener, L. G., Beuker, E., & Tigerstedt, P. M. A. (2001). Genetic control of wood physicochemical properties, growth, and phenology in hybrid aspen clones. Canadian Journal of Forest Research, 31, 1348–1356.
Acknowledgements
Järvselja Training and Experimental Forest Centre is acknowledged for providing territory and financial support for establishment of the hybrid aspen progeny trial in Agali. Georg von Wühlisch, Guntis Grandans, Anders Håkansson, Tiit Juhani, Lars-Göran Stener and Raimo Jaatinen are acknowledged for providing the plants and background information. We also thank Raul Pihu, Endel Jänes and Andres Jäärats for their help in establishment of the trial, Edvard Eelsalu and Marten Merdikes for their help in conducting tree growth measurements and phenological surveys, and Arno Kanal for the soil description in the trial site. This work was supported by institutional research funding IUT (grants IUT21-4 and IUT34-9) of the Estonian Ministry of Education and Research.
Authors’ contributions
All authors carried out the field work and data analysis and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
§ Based on a paper presented at the Forest Genetics for Productivity conference, Rotora, New Zealand, 14-18 March 2016.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lutter, R., Tullus, A., Tullus, T. et al. Spring and autumn phenology of hybrid aspen (Populus tremula L. × P. tremuloides Michx.) genotypes of different geographic origin in hemiboreal Estonia§ . N.Z. j. of For. Sci. 46, 20 (2016). https://doi.org/10.1186/s40490-016-0078-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40490-016-0078-7