Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder with neuronal and synaptic losses due to the accumulation of toxic amyloid β (Αβ) peptide oligomers, plaques, and tangles containing tau (tubulin-associated unit) protein. While familial AD is caused by specific mutations, the sporadic disease is more common and appears to result from a complex chronic brain neuroinflammation with mitochondriopathies, inducing free radicals’ accumulation. In aged brain, mutations in DNA and several unfolded proteins participate in a chronic amyloidosis response with a toxic effect on myelin sheath and axons, leading to cognitive deficits and dementia. Αβ peptides are the most frequent form of toxic amyloid oligomers. Accumulations of misfolded proteins during several years alters different metabolic mechanisms, induce chronic inflammatory and immune responses with toxic consequences on neuronal cells. Myelin composition and architecture may appear to be an early target for the toxic activity of Aβ peptides and others hydrophobic misfolded proteins. In this work, we describe the possible role of early myelin alterations in the genesis of neuronal alterations and the onset of symptomatology. We propose that some pathophysiological and clinical forms of the disease may arise from structural and metabolic disorders in the processes of myelination/demyelination of brain regions where the accumulation of non-functional toxic proteins is important. In these forms, the primacy of the deleterious role of amyloid peptides would be a matter of questioning and the initiating role of neuropathology would be primarily the fact of dysmyelination.
Similar content being viewed by others
Introduction
Sporadic AD typically occurs after the age of 65 and is the most common cause of dementia in older people. We consider here the disease under its main pathophysiological definition that classically consists of extracellular amyloid plaques and intracellular neurofibrillary tangles [69, 70]. These abnormalities lead to a cascade of events eventually conducting to cognitive disorders and dementia. There are, however, a significant number of diverse clinical presentations with ages of onset and evolution in the disease that suggest non-univocal pathophysiological mechanisms [61]. It seems that some cases of sporadic AD involve changes in the constitution and architecture of myelin, and this early in the life of the future patient. In general, extracellular abnormalities of the amyloid cascade predominate, followed most often by other Tau mediated biological mechanisms at the intracellular level, accompanied by inflammatory, neuroimmune and neurochemical disorders that can put dysmyelination at the forefront of neurodegenerative disorders.
Myelin consists of a multilayered membrane wrapped around the axons of most central nervous system (CNS) neurons. This membrane is produced by expansions of specialized glial cells of the brain, “the mature oligodendrocytes”, derived from oligodendrocytes progenitor cells (OPCs) [21]. This cell line constitutes the precursor cells for the constitution of the myelin sheath through a well-defined program of proliferation, migration, and differentiation to lead to the myelination of neuronal axons [100]. Among the properties of myelin, the best known is the saltatory conduction of nerve impulses, which gives it more speed and efficiency. Furthermore, it is now well acknowledged that oligodendrocytes’ expansions display a trophic, plastic, and metabolic influence on the axons they envelop (Fig. 1) [130, 139]. Myelin is constantly reshaping and its alteration in degenerative phenomena such as Alzheimer’s disease (AD) may be a fundamental element for the genesis of pathophysiological and clinical disorders observed in the early stages of the disease [22].

(modified from [175]). The differentiated oligodendrocytes of OPCs migrate to different axons via positive chemotactism [216]. A variety of growth and trophic factors regulate the development of oligodendrocytes and their temporal and geographical attractions [17]. Many of these factors are produced by both neurons and astrocytes, regulating the proliferation, survival, or degeneration of OPCs. The neuroregulin, which activates Erb-tyrosines kinases receptors, promotes the survival and proliferation of oligodendrocytes. The activation of the Notch 1 cascade inhibits the differentiation of oligodendrocytes, and an integrin/contactin complex coordinates signals from the extracellular matrix and the axonal surface to regulate oligodendrocyte survival and myelination. This also depends closely on the electrical activity propagated in the axons. OPCs express functional adenosine receptors, activated by action potential [149, 183]. Adenosine acts as a powerful transmitter between glia and neurons to inhibit the proliferation of OPCs, stimulate their differentiation and stimulate myelin production. The LIF (leukemia inhibitory factor) is heavily involved in oligodendrocyte development kinetics and in the overall myelination process [133, 198]. Abbreviations: CNTF, ciliary neurotrophic factor; FGF, fibroblast growth factor; IGF, insulin-like growth factor; LIF, leukemia inhibitory factor; NCAM, neural cell adhesion molecule; NT-3, neurotrophin 3; OPCs, oligodendrocyte precursor cells; PDGF-A, platelet-derived growth factor-A
Oligodendrocytes are derived from the differentiation of oligodendrocyte precursor cells (OPCs) and are the main cell for remyelination.
AD involves progressive neurodegeneration with neuronal losses leading to cognitive, memory, emotional, behavioral disorders, and a progressive dependence [210]. Long considered to primarily affect the grey matter, many studies have described early lesions of the white matter in patients with nascent and moderate intensity [55]. The main molecular alterations of the disease are considered to contain essentially a pathology of the production/degradation and an intra-brain accumulation of amyloid β (Aβ) peptides producing deposits in the form of hydrophobic plates of aggregated toxic peptides (senile plaques) [69, 159]. Another proteinopathy usually accompanies the previous protein in the form of hyperphosphorylated tau proteins and deposits of neurofibrillary tangles. These toxic proteins maintain chronic inflammation and oxidative stress accompanied by neuronal and synaptic losses at the origin of the symptomatology [31]. This paper reviews the fundamental importance of myelin and correct CNS myelination for its development, functional adaptations, and permanent reshuffling. During aging in the human patient, sporadic AD has plurifocal impairments that induces various clinical presentations depending on the intensity of inflammatory and immune reactions, and ischemic, mitochondrial, and free radical disorders. In many cases, the hypothesis of an alteration of the amyloid cascade Aβ as a primitive mechanistic etiology is questionable and multiple proteinopathies can be implicated, which depend on somatic mosaicism, transcriptional and translational alterations. In many cases, myelin and its integrity appear to be a preferential and early target in multiple forms of AD, and oligodendrocytes represent a cell population highly sensitive to Aβ and other proteinopathies. These misfolded proteins result in multiple dysmetabolism that accentuate and modify the course and clinical forms of the disease.
Multiple causes, multiple targets
AD as an heterogeneous disease
The amyloid hypothesis as the essential cause of neuronal loss and brain atrophy is a matter of discussion mainly because removal or reduction of amyloid plaques by immunological treatments display no significant effect on clinical symptoms of AD [39, 140]. However, before aggregation, the soluble oligomeric forms of Aβ peptides possess strong toxic properties against myelin integrity and neuronal survival [160, 188]. White matter lesions are commonly found in magnetic resonance imaging (MRI) scans of elderly people and are associated with cognitive decline [128]. Whether or not a primary role of Aβ peptides is fundamental in these lesions is a matter of debate.
Increase in the concentration of Aβ peptides in brain has also been described because of head trauma or cerebral ischemia [178, 220]. This increase in the concentration of Aβ peptides is additional evidence for the existence of various forms of Alzheimer’s like diseases with various clinical pictures and various mechanisms of neurodegenerative processes in term of pathophysiological evolution and biological markers [43, 214]. In addition to Aβ peptides accumulation and toxicity, hyperphosphorylation of tau proteins, which disturbs microtubules assembly and axonal transport, could have some impact on the trophic effect on the myelin envelop [209]. These basic alterations are also supplemented with several others biological modifications of many molecular pathways and functions namely in the domain of energy metabolism and cholesterol transports [19]. Cholesterol is fundamental for oligodendrocytes survival and for synthetis of myelin, this compounds represent a large proportion of the human brain and abnormalities in cholesterol metabolism are present and associated with brain age and in Alzheimer’s disease [12, 105]. Regarding cholesterol delivery to axons and synapses, the ε4 allele of the apolipoprotein E (APOE) gene is the less effective factor for cholesterol transport compared to ε3 and ε2. Interestingly, normal adults at the cognitive level show microstructural changes in myelin architecture when carrying homozygous alleles ε4 that is a major marker for late AD [128, 145, 146].
Sporadic AD is a multifactorial disease
While familial AD has essentially genetic causes expressed in amyloid precursor protein (APP) and presenilin leading early in life to specific proteinopathies and inducing neurodegenerative pathologies [63], these mechanisms are less consistent in the sporadic AD of elderly subjects. In these patients displaying accumulations of cerebral amyloid peptides, the question arises as to whether this accumulation is the cause or the consequence of other factors inducing neurodegeneration. Over the years, many mutations are present in brain neurons, generating multiple proteinopathies after transcriptional, translational, or post-translational errors. Some of these are of exogenous origin and enter the CNS due to the pathological porosity of the blood-brain barrier (BBB). The accumulation of these abnormal proteins generates inflammatory and immune responses that lasts for many years. Such accumulation is increased by mitochondriopathies, and the genesis of free radicals related to the disruption of oxidative phosphorylation and the depreciation of energy metabolism. The most deleterious and widespread proteinopathy is that affecting the regulated proteolysis of the APP, giving rise to toxic peptides interfering with many neuronal functions and leading to synaptic and neuronal losses, as well as inducing profound cognitive and behavioral functional abnormalities. These peptides exhibit amyloid properties and accumulate over time into hydrophobic plaques, which can be detected by PET scan ligands or post-mortem histology.
Accumulating evidence supports a multi-factor for the origin of many forms of sporadic AD. Multi-organ alterations could initiate or worsen neurodegeneration [8, 193, 208]. Developing heart failure promoting hypoxia, intestinal and hepatic disorders altering brain metabolism through the microbiome, ischemic symptoms due to vascular deposits and chronic inflammation, could also contribute to the decrease in neuronal survival [114, 197].
Metabolic disorders and AD
Many metabolic alterations have often been encountered in AD with varying severities. Most of these alterations concern or affect brain energy metabolism, these phenomena being aggravated by cerebral hypoperfusion and blood sugar abnormalities [10]. Carbohydrate metabolism and dysfunctions in intestinal absorption phenomena, nutritional abnormalities and deficiencies, resistance to glucose utilization via decreased insulin sensitivities combined with fatty acid metabolism disorders, induce energy deficiencies deleterious to neuronal functioning and survival [85]. The slowing down of the tricarboxylic cycle generates the accumulation of acetyl-CoA coming from the increased fatty acids degradation and the synthesis of ketone bodies that could have a positive role on neuronal survival. White matter degeneration in AD could be in part due to the accelerated degradation of lipids in this context of decreased energetic metabolism coming from reduced glucose utilization (Fig. 2) [141, 206].
Dysregulation in multiple biochemical pathways underlie the pathogenesis of AD. Metabolomic approaches conducted from the blood or CSF of AD patients compared to controls highlighted abnormalities in the energy metabolism of patients. A diabetic-type pathology is often evoked with a decrease in insulin sensitivity. In addition to disorders in glycolysis and the respiratory chain, abnormalities involving accumulations of ketone bodies resulting from the metabolism of acetyl-CoA residues, a product of the accelerated degradation of fatty acids by β-oxidation, have been described. The bioavailability and metabolism of several amino acids could also be affected, especially concerning tryptophan degraded in the kynurenine cycle and resulting in the formation of neuroprotective (kynurenic acid) or neurotoxic (quinolinic acid) compounds. Abbreviations: AD, Alzheimer’s disease; CSF, cerebrospinal fluid; NADH, nicotinamide-adenine-dinucleotide; ROS, reactive oxygen species; TCA, tricarboxylic acid; βOHD, beta-hydroxybutyrate
At the hepatic level, functional alterations (cirrhosis, hepatitis) could aggravate the elimination of deleterious proteins including Aβ [201]. Modifications of bile acids synthesized by the altered liver tissue exhibit impaired neuroprotective functions. Frequently, the accumulation of mutations in the mitochondrial genome accelerates pathological phenomena at the level of the tricarboxylic cycle or the respiratory chain and increase ROS production [73]. Metabolic disorders of the periphery of the body often affect brain metabolism via abnormal permeability of BBB and the presence of abnormal metabolites in the cerebrospinal fluid (CSF). This mainly concerns certain intermediates of amino acids metabolism, particularly regarding the catabolites of tryptophan degradation [167]. This essential amino acid is the precursor not only of melatonin and serotonin, but also of the intermediates of the kynurenine cycle, some of which possess neuroprotective or neurotoxic properties or interfere with the elimination of amyloid peptides from the brain [116].
Myelin has morphological alterations in the early stages of AD
Change of the lipid composition of myelin over time [127]
Oligodendrocytes-derived myelin accounts for about 40% of CNS lipids, consisting of 50% phospholipids, 40% glycolipids, 10% cholesterol and cholesterol esters, and polyunsaturated long-chain fatty acids (Fig. 3) [82] .
Myelin composition and organization. The myelin wrapping around most of the CNS axons includes a large majority of complex lipids and 15–30% of specific proteins. Lipids are essentially made up of cholesterol, galactocerebrosides and phospholipids. This envelope is constantly reshuffled in time and space from the oligodendrocytes that make up the bulk of the glial cells of the CNS. Chronic inflammatory and autoimmune reactions, mutations in certain constituent proteins, attacks by free radicals or ischemic, and metabolic problems related to aging alter the myelin sheath that releases its constituents into the CSF and the bloodstream. In AD, myelin is one of the first bulwarks for the anatomical and functional integrity of the axons it surrounds and undergoes early toxic action of misfolded extracellular toxic proteins or peptides. Abbreviations: AD, Alzheimer’ disease; CNP, C-type natriuretic peptide; CNS, central nervous system; CSF, cerebrospinal fluid; MAG, myelin-associated glycoprotein; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; PLP, proteolipid protein
Synthesized by oligodendrocytes, cholesterol comes almost exclusively from ketone bodies as precursors. This lipid has structural functions at the level of the myelin by regulating the fluidity and permeability of this membrane around the axons, and it regulates the speed of myelination according to its uptake by the membrane in formation. The typical lipids of myelin are essentially galactosyl ceramides and sulfatides. They stabilize and organize myelin in direct association with the basic protein.
Changes in the configuration of myelin are observed with age but are more accentuated in AD. Not all regions of the brain are affected in the same way. In general, the volume of white matter decreases over time and the phenomena of demyelination/remyelination accentuated by pathology leads to the decrease in the size of axons and the reduction in the size of the internodal distances. These structural changes induce functional consequences for conduction rates and vulnerability to traumatic, ischemic, dysmetabolic conditions and toxic factors such as oligomers Aβ peptides. These processes are commonly encountered as factors favoring Alzheimer’s genesis and pathology.
A step for the conversion of mild cognitive impairment (MCI) into dementia?
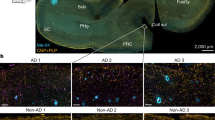
Studies of myelin sheath’s conformation in AD were mostly conducted by electron microscopy and MRI both in animals and humans. The 5XFAD mouse is a transgenic model that expresses three different mutations in the APP and two in presinilin 1 (PS1). In this mouse, amyloid deposits can be detected with synaptic losses at an age as early as 1.5 months [142] and myelin abnormalities can be seen even earlier accompanying the first alterations in spatial memory occurring around the age of 1 month [67]. Several studies conducted in humans suggest that myelin disorders strongly contribute to the onset of AD symptoms. Neuroimaging shows myelination defects in several brain regions, but especially and firstly in the hippocampus and corpus callosum [55, 137, 147, 202]. Conformation abnormalities accompanied by thinning of the myelin sheath are frequently encountered even before the onset of axonal lesions, which may indicate premises for demyelination. In the pre-clinical stages of the disease, MRI shows altered longitudinal and transverse relaxation times and increased myelin hydration degrees [18]. In general, abnormalities in the structure and formation of the cerebral white matter have been identified in many presentations of Alzheimer’s disease that can be warning signs for a disease in progress [156]. Variations in T1w/T2w ratios in patients with risk factors (close family history, APOE4 phenotypes) were identified compared to control individuals. In addition, individuals at risk had an association with altered patterns of resting-state functional connectivity (rs-FC) [52]. These abnormalities support the idea of significant alterations in myelin developing with age and constituting signals of vulnerability [53]. Interestingly, some studies have shown a relationship between structural abnormalities of myelin in ADs in the pre-clinical period and peptide concentrations of Aβ1–42 in patients’ CSF [34, 38].
Studies of cortical stratification in the human brain provide important knowledge on the level of degeneration, in addition to the information given by the level of volumetric atrophy [147, 155]. MRI studies show hyper-densities in the white matter with volume increase consistent with the abnormalities of amyloid peptides and tau proteins in CSF. At the histological level, it appears that these stratification disorders are mainly due to alterations in myelin architecture in which iron ions could play an important role [194]. Vascularization and oxygen supply in injured hyper-dense regions are decreased and are related to axonal lesions and inflammatory disorders, BBB permeability abnormalities and multiple disseminated micro-hemorrhagic structures [88, 102].
Potential involvements of epigenetic mechanisms in myelin reshuffle
GWAS (genome-wide association study) has identified about 40 loci associated with AD in the European population and in these respective loci, several genes involved directly in the causative mechanism of the disease have been described (APOE, CR1, BIN1, TREM2, CLU SORL1, ADAM10, ABCA7, CD33, SP11, PIRLA). It remains to identify the functions of many genes in the identified loci. Many risk genes are involved in the innate immune response and neuroinflammation. The CD33 and TREM2 microglia receptors, implicated in microglial pathology, represent new targets for the development of therapeutic tools. It is possible in many cases that the activation of innate immunity, like that encountered in other myelin pathologies, associated with long-term inflammatory mechanisms, is responsible for subtle alterations of myelin during the incubation period of the disease [5, 65].
Over 20 AD risk loci falling mainly in noncoding regions of the genome have been identified by genome-wide association studies, explaining the complexity of the disease at the genetic level [81, 98, 126]. The regulation of gene expression by microRNA is a promising issue for the diagnostic and treatment of several kind of MCI and AD at the beginning of the symptoms, as well as to discriminate with other myelin pathologies like multiple sclerosis [103, 226]. In such diseases, the dynamics of the myelination/demyelination/remyelination balance is continuously evolving under normal conditions of plasticity of the nervous system, but also under pathological conditions, where this balance is affected [47]. In this respect, the process of myelin degeneration are particularly concerned both in multiple sclerosis and in the early phases of AD. Oligodendrocytes and their progenitors are directly involved in membrane and metabolic interactions with neurons during the different phases of destruction and regeneration of the myelin sheath, driven by the dynamic and fluctuating expression of many transcription factors [179]. The activity of the nervous system is intimately linked to the epigenetic regulation of the activity of these factors and to the neo-expression of certain genes involved in the functional dynamics of the production/destruction of myelin [158]. Correct myelination is essential for the proper development and evolution of neuronal connections and the adaptation of brain function to the environment. It constantly reshapes neuron/oligodendrocytes interactions following many factors such as learning, social relationships, emotional stimuli (emotions, anxiety) [166, 217]. These stimuli can induce epigenetic modifications that alter the physiology and functionality of precursors and oligodendrocytes [165].
Changes in the epigenome have a role in the manifestations of AD [13]. Social isolation impacts the intensity of neuronal activity and reduces the importance of myelination [6, 139]. Modifications in the acetylation and methylation of histones were detected, as well as in DNA [131]. These adaptations participate in the regulation of genes involved in the processes of myelination/demyelination and in the pathophysiology of certain neurodegenerative diseases where these processes play a central role (AD, but also multiple sclerosis) [30]. This last pathology of myelin includes analogies with those existing in some phenotypic form of AD and may be the consequence of a combined alteration of genetic and epigenetic factors, the latter involving DNA methylations, histone modifications, chromatin remodeling and modified regulation of non-coding RNA [16].
Brain markers of myelin in Alzheimer’s patients
Looking for myelin components in biological fluids
At the genetic markers level, several genes associated with the corpus of the oligodendrocyte ecosystem have been described as risk factors in late-onset AD [124]. In genomic association studies, the BIN1 (bridging integrator 1) gene is considered to be significantly involved in late AD behind the APOE gene [169]. It is mainly expressed in mature oligodendrocytes and white matter in rodents and humans, where it regulates membrane dynamics in the phenomena of endocytosis and membrane remodeling [37]. Histologically, BIN1 is mostly expressed at the Ranvier nodes.
BACE1 (beta-site amyloid precursor protein cleaving enzyme 1) codes for a transmembrane β secretase expressed in several cell types including oligodendrocytes. It cleaves APP giving birth to amyloid peptides, but also neuroregulin 1, which modulates the myelination and differentiation of oligodendrocytes [50, 195]. Many β-secretase inhibitors have effects on myelin abnormalities caused by AD. Finally, several other genes that are also expressed in oligodendrocytes (PICALM, NME8, PSEN, for example) possess a special responsibility as genetic factors associated with the development of AD [123].
LINGO 1 (leucine rich repeat and Immunoglobulin-like domain-containing protein 1) codes for a transmembrane protein primarily expressed in the cortex, hippocampus, thalamus, and amygdala. The protein acts primarily as a negative regulator of myelination and its inhibition may have potential applications for the treatment of myelin damage in neurodegenerative diseases [204]. As such, anti-LINGO 1 antibodies promote the action of oligodendrocytes and the repair of myelin disease [212].
The biochemical markers of the white matter indicating the evolution of late AD are of many natures and depend on the stage of the disease. Since myelin is mainly composed of complex lipids synthesized by oligodendrocytes, reduced levels of galactosyl ceramide (cerebroside) and sulfatide can be found in both the grey and white matters of AD brains [89]. These compounds are the most specific lipids of myelin, decreasing in parallel with the severity of the disease and altering long before fibrillary deposits of tau protein [28, 87]. Cholesterol concentrations, another majority lipid compound of myelin sheaths, is known to decrease with the onset of cerebral atrophy [44].
Myelin proteins are also involved in relatively early stages of the disease (Braak stage I and II), in which alterations of oligodendrocytes and myelin are noted even before the onset of clinically detectable cognitive disorders [55]. The level of most myelin proteins is likewise decreased in more advanced stages of AD (Braak stages V and VI). Lowered concentrations of basic myelin protein (MBP), proteolipid (PLP) and 2’-3’ cyclic nucleotide phosphodiesterase (CNPase) are observed, specifically in several regions of the cerebral cortex.
In the field of protein markers present in patients’ CSF, there is a wide heterogeneity and variability, which confirm the impression that sporadic AD may be the consequence of multiple and varied alterations in many metabolic circuits. This reinforces the idea that the pathophysiological mechanisms leading to late AD are multifactorial and reveal a disease of great complexity [205]. Many cognitive pathologies with MCI are often accompanied in a non-specific way by the presence of inflammatory markers and proteins associated with the complement cascade in the CSF or blood of patients.
Oligodendrocyte’s dysfunction: A major risk factor in AD and a process in the onset of the disease?
Before the appearance of amyloid and tau pathology, many forms of AD showed a breakdown of myelin due to the vulnerability of oligodendrocytes under this neurodegenerative pathology. In many cases, the loss of myelin sheaths appears to be the initiating step in the earliest stages of the disease. Extensive evidence has indicated that the breakdown of myelin is associated with AD since the vulnerability of oligodendrocytes under Alzheimer’s pathology easily induces the myelin breakdown and the loss of the myelin sheath.
Aging itself is already an important factor of myelin alterations and multiple cellular partners are involved in this process. Brain MRI often reveals signs including several hyper signal outbreaks in T2-weighted images (T2WI) with chronic cerebral hypoperfusion often associated with carotid stenosis [112]. These alterations appear more massive at the stage of MCI both in animal models and in human pathology than in established AD. In myelin abnormalities, association of oligodendrocytes’ losses with axonal alterations are commonly encountered in post-mortem patients [137]. The accumulation of Aβ peptides is considered a princeps factor in the neurodegenerative process even before the appearance of aggregates in the form of amyloid plaques [23, 75]. The response of oligodendrocytes to the presence of amyloid peptides or plaques has been the subject of several studies. During aging, the spontaneous involution of these cells is important, and their disappearance is close to 25% from the age of 50 years, this phenomenon being potentiated by the presence of the APOE ε4 allele in the genomic baggage of the individual [107, 132, 134]. Furthermore, the importance of increase in the expression of myelinating genes in oligodendrocytes from Alzheimer’s patients is related to the severity of the disease [64, 79]. In transgenic animals over-expressing the APP, the myelin sheath has an increased thickness and a modified architecture [54, 67, 211].
Originating mostly from the ventricular and subventricular regions of the brain, OPCs are present in the brain, even in the adult stage [36, 164, 187, 218]. OPCs control the angiogenesis of the white matter, its vascularization/oxygenation, and the myelination of axons according to spatial-temporal parameters, which contribute to their stability, functions, and integrity [96, 153]. Intimate exchanges between neurons regulate ionic homeostasis, making of real synaptic connections and activity of OPCs via numerous neurotransmitters [58, 68, 172]. These cells are also the target for several mitogens produced by neurons and trophic factors like neuroregulin 1 and brain-derived neurotrophic factors (BDNF), whose release depends on neuronal activity [77, 199]. Many studies have been conducted to explore early oligodendrocyte alterations in AD in association with changes in myelination and early symptoms of the disease. Most commonly, oligodendrocyte differentiation abnormalities are associated with disruption of oxidative stress phenomena associated with excitotoxicity, mediated by glutamatergic metabotropic receptors in large amounts in oligodendrocyte precursors [22, 137]. Other factors, such as high iron ion levels and disorders in the glutathione cycle, would accentuate the presence of free radicals, without forgetting the mitochondrial chain disorders induced by the toxicity of Aβ peptides [190]. Mitochondria pathologies are at the forefront of axonal survival for functional and metabolic exchanges with the myelin envelope [66, 219].
Myelination is directly related to the intensity of neural activity, which affects the electrical properties of axons. The toxicity of Aβ peptides proteinopathy affects immediately the whole myelin-axon, which forms a couple with multiple functional and metabolic relationships [181].
The toxicity of proteinopathies, which causes degenerations in AD, mainly concerns oligomeric Aβ peptides and hyperphosphorylated tau proteins [2, 41, 82]. Senile plaques are rarely seen in the hydrophobic white matter and does not lend itself to the aggregation of toxic oligomers. As a result, it is mainly these latter that exert toxicity on myelin. The therapeutic strategies currently developed for plaque removal seem not to display huge impact on clinical symptomatology [196]. In fact, the degree to which the therapeutic strategies for plaque removal have clinical effects remains an open question, as the reason why such therapies are not working well. For more than twenty years, therapeutic research against AD has focused on reducing the accumulation of pathological amyloid peptides and the substances studied have made it possible to achieve this goal without significant improvement in cognitive impairment in patients. The amyloid hypothesis revised in many cases is questioned, sometimes in favor of primitive alterations of the myelin envelope [61]. Oligodendrocytes are very active cells from a metabolic point of view, especially during the process of myelination or remyelination. A cellular respiratory abnormality that may be related to hypo-vascularization or ischemia may be the source of a myelination disorder [137]. Vascular pathologies affecting the white matter are common in the elderly or with symptomatologically occurring early AD [78, 109]. The human brain is largely myelinated, which may partly explain its vulnerability to neurodegeneration [192].
Oligodendrocytes are widely represented in many areas of the human CNS, especially in the neocortex, where they account for about 75% of glial cells [45]. They are considered very fragile, and their density decreases sharply in the brain of the elderly person from 50 years of age [223]. Various methods of labeling these cells have shown a severe loss of oligodendrocytes in several regions of the hippocampus, not correlated with the density of amyloid β deposits [40]. These specific depopulations probably precede the disorganization of the neural connectome that precedes the appearance of AD, and a contemporary demyelination around the outbreaks of amyloid peptide deposits [94]. These dysfunctions are strongly associated with abnormalities in lipoprotein metabolism given that the amyloid oligodendrocytes actively participate in the synthesis of cholesterol constituting synaptic contacts [120]. They secrete apolipoproteins E and J, which are severe risk factors depending on the alleles involved in the onset of AD [85]. Interestingly, the production of new oligodendrocytes seems fundamental for motor learning in mice [111].
During the development of AD, including in the early stages characterized by mild, worsening memory disorders (MCI), numerous studies have been performed to characterize the changes observable by MRI techniques in the structure and architecture of myelin. Schematically, the results obtained showed very early the existence of a reduction in cerebral myelin levels, with losses of oligodendrocytes and axons, microglial activations accompanied by dilated perivascular regions in the white matter. These studies are essentially based on the contrasts between the aqueous contents of the intra- and extra-cellular spaces at the periventricular level, comparing MCI patients and control persons. It seems that progressive ischemia with vascular and energy losses associated with the toxicity of certain proteinopathies (especially amyloidosis Aβ) alters the myelin structure very early and hinders proliferation and oligodendrocytic re-myelination [88, 137, 148].
Adaptative immunity to myelin components in AD
An auto-immune process for degeneration?
Many results support the existence of mutual interactions between immune processes (innate and acquired) and neurodegenerative events, especially those occurring during the incubation of AD [33, 51, 144].
Neuroinflammation phenomena are considered to pre-exist for a very long time in the brain before the onset of cellular stigmas of neurodegeneration and clinical symptomatology. Chronic inflammation, microglia activation and lymphocytic infiltration are thought to be the result of intracerebral accumulation of misfolded proteins and/or multiple exogenous attacks of various infectious agents during the individual’s lifetime [42]. Amyloid peptides and hyperphosphorylated tau are particularly involved in the inflammatory reaction and progressive onset of autoimmunity [207]. Changes in circulating cytokines as well as disorders in the cascades of the complement and clotting factors testify to changes in the immune response at the periphery [143]. Cleavage fragments of abnormal proteins, numerous glycated proteins and a large population of phosphoproteins contribute to microglial activation in the brain and alteration of many resident proteins [24]. This include myelin constituent proteins that are presented as new antigens to the immune system. Studies have shown significant accumulation of autoantibodies in the serum of patients with AD, especially directed against myelin proteins [62, 115]. IgG and IgM immunoglobulins directed against the MOG, MBP, MAG and PLP proteins are frequently present in the CSF and circulating blood [152]. This strongly suggests the involvement of the immune system in myelin alterations observed in many AD patients and in some animal models of the disease.
Since the discovery of mutations in APP, PSEN1 and PSEN2 genes, which induce familial ADs, the hypothesis of the amyloid cascade at the origin of the pathophysiology of AD remains the preferred mechanism of this type of neurodegeneration [136]. The problem is that sporadic AD does not usually present this type of mutation, although similar pathologies of Aβ peptides and tau proteins are encountered in familial and late forms of the disease [106]. The main hypothesis remains those long-term abnormalities in Aβ peptide metabolism are the starting point of tau dysfunction and a series of toxic phenomena inducing neuronal and cognitive losses. It appears that during aging, multiple mutations accumulate in the nuclear and mitochondrial DNA of neural cells that add up to the increased loss of editing and quality control of translated proteins [76, 108]. It is estimated that in the normal individual, about 20% of the proteins synthesized by ribosomes have structural and folding abnormalities and must be eliminated by the proteasome, lysosome, or resident proteases of the cell membrane [97, 157, 180]. Overloading these mechanisms leads to chronic neuroinflammation, microglial and macrophagic activation, and immune responses against abnormal non-functional proteins over the long-term [122]. Among the oligomeric peptides that accumulate in the brain and display significant cellular toxicity are Aβ peptides and particularly the peptide Aβ1–42. This accumulation most often comes from a drop in the clearance to the vascular compartment and the CSF.
The rupture of the myelin envelope appears to be an early phenomenon in the pathophysiology of AD [38, 150, 202]. In humans, the vulnerability of myelin materializes on MRI through morphological changes, thinning and hydration swelling [49, 229]. At the same time, there are elevations of tau, phosphotau, soluble APPβ (sAPPβ) peptides and Aβ1–42 peptides. The latter peptide has a high toxicity to myelin in oligodendrocyte cultures and in animal models of familial AD (e.g. 5XFAD mice), where morphological alterations of myelin are the first pathological stigmas to appear in animals at 1 month of age [67]. The mechanisms of toxicity of peptides Aβ are still the subject of speculation; it seems that the oligomeric Aβ peptides are the main culprits of this toxicity [75]. Several cellular receptors (glutamates, ephrin’s, adrenergic, cholinergic, and immunoglobulins) bind oligomeric Aβ peptides and could mediate the toxicity of amyloid oligomers during the years of incubation of the disease [171, 215].
Multi-proteinopathies are associated with aging brains
During lifetime, mutations accumulate in the post-mitotic cells of neurons due to non-replication of DNA, but also within mitochondrial DNA [86, 95]. This results in harmful mitochondriopathies for neuronal survival, as well as increased production of misfolded and non-functional proteins. Deficiencies in quality control and protein structure editing also contribute to the intra-brain accumulation of protease-resistant hydrophobic deposits with intrinsic toxicity [1, 151]. These chronic accumulations lead to long-term inflammatory and phagocytic reactions, as well as immune responses accompanied by infiltration of immunocompetent cells. Gradually, an amyloid reaction develops, in which peptides Aβ participates largely because of their cerebral accumulation. The elimination of these peptides from the brain is largely conditioned by the effectiveness of the enzymes that degrade them and allow their clearance [222]. The peptide Aβ1–42 is particularly toxic to myelin sheaths, axonal and synaptic endings that finally degenerate [75, 137]. In several transgenic models of familial forms of Alzheimer’s, the first stigmas of the disease result in morphological abnormalities of myelin sheaths, in the form of edema and thinning of the envelope surrounding myelinated axons. At the same time, disturbances in animal behavior appear manifesting as reduced anxiety manifestations and reduction of memory and spatial recognition [56, 57].
Depending on the individual, brain aging does not occur unequivocally but depends on multiple factors related to specific genes and environmental situations (Fig. 4) [176]. The accumulation of mutations in the nuclear DNA of post-mitotic cells and mitochondrial DNA induce deleterious mitochondriopathies [121, 186], promote the production of abnormal proteins, impair respiratory and energy functions, and amplify cellular and oxidative stress [91]. Toxicity of abnormal oligomers seems to be the result of their misfolded nature, which exposes hydrophobic residues leading to aggregation and abnormal interactions with a large range of cellular components [4]. Membranes like myelin constituted mainly by complex hydrophobic lipids could be an important target for amyloid oligomers for direct interactions andymes modifications inducing inflammatory and immune responses.
Multiple Alzheimer’s disease etiologies and many cellular partners. Deleterious proteinopathies (in the first-place amyloid peptides) are to be integrated into the complex cellular environment of the brain. These multiple cellular elements participate in progressive multi-focal neuro-axonal degeneration leading to the irreversible symptomatology of AD. This is expressed when the toxic peptide removal systems are overwhelmed, which appears only after a long incubation period. Altered neurons express phases of hypo- and hyperexcitability with deficits in axonal transport and synaptic activity that affects myelination/remyelination activity and oligodendrocyte trophism. These are very vulnerable cells whose density decreases sharply with age. There seems to be a link between the intensity of neuronal involvement and the extent of demyelination. This is strongly accentuated in AD in which remyelination processes seem deficient. The activation of astrocytes participates in the elimination of deficient neurons and synapses. They actively participate in the elimination of abnormal proteins and inflammation processes, in the same way that the activation of microglia facilitates the phagocytosis of cellular debris. In the same way, these cells participate in the activation of the innate immune responses, the activation of the complement and the secretion of inflammatory cytokines. Abbreviations: AD, Alzheimer’s disease; ROS, reactive oxygen species
Several evidence from studies of the population of abnormal proteins in the CSF showed that abnormal proteins in CSF represent a picture close to that of abnormal proteins in the brain [11]. This methodology can provide information on the biochemical and metabolic changes that occur in the CNS of patients with neurodegeneration. CSF amyloid peptides and tau proteins are used for the diagnosis and evolution of AD [154]. Aβ1–42 peptides correlation has been described with several CSF proteins belonging to the endocannabinoid and the somatostatin systems [71] with the latter regulating the proteolytic degradation of the amyloid peptide. The presence of other proteins has been linked to the degradation of the myelin [170].
The quality control processes of in vivo newly formed proteins and the elimination of abnormal proteins are phenomena with growing alterations with age [92, 138]. This results in the cellular and extra-cellular accumulation of an increasing number of non-functional proteins that tend to form hydrophobic aggregates [117]. At the brain level, these toxic aggregates induce significant cellular and functional losses that are the basis of many neurodegenerative diseases [14]. In addition to the amyloid peptides and tau protein that are the canonical proteins of early AD and whose toxic deposits in brain tissue are the basis of mechanistic theories of neurodegeneration, it has been shown that a wide range of protein aggregates from other sources exist in the brain of elderly patients displaying a cognitive impairment or at first stages of AD [162]. Among the proteins significantly altered compared to controls, many are found in the biochemical cascade of glycolysis that primarily feeds cellular energy and whose intensity decreases with age, even faster in patients with AD [135]. Other strategic proteins form larger insoluble aggregates depending on symptomatologic impairment. These include glucose 6 phosphate isomerase creatine kinase B, certain forms of adenylate cyclase and calcium/calmodulin protein kinase 2. This list is not exhaustive but reflects the importance of metabolic and functional disorders that develop over time in the brains of patients with mild cognitive impairments that worsen in AD [91].
It could be speculated that the accumulation of misfolded proteins during old age in multiple regions of the brain alters mitochondrial and metabolic functions, saturates the processes of cleaning and elimination of senescent cells, and slows down the neurogenesis that persists in the older brain [20, 80]. Inflammatory vasculitis, hypoxia, and oxidative stress due to the accumulation of non-functional deleterious proteins are considered the primary factors in myelin envelope impairment. The decrease in electrical and metabolic activity of axons contributes to the decrease in myelin density that surrounds them leading to its gradual dislocation. The toxicity of Aβ peptides has been demonstrated in vitro against neurons, endothelial cells, astrocytes, vascular smooth muscle cells and oligodendrocytes [224]. Aβ peptides cytotoxicity might involve the susceptibility of oligodendrocytes to oxidative stress because of its low content of reduced glutathione and high concentration of iron [190]. The Aβ peptides activation of the neutral sphingomyelinase-ceramide pathway has been reported to induce oligodendrocyte death [101]. In addition, inhibition of neutral sphingomyelinase 2 in these cells reduces their ceramide content and favor the myelination process by improving the quality of myelin structure [221].
Microbiome and myelin dysregulation in the neurodegenerative brain
A role for the gut-brain axis and for hepatic metabolism
Some arguments favor a view of AD as a disease that is not limited to the CNS alone but reflects multi-organ dysfunctions that contribute to or influence brain neurodegeneration [200]. Multiple proteinopathies, including the Aβ cascade, may come from peripheral organs that no longer metabolize abnormal proteins properly and allow their dissemination through a permeable BBB. Abnormal communication between various compartments of amyloid proteins can contribute to altering brain disease. Chronic peripheral metabolic abnormalities are suspected to participate or to worse neurodegeneration. In this regard, intestinal metabolism is often questioned.
The population of microorganisms of the gut microbiota constitutes a true symbiotic organ that has a great inter-individual heterogeneity due to many intrinsic and extrinsic factors dependent on genetic, medication (e.g. antibiotics), physical and hormonal activity, and infectious factors [185]. The composition of the microbiome changes with age and the reactivity of the immune system [129]. An active exchange via the bloodstream and intestinal innervation between the microbiome and the nervous system exist, whose influence is important during the neurogenesis, the molecular organization of the connectome, and the variations of CNS myelination. These phenomena are especially different during periods of brain development or during aging [228].
In various transgenic models of familial AD, disturbances in the composition and diversity of the intestinal microbiome compared to healthy animals are observed [174]. In humans, it has also been described qualitative and quantitative changes in the population of intestinal bacteria in patients with cognitive disorders associated with cerebral amyloidosis [59, 118]. These disturbances can be the source of chronic neuroinflammation targeting several organs including the brain and a decrease in the immune response inducing neurodegeneration over the long term [26, 74]. These phenomena are increased by the leaky permeability of the BBB as a function of age, allowing the passage at the cerebral level of many toxics present at the periphery (Fig. 5) [213].
Multiple communication system that includes neural, immune, endocrine, and metabolic pathways lead to degeneration. Continuous fluctuation of the microbiota due to the environment constantly influences the inflammatory, immune, and metabolic responses of the CNS [110]. With age, the permeability of intestinal and BBB is often impaired [84, 161]. The gut microbiota metabolizes and release many growths, metabolic and inflammatory factors which could penetrate the brain via the circulating blood. These substances contribute to increase the inflammatory, immune, and oxidative phenomena that exist in the elderly brain due to the accumulation over time of many abnormal proteins due to their hydrophobic conformation. The very likely origin of these malformed proteins is found in the accumulation during senescence of many mutations in post-mitotic cells that are neurons [9, 76]. In addition, the role of epigenetic dysregulation of gene expression induced by aging or abnormal environmental stimulation is also considered to be an important factor in neurodegeneration and cognitive alterations [125]. Abbreviations: BBB, blood-brain barrier; CNS, central nervous system
The problems of pathological cerebral aging are probably the result either of the evolution of protein targets at the central level, or the modification of a peripheral immune response [60, 182], the two mechanisms can combine over time to lead to an autoimmune alteration colonizing the CNS and involving, in the first place, the components of myelin. Many environmental factors can promote this chronic process by perpetuating the homeostasis of the intestinal flora and at the origin of certain metabolic and cytotoxic disorders [15, 90]. This primarily affect oligodendrocytes, which are fragile cells of the CNS, and which adapt their functions throughout the life of the individual. Many factors contribute to the activity of oligodendrocytes, intrinsic and environmental factors that modify the status of the epigenome [165]. Among these factors, the composition and activity of the microbiome plays a reweighting role and interferes with the spatio-temporal character of myelination in the brain. In general, the relationships between the intestinal sphere and the brain are of primary importance for myelination. This sphere includes not only the intestinal epithelium, but also hepatic metabolism, sympathetic and parasympathetic nerve activity, endocrine, and cytokine secretions and metabolites of microbial origin [46]. The microbiota has an important role in the regulation of myelin plasticity as the existence of hyper-myelinated axons has been demonstrated in germ-free mice or treated chronically with antibiotics [72]. This abnormality could be a consequence of neuronal hyperactivity in certain regions of the brain of these mice, such as the amygdala or the prefrontal cortex. The development of myelinating oligodendrocytes is controlled by a set of transcription factors (Sox 10 and Myrf for example) that drive the steps of myelination and re-myelination [7]. The anomalies of these phenomena alter certain brain functions, those concerning cognitive functions. Restoring a normal microbiome in germ-free mice greatly improves their social and executive performance [173, 189, 191].
It is now recognized that disorders intestinal physiology can influence the risk of Alzheimer’s and its rate of progression. Deposits of aggregate of Aβ peptides at the intestinal level have been detected in AD patients [74], but most of the results involving the intestinal sphere and the progression of AD have been obtained in animal models. The ratios between Firmicutes and Bacteroidetes are considered strategic in the composition of the human intestinal microbiota [168]. The fecal microbiota is the product of a very complex and diverse ecosystem, and its composition can modify the accumulation of intestinal APP in the early phases of AD [29, 119]. In transgenic APP/PS1 animals, an increase in Aβ peptides levels have been observed in the CNS in relation to changes in the intestinal flora, accompanied by disorders of spatial memory [225]. Oligodendrocytes and myelin sheaths may be the first to be affected by these deleterious deposits. A parallel can be observed between the myelin alterations observed in AD and during normal aging in the elderly. In the latter case, the installation of progressive ischemia could be the cause of this demyelination [137, 177]. The lesions often appear disseminated with a predilection for intracortical axons of small diameters that are myelinated late during development. Myelin dystrophies lead to axonal alterations and neuronal death with different rate in individuals [163].
Conclusion and perspectives
To conclude, myelin damage and its several possible outcomes (Table 1) is one of the early lesions observed in many clinical forms of AD. Even though many differences exist in the presentations and structural alterations between multiple sclerosis and AD, neurodegenerative alterations between both pathologies have common etiologies and mechanisms [113]: long-standing inflammatory disorders, some autoimmune reactions, cognitive impairments, and mitochondrial alterations [104, 184]. Amyloid disorders are not absent from the pathophysiology of multiple sclerosis and Aβ peptides levels are generally lower in the CSF of patients with multiple sclerosis who have cognitive impairment [93]. The accumulation of APP in the brain of these patients appears parallel to the worsening of symptomatology and dynamic processes of demyelination/remyelination [25]. These parallels remain hypotheses at present, but there are indications that some mechanistic similarities exist.
Current mechanistic hypothesis favors long-term dysfunctions in the proteolysis of APP and in the accumulation of hydrophobic Aβ peptides with multiple toxicities. These lead to inflammatory, oxidative, and immune reactions leading to massive cellular apoptosis accompanied by post-translational modifications on target proteins inducing profound functional alterations in brain cells activities. It seems possible that a multiplicity of mutations and epigenetic alterations of neuronal genomes, associated with intrinsic or extrinsic predisposing factors, generate metabolic and inflammatory alterations over the long term, inducing a multiplicity of phenotypic and clinical presentations involving secondarily multiple deleterious proteinopathies, including amyloidosis of Aβ types.
Data Availability
Not applicable.
References
Adav SS, Sze SK (2016) Insight of brain degenerative protein modifications in the pathology of neurodegeneration and dementia by proteomic profiling. Mol Brain 9:92. https://doi.org/10.1186/s13041-016-0272-9
Allegri RF (2020) Moving from neurodegenerative dementias, to cognitive proteinopathies, replacing “where” by “what”…. Dement Neuropsychol 14:237–242. https://doi.org/10.1590/1980-57642020dn14-030005
Allen M, Wang X, Burgess JD, Watzlawik J, Serie DJ, Younkin CS, Nguyen T, Malphrus KG, Lincoln S, Carrasquillo MM, Ho C, Chakrabarty P, Strickland S, Murray ME, Swarup V, Geschwind DH, Seyfried NT, Dammer EB, Lah JJ, Levey AI, Golde TE, Funk C, Li H, Price ND, Petersen RC, Graff-Radford NR, Younkin SG, Dickson DW, Crook JR, Asmann YW, Ertekin-Taner N (2018) Conserved brain myelination networks are altered in Alzheimer’s and other neurodegenerative diseases. Alzheimers Dement J Alzheimers Assoc 14:352–366. https://doi.org/10.1016/j.jalz.2017.09.012
Almeida ZL, Brito RMM (2020) Structure and aggregation mechanisms in Amyloids. Mol Basel Switz 25:1195. https://doi.org/10.3390/molecules25051195
Andrews SJ, Fulton-Howard B, Goate A (2020) Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol 19:326–335. https://doi.org/10.1016/S1474-4422(19)30435-1
Antontseva E, Bondar N, Reshetnikov V, Merkulova T (2020) The Effects of chronic stress on Brain Myelination in humans and in various Rodent Models. Neuroscience 441:226–238. https://doi.org/10.1016/j.neuroscience.2020.06.013
Aprato J, Sock E, Weider M, Elsesser O, Fröb F, Wegner M (2020) Myrf guides target gene selection of transcription factor Sox10 during oligodendroglial development. Nucleic Acids Res 48:1254–1270. https://doi.org/10.1093/nar/gkz1158
Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK, Akram M, Jabir NR, Firoz CK, Naeem A, Alhazza IM, Damanhouri GA, Kamal MA (2014) Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets 13:1280–1293. https://doi.org/10.2174/1871527313666140917095514
Atwood CS, Bowen RL (2015) A unified hypothesis of early- and late-onset Alzheimer’s Disease Pathogenesis. J Alzheimers Dis JAD 47:33–47. https://doi.org/10.3233/JAD-143210
Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, Prabhakaran V (2011) Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis JAD 26(Suppl 3):123–133. https://doi.org/10.3233/JAD-2011-0010
Bader JM, Geyer PE, Müller JB, Strauss MT, Koch M, Leypoldt F, Koertvelyessy P, Bittner D, Schipke CG, Incesoy EI, Peters O, Deigendesch N, Simons M, Jensen MK, Zetterberg H, Mann M (2020) Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Mol Syst Biol 16:e9356. https://doi.org/10.15252/msb.20199356
Bartzokis G (2004) Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 25:5–18 author reply 49–62. https://doi.org/10.1016/j.neurobiolaging.2003.03.001
Basavarajappa BS, Subbanna S (2021) Histone methylation regulation in neurodegenerative Disorders. Int J Mol Sci 22:4654. https://doi.org/10.3390/ijms22094654
Bayer TA (2015) Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 25:713–724. https://doi.org/10.1016/j.euroneuro.2013.03.007
Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479:538–541. https://doi.org/10.1038/nature10554
Berry K, Wang J, Lu QR (2020) Epigenetic regulation of oligodendrocyte myelination in developmental disorders and neurodegenerative diseases. F1000Research 9:F1000 Faculty Rev-105. https://doi.org/10.12688/f1000research.20904.1
Bonetto G, Kamen Y, Evans KA, Káradóttir RT (2020) Unraveling myelin plasticity. Front Cell Neurosci 14:156. https://doi.org/10.3389/fncel.2020.00156
Bouhrara M, Reiter DA, Bergeron CM, Zukley LM, Ferrucci L, Resnick SM, Spencer RG (2018) Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement J Alzheimers Assoc 14:998–1004. https://doi.org/10.1016/j.jalz.2018.03.007
Braak H, Del Tredici K (2015) The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain J Neurol 138:2814–2833. https://doi.org/10.1093/brain/awv236
Briston T, Hicks AR (2018) Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention. Biochem Soc Trans 46:829–842. https://doi.org/10.1042/BST20180025
Butt AM, Papanikolaou M, Rivera A (2019) Physiology of Oligodendroglia. Adv Exp Med Biol 1175:117–128. https://doi.org/10.1007/978-981-13-9913-8_5
Butt AM, De La Rocha IC, Rivera A (2019) Oligodendroglial cells in Alzheimer’s Disease. Adv Exp Med Biol 1175:325–333. https://doi.org/10.1007/978-981-13-9913-8_12
Cai Z, Xiao M (2016) Oligodendrocytes and Alzheimer’s disease. Int J Neurosci 126:97–104. https://doi.org/10.3109/00207454.2015.1025778
Cao W, Zheng H (2018) Peripheral immune system in aging and Alzheimer’s disease. Mol Neurodegener 13:51. https://doi.org/10.1186/s13024-018-0284-2
Chandra A (2015) Role of amyloid from a multiple sclerosis perspective: a literature review. Neuroimmunomodulation 22:343–346. https://doi.org/10.1159/000375309
Chen Y, Fang L, Chen S, Zhou H, Fan Y, Lin L, Li J, Xu J, Chen Y, Ma Y, Chen Y (2020) Gut microbiome alterations precede cerebral amyloidosis and Microglial Pathology in a mouse model of Alzheimer’s Disease. BioMed Res Int 2020:8456596. https://doi.org/10.1155/2020/8456596
Chen J-F, Liu K, Hu B, Li R-R, Xin W, Chen H, Wang F, Chen L, Li R-X, Ren S-Y, Xiao L, Chan JR, Mei F (2021) Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 109:2292–2307e5. https://doi.org/10.1016/j.neuron.2021.05.012
Chew H, Solomon VA, Fonteh AN (2020) Involvement of lipids in Alzheimer’s Disease Pathology and potential therapies. Front Physiol 11:598. https://doi.org/10.3389/fphys.2020.00598
Chi H, Cao W, Zhang M, Su D, Yang H, Li Z, Li C, She X, Wang K, Gao X, Ma K, Zheng P, Li X, Cui B (2021) Environmental noise stress disturbs commensal microbiota homeostasis and induces oxi-inflammmation and AD-like neuropathology through epithelial barrier disruption in the EOAD mouse model. J Neuroinflammation 18:9. https://doi.org/10.1186/s12974-020-02053-3
Chomyk AM, Volsko C, Tripathi A, Deckard SA, Trapp BD, Fox RJ, Dutta R (2017) DNA methylation in demyelinated multiple sclerosis hippocampus. Sci Rep 7:8696. https://doi.org/10.1038/s41598-017-08623-5
Ciccocioppo F, Bologna G, Ercolino E, Pierdomenico L, Simeone P, Lanuti P, Pieragostino D, Del Boccio P, Marchisio M, Miscia S (2020) Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen Res 15:850–856. https://doi.org/10.4103/1673-5374.268971
Couttas TA, Kain N, Suchowerska AK, Quek L-E, Turner N, Fath T, Garner B, Don AS (2016) Loss of ceramide synthase 2 activity, necessary for myelin biosynthesis, precedes tau pathology in the cortical pathogenesis of Alzheimer’s disease. Neurobiol Aging 43:89–100. https://doi.org/10.1016/j.neurobiolaging.2016.03.027
Das R, Chinnathambi S (2019) Microglial priming of antigen presentation and adaptive stimulation in Alzheimer’s disease. Cell Mol Life Sci CMLS 76:3681–3694. https://doi.org/10.1007/s00018-019-03132-2
Dayon L, Núñez Galindo A, Wojcik J, Cominetti O, Corthésy J, Oikonomidi A, Henry H, Kussmann M, Migliavacca E, Severin I, Bowman GL, Popp J (2018) Alzheimer disease pathology and the cerebrospinal fluid proteome. Alzheimers Res Ther 10:66. https://doi.org/10.1186/s13195-018-0397-4
de Faria O, Pivonkova H, Varga B, Timmler S, Evans KA, Káradóttir RT (2021) Periods of synchronized myelin changes shape brain function and plasticity. Nat Neurosci 24:1508–1521. https://doi.org/10.1038/s41593-021-00917-2
de la Fuente AG, Queiroz RML, Ghosh T, McMurran CE, Cubillos JF, Bergles DE, Fitzgerald DC, Jones CA, Lilley KS, Glover CP, Franklin RJM (2020) Changes in the Oligodendrocyte Progenitor Cell Proteome with Ageing. Mol Cell Proteomics MCP 19:1281–1302. https://doi.org/10.1074/mcp.RA120.002102
De Rossi P, Buggia-Prévot V, Clayton BLL, Vasquez JB, van Sanford C, Andrew RJ, Lesnick R, Botté A, Deyts C, Salem S, Rao E, Rice RC, Parent A, Kar S, Popko B, Pytel P, Estus S, Thinakaran G (2016) Predominant expression of Alzheimer’s disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Mol Neurodegener 11:59. https://doi.org/10.1186/s13024-016-0124-1
Dean DC, Hurley SA, Kecskemeti SR, O’Grady JP, Canda C, Davenport-Sis NJ, Carlsson CM, Zetterberg H, Blennow K, Asthana S, Sager MA, Johnson SC, Alexander AL, Bendlin BB (2017) Association of amyloid Pathology with myelin alteration in preclinical Alzheimer Disease. JAMA Neurol 74:41–49. https://doi.org/10.1001/jamaneurol.2016.3232
Decourt B, Boumelhem F, Pope ED, Shi J, Mari Z, Sabbagh MN (2021) Critical Appraisal of amyloid lowering agents in AD. Curr Neurol Neurosci Rep 21:39. https://doi.org/10.1007/s11910-021-01125-y
DeFlitch L, Gonzalez-Fernandez E, Crawley I, Kang SH (2022) Age and Alzheimer’s Disease-Related oligodendrocyte changes in hippocampal subregions. Front Cell Neurosci 16:847097. https://doi.org/10.3389/fncel.2022.847097
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32. https://doi.org/10.1186/s13024-019-0333-5
Di Domenico F, Pupo G, Giraldo E, Lloret A, Badia M-C, Schinina ME, Giorgi A, Butterfield DA, Vina J, Perluigi M (2016) Autoantibodies Profile in matching CSF and serum from AD and aMCI patients: potential pathogenic role and link to oxidative damage. Curr Alzheimer Res 13:112–122. https://doi.org/10.2174/1567205013666151218131424
Di Fede G, Giaccone G, Tagliavini F (2013) Hereditary and sporadic beta-amyloidoses. Front Biosci Landmark Ed 18:1202–1226. https://doi.org/10.2741/4173
Di Paolo G, Kim T-W (2011) Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 12:284–296. https://doi.org/10.1038/nrn3012
Edwards LJ, Kirilina E, Mohammadi S, Weiskopf N (2018) Microstructural imaging of human neocortex in vivo. NeuroImage 182:184–206. https://doi.org/10.1016/j.neuroimage.2018.02.055
Eiser AR, Fulop T (2020) Extra-cranial factors in the development of Alzheimer’s disease. Brain Res 1748:147076. https://doi.org/10.1016/j.brainres.2020.147076
Ettle B, Schlachetzki JCM, Winkler J (2016) Oligodendroglia and myelin in neurodegenerative diseases: more than just bystanders? Mol Neurobiol 53:3046–3062. https://doi.org/10.1007/s12035-015-9205-3
Faizy TD, Thaler C, Broocks G, Flottmann F, Leischner H, Kniep H, Nawabi J, Schön G, Stellmann J-P, Kemmling A, Reddy R, Heit JJ, Fiehler J, Kumar D, Hanning U (2020) The myelin Water Fraction serves as a marker for age-related myelin alterations in the cerebral White Matter – A Multiparametric MRI Aging Study. Front Neurosci 14:136. https://doi.org/10.3389/fnins.2020.00136
Falangola MF, Nie X, Ward R, McKinnon ET, Dhiman S, Nietert PJ, Helpern JA, Jensen JH (2020) Diffusion MRI detects early brain microstructure abnormalities in 2-month-old 3×Tg-AD mice. NMR Biomed 33:e4346. https://doi.org/10.1002/nbm.4346
Farah MH, Pan BH, Hoffman PN, Ferraris D, Tsukamoto T, Nguyen T, Wong PC, Price DL, Slusher BS, Griffin JW (2011) Reduced BACE1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system. J Neurosci Off J Soc Neurosci 31:5744–5754. https://doi.org/10.1523/JNEUROSCI.6810-10.2011
Feng Y-S, Tan Z-X, Wu L-Y, Dong F, Zhang F (2020) The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res Rev 64:101192. https://doi.org/10.1016/j.arr.2020.101192
Fernandez-Alvarez M, Atienza M, Zallo F, Matute C, Capetillo-Zarate E, Cantero JL (2022) Linking plasma amyloid Beta and neurofilament light chain to intracortical myelin content in cognitively normal older adults. Front Aging Neurosci 14:896848. https://doi.org/10.3389/fnagi.2022.896848
Fernandez-Alvarez M, Atienza M, Cantero JL (2022) Effects of non-modifiable risk factors of Alzheimer’s disease on intracortical myelin content. Alzheimers Res Ther 14:202. https://doi.org/10.1186/s13195-022-01152-y
Ferreira S, Pitman KA, Wang S, Summers BS, Bye N, Young KM, Cullen CL (2020) Amyloidosis is associated with thicker myelin and increased oligodendrogenesis in the adult mouse brain. J Neurosci Res 98:1905–1932. https://doi.org/10.1002/jnr.24672
Ferrer I, Andrés-Benito P (2020) White matter alterations in Alzheimer’s disease without concomitant pathologies. Neuropathol Appl Neurobiol 46:654–672. https://doi.org/10.1111/nan.12618
Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116:2093–2104. https://doi.org/10.1152/jn.00221.2016
Filley CM (2021) Cognitive dysfunction in White Matter Disorders: New Perspectives in Treatment and Recovery. J Neuropsychiatry Clin Neurosci 33:349–355. https://doi.org/10.1176/appi.neuropsych.21030080
Fontenas L, Welsh TG, Piller M, Coughenour P, Gandhi AV, Prober DA, Kucenas S (2019) The Neuromodulator Adenosine regulates Oligodendrocyte Migration at Motor Exit Point transition zones. Cell Rep 27:115–128e5. https://doi.org/10.1016/j.celrep.2019.03.013
Fotiadis P, Reijmer YD, Van Veluw SJ, Martinez-Ramirez S, Karahanoglu FI, Gokcal E, Schwab KM, Alzheimer’s Disease Neuroimaging Initiative study group, Goldstein JN, Rosand J, Viswanathan A, Greenberg SM, Gurol ME (2020) White matter atrophy in cerebral amyloid angiopathy. Neurology 95:e554–e562. https://doi.org/10.1212/WNL.0000000000010017
Friedland RP, McMillan JD, Kurlawala Z (2020) What are the Molecular Mechanisms by which functional bacterial amyloids influence amyloid Beta deposition and neuroinflammation in neurodegenerative Disorders? Int J Mol Sci 21:1652. https://doi.org/10.3390/ijms21051652
Frisoni GB, Altomare D, Thal DR, Ribaldi F, van der Kant R, Ossenkoppele R, Blennow K, Cummings J, van Duijn C, Nilsson PM, Dietrich P-Y, Scheltens P, Dubois B (2022) The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci 23:53–66. https://doi.org/10.1038/s41583-021-00533-w
Ge W, Cheng Y, Zhang S, Ye S, Chen J, Wang R, Li C (2000) Variation of myelin basic protein and its antibody in serum in senile dementia patients. Chin Med Sci J Chung-Kuo Hsueh Ko Hsueh Tsa Chih 15:28
Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349:704–706. https://doi.org/10.1038/349704a0
Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJG (2008) Heterogeneity of white matter hyperintensities in Alzheimer’s disease: post-mortem quantitative MRI and neuropathology. Brain J Neurol 131:3286–3298. https://doi.org/10.1093/brain/awn265
Griciuc A, Tanzi RE (2021) The role of innate immune genes in Alzheimer’s disease. Curr Opin Neurol 34:228–236. https://doi.org/10.1097/WCO.0000000000000911
Griggs RB, Yermakov LM, Susuki K (2017) Formation and disruption of functional domains in myelinated CNS axons. Neurosci Res 116:77–87. https://doi.org/10.1016/j.neures.2016.09.010
Gu L, Wu D, Tang X, Qi X, Li X, Bai F, Chen X, Ren Q, Zhang Z (2018) Myelin changes at the early stage of 5XFAD mice. Brain Res Bull 137:285–293. https://doi.org/10.1016/j.brainresbull.2017.12.013
Hamilton NB, Clarke LE, Arancibia-Carcamo IL, Kougioumtzidou E, Matthey M, Káradóttir R, Whiteley L, Bergersen LH, Richardson WD, Attwell D (2017) Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65:309–321. https://doi.org/10.1002/glia.23093
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12:383–388. https://doi.org/10.1016/0165-6147(91)90609-v
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. https://doi.org/10.1126/science.1072994
Heilig M, Sjögren M, Blennow K, Ekman R, Wallin A (1995) Cerebrospinal fluid neuropeptides in Alzheimer’s disease and vascular dementia. Biol Psychiatry 38:210–216. https://doi.org/10.1016/0006-3223(94)00239-Y
Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF (2016) Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6:e774. https://doi.org/10.1038/tp.2016.42
Holubiec MI, Gellert M, Hanschmann EM (2022) Redox signaling and metabolism in Alzheimer’s disease. Front Aging Neurosci 14:1003721. https://doi.org/10.3389/fnagi.2022.1003721
Honarpisheh P, Reynolds CR, Blasco Conesa MP, Moruno Manchon JF, Putluri N, Bhattacharjee MB, Urayama A, McCullough LD, Ganesh BP (2020) Dysregulated gut homeostasis observed prior to the Accumulation of the Brain Amyloid-β in Tg2576 mice. Int J Mol Sci 21:1711. https://doi.org/10.3390/ijms21051711
Horiuchi M, Maezawa I, Itoh A, Wakayama K, Jin L-W, Itoh T, Decarli C (2012) Amyloid β1–42 oligomer inhibits myelin sheet formation in vitro. Neurobiol Aging 33:499–509. https://doi.org/10.1016/j.neurobiolaging.2010.05.007
Hou Y, Song H, Croteau DL, Akbari M, Bohr VA (2017) Genome instability in Alzheimer disease. Mech Ageing Dev 161:83–94. https://doi.org/10.1016/j.mad.2016.04.005
Hu J-G, Wu X-J, Feng Y-F, Xi G-M, Wang Z-H, Zhou J-S, Lü H-Z (2012) PDGF-AA and bFGF mediate B104CM-induced proliferation of oligodendrocyte precursor cells. Int J Mol Med 30:1113–1118. https://doi.org/10.3892/ijmm.2012.1110
Ihara M, Polvikoski TM, Hall R, Slade JY, Perry RH, Oakley AE, Englund E, O’Brien JT, Ince PG, Kalaria RN (2010) Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol (Berl) 119:579–589. https://doi.org/10.1007/s00401-009-0635-8
Iordanishvili E, Schall M, Loução R, Zimmermann M, Kotetishvili K, Shah NJ, Oros-Peusquens A-M (2019) Quantitative MRI of cerebral white matter hyperintensities: a new approach towards understanding the underlying pathology. NeuroImage 202:116077. https://doi.org/10.1016/j.neuroimage.2019.116077
Irwin RW, Wang JM, Chen S, Brinton RD (2011) Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Front Endocrinol 2:117. https://doi.org/10.3389/fendo.2011.00117
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, Sealock J, Karlsson IK, Hägg S, Athanasiu L, Voyle N, Proitsi P, Witoelar A, Stringer S, Aarsland D, Almdahl IS, Andersen F, Bergh S, Bettella F, Bjornsson S, Brækhus A, Bråthen G, de Leeuw C, Desikan RS, Djurovic S, Dumitrescu L, Fladby T, Hohman TJ, Jonsson PV, Kiddle SJ, Rongve A, Saltvedt I, Sando SB, Selbæk G, Shoai M, Skene NG, Snaedal J, Stordal E, Ulstein ID, Wang Y, White LR, Hardy J, Hjerling-Leffler J, Sullivan PF, van der Flier WM, Dobson R, Davis LK, Stefansson H, Stefansson K, Pedersen NL, Ripke S, Andreassen OA, Posthuma D (2019) Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 51:404–413. https://doi.org/10.1038/s41588-018-0311-9
Jellinger KA (2020) Neuropathological assessment of the Alzheimer spectrum. J Neural Transm Vienna Austria 1996 127:1229–1256. doi: https://doi.org/10.1007/s00702-020-02232-9
Jean Harry G, Toews AD (1998) Myelination, dysmyelination, and demyelination. Handb Dev Neurotoxicology. Handb Dev Neurotoxicology 87–115. https://doi.org/10.1016/B978-012648860-9.50007-8
Jin L, Pan Y, Tran NLL, Polychronopoulos LN, Warrier A, Brouwer KLR, Nicolazzo JA (2020) Intestinal permeability and oral absorption of selected drugs are reduced in a mouse model of familial Alzheimer’s Disease. Mol Pharm 17:1527–1537. https://doi.org/10.1021/acs.molpharmaceut.9b01227
Johnson LA (2020) APOE and metabolic dysfunction in Alzheimer’s disease. Int Rev Neurobiol 154:131–151. https://doi.org/10.1016/bs.irn.2020.02.002
Kaeser GE, Chun J (2020) Mosaic somatic gene recombination as a potentially unifying hypothesis for Alzheimer’s Disease. Front Genet 11:390. https://doi.org/10.3389/fgene.2020.00390
Kao Y-C, Ho P-C, Tu Y-K, Jou I-M, Tsai K-J (2020) Lipids and Alzheimer’s Disease. Int J Mol Sci 21:1505. https://doi.org/10.3390/ijms21041505
Kavroulakis E, Simos PG, Kalaitzakis G, Maris TG, Karageorgou D, Zaganas I, Panagiotakis S, Basta M, Vgontzas A, Papadaki E (2018) Myelin content changes in probable Alzheimer’s disease and mild cognitive impairment: Associations with age and severity of neuropsychiatric impairment. J Magn Reson Imaging JMRI 47:1359–1372. https://doi.org/10.1002/jmri.25849
Kaya I, Jennische E, Lange S, Tarik Baykal A, Malmberg P, Fletcher JS (2020) Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer’s disease mice. J Neurochem 154:84–98. https://doi.org/10.1111/jnc.14999
Keogh CE, Kim DHJ, Pusceddu MM, Knotts TA, Rabasa G, Sladek JA, Hsieh MT, Honeycutt M, Brust-Mascher I, Barboza M, Gareau MG (2021) Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav Immun 91:437–450. https://doi.org/10.1016/j.bbi.2020.11.001
Kepchia D, Huang L, Dargusch R, Rissman RA, Shokhirev MN, Fischer W, Schubert D (2020) Diverse proteins aggregate in mild cognitive impairment and Alzheimer’s disease brain. Alzheimers Res Ther 12:75. https://doi.org/10.1186/s13195-020-00641-2
Kim D-K, Kim TH, Lee S-J (2016) Mechanisms of aging-related proteinopathies in Caenorhabditis elegans. Exp Mol Med 48:e263. https://doi.org/10.1038/emm.2016.109
Kobylarek D, Iwanowski P, Masolak D, Limphaibool N, Chou JT-T, Kozubski W (2020) A case report: co-occurrence of cerebral amyloid angiopathy and multiple sclerosis. Mult Scler Relat Disord 46:102517. https://doi.org/10.1016/j.msard.2020.102517
Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P (2007) Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology 68:927–931. https://doi.org/10.1212/01.wnl.0000257094.10655.9a
Krishnan KJ, Ratnaike TE, De Gruyter HLM, Jaros E, Turnbull DM (2012) Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer’s disease. Neurobiol Aging 33:2210–2214. https://doi.org/10.1016/j.neurobiolaging.2011.08.009
Kuhn S, Gritti L, Crooks D, Dombrowski Y (2019) Oligodendrocytes in development, myelin Generation and Beyond. Cells 8:1424. https://doi.org/10.3390/cells8111424
Kundu D, Prerna K, Chaurasia R, Bharty MK, Dubey VK (2020) Advances in protein misfolding, amyloidosis and its correlation with human diseases. 3 Biotech 10:193. https://doi.org/10.1007/s13205-020-2166-x
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ et al (2019) Alzheimer Disease Genetics Consortium (ADGC), European Alzheimer’s Disease Initiative (EADI), Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE), Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES), Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51:414–430. doi: https://doi.org/10.1038/s41588-019-0358-2
Laule C, Leung E, Lis DKB, Traboulsee AL, Paty DW, MacKay AL, Moore GRW (2006) Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler Houndmills Basingstoke Engl 12:747–753. https://doi.org/10.1177/1352458506070928
Lebel C, Deoni S (2018) The development of brain white matter microstructure. NeuroImage 182:207–218. https://doi.org/10.1016/j.neuroimage.2017.12.097
Lee J-T, Xu J, Lee J-M, Ku G, Han X, Yang D-I, Chen S, Hsu CY (2004) Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol 164:123–131. https://doi.org/10.1083/jcb.200307017
Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, Marcus DS, Fagan AM, Goate A et al (2016) Dominantly Inherited Alzheimer Network White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol 79:929–939. doi: https://doi.org/10.1002/ana.24647
Leidinger P, Backes C, Deutscher S, Schmitt K, Mueller SC, Frese K, Haas J, Ruprecht K, Paul F, Stähler C, Lang CJG, Meder B, Bartfai T, Meese E, Keller A (2013) A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol 14:R78. https://doi.org/10.1186/gb-2013-14-7-r78
Leszek J, Barreto GE, Gąsiorowski K, Koutsouraki E, Ávila-Rodrigues M, Aliev G (2016) Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of Brain Innate Immune System. CNS Neurol Disord Drug Targets 15:329–336. https://doi.org/10.2174/1871527315666160202125914
Li D, Zhang J, Liu Q (2022) Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci 45:401–414. https://doi.org/10.1016/j.tins.2022.01.002
Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, Zhao K, Tang D (2020) Contributions of DNA damage to Alzheimer’s Disease. Int J Mol Sci 21:1666. https://doi.org/10.3390/ijms21051666
Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–118. https://doi.org/10.1038/nrneurol.2012.263
Lodato MA, Walsh CA (2019) Genome aging: somatic mutation in the brain links age-related decline with disease and nominates pathogenic mechanisms. Hum Mol Genet 28:R197–R206. https://doi.org/10.1093/hmg/ddz191
Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X (2017) Aging of cerebral white matter. Ageing Res Rev 34:64–76. https://doi.org/10.1016/j.arr.2016.11.006
Liu P, Jia X-Z, Chen Y, Yu Y, Zhang K, Lin Y-J, Wang B-H, Peng G-P (2021) Gut microbiota interacts with intrinsic brain activity of patients with amnestic mild cognitive impairment. CNS Neurosci Ther 27:163–173. https://doi.org/10.1111/cns.13451
Long P, Corfas G (2014) Neuroscience. To learn is to myelinate. Science 346:298–299. https://doi.org/10.1126/science.1261127
Lorenzini L, Fernandez M, Baldassarro VA, Bighinati A, Giuliani A, Calzà L, Giardino L (2020) White Matter and Neuroprotection in Alzheimer’s dementia. Mol Basel Switz 25:503. https://doi.org/10.3390/molecules25030503
Luczynski P, Laule C, Hsiung G-YR, Moore GRW, Tremlett H (2019) Coexistence of multiple sclerosis and Alzheimer’s disease: a review. Mult Scler Relat Disord 27:232–238. https://doi.org/10.1016/j.msard.2018.10.109
Maarouf CL, Walker JE, Sue LI, Dugger BN, Beach TG, Serrano GE (2018) Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS ONE 13:e0203659. https://doi.org/10.1371/journal.pone.0203659
Maetzler W, Berg D, Synofzik M, Brockmann K, Godau J, Melms A, Gasser T, Hörnig S, Langkamp M (2011) Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J Alzheimers Dis JAD 26:171–179. https://doi.org/10.3233/JAD-2011-110221
Maitre M, Klein C, Patte-Mensah C, Mensah-Nyagan G (2020) Tryptophan metabolites modify brain Aβ peptide degradation: a role in Alzheimer’s disease? Prog Neurobiol 190. https://doi.org/10.1016/j.pneurobio.2020.101800
Malampati S, Song J-X, Chun-Kit Tong B, Nalluri A, Yang C-B, Wang Z, Gopalkrishnashetty Sreenivasmurthy S, Zhu Z, Liu J, Su C, Krishnamoorthi S, Iyaswamy A, Cheung K-H, Lu J-H, Li AM (2020) Targeting Aggrephagy for the treatment of Alzheimer’s Disease. Cells 9:311. https://doi.org/10.3390/cells9020311
Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ (2020) Braak Stage, cerebral amyloid Angiopathy, and Cognitive decline in early Alzheimer’s Disease. J Alzheimers Dis JAD 74:189–197. https://doi.org/10.3233/JAD-191151
Manocha GD, Floden AM, Miller NM, Smith AJ, Nagamoto-Combs K, Saito T, Saido TC, Combs CK (2019) Temporal progression of Alzheimer’s disease in brains and intestines of transgenic mice. Neurobiol Aging 81:166–176. https://doi.org/10.1016/j.neurobiolaging.2019.05.025
Marangon D, Boccazzi M, Lecca D, Fumagalli M (2020) Regulation of oligodendrocyte functions: targeting lipid metabolism and extracellular matrix for myelin repair. J Clin Med 9:470. https://doi.org/10.3390/jcm9020470
Martin LJ, Wong M, Hanaford A (2019) Neonatal brain Injury and genetic causes of adult-onset neurodegenerative disease in mice interact with Effects on Acute and late outcomes. Front Neurol 10:635. https://doi.org/10.3389/fneur.2019.00635
Masters SL, O’Neill LAJ (2011) Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med 17:276–282. https://doi.org/10.1016/j.molmed.2011.01.005
Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai L-H (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570:332–337. https://doi.org/10.1038/s41586-019-1195-2
McKenzie AT, Moyon S, Wang M, Katsyv I, Song W-M, Zhou X, Dammer EB, Duong DM, Aaker J, Zhao Y, Beckmann N et al (2017) Multiscale network modeling of oligodendrocytes reveals molecular components of myelin dysregulation in Alzheimer’s disease. Mol Neurodegener 12:82. https://doi.org/10.1186/s13024-017-0219-3
Mohd Murshid N, Aminullah Lubis F, Makpol S (2022) Epigenetic changes and its intervention in Age-Related neurodegenerative Diseases. Cell Mol Neurobiol 42:577–595. https://doi.org/10.1007/s10571-020-00979-z
Morabito S, Miyoshi E, Michael N, Shahin S, Martini AC, Head E, Silva J, Leavy K, Perez-Rosendahl M, Swarup V (2021) Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat Genet 53:1143–1155. https://doi.org/10.1038/s41588-021-00894-z
Morell P, Quarles RH (1999) Myelin Formation, Structure and Biochemistry. In: Basic Neurochemistry, 6th edition, 6th edition. Raven Press, New York, pp 70–93
Mortamais M, Reynes C, Brickman AM, Provenzano FA, Muraskin J, Portet F, Berr C, Touchon J, Bonafé A, le Bars E, Maller JJ, Meslin C, Sabatier R, Ritchie K, Artero S (2013) Spatial distribution of cerebral white matter lesions predicts progression to mild cognitive impairment and dementia. PLoS ONE 8:e56972. https://doi.org/10.1371/journal.pone.0056972
Mossad O, Blank T (2021) Getting on in Old Age: how the gut microbiota interferes with brain innate immunity. Front Cell Neurosci 15:698126. https://doi.org/10.3389/fncel.2021.698126
Mot AI, Depp C, Nave K-A (2018) An emerging role of dysfunctional axon-oligodendrocyte coupling in neurodegenerative diseases. Dialogues Clin Neurosci 20:283–292
Moyon S, Frawley R, Marechal D, Huang D, Marshall-Phelps KLH, Kegel L, Bøstrand SMK, Sadowski B, Jiang Y-H, Lyons DA, Möbius W, Casaccia P (2021) TET1-mediated DNA hydroxymethylation regulates adult remyelination in mice. Nat Commun 12:3359. https://doi.org/10.1038/s41467-021-23735-3
Mueller SG, Weiner MW (2009) Selective effect of age, apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus 19:558–564. https://doi.org/10.1002/hipo.20614
Murphy M, Dutton R, Koblar S, Cheema S, Bartlett P (1997) Cytokines which signal through the LIF receptor and their actions in the nervous system. Prog Neurobiol 52:355–378. https://doi.org/10.1016/s0301-0082(97)00020-8
Najm R, Jones EA, Huang Y (2019) Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer’s disease. Mol Neurodegener 14:24. https://doi.org/10.1186/s13024-019-0324-6
Nalbandian A, Llewellyn KJ, Gomez A, Walker N, Su H, Dunnigan A, Chwa M, Vesa J, Kenney MC, Kimonis VE (2015) In vitro studies in VCP-associated multisystem proteinopathy suggest altered mitochondrial bioenergetics. Mitochondrion 22:1–8. https://doi.org/10.1016/j.mito.2015.02.004
Nardini E, Hogan R, Flamier A, Bernier G (2021) Alzheimer’s disease: a tale of two diseases? Neural Regen Res 16:1958–1964. https://doi.org/10.4103/1673-5374.308070
Nasrabady SE, Rizvi B, Goldman JE, Brickman AM (2018) White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun 6:22. https://doi.org/10.1186/s40478-018-0515-3
Noor A, Zafar S, Zerr I (2021) Neurodegenerative Proteinopathies in the Proteoform Spectrum-Tools and Challenges. Int J Mol Sci 22:1085. https://doi.org/10.3390/ijms22031085
Nickel M, Gu C (2018) Regulation of Central Nervous System Myelination in higher brain functions. Neural Plast 2018:6436453. https://doi.org/10.1155/2018/6436453
Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, Neal JW, Holmes C, Boche D (2019) Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain J Neurol 142:2113–2126. https://doi.org/10.1093/brain/awz142
Novotny BC, Fernandez MV, Wang C, Budde JP, Bergmann K, Eteleeb AM, Bradley J, Webster C, Ebl C, Norton J, Gentsch J, Dube U, Wang F, Morris JC, Bateman RJ, Perrin RJ, McDade E, Xiong C, Chhatwal J, Dominantly Inherited Alzheimer Network (DIAN) Study Group, Alzheimer’s Disease Neuroimaging Initiative, and the Alzheimer’s Disease Metabolomics Consortium (ADMC), Goate A, Farlow M, Schofield P, Chui H, Karch CM, Cruchaga C, Benitez BA, Harari O (2022) Metabolomic and lipidomic signatures in autosomal dominant and late-onset Alzheimer’s disease brains. Alzheimers Dement J Alzheimers Assoc. doi: https://doi.org/10.1002/alz.12800
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci Off J Soc Neurosci 26:10129–10140. https://doi.org/10.1523/JNEUROSCI.1202-06.2006
Ogunmokun G, Dewanjee S, Chakraborty P, Valupadas C, Chaudhary A, Kolli V, Anand U, Vallamkondu J, Goel P, Paluru HPR, Gill KD, Reddy PH, De Feo V, Kandimalla R (2021) The potential role of cytokines and growth factors in the pathogenesis of Alzheimer’s Disease. Cells 10:2790. https://doi.org/10.3390/cells10102790
Olsen I, Singhrao SK (2016) Inflammasome involvement in Alzheimer’s Disease. J Alzheimers Dis JAD 54:45–53. https://doi.org/10.3233/JAD-160197
Operto G, Cacciaglia R, Grau-Rivera O, Falcon C, Brugulat-Serrat A, Ródenas P, Ramos R, Morán S, Esteller M, Bargalló N, Molinuevo JL, Gispert JD, ALFA Study (2018) White matter microstructure is altered in cognitively normal middle-aged APOE-ε4 homozygotes. Alzheimers Res Ther 10:48. https://doi.org/10.1186/s13195-018-0375-x
Operto G, Molinuevo JL, Cacciaglia R, Falcon C, Brugulat-Serrat A, Suárez-Calvet M, Grau-Rivera O, Bargalló N, Morán S, Esteller M, Study ALFA, Gispert JD (2019) Interactive effect of age and APOE-ε4 allele load on white matter myelin content in cognitively normal middle-aged subjects. NeuroImage Clin 24:101983. https://doi.org/10.1016/j.nicl.2019.101983
Ota K, Oishi N, Ito K, Fukuyama H, Study Group SEAD-J, Alzheimer’s Disease Neuroimaging Initiative (2016) Prediction of Alzheimer’s disease in amnestic mild cognitive impairment subtypes: Stratification based on imaging biomarkers. J Alzheimers Dis JAD 52:1385–1401. https://doi.org/10.3233/JAD-160145
Ota M, Sato N, Kimura Y, Shigemoto Y, Kunugi H, Matsuda H (2019) Changes of myelin Organization in patients with Alzheimer’s Disease shown by q-Space myelin map imaging. Dement Geriatr Cogn Disord Extra 9:24–33. https://doi.org/10.1159/000493937
Othman T, Yan H, Rivkees SA (2003) Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia 44:166–172. https://doi.org/10.1002/glia.10281
Otto G (2021) Myelin loss in AD. Nat Rev Neurosci 22:456–457. https://doi.org/10.1038/s41583-021-00492-2
Pace MC, Xu G, Fromholt S, Howard J, Crosby K, Giasson BI, Lewis J, Borchelt DR (2018) Changes in proteome solubility indicate widespread proteostatic disruption in mouse models of neurodegenerative disease. Acta Neuropathol (Berl) 136:919–938. https://doi.org/10.1007/s00401-018-1895-y
Papuć E, Kurys-Denis E, Krupski W, Tatara M, Rejdak K (2015) Can antibodies against glial derived antigens be early biomarkers of hippocampal demyelination and memory loss in Alzheimer’s Disease? J Alzheimers Dis JAD 48:115–121. https://doi.org/10.3233/JAD-150309
Papuć E, Rejdak K (2020) The role of myelin damage in Alzheimer’s disease pathology. Arch Med Sci AMS 16:345–351. https://doi.org/10.5114/aoms.2018.76863
Pedrero-Prieto CM, Frontiñán-Rubio J, Alcaín FJ, Durán-Prado M, Peinado JR, Rabanal-Ruiz Y (2021) Biological significance of the protein changes occurring in the Cerebrospinal Fluid of Alzheimer’s Disease Patients: getting clues from Proteomic Studies. Diagn Basel Switz 11:1655. https://doi.org/10.3390/diagnostics11091655
Piersson AD, Ibrahim B, Suppiah S, Mohamad M, Hassan HA, Omar NF, Ibrahim MI, Yusoff AN, Ibrahim N, Saripan MI, Razali RM (2021) Multiparametric MRI for the improved diagnostic accuracy of Alzheimer’s disease and mild cognitive impairment: Research protocol of a case-control study design. PLoS ONE 16:e0252883. https://doi.org/10.1371/journal.pone.0252883
Poggi G, Boretius S, Möbius W, Moschny N, Baudewig J, Ruhwedel T, Hassouna I, Wieser GL, Werner HB, Goebbels S, Nave K-A, Ehrenreich H (2016) Cortical network dysfunction caused by a subtle defect of myelination. Glia 64:2025–2040. https://doi.org/10.1002/glia.23039
Polychronidou E, Avramouli A, Vlamos P (2020) Alzheimer’s Disease: the role of mutations in protein folding. Adv Exp Med Biol 1195:227–236. https://doi.org/10.1007/978-3-030-32633-3_31
Pruvost M, Moyon S (2021) Oligodendroglial epigenetics, from lineage specification to activity-dependent myelination. Life Basel Switz 11:62. https://doi.org/10.3390/life11010062
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344. https://doi.org/10.1056/NEJMra0909142
Resende R, Ferreiro E, Pereira C, Resende de Oliveira C (2008) Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1–42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience 155:725–737. https://doi.org/10.1016/j.neuroscience.2008.06.036
Rutsch A, Kantsjö JB, Ronchi F (2020) The gut-brain Axis: how microbiota and host Inflammasome Influence Brain Physiology and Pathology. Front Immunol 11:604179. https://doi.org/10.3389/fimmu.2020.604179
Saez-Atienzar S, Masliah E (2020) Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat Rev Neurosci 21:433–444. https://doi.org/10.1038/s41583-020-0325-z
Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, Schifferer M, Gouna G, Usifo F, Kannaiyan N, Fitzner D, Xiang X, Rossner MJ, Brendel M, Gokce O, Simons M (2021) White matter aging drives microglial diversity. Neuron 109:1100–1117e10. https://doi.org/10.1016/j.neuron.2021.01.027
Sams EC (2021) Oligodendrocytes in the aging brain. Neuronal Signal 5:NS20210008. https://doi.org/10.1042/NS20210008
Samudyata null, Castelo-Branco G, Liu J (2020) Epigenetic regulation of oligodendrocyte differentiation: from development to demyelinating disorders. Glia 68:1619–1630. https://doi.org/10.1002/glia.23820
Sancho L, Contreras M, Allen NJ (2021) Glia as sculptors of synaptic plasticity. Neurosci Res 167:17–29. https://doi.org/10.1016/j.neures.2020.11.005
Savonije K, Weaver DF (2023) The role of Tryptophan Metabolism in Alzheimer’s Disease. Brain Sci 13:292. https://doi.org/10.3390/brainsci13020292
Sanguinetti E, Collado MC, Marrachelli VG, Monleon D, Selma-Royo M, Pardo-Tendero MM, Burchielli S, Iozzo P (2018) Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci Rep 8:4907. https://doi.org/10.1038/s41598-018-23261-1
Santos LRD, Almeida JFF, Pimassoni LHS, Morelato RL, de Paula F (2020) The combined risk effect among BIN1, CLU, and APOE genes in Alzheimer’s disease. Genet Mol Biol 43:e20180320. https://doi.org/10.1590/1678-4685-GMB-2018-0320
Sathe G, Albert M, Darrow J, Saito A, Troncoso J, Pandey A, Moghekar A (2021) Quantitative proteomic analysis of the frontal cortex in Alzheimer’s disease. J Neurochem 156:988–1002. https://doi.org/10.1111/jnc.15116
Sciaccaluga M, Megaro A, Bellomo G, Ruffolo G, Romoli M, Palma E, Costa C (2021) An unbalanced synaptic transmission: cause or consequence of the amyloid oligomers neurotoxicity? Int J Mol Sci 22:5991. https://doi.org/10.3390/ijms22115991
Serrano-Regal MP, Luengas-Escuza I, Bayón-Cordero L, Ibarra-Aizpurua N, Alberdi E, Pérez-Samartín A, Matute C, Sánchez-Gómez MV (2020) Oligodendrocyte differentiation and myelination is potentiated via GABAB receptor activation. Neuroscience 439:163–180. https://doi.org/10.1016/j.neuroscience.2019.07.014
Sheng C, Lin L, Lin H, Wang X, Han Y, Liu S-L (2021) Altered gut microbiota in adults with subjective cognitive decline: the SILCODE Study. J Alzheimers Dis JAD 82:513–526. https://doi.org/10.3233/JAD-210259
Shukla PK, Delotterie DF, Xiao J, Pierre JF, Rao R, McDonald MP, Khan MM (2021) Alterations in the gut-microbial-inflammasome-brain Axis in a mouse model of Alzheimer’s Disease. Cells 10:779. https://doi.org/10.3390/cells10040779
Simons M, Trajkovic K (2006) Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci 119:4381–4389. https://doi.org/10.1242/jcs.03242
Sinclair P, Baranova A, Kabbani N (2021) Mitochondrial disruption by amyloid Beta 42 identified by Proteomics and Pathway Mapping. Cells 10:2380. https://doi.org/10.3390/cells10092380
Singh DK, Ling E-A, Kaur C (2018) Hypoxia and myelination deficits in the developing brain. Int J Dev Neurosci Off J Int Soc Dev Neurosci 70:3–11. https://doi.org/10.1016/j.ijdevneu.2018.06.012
Smith DH, Nakamura M, McIntosh TK, Wang J, Rodríguez A, Chen XH, Raghupathi R, Saatman KE, Clemens J, Schmidt ML, Lee VM, Trojanowski JQ (1998) Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol 153:1005–1010. https://doi.org/10.1016/s0002-9440(10)65643-x
Sock E, Wegner M (2019) Transcriptional control of myelination and remyelination. Glia 67:2153–2165. https://doi.org/10.1002/glia.23636
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21:1332–1340. https://doi.org/10.1038/s41593-018-0235-9
Stadelmann C, Timmler S, Barrantes-Freer A, Simons M (2019) Myelin in the Central Nervous System: structure, function, and Pathology. Physiol Rev 99:1381–1431. https://doi.org/10.1152/physrev.00031.2018
Stephenson J, Nutma E, van der Valk P, Amor S (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154:204–219. https://doi.org/10.1111/imm.12922
Stevens B, Porta S, Haak LL, Gallo V, Fields RD (2002) Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36:855–868. https://doi.org/10.1016/s0896-6273(02)01067-x
Stojić-Vukanić Z, Hadžibegović S, Nicole O, Nacka-Aleksić M, Leštarević S, Leposavić G (2020) CD8 + T cell-mediated mechanisms contribute to the progression of neurocognitive impairment in both multiple sclerosis and Alzheimer’s Disease? Front Immunol 11:566225. https://doi.org/10.3389/fimmu.2020.566225
Stopińska K, Radziwoń-Zaleska M, Domitrz I (2021) The Microbiota-Gut-Brain Axis as a Key to Neuropsychiatric Disorders: a Mini Review. J Clin Med 10:4640. https://doi.org/10.3390/jcm10204640
Sukhorukov VS, Mudzhiri NM, Voronkova AS, Baranich TI, Glinkina VV, Illarioshkin SN (2021) Mitochondrial Disorders in Alzheimer’s Disease. Biochem Biokhimiia 86:667–679. https://doi.org/10.1134/S0006297921060055
Sulehria T, Corbett AM, Sharma N, Nagarajan D, Abushamma A, Gagle S, Johnson A (2020) Increasing Progenitor Cell Proliferation in the Sub-Ventricular Zone: a therapeutic treatment for progressive multiple sclerosis? Recent Pat Drug Deliv Formul 14:233–241. https://doi.org/10.2174/1872211314999201117130123
Tamagno E, Bardini P, Guglielmotto M, Danni O, Tabaton M (2006) The various aggregation states of beta-amyloid 1–42 mediate different effects on oxidative stress, neurodegeneration, and BACE-1 expression. Free Radic Biol Med 41:202–212. https://doi.org/10.1016/j.freeradbiomed.2006.01.021
Tang W, Meng Z, Li N, Liu Y, Li L, Chen D, Yang Y (2020) Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and Hippocampus-Dependent Behaviors. Front Cell Infect Microbiol 10:611014. https://doi.org/10.3389/fcimb.2020.611014
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67:1014–1022. https://doi.org/10.1046/j.1471-4159.1996.67031014.x
Ticinesi A, Tana C, Nouvenne A (2019) The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care 22:4–12. https://doi.org/10.1097/MCO.0000000000000521
Timmler S, Simons M (2019) Grey matter myelination. Glia 67:2063–2070. https://doi.org/10.1002/glia.23614
Tini G, Scagliola R, Monacelli F, La Malfa G, Porto I, Brunelli C, Rosa GM (2020) Alzheimer’s Disease and Cardiovascular Disease: a Particular Association. Cardiol Res Pract 2020:2617970. https://doi.org/10.1155/2020/2617970
van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L, Natté R (2017) Cortical Iron reflects severity of Alzheimer’s Disease. J Alzheimers Dis JAD 60:1533–1545. https://doi.org/10.3233/JAD-161143
Vassar R, Kuhn P-H, Haass C, Kennedy ME, Rajendran L, Wong PC, Lichtenthaler SF (2014) Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem 130:4–28. https://doi.org/10.1111/jnc.12715
Vaz M, Silvestre S (2020) Alzheimer’s disease: recent treatment strategies. Eur J Pharmacol 887:173554. https://doi.org/10.1016/j.ejphar.2020.173554
Vegas-Suárez S, Simón J, Martínez-Chantar ML, Moratalla R (2022) Metabolic diffusion in neuropathologies: the relevance of Brain-Liver Axis. Front Physiol 13:864263. https://doi.org/10.3389/fphys.2022.864263
Vos JP, Gard AL, Pfeiffer SE (1996) Regulation of oligodendrocyte cell survival and differentiation by ciliary neurotrophic factor, leukemia inhibitory factor, oncostatin M, and interleukin-6. Perspect Dev Neurobiol 4:39–52
Wang Z, Colognato H, Ffrench-Constant C (2007) Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia 55:537–545. https://doi.org/10.1002/glia.20480
Wang J, Gu BJ, Masters CL, Wang Y-J (2017) A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 13:612–623. https://doi.org/10.1038/nrneurol.2017.111
Wang Y-R, Wang Q-H, Zhang T, Liu Y-H, Yao X-Q, Zeng F, Li J, Zhou F-Y, Wang L, Yan J-C, Zhou H-D, Wang Y-J (2017) Associations between hepatic functions and plasma amyloid-Beta levels-implications for the capacity of liver in Peripheral amyloid-Beta clearance. Mol Neurobiol 54. https://doi.org/10.1007/s12035-016-9826-1
Wang S-S, Zhang Z, Zhu T-B, Chu S-F, He W-B, Chen N-H (2018) Myelin injury in the central nervous system and Alzheimer’s disease. Brain Res Bull 140:162–168. https://doi.org/10.1016/j.brainresbull.2018.05.003
Wang F, Ren S-Y, Chen J-F, Liu K, Li R-X, Li Z-F, Hu B, Niu J-Q, Xiao L, Chan JR, Mei F (2020) Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat Neurosci 23:481–486. https://doi.org/10.1038/s41593-020-0588-8
Wang X-L, Li L (2021) Cell type-specific potential pathogenic genes and functional pathways in Alzheimer’s Disease. BMC Neurol 21:381. https://doi.org/10.1186/s12883-021-02407-1
Wesenhagen KEJ, Teunissen CE, Visser PJ, Tijms BM (2020) Cerebrospinal fluid proteomics and biological heterogeneity in Alzheimer’s disease: a literature review. Crit Rev Clin Lab Sci 57:86–98. https://doi.org/10.1080/10408363.2019.1670613
Westi EW, Jakobsen E, Voss CM, Bak LK, Pinborg LH, Aldana BI, Andersen JV (2022) Divergent Cellular Energetics, Glutamate Metabolism, and mitochondrial function between human and mouse cerebral cortex. Mol Neurobiol 59:7495–7512. https://doi.org/10.1007/s12035-022-03053-5
Westwell-Roper C, Verchere CB (2019) Modulation of Innate immunity by amyloidogenic peptides. Trends Immunol 40:762–780. https://doi.org/10.1016/j.it.2019.06.005
Wiatrak B, Balon K, Jawień P, Bednarz D, Jęśkowiak I, Szeląg A (2022) The role of the Microbiota-Gut-Brain Axis in the development of Alzheimer’s Disease. Int J Mol Sci 23:4862. https://doi.org/10.3390/ijms23094862
Wirths O, Weis J, Kayed R, Saido TC, Bayer TA (2007) Age-dependent axonal degeneration in an Alzheimer mouse model. Neurobiol Aging 28:1689–1699. https://doi.org/10.1016/j.neurobiolaging.2006.07.021
Wissler Gerdes EO, Zhu Y, Weigand BM, Tripathi U, Burns TC, Tchkonia T, Kirkland JL (2020) Cellular senescence in aging and age-related diseases: implications for neurodegenerative diseases. Int Rev Neurobiol 155:203–234. https://doi.org/10.1016/bs.irn.2020.03.019
Wu Y, Ma Y, Liu Z, Geng Q, Chen Z, Zhang Y (2017) Alterations of myelin morphology and oligodendrocyte development in early stage of Alzheimer’s disease mouse model. Neurosci Lett 642:102–106. https://doi.org/10.1016/j.neulet.2017.02.007
Wu D, Tang X, Gu L-H, Li X-L, Qi X-Y, Bai F, Chen X-C, Wang J-Z, Ren Q-G, Zhang Z-J (2018) LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in the early stage of 5XFAD mice. CNS Neurosci Ther 24:381–393. https://doi.org/10.1111/cns.12809
Wu S, Liu X, Jiang R, Yan X, Ling Z (2021) Roles and mechanisms of gut microbiota in patients with Alzheimer’s Disease. Front Aging Neurosci 13:650047. https://doi.org/10.3389/fnagi.2021.650047
Wu HM, Goate AM, O’Reilly PF (2021) Heterogeneous effects of genetic risk for Alzheimer’s disease on the phenome. Transl Psychiatry 11:406. https://doi.org/10.1038/s41398-021-01518-0
Xia M, Cheng X, Yi R, Gao D, Xiong J (2016) The binding receptors of Aβ: an alternative therapeutic target for Alzheimer’s Disease. Mol Neurobiol 53:455–471. https://doi.org/10.1007/s12035-014-8994-0
Xia W, Fancy SPJ (2021) Mechanisms of oligodendrocyte progenitor developmental migration. Dev Neurobiol 81:985–996. https://doi.org/10.1002/dneu.22856
Xin W, Chan JR (2020) Myelin plasticity: sculpting circuits in learning and memory. Nat Rev Neurosci 21:682–694. https://doi.org/10.1038/s41583-020-00379-8
Xing YL, Röth PT, Stratton JAS, Chuang BHA, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD (2014) Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci Off J Soc Neurosci 34:14128–14146. https://doi.org/10.1523/JNEUROSCI.3491-13.2014
Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, Hsu CY (2001) Amyloid-beta peptides are cytotoxic to oligodendrocytes. J Neurosci Off J Soc Neurosci 21:RC118. https://doi.org/10.1523/JNEUROSCI.21-01-j0001.2001
Yokota M, Saido TC, Tani E, Yamaura I, Minami N (1996) Cytotoxic fragment of amyloid precursor protein accumulates in hippocampus after global forebrain ischemia. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 16:1219–1223. https://doi.org/10.1097/00004647-199611000-00016
Yoo S-W, Agarwal A, Smith MD, Khuder SS, Baxi EG, Thomas AG, Rojas C, Moniruzzaman M, Slusher BS, Bergles DE, Calabresi PA, Haughey NJ (2020) Inhibition of neutral sphingomyelinase 2 promotes remyelination. Sci Adv 6:eaba5210. https://doi.org/10.1126/sciadv.aba5210
Yoon S-S, Jo SA (2012) Mechanisms of Amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s Disease. Biomol Ther 20:245–255. https://doi.org/10.4062/biomolther.2012.20.3.245
You Y, Joseph C, Wang C, Gupta V, Liu S, Yiannikas C, Chua BE, Chitranshi N, Shen T, Dheer Y, Invernizzi A, Borotkanics R, Barnett M, Graham SL, Klistorner A (2019) Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain J Neurol 142:426–442. https://doi.org/10.1093/brain/awy338
Yu SP, Farhangrazi ZS, Ying HS, Yeh CH, Choi DW (1998) Enhancement of outward potassium current may participate in beta-amyloid peptide-induced cortical neuronal death. Neurobiol Dis 5:81–88. https://doi.org/10.1006/nbdi.1998.0186
Yu W, Gao D, Jin W, Wang Z, Li Y, Peng X, Cong Y, Li C, Zhao A, Liu S, Qi S (2020) Intestinal Flora Dysbiosis aggravates cognitive dysfunction Associated with Neuroinflammation in Heart failure. J Card Fail 26:885–894. https://doi.org/10.1016/j.cardfail.2020.02.002
Zendjabil M (2018) Circulating microRNAs as novel biomarkers of Alzheimer’s disease. Clin Chim Acta Int J Clin Chem 484:99–104. https://doi.org/10.1016/j.cca.2018.05.039
Zhan X, Jickling GC, Ander BP, Stamova B, Liu D, Kao PF, Zelin MA, Jin L-W, DeCarli C, Sharp FR (2015) Myelin Basic Protein Associates with AβPP, Aβ1–42, and amyloid plaques in cortex of Alzheimer’s Disease Brain. J Alzheimers Dis JAD 44:1213–1229. https://doi.org/10.3233/JAD-142013
Zhu X, Li B, Lou P, Dai T, Chen Y, Zhuge A, Yuan Y, Li L (2021) The Relationship between the gut microbiome and neurodegenerative Diseases. Neurosci Bull 37:1510–1522. https://doi.org/10.1007/s12264-021-00730-8
Zhang X, Sun Y, Li W, Liu B, Wu W, Zhao H, Liu R, Zhang Y, Yin Z, Yu T, Qing Z, Zhu B, Xu Y, Nedelska Z, Hort J, Zhang B, Alzheimer’s Disease Neuroimaging Initiative (2019) Characterization of white matter changes along fibers by automated fiber quantification in the early stages of Alzheimer’s disease. NeuroImage Clin 22:101723. https://doi.org/10.1016/j.nicl.2019.101723
Acknowledgements
This work was supported by recurring grants from the Institut National de la Santé et de la Recherche Médicale (INSERM, France, grant #U1119) and Université de Strasbourg, France (grant #UMR_S1119).
Funding
Funding provided by the University of Strasbourg and by the INSERM.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Michel Maître and Hélène Jeltsch-David, who performed the literature search and data analysis. Idea for the article: Michel Maitre and Ayikoe-Guy Mensah-Nyagan. Table and pictures design and editing: Christian Klein, Michel Maitre and Hélène Jeltsch-David. All authors commented on previous versions of the manuscript and have approved the manuscript for submission; the content of which is not submitted or published elsewhere. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Financial interests
The authors declare they have no financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maitre, M., Jeltsch-David, H., Okechukwu, N.G. et al. Myelin in Alzheimer’s disease: culprit or bystander?. acta neuropathol commun 11, 56 (2023). https://doi.org/10.1186/s40478-023-01554-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-023-01554-5