Abstract
Clinico-pathological correlation studies show that some otherwise healthy elderly individuals who never developed cognitive impairment harbor a burden of Alzheimer’s disease lesions (plaques and tangles) that would be expected to result in dementia. In the absence of comorbidities explaining such discrepancies, there is a need to identify other brain changes that meaningfully contribute to the cognitive status of an individual in the face of such burdens of plaques and tangles. Glial inflammatory responses, a universal phenomenon in symptomatic AD, show robust association with degree of cognitive impairment, but their significance in early tau pathology stages and contribution to the trajectory of cognitive decline at an individual level remain widely unexplored. We studied 55 brains from individuals at intermediate stages of tau tangle pathology (Braak III-IV) with diverging antemortem cognition (demented vs. non-demented, here termed `resilient’), and age-matched cognitively normal controls (Braak 0-II). We conducted quantitative assessments of amyloid and tau lesions, cellular vulnerability markers, and glial phenotypes in temporal pole (Braak III-IV region) and visual cortex (Braak V-VI region) using artificial-intelligence based semiautomated quantifications. We found distinct glial responses with increased proinflammatory and decreased homeostatic markers, both in regions with tau tangles (temporal pole) and without overt tau deposits (visual cortex) in demented but not in resilient. These changes were significantly associated with markers of cortical cell damage. Similar phenotypic glial changes were detected in the white matter of demented but not resilient and were associated with higher burden of overlying cortical cellular damage in regions with and without tangles. Our data suggest that changes in glial phenotypes in cortical and subcortical regions represent an early phenomenon that precedes overt tau deposition and likely contributes to cell damage and loss of brain function predicting the cognitive status of individuals at intermediate stages of tau aggregate burden (Braak III-IV).
Similar content being viewed by others
Introduction
The frequency of both amyloid plaques and neurofibrillary tangles (the classic neuropathologic hallmarks of AD) and dementia increases with age [14, 26, 72]. Yet, multiple clinicopathological correlation studies have shown that amyloid plaques and neurofibrillary tangles (NFTs) do not suffice to fully explain the extent of brain injury responses (e.g. neuronal and synaptic loss) observed in AD brains [3, 20, 28, 87] nor do inevitably lead to cognitive impairment in all individuals [18, 21, 73, 77]. In cohort studies of aging and dementia that include brain donation, about 5–7% of brains from older people without cognitive impairment demonstrate a high stage of tau tangle pathology (Braak V-VI), and up to 45% exhibit an intermediate stage (Braak III-IV) at postmortem (reviewed in [79]). In agreement with these observations, up to 30% of cognitively unimpaired elderly volunteers are amyloid-positive in PET imaging studies [1, 42, 54, 59, 75], and around 13% are tau-positive by PET as well [9]. Moreover, recent studies have highlighted that the vast majority of post-mortem brains of individuals 85 years of age and older show some degree of classic AD neuropathologic changes, which by large exceeds the expected antemortem prevalence of dementia in these and subsequent age groups which is cumulatively estimated to be at around 50% [10, 52, 53].The phenomenon of absence of overt cognitive deterioration in the face of neuropathologic lesions expected to have had a deleterious impact on cognition is the process we and others have termed 'resilience' to AD pathology [5, 68]. The discrepancy remains even after adjusting for the presence of other age-related common co-morbidities such as TDP-43 and alpha-synuclein aggregates or vascular lesions that also contribute to clinical disease expression [5, 68]. These observations from resilient brains suggest that additional mechanisms beyond plaques and tangles must be the real culprits driving the tissue injury responses (e.g. neuronal and synaptic loss) that ultimately result in brain functional changes and impaired cognition in AD, and may contribute to the wide inter-individual heterogeneity in the clinical expression of amyloid and tau containing lesions.
Detailed examination of high tangle burden (Braak V-VI) demented and resilient brains by our group and others has shown that one of the most robust and significant differences is the presence of substantially increased glial responses, comprising inflammatory microglia and astrocytes [56, 68], alongside a distinct proinflammatory cytokine expression profile in the demented [5]. Glial involvement in AD pathogenesis has only recently gained considerable interest fueled by genome-wide association studies (GWAS) that identified risk loci in genes related to the immune system in human [7, 30, 31, 34, 51, 98] along with novel insights of the unexpected involvement of microglia in synaptic pruning and modulation of synaptic activity in mouse models [35, 86, 96] and in the human AD brain [89]. Importantly, brain glial responses are present early and increase around amyloid plaques and tau tangles with progression of symptoms in clinically manifest AD [80].
Emerging studies are highlighting the broad heterogeneity in phenotypes of astrocytes and microglial cells in AD but the role of each of these glial phenotypes (e.g. pro-inflammatory and homeostatic) in the clinical expression of plaques and tangles and other common neurodegenerative lesions, and in the tissue injury responses (synaptic and neuronal damage) and trajectory of cognitive impairment are incompletely understood [4, 44, 78, 82, 83]. Some studies have suggested that increased proinflammatory glia in early mild cognitive impairment (MCI) stages may exert a beneficial role on preserving brain structure and function [20], while identical phenotypes in subsequent phases of the dementing disorder are associated with increased severity and faster rates of cognitive dysfunction [37, 57, 65]. This suggests that glial phenotypes may follow a non-linear trajectory and likely switch their function during disease progression [24]. Recent data in animal models of AD and human AD brains have pointed to the potentially even more relevant loss of homeostatic glial markers early in the disease process and to their correlation with the extent of synaptic loss [33, 62, 85, 91].
In addition, recent evidence from neuroimaging and autopsy studies has suggested that white matter (WM) damage comprising axonal loss, astrogliosis, and oligodendrocyte dysfunction accounts for one of the earliest pathologic changes that may be implicated in the risk and progression of AD [23, 62]. Early axonal dysfunction and WM lesions [49] have both been associated with a higher burden of overlying cortical AD lesions [58], and microstructural WM changes have been correlated with the degree of cognitive impairment in AD [17], with imaging studies indicating that significant loss of WM volume and axonal connectivity is present very early from MCI stages of the condition [48]. Moreover, in familial early-onset AD mutation carriers, functional and structural changes in the WM have been found 5–10 years prior to dementia onset and have been associated with increased pro-inflammatory microglial markers in these individuals [2]. Postmortem studies in sporadic late-onset AD have endorsed that inflammatory changes in WM occur early [70], and in vivo PET imaging studies suggest that microglial inflammation in WM is associated with higher burden of small vessel disease markers (WM hyperintensities) and cognitive impairment [55].
Whether an aberrant activation and/or lack of homeostatic properties of glial elements in cortical and juxtacortical WM regions substantially contribute to neuronal damage and loss of brain function in AD or reflect a mere response to neuronal injury remains controversial. The study of brains at intermediate stages of tau burden (Braak III-IV) with divergent ante-mortem cognitive status (demented vs. resilient) provides a valuable opportunity to assess early presence and interaction(s) between glial cell responses and brain changes other than Aβ and tau that occur in regions yet devoid of NFTs and that could be relevant to the earliest and most preventable phases of neurodegeneration in AD. We hypothesized that changes in cortical and subcortical glial phenotypes precede overt NFT formation and correlate with early markers of cortical cell damage predicting the cognitive fate of individuals at intermediate stages of tau burden (Braak III-IV).
Material and methods
Human brain samples
The study included 55 cases from the Massachusetts General Hospital ADRC Brain Bank. Cases were scored by Thal phases for Aβ deposition (0–5) [88], Braak stage for NFTs (0–VI) [13], and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) for neuritic plaques (A-C) [60], and divided into three groups: (1) non-demented individuals before death whose post-mortem exam demonstrated a Braak stage 0-II (controls) (n = 8); (2) non-demented individuals before death whose post-mortem examination demonstrated a Braak stage III-IV (resilient) (n = 20); and (3) demented individuals before death whose post-mortem examination demonstrated a Braak stage III-IV (demented) (n = 27) [13].
The majority of both demented and resilient brains in these series also harbored intermediate loads of amyloid neuritic plaques (moderate CERAD scores) meeting criteria for an intermediate probability of AD at autopsy according to NIA-AA guidelines [39]. A smaller subset of brains with tangle Braak stage III-IV and a CERAD neuritic plaque score `none’ but different cognitive outcomes (impaired vs. cognitively normal `resilient’) meeting criteria for primary age-related tauopathy (PART) [19] were also included in the demented and resilient group categories (n = 3 and 5, respectively). NFTs in PART are indistinguishable from those of AD, in the absence of amyloid beta (Aβ) plaques, and symptoms in these individuals usually range from normal to amnestic cognitive changes. In order to align with the Braak stage III-IV tangle burden of the cases with plaques, these cases of presumed PART had only mesial temporal lobe involvement. Of all cases, 24 (including 4 controls, 6 resilient and 14 demented) had undergone a formal cognitive evaluation within 2 years of death including measures of attention, processing speed, executive function, episodic memory and language, as part of their longitudinal enrollment in the Uniform Data Set (UDS) of the Alzheimer’s Disease Centers (ADC) program of the National Institute on Aging (NIA) [97]. The rest were individuals with either no reported dementia or dementia according to their clinical records and death certificates. Histological evaluation was performed using a set of regional tissue blocks representative of the spectrum of neurodegenerative diseases. All blocks were routinely stained with Luxol fast blue, haematoxylin, and eosin and selected blocks were stained for Bielschowsky silver stain and Aβ, phospho-TDP-43, alpha-synuclein, ubiquitin and phospho-tau immunoreactivity. Neuropathological diagnosis was recorded, and cases with evidence of cortical Lewy body pathology, phospho-TDP-43 aggregates, or other lesions different to classic AD pathology were excluded. The human demographics, neuropathological data and Mini-Mental State Exam (MMSE) scores of the cases prior to death are summarized in Table 1. The three groups were carefully matched for age, gender, co-morbidities, as well as additional systemic factors like terminal septicemia or prolonged hypoxia. Resilient and demented groups were matched for Thal phase, Braak stage, CERAD neuritic plaque score, ApoE genotype and postmortem intervals (PMIs). A composite vascular score was also calculated based on hypertensive cerebrovascular, atherosclerosis, occlusive atherosclerosis and cerebral amyloid scores as evaluated by neuropathologists on a rating scale from 0–3 (none, mild, moderate, severe) [94]. All autopsies were performed according to standardized protocols [93] and tissue collection and use was approved by the local Institutional Review Boards.
Semi-automated quantitative neuropathology using artificial intelligence-based analyses
Quantitative neuropathological assessments were derived from two regions of interest, temporal pole (Brodmann’s area 38) and visual cortex (Brodmann’s area 17/18), representing regions known to be affected by NFT pathology at Braak stages III-IV and V-VI respectively. Brain tissue sections derived from formalin-fixed paraffin-embedded tissue blocks were cut at a thickness of 7 µm. Immunohistochemical staining was conducted using a Bond RX autostainer from Leica Biosystems. We used primary antibodies to label Aβ deposits (4G8, Bio Legend, 1:8000), phospho-tau (AT8, Thermo Fisher, 1:500), phospho-TDP43 (Fisher Scientific, 1:3000), alpha-synuclein (Fisher Scientific, 1:200), cellular DNA damage (γH2AX, Abcam, 1:1000), constitutive and inflammatory astrocytic markers (ALDH1L1, Abcam, 1:200; GFAP, Sigma Aldrich, 1:20,000), constitutive oligodendrocyte markers (Olig2, Abcam, 1:100), and constitutive, inflammatory and homeostatic microglial markers (IBA1, Abcam, 1:500; CD68, Dako, 1:500; HLA-DR, Abcam, 1:50; TMEM119, Sigma Aldrich, 1:50; P2RY12, Fisher Scientific, 1:100) followed by secondary anti-mouse and anti-rabbit horseradish peroxidase (HRP) antibody conjugation (BOND Polymer Refine Detection, catalog number DS9800, Leica Biosystems Newcastle, UK) following the Leica autostainer protocol. Immunolabeling was visualized using 3,3'-diaminobenzidine (DAB) and slides were counterstained with the nuclear marker haematoxylin using the Leica Biosystems autostainer protocol. Slides were manually dehydrated, cover-slipped using Permount mounting medium and scanned with a Nanozoomer XR (NDP.scan 3.3.3, Hamamatsu Photonics, Hertfordhsire, UK) using a 20X objective.
To quantify positively labelled cells and lesions, we used an artificial intelligence (AI) based semi-automated quantification method (Aiforia version 5.2, Aiforia Inc, Cambridge, MA), which allowed us to train 2-dimensional AI models, perform human-based validation(s) and apply individual AI models for accurate cell and lesion quantification(s) in predetermined regions of interest. To this end, scanned slides were uploaded to the Aiforia cloud-based platform and stored in folders. Two regions of interest (ROIs) were selected for each brain slide in cortical and adjacent subcortical brain regions respectively. Cortical ROIs were determined based on representative and well-preserved brain regions containing the 6 cortical layers and measuring a total of 6 mm in length along the pial surface (Fig. 1a, b). Subcortical ROIs were selected in the juxtacortical WM immediately adjacent to the cortical ROI region and were drawn as circular or oval sections, avoiding inclusion of major vessels or gross artifacts (Fig. 1a, b). Two ROIs were selected for each individual in cortical and adjacent subcortical brain regions respectively. Total number of cells and lesions, average density (cells or lesions/mm2), and lesion burdens (percentage of cortex and WM occupied by lesions) were quantified within the sum of the two cortical and subcortical ROIs (representative examples of AI model performance for P2RY12 and IBA1 stainings are shown in Fig. 1c–h).
Example of the artificial intelligence based semiautomated quantification method used. Selection of cortical and subcortical regions of interest (a, b), tissue layer detection in green (b, c, f), P2RY12 counter in purple (c, d), IBA1 area detector in blue (f, g) and unannotated images stained with P2RY12 (e) and IBA1 (h)
Aiforia-based AI models and quantifications were performed in the cortex for the antibodies 4G8, AT8, γH2AX, ALDH1L1, GFAP, IBA1, CD68, HLA-DR, TMEM119 and P2RY12 and in subcortical WM for Olig2, IBA1, CD68, HLA-DR, TMEM119 and P2RY12. Models were created to quantify `objects’ (positive cells) for AT8, γH2AX, ALDH1L1, GFAP, CD68, HLA-DR, TMEM119 and P2RY12 antibodies, and `burdens’ (% of cortex/juxtacortical WM labelled by the antibody of interest) for 4G8 and IBA1. No AI model for formal quantification of alpha synuclein or pTDP43 was performed since absence of those comorbidities was a prerequisite when selecting the brains included in the present study.
To better assess colocalization of γH2AX with glial markers (IBA1 and GFAP) with increased resolution at the cellular level, we expanded human brain sections following a previously published expansion microscopy (ExM) protocol [16]. We used primary antibodies to label IBA1 (Synaptic Systems, 1:100), GFAP (G3893, 1:100) and γH2AX (Abcam, 1:100), followed by the appropriate secondary antibodies (Alexa Fluor 488, 555, and 647, 1:100). It has been previously demonstrated that the ExM technique attains increased epitope exposure and isotropic tissue magnification, substantially increasing image resolution to about 4-5X [27].
A summary of the antibodies used in this study is displayed in Table 2.
Statistical analyses
D’Agostino-Pearson normality test was applied to test for Gaussian distribution. Multiple group analyses were then performed using one-way ANOVA for parametric variables and Kruskall-Wallis for non-parametric variables. Posthoc analyses to assess for between group differences were evaluated with Holm-Šídák’s test.
Correlation analyses were performed using Pearson test when both variables were normally distributed and Spearman test when at least one of the variables was not normally distributed. Significance level was set at p < 0.05. All statistical analyses and graphs were generated using Graphpad Prism version 9.3.0 (Graphpad Software Inc, La Jolla, CA).
Data are presented as mean ± standard error, unless otherwise indicated.
Results
Regional Aβ-plaque burden and number of neurofibrillary tangles did not show significant differences between resilient and demented brains at Braak III-IV stages.
Recent studies have stressed that regional burden of amyloid plaques and NFTs may be a better predictor of cognition than the mere presence or absence of those lesions [52]. It is well known that amyloid burden increases in a linear fashion over a period of up to 15 years in advance of clinical symptoms before reaching a plateau [41]. Tangles, however, continue to accumulate linearly within brain regions as tau pathology progresses along well-defined tau spreading pathways (from Braak I-II, transentorhinal/entorhinal cortex, to Braak VI, primary visual cortex) correlating with the severity of cognitive decline and the amount of neuronal loss in symptomatic individuals [32, 40, 41, 90].
Thus, even though demented and resilient brains were well matched for Thal phases, Braak stages, and CERAD scores, we sought to carefully quantify Aβ plaque and tau NFT load in the ROIs. Aβ-plaque burden (defined as the percentage of cortex in the ROIs occupied by deposits immunolabeled with 4G8 antibody) did not show statistically significant differences between resilient and demented brains in either temporal pole or visual cortex, suggesting that neither plaque scores nor regional Aβ-plaque loads were predictive of the different cognitive fate of those two groups (Fig. 2a). Quantification of number of NFTs (as visualized by immunolabeling with AT8 antibody) in the temporal pole did not show significant differences between resilient and demented either (p = 0.33), even though a trend towards higher NFT counts was observed in this region in the demented group (Fig. 2b). As expected at tangle Braak III-IV stages, no NFTs were detected in the visual cortex of either demented or resilient brains (Fig. 2c).
Regional Aβ plaque burden (defined as the percentage of cortex occupied by amyloid beta (Aβ) deposits immunostained with 4G8 antibody) (a) and number of neurofibrillary tangles (as reported by immunolabeling with AT8 antibody) (b, c) did not significantly differ in demented compared to resilient. Representative photomicrographs of plaques (a) and tangles (b, c) on sections from control, resilient and demented cases containing temporal pole (a and b) and visual cortex (c). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); *p < 0.05; **p < 0.01. Scale bars 100 μm and 50 μm
Total number of astrocytes, microglial cells and oligodendrocytes in the cortical and subcortical ROIs did not differ between control, resilient, and demented brains at Braak III-IV stages.
Our results showed that total number and density of astrocytes (ALDH1L1), microglial cells (IBA1) and oligodendrocytes (Olig2), as reported by their respective constitutive markers in cortical and subcortical ROIs did not significantly differ between controls, resilient and demented groups (Fig. 3). These data are in agreement with previous reports [36] and suggest that phenotypic changes of existing resting glia rather than proliferation underlie glial responses in AD.
Quantifications for total IBA1 burden (percentage of cortex or white matter covered by IBA1 + microglia), total number of astrocytes (ALDH1L1), and total number of oligodendrocytes (Olig2) in temporal and visual cortex (microglia and astrocytes) and white matter (microglia and oligodendrocytes) (b) did not show statistically significant differences between demented, resilient and control brains. Representative photomicrographs of IBA1 + microglia, ALDH1L1 + astrocytes and OLIG2 + oligodendrocytes on sections from control, resilient and demented cases containing temporal pole are displayed on the top (a). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV). Scale bar 50 μm
Phenotypic changes in glial cells are an early phenomenon that precede overt tau tangle pathology in demented individuals at Braak III-IV stages.
Although total cell numbers remained stable, there was a phenotypic change in astrocytes in the temporal pole in the setting of dementia with a significantly higher number of GFAP + activated astrocytes compared to both controls and resilient brains (Fig. 4). Interestingly, a significantly higher number of GFAP + astrocytes was also observed in the visual cortex of demented compared to resilient brains despite absence of NFTs in this brain region and equivalent Aβ plaque burdens in both groups (Fig. 2).
Number of reactive astrocytes as labelled by GFAP antibody were significantly higher in the temporal pole and visual cortex of demented but not resilient when compared to control brains (a). Representative photomicrographs of GFAP + astrocytes on sections from control, resilient and demented cases containing temporal pole are displayed on the right (b). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); **p < 0.01; ****p < 0.0001. Scale bar 50 μm
A significantly higher number of pro-inflammatory microglial cells, as reported by CD68 antibody (Fig. 5), and a decreased number of homeostatic microglia, as reported by TMEM119 and P2RY12 immunolabeling (Fig. 6), were also found in the temporal pole of demented while no differences were found in resilient brains when compared to controls. Interestingly, similar changes in microglial phenotypes were found in the visual cortex of demented but not resilient individuals (Figs. 5 and 6) further suggesting that changes in glial phenotypes in demented individuals at Braak III-IV stages not only increase in parallel to tangle formation in regions already displaying tau aggregates (temporal pole) but also occur in advance of overt tau deposition in brain regions along the Braak tau pathway that are not yet impacted by tau pathology (visual cortex). Of note, no significant correlations were found between PMIs and pro-inflammatory (CD68 p = 0.40 and p = 0.51 in temporal pole and visual cortex respectively; HLA-DR p = 0.46 and p = 0.60 in temporal pole and visual cortex, respectively) or homeostatic microglial markers (TMEM119 p = 0.55 and p = 0.47 in temporal pole and visual cortex, respectively; P2RY12 p = 0.46 and p = 0.69 in temporal pole and visual cortex, respectively).
Number of CD68 + microglia was significantly higher in the temporal pole and visual cortex of demented but not in resilient when compared to control brains (a). A similar trend that did not reach statistical significance was observed in the number of HLA-DR + microglia (a). Representative photomicrographs of CD68 + and HLD-DR + microglia on sections from control, resilient and demented cases containing temporal pole are displayed on the right (b). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); *p < 0.05; **p < 0.01; ****p < 0.0001. Scale bars 50 μm
Homeostatic microglia stained with TMEM119 and P2RY12 antibodies in temporal pole and visual cortex was significantly decreased in the temporal pole and visual cortex of demented but not in resilient when compared to control brains (a). Representative photomicrographs of TMEM119 + and P2RY12 + microglia on sections from control, resilient and demented cases containing visual cortex are displayed on the right (b). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); *p < 0.05; **p < 0.01; ****p < 0.0001. Scale bars 50 μm
Whether these early glial phenotypic changes observed in visual cortex of demented individuals may set a path that facilitates subsequent pathogenic tau conversion/deposition and neuronal damage or represent a response to the presence of soluble pathogenic tau aggregates in Braak V-VI regions prior to overt tau tangle pathology remains uncertain [21]. It is possible that the absence of such aberrant glial cell responses in resilient brains may be capable of maintaining a homeostatic regulation of neuroinflammation and contribute to preserve neuronal cell integrity and thus brain function in these individuals.
Early markers of cellular DNA damage precede overt tau tangle pathology and correlate with phenotypic changes in glia of demented individuals at Braak III-IV stages.
Gamma H2AX (ɣH2AX) is a well-established biomarker of early cellular damage. Previous studies have shown that DNA damage resulting in double stranded DNA breakages initiates the phosphorylation of histone variant H2A at the Serine 139 site to generate ɣH2AX [81]. We quantified the number of ɣH2AX positive cells in the cortical ROIs of control, resilient and demented brains and found an increase in the number of ɣH2AX positive cells in the demented brains in both, temporal pole and visual cortex, but not in resilient brains (Fig. 7a, b). Importantly, a significant correlation between ɣH2AX and markers of cortical pro-inflammatory glia (GFAP, CD68, HLA-DR) were observed (Spearman R and p values for correlation analyses between cortical GFAP, CD68, HLA-DR and cortical ɣH2AX in temporal/visual regions: GFAP: R = 0.57/0.45 and p = 0.0006/ < 0.0001; CD68: R = 0.44/0.46 and p = 0.0009/0.0005; HLA-DR: R = 0.32/0.07 and p = 0.02/0.6, respectively).
Number of γH2AX + cells was significantly increased in temporal pole and visual cortex of demented but not in resilient when compared to control brains (a). Representative photomicrographs of γH2AX + cells on sections from control, resilient and demented cases containing visual cortex (b). Representative immunofluorescence images of colocalization of γH2AX + cells (brown) with neurons (red) (7c 1–3, showing a progressively zoomed-in brain region), GFAP + astrocytes (green) (c 4–6, showing GFAP alone, 7c4, γH2AX alone, 7c5, both GFAP-γH2AX, 7c6), and IBA1 + microglia (green) (7c 7–9, showing IBA1 alone, 7c7, γH2AX alone, 7c8, both IBA1-γH2AX, 7c9). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); **p < 0.01; ***p < 0.001. Scale bars 1 mm, 50 μm, and 20 μm. White arrows indicate colocalization between γH2AX+ cells and neurons, astrocytes, and microglia, respectively
Interestingly, the differences in the number of ɣH2AX+cells between demented and resilient were more pronounced in visual cortex than in temporal pole, demonstrating that cellular damage precedes overt NFT deposition, and pointing to the readily observed aberrant glial responses in the same brain regions as a potential contributor to early cellular vulnerability and brain dysfunction in demented individuals (Fig. 7a). Importantly, no significant correlation was found between number of ɣH2AX + cells and number of NFTs (p = 0.43 in temporal pole) or PMIs (p = 0.26 and p = 0.09 in temporal pole and visual cortex respectively). Of note, ɣH2AX immunolabelling was observed in neurons as well as in glial cells in demented brains (Fig. 7c) suggesting that both, neuronal and glial damage are part of the brain injury responses that ultimately result in functional changes and impaired cognition, recognized as the dementing disorder in AD.
Glial changes in subcortical white matter precede overt tau tangle pathology and correlate with markers of cortical cellular damage in demented brains at Braak III-IV stages.
The changes in glia within subcortical WM led us to examine whether changes in the subcortical glial phenotype could also be present at early tau pathological stages and predict cellular vulnerability and cognitive outcomes. We hypothesized that dysfunctional glial responses in juxtacortical WM tracts occurred prior to overt tau tangle deposition in the corresponding cortical regions and that they correlated with markers of early cortical cell damage in these brains.
In our analyses of juxtacortical WM we found a significant increase in activated pro-inflammatory microglia (CD68, HLA-DR) (Fig. 8) accompanied by a loss of homeostatic microglia (TMEM119, P2RY12) in WM of demented compared to resilient and control brains (Fig. 9). These WM changes were not only present in brain regions where cortical NFTs were already present (temporal pole), but also in areas without detectable tau burden (occipital cortex) suggesting that dysfunctional glial profiles in WM are present early in the disease course and precede overt tau deposition in the overlying brain cortices. In agreement with the results above, we also found that total number of oligodendrocytes (Olig2) and total microglial (IBA1) cell numbers and burden in WM remained unchanged across groups regardless of presence and/or absence of tau deposition in the immediately adjacent cortex (Fig. 3), further reinforcing the idea of qualitative rather than quantitative glial changes in demented AD brains.
Density of reactive microglia (CD68+ , HLA-DR +) in temporal and occipital white matter (WM) was significantly increased in demented but not in resilient when compared to control brains (a). Representative photomicrographs of CD68+ and HLA-DR+ microglia on sections from control, resilient and demented cases containing temporal WM are displayed on the right (b). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); **p < 0.01; ****p < 0.0001. Scale bar 50 μm
Density of homeostatic microglia (TMEM119 + , P2RY12 +) in temporal and occipital white matter (WM) was significantly decreased in demented but not in resilient when compared to control brains (a). Representative photomicrographs of TMEM119 + and P2RY12 + microglia on sections from control, resilient and demented cases containing temporal WM are displayed on the right (b). C Control (Braak 0-II); R Resilient (Braak III/IV); D Demented (Braak III/IV); *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Scale bar 50 μm
Because the clinical symptoms characterizing the dementing disorder of AD are predominantly cortical rather than subcortical in nature, we evaluated whether the subcortical glial changes observed were associated with cortical cellular vulnerability (ɣH2AX). Interestingly, we found a significant inverse association between number of TMEM119 + microglial cells in the WM and number of ɣH2AX + cells in immediately adjacent temporal and visual cortical regions (Spearman R and p values for correlation analyses between WM TMEM119 and cortical ɣH2AX in temporal/visual regions: R = − 0.36/ − 0.29 and p = 0.007/0.03 respectively), which did not reach significance when correlating TMEM119 and ɣH2AX positive cells in the cortex. This suggests that loss of homeostatic microglia in the WM is an early brain change that occurs in advance of classic tau tangle pathology and may potentially contribute to cortical cell damage and loss of brain function in demented brains. It is also possible that the higher burden of overall cerebrovascular risk observed in the demented individuals might be contributing to early glial derangements in the WM of these individuals even in the absence of significant differences in vascular insults across groups. Of note, identical changes in cortical and subcortical glial phenotypes and their correlation with markers of cortical cell damage were also present when analyses were limited to the subset of individuals with PART (not shown).
Discussion
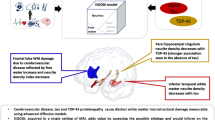
In this study we have found that early changes in the phenotype of glial cells in the cortex and white matter (WM) – involving both increased pro-inflammatory and decreased homeostatic responses – occur in advance of overt NFT formation along the pathologically defined tau pathway, strongly correlate with markers of cortical cell damage, and more accurately predict than tangles themselves the cognitive status of individuals who are at tau Braak III-IV stages at autopsy. These results favor a model where early glial dysfunction in cortical and subcortical regions may be a key element of the tissue injury response that determines the cognitive fate of individuals who harbor intermediate stages of classic AD tau tangle pathology in their brains. Our data also suggest that the absence of such aberrant glial cell responses in resilient brains may reflect maintenance of homeostatic regulation of neuroinflammation and contribute to the preservation of cell integrity as a mechanism for resilience. If true, this would highlight possible new targets for intervention in the face of plaques and tangles.
Current schemes for assessing disease burden in AD examine the distribution, abundance and characteristics of plaques and tangles, yielding an estimate of the likelihood of cognitive impairment. While this is highly predictive for most individuals, in some instances there is a mismatch between lesions and symptoms. Some individuals harbor a substantial burden of these lesions at autopsy which would be expected to have had deleterious consequences on their brain function, but remain at their cognitive baseline. This phenomenon of absence of overt cognitive deterioration in the face of neuropathologic lesions expected to have had substantial clinical consequences is the process we and others have termed ‘resilience’ to AD pathology (and we refer to the tissue from such individuals as ‘resilient brains’). While resilient brains with a high burden of AD pathology meeting criteria for high probability of AD at autopsy (moderate-frequent neuritic plaques and tangle Braak stage V-VI) according to NIA-AA guidelines [39] represent only a small subset of outliers [18, 68], the percentage of cognitively unimpaired individuals meeting an intermediate probability of AD at autopsy (moderate-frequent neuritic plaques and tangle Braak III-IV stage) is substantially higher at around 40% [63, 64]. This evident disconnect between dementia status and classic AD neuropathologic changes (e.g. plaques and tangles) [38] sets the stage for investigating additional brain changes that could be meaningful to more accurately predict at the individual level the presence or absence of dementia in the setting of amyloid and tau lesions.
Trying to understand the relationship between plaques, tangles and cognition can be confounded by a wide range of variables, including individual differences in the regional burden of lesions [38], genetic risk and protective factors [61], presence of distinct tau strains with different propensity to induce tau aggregation [22], as well as life style factors including educational attainment [8] and cognitive activity across the life span [99], that likely contribute to interindividual variation. Moreover, there is the complicating impact of other concomitant neurodegenerative processes (including those marked by inclusions containing alpha-synuclein and TAR DNA-binding protein 43 (TDP-43)) and vascular brain lesions (including small vessel disease such as arteriolar sclerosis and cerebral amyloid angiopathy (CAA), as well as stroke) whose prevalence also increases with age [6, 25, 46]. Clinical-pathological correlation analyses demonstrate that the burden of these lesions lower the threshold for the clinical diagnosis of dementia in the face of low levels of classic AD neuropathological changes [45, 74], yet a recent study showed that over one third of cases with clinically manifest AD cannot be attributable to all these age-related neuropathologic indices (AD pathology and comorbidities) combined [12]. We and others have previously demonstrated that even after excluding individuals with significant concomitant neurodegenerative and vascular lesions and carefully matching burden and regional distribution of plaques and tangles, there is a small subset of individuals who appear capable of tolerating the insult of a full burden of plaques and tangles (moderate-frequent neuritic plaques and tangle Braak V-VI stage) in their brain without developing dementia [18, 68, 73, 77]. Intriguingly, the study of these unique brains revealed that distinct glial cell responses and brain cytokine expression profiles may serve to more accurately predict future brain cell damage and cognitive fate than plaques and tangles themselves [5, 56, 68], pointing to neuroinflammation as a potentially significant contributor to cell damage and loss of brain function in AD rather than an innocent bystander response to neuronal injury.
In keeping with the above literature, the present study primarily focused on detailed assessments of glial phenotypes of brains from individuals who harbor intermediate stages of NFT pathology at autopsy (Braak stage III-IV) but exhibited opposite antemortem clinical outcomes (demented vs. cognitive normal `resilient’). It is well known that tangle pathology appears in human AD brains in a hierarchically spatial consistent manner, starting in the transenthorinal/entorhinal cortex (Braak stage I-II), and ultimately culminating in the primary visual cortex (Braak stage VI), resulting in overt tau pathology and cell damage along the same pathway. Thus, intermediate Braak stages (Braak stages III-IV) provide a unique window of opportunity to investigate other brain changes occurring in later regions in the Braak tau pathway (visual cortex) that precede tau tangle accumulation and may ultimately drive, rather than tangles themselves, neurodegeneration and change from baseline function.
Importantly, brains with comorbidities like TDP43 and alpha-synuclein were excluded from the present study to avoid additional potential confounders contributing to the heterogeneity in the clinical expression of tangle Braak III-IV stages. We confirmed that the regional burdens of both plaques and tangles in the ROIs studied (temporal pole, Brodmann’s area 38, and visual cortex, Brodmann’s area 17/18) were well matched between demented and resilient brains thus ruling out the most parsimonious explanation that a higher regional load of lesions in the demented could explain the divergent clinical expression of the two groups. Even though multiple studies, including our own, have shown that the number of NFTs tends to correlate much better than Aβ plaques with the severity and duration of clinical symptoms and the amount of neuronal loss [3, 28], some neuronal populations are vulnerable to cell loss with limited tangle formation [11]. Even in areas that develop abundant tangles, the vast majority of neuronal loss in patients with clinically manifest AD cannot be explained by the number of tangles present at autopsy [28, 29, 92]. Moreover, in mice overexpressing mutant tau to mimic AD neuronal loss, tangles on their own are not sufficient to permanently disrupt cognitive function and, similarly to human AD, the extent of neuronal loss in their brains exceeds by far tangle numbers [76]. Increasing evidence from mouse models and human brains supports a role of soluble pathological tau assemblies leading to synapse and neuronal damage [50]. We and others have previously observed a robust aberrant accumulation of soluble species of hyperphosphorylated tau within the synaptic compartment in clinically manifest AD brains compared to very small quantities or absence of those in resilient brains with comparable tangle burdens [68, 84], suggesting that soluble pathological species of tau, rather than tau tangles, might be the critical toxic moiety leading to neurodegeneration and clinical expression of AD. So even though amyloid and tau are likely instigators, other downstream mechanisms must be the real culprits driving brain cell damage and dementia in individuals who harbor these proteins in their brains.
Previous studies have convincingly shown that activation of astrocytes and microglial cells are present early and linearly increase around plaques and tangles with disease progression in clinically manifest AD brains [80]. Additionally, recent studies have also identified a loss of homeostatic glial gene expression that can exert detrimental effects in early steps of AD pathogenesis [62, 85]. In the present study, we used a panel of well characterized antibodies against constitutive as well as pro-inflammatory and homeostatic glial markers. GFAP is the main intermediate filament protein found in astrocytes and gets upregulated upon abnormal astrocytic activation. In AD, it has been found to increase from mild cognitive impairment (MCI) stages and to be particularly expressed in the vicinity of Aβ deposits [15]. In addition, recent studies have found higher GFAP levels in cerebrospinal fluid and plasma of clinically symptomatic AD patients, and an association between plasma GFAP and a higher burden of WM lesions and degree of cognitive impairment [66]. Activated proinflammatory microglia is characterized by increases in CD68, a lysosomal associated membrane protein (LAMP) that gets upregulated upon increased phagocytic activity, and HLA-DR (human leukocyte antigen class II molecule), a membrane bound glycoprotein used by antigen-presenting cells to expose fragments of phagocytosed pathogens and trigger additional inflammatory responses [95]. Studies of postmortem human brains have shown that both, CD68 and HLA-DR, robustly increase in microglia of demented AD brains across brain regions [36], and that these effects go beyond the pro-inflammatory state observed in normal ageing [43]. Although much of microglial characterization in AD has focused on the overexpression of pro-inflammatory genes, recent studies have also identified that loss of homeostatic microglial genes can exert detrimental effects in early steps of AD pathogenesis [62, 85]. Loss of the homeostatic phenotype of microglia is in fact a hallmark of proinflammatory states in general and a key element across various neurological conditions [47, 100]. Interestingly, a recent study found a significant association between the amount of neuronal loss and the degree of reduced homeostatic microglial gene expression profiles in AD mouse models and in RNAseq analyses of the precuneus in human brains [85]. To date, the most specific microglial homeostatic markers are TMEM119 and P2RY12 [43]. While the function of TMEM119, a transmembrane protein that distinguishes resident microglia from blood-derived macrophages, remains largely unknown, P2RY12 is involved in sensing external injury signals through its purinoreceptor and in chemotaxis upon stimulation through external injury signals. We found a distinct change in the phenotype of glial cells in demented brains at Braak III-IV stages with significantly increased pro-inflammatory astrocyte and microglial markers (GFAP, CD68), and decreased homeostatic microglial markers (TMEM119, P2RY12) that distinguished them from age-matched resilient and control brains. Importantly, these glial phenotypic changes in demented brains were present not only in brain areas that already contained NFTs at Braak III-IV stage (temporal pole), but also in areas with no overt tangle formation by immunohistochemistry (visual cortex). Identical glial phenotypic changes were found in demented in the adjacent subcortical WM in the same brain regions but not in resilient or control brains. Moreover, both grey and white matter glial cell abnormalities in demented significantly correlated with an increased expression of ɣH2AX, a marker of early cellular damage, both in neurons and glia. These novel insights favor a model where glial responses, and in particular microglia—the immunocompetent cells of the central nervous system—and immune-related mechanisms may be real culprits of cell injury and impaired brain function in the setting of plaques and tangles. Of note, after matching the three groups for cerebrovascular pathology, demented brains still showed an overall higher composite cerebrovascular risk score when compared to resilient and control brains. All these observations are in agreement with previous findings from radiological and genetic studies that point to the contribution of elevated vascular risk biomarkers and genetic variants that promote inflammation to age-related cellular/vascular changes in the cerebral WM (considered to be the substrate of WM hyperintensities) and increased risk and faster progression of cognitive impairment in the face of classic AD pathology (e.g. plaques and tangles) [67, 69, 71].
This study is not without caveats. First, while observations derived from autopsy studies like the present one may allow to infer information about the hierarchical order of pathological brain changes, their temporo-spatial relationships, and the resulting impact on antemortem brain function, the cross-sectional nature of such studies precludes drawing conclusions on specific underlying molecular mechanisms involved. Secondly, we cannot rule out the possibility that distinct tau strains may exist in demented and resilient brains with different biological properties including their propensity to form toxic soluble tau species (tau seeding activity) prior to the development of overt tau pathology, or the intensity of phosphorylation of different tau phosphor-epitopes that may result in their differential glial cell responses and clinical disease expression. Alternatively, the possibility also exists that the aberrant early changes in the glial phenotype in the grey and white matter of demented brains that precede tangle formation may be the real culprits of neuronal and glial damage and impaired brain function, and that preservation of physiological phenotypes of astrocytes and microglial cells would be needed for a human brain to cope with the challenges of Aβ and tau aggregation and deposition and thus preserve its normal function. Future studies will be conducted to address these important questions.
In sum, the present study demonstrates that loss of homeostatic phenotypes of microglia and rise in pro-inflammatory astrocytes and microglial cells in the cortex and WM precede NFT formation along the well-defined tau pathological pathways in demented individuals who are at intermediate stages of classic tangle burden (Braak stages III-IV). These changes in glial phenotypes closely correlate with early markers of DNA damage in neurons and glia and accurately distinguish demented individuals from those exhibiting preserved cognition at a similar tau Braak stage at autopsy. These novel findings have potential important implications to guide the development of new biomarkers and also to develop novel therapies that can prevent cognitive impairment in the presence of classic AD lesion burden (e.g. plaques and tangles). The development of surrogate in vivo markers capable of detecting early changes in the glial phenotypes and signs of early neuronal and glial vulnerability in advance of the predicted spreading of tau aggregation between anatomically connected brain regions has the potential to more precisely identify who will develop clinical symptoms of dementia among asymptomatic amyloid and tau positive elderly at intermediate stages of pathology and over what time frame, providing valuable guidance on the need and optimal timing for personalized preventive intervention.
Availability of data and materials
Original slides and diagnostic material are retained. There are no novel reagents or materials for others to request.
References
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65:1509–1517. https://doi.org/10.1001/archneur.65.11.1509
Araque Caballero MÁ, Suárez-Calvet M, Duering M, Franzmeier N, Benzinger T, Fagan AM, Bateman RJ, Jack CR, Levin J, Dichgans M, Jucker M, Karch C, Masters CL, Morris JC, Weiner M, Rossor M, Fox NC, Lee J-H, Salloway S, Danek A, Goate A, Yakushev I, Hassenstab J, Schofield PR, Haass C, Ewers M (2018) White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease. Brain 141:3065–3080. https://doi.org/10.1093/brain/awy229
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681. https://doi.org/10.1212/WNL.42.9.1681
Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Patel E, Abner EL, Kryscio RJ, Nelson PT (2015) Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 3:32–32. https://doi.org/10.1186/s40478-015-0209-z
Barroeta-Espar I, Weinstock LD, Perez-Nievas BG, Meltzer AC, Siao Tick Chong M, Amaral AC, Murray ME, Moulder KL, Morris JC, Cairns NJ, Parisi JE, Lowe VJ, Petersen RC, Kofler J, Ikonomovic MD, López O, Klunk WE, Mayeux RP, Frosch MP, Wood LB, Gomez-Isla T (2019) Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol Dis 121:327–337. https://doi.org/10.1016/j.nbd.2018.10.009
Beach TG, Malek-Ahmadi M (2021) Alzheimer’s disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis JAD 79:389–400. https://doi.org/10.3233/JAD-201213
Bellenguez C, Küçükali F, Jansen I, Andrade V, Moreno-Grau S, Amin N, Naj AC, Grenier-Boley B, Campos-Martin R, Holmans PA, Boland A, Kleineidam L, Damotte V, van der Lee SJ, Kuulasmaa T, Yang Q, de Rojas I, Bis JC, Yaqub A, Prokic I, Costa MR, Chapuis J, Ahmad S, Giedraitis V, Boada M, Aarsland D, García-González P, Abdelnour C, Alarcón-Martín E, Alegret M, Alvarez I, Álvarez V, Armstrong NJ, Tsolaki A, Antúnez C, Appollonio I, Arcaro M, Archetti S, Pastor AA, Arosio B, Athanasiu L, Bailly H, Banaj N, Baquero M, Belén Pastor A, Benussi L, Berr C, Besse C, Bessi V, Binetti G, Bizzarro A, Alcolea D, Blesa R, Borroni B, Boschi S, Bossù P, Bråthen G, Bresner C, Brookes KJ, Brusco LI, Bûrger K, Bullido MJ, Burholt V, Bush WS, Calero M, Dufouil C, Carracedo Á, Cecchetti R, Cervera-Carles L, Charbonnier C, Chillotti C, Brodaty H, Ciccone S, Claassen JAHR, Clark C, Conti E, Corma-Gómez A, Costantini E, Custodero C, Daian D, Dalmasso MC, Daniele A, Dardiotis E, Dartigues J-F, de Deyn PP, de Paiva Lopes K, de Witte LD, Debette S, Deckert J, del Ser T, Denning N, DeStefano A, Dichgans M, Diehl-Schmid J, Diez-Fairen M, Rossi PD, Djurovic S, Duron E, Düzel E, Engelborghs S, Escott-Price V, Espinosa A, Buiza-Rueda D, Ewers M, Tagliavini F, Nielsen SF, Farotti L, Fenoglio C, Fernández-Fuertes M, Hardy J, Ferrari R, Ferreira CB, Ferri E, Fin B, Fischer P, Fladby T, Fließbach K, Fortea J, Fostinelli S, Fox NC, Franco-Macías E, Frank-García A, Froelich L, Galimberti D, García-Alberca JM, Garcia-Madrona S, García-Ribas G, Chene G, Ghidoni R, Giegling I, Giaccone G, Goldhardt O, González-Pérez A, Graff C, Grande G, Green E, Grimmer T, Grünblatt E, Guetta-Baranes T, Haapasalo A, Hadjigeorgiou G, Haines JL, Hamilton-Nelson KL, EADB, Gra@ce, ADGC, Charge, DemGen, FinnGen, EADI, GERAD, Hampel H, Hanon O, Hartmann AM, Hausner L, Harwood J, Heilmann-Heimbach S, Helisalmi S, Heneka MT, Hernández I, Herrmann MJ, Hoffmann P, Holmes C, Holstege H, Vilas RH, Hulsman M, Humphrey J, Biessels GJ, Johansson C, Kehoe PG, Kilander L, Ståhlbom AK, Kivipelto M, Koivisto A, Kornhuber J, Kosmidis MH, Kuksa PP, Kunkle BW, Lage C, Laukka EJ, Lauria A, Lee C-Y, Lehtisalo J, Satizabal CL, Lerch O, Lleó A, Lopez R, Lopez O, de Munain AL, Love S, Löwemark M, Luckcuck L, Macías J, MacLeod CA, Maier W, Mangialasche F, Spallazzi M, Marquié M, Marshall R, Martin ER, Martín Montes A, Rodríguez CM, Masullo C, Mayeux R, Mead S, Mecocci P, Medina M, Meggy A, Mendoza S, Menéndez-González M, Mir P, Periñán MT, Mol M, Molina-Porcel L, Montrreal L, Morelli L, Moreno F, Morgan K, Nöthen MM, Muchnik C, Nacmias B, Ngandu T, Nicolas G, Nordestgaard BG, Olaso R, Orellana A, Orsini M, Ortega G, Padovani A, Caffarra P, Papenberg G, Parnetti L, Pasquier F, Pastor P, Pérez-Cordón A, Pérez-Tur J, Pericard P, Peters O, Pijnenburg YAL, Pineda JA, Piñol-Ripoll G, Pisanu C, Polak T, Popp J, Posthuma D, Priller J, Puerta R, Quenez O, Quintela I, Thomassen JQ, Rábano A, Rainero I, Ramakers I, Real LM, Reinders MJT, Riedel-Heller S, Riederer P, Rodriguez-Rodriguez E, Rongve A, Allende IR, Rosende-Roca M, Royo JL, Rubino E, Rujescu D, Sáez ME, Sakka P, Saltvedt I, Sanabria Á, Sánchez-Arjona MB, Sanchez-Garcia F, Mehrabian S, Sánchez-Juan P, Sánchez-Valle R, Sando SB, Scamosci M, Scarmeas N, Scarpini E, Scheltens P, Scherbaum N, Scherer M, Schmid M, Schneider A, Schott JM, Selbæk G, Sha J, Shadrin AA, Skrobot O, Snijders GJL, Soininen H, Solfrizzi V, Solomon A, Sorbi S, Sotolongo-Grau O, Spalletta G, Spottke A, Squassina A, Tartari JP, Tárraga L, Tesí N, Thalamuthu A, Tegos T, Traykov L, Tremolizzo L, Tybjærg-Hansen A, Uitterlinden A, Ullgren A, Ulstein I, Valero S, Van Broeckhoven C, van der Lugt A, Van Dongen J, van Rooij J, van Swieten J, Vandenberghe R, Verhey F, Vidal J-S, Vogelgsang J, Vyhnalek M, Wagner M, Wallon D, Wang L-S, Wang R, Weinhold L, Wiltfang J, Windle G, Woods B, Yannakoulia M, Zhao Y, Zulaica M, Serrano-Rios M, Seripa D, Stordal E, Farrer LA, Psaty BM, Ghanbari M, Raj T, Sachdev P, Mather K, Jessen F, Ikram MA, de Mendonça A, Hort J, Tsolaki M, Pericak-Vance MA, Amouyel P, Williams J, Frikke-Schmidt R, Clarimon J, Deleuze J-F, Rossi G, Seshadri S, Andreassen OA, Ingelsson M, Hiltunen M, Sleegers K, Schellenberg GD, van Duijn CM, Sims R, van der Flier WM, Ruiz A, Ramirez A, Lambert J-C (2020) New insights on the genetic etiology of Alzheimer’s and related dementia. medRxiv 2020.10.01.20200659.https://doi.org/10.1101/2020.10.01.20200659
Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL (2003) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60:1909–1915. https://doi.org/10.1212/01.WNL.0000069923.64550.9F
Biel D, Brendel M, Rubinski A, Buerger K, Janowitz D, Dichgans M, Franzmeier N, for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2021) Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheim Res Ther, 13:137. https://doi.org/10.1186/s13195-021-00880-x
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535. https://doi.org/10.1038/nature08983
Bondareff W, Mountjoy CQ, Roth M, Hauser DL (1989) Neurofibrillary degeneration and neuronal loss in alzheimer’s disease. Neurobiol Aging 10:709–715. https://doi.org/10.1016/0197-4580(89)90007-9
Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, Bennett DA (2019) Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol 85:114–124. https://doi.org/10.1002/ana.25380
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259. https://doi.org/10.1007/BF00308809
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in alzheimer disease: age categories from 1 to 100 Years. J Neuropathol Exp Neurol 70:960–969. https://doi.org/10.1097/NEN.0b013e318232a379
Chatterjee P, Pedrini S, Stoops E, Goozee K, Villemagne VL, Asih PR, Verberk IMW, Dave P, Taddei K, Sohrabi HR, Zetterberg H, Blennow K, Teunissen CE, Vanderstichele HM, Martins RN (2021) Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry 11:27. https://doi.org/10.1038/s41398-020-01137-1
Chen F, Tillberg PW, Boyden ES (2015) Expansion microscopy. Science 347:543–548. https://doi.org/10.1126/science.1260088
Coelho A, Fernandes HM, Magalhães R, Moreira PS, Marques P, Soares JM, Amorim L, Portugal-Nunes C, Castanho T, Santos NC, Sousa N (2021) Signatures of white-matter microstructure degradation during aging and its association with cognitive status. Sci Rep 11:4517. https://doi.org/10.1038/s41598-021-83983-7
Corrada MM, Berlau DJ, Kawas CH (2012) A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res 9:709–717. https://doi.org/10.2174/156720512801322537
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol (Berl) 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464. https://doi.org/10.1002/ana.410270502
DeVos SL, Corjuc BT, Oakley DH, Nobuhara CK, Bannon RN, Chase A, Commins C, Gonzalez JA, Dooley PM, Frosch MP, Hyman BT (2018) Synaptic tau seeding precedes tau pathology in human alzheimer’s disease brain. Front Neurosci 12:267. https://doi.org/10.3389/fnins.2018.00267
Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, De Los Santos MB, Klickstein N, Corjuc DL, Corjuc BT, Dooley PM, Viode A, Oakley DH, Moore BD, Mullin K, Jean-Gilles D, Clark R, Atchison K, Moore R, Chibnik LB, Tanzi RE, Frosch MP, Serrano-Pozo A, Elwood F, Steen JA, Kennedy ME, Hyman BT (2020) Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat Med 26:1256–1263. https://doi.org/10.1038/s41591-020-0938-9
Duncan GJ, Simkins TJ, Emery B (2021) Neuron-oligodendrocyte interactions in the structure and integrity of axons. Front Cell Dev Biol 9:460. https://doi.org/10.3389/fcell.2021.653101
Edison P, Donat CK, Sastre M (2018) In vivo imaging of glial activation in Alzheimer’s Disease. Front Neurol 9:625. https://doi.org/10.3389/fneur.2018.00625
Farfel JM, Yu L, Boyle PA, Leurgans S, Shah RC, Schneider JA, Bennett DA (2019) Alzheimer’s disease frequency peaks in the tenth decade and is lower afterwards. Acta Neuropathol Commun 7:104. https://doi.org/10.1186/s40478-019-0752-0
van der Flier WM, Scheltens P (2005) Epidemiology and risk factors of dementia. J Neurol Neurosurg Amp Psychiatry 76:v2. https://doi.org/10.1136/jnnp.2005.082867
Gallagher B, Zhao Y (2019) Nanoscale imaging of synaptic connections with expansion microscopy. Discov Craiova Rom 7:e101–e101. https://doi.org/10.15190/d.2019.14
Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 41:17–24. https://doi.org/10.1002/ana.410410106
Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild alzheimer’s disease. J Neurosci 16:4491. https://doi.org/10.1523/JNEUROSCI.16-14-04491.1996
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert J-C, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St. George-Hyslop P, Singleton A, Hardy J (2013) TREM2 variants in alzheimer’s disease. N Engl J Med 368:117–127. https://doi.org/10.1056/NEJMoa1211851
Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41:1088–1093. https://doi.org/10.1038/ng.440
Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, Mellinger TJ, Edwards L, Pham J, Miller BL, Rabinovici GD, Jagust WJ (2019) Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol 85:229–240. https://doi.org/10.1002/ana.25406
Henstridge CM, Tzioras M, Paolicelli RC (2019) Glial contribution to excitatory and inhibitory synapse loss in neurodegeneration. Front Cell Neurosci 13:63. https://doi.org/10.3389/fncel.2019.00063
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ERLC, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, Alzheimer’s Disease Neuroimaging Initiative, van Duijn CM, Breteler MMB, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, CHARGE consortium, Berr C, Campion D, Epelbaum J, Dartigues J-F, Tzourio C, Alpérovitch A, Lathrop M, EADI1 consortium, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43:429–435. https://doi.org/10.1038/ng.803
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712–716. https://doi.org/10.1126/science.aad8373
Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, Bazinet RP (2018) Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry 23:177–198. https://doi.org/10.1038/mp.2017.246
Hu WT, Ozturk T, Kollhoff A, Wharton W, Christina Howell J, Weiner M, Aisen P, Petersen R, Jack CR, Jagust W, Trojanowki JQ, Toga AW, Beckett L, Green RC, Saykin AJ, Morris J, Perrin RJ, Shaw LM, Kachaturian Z, Carrillo M, Potter W, Barnes L, Bernard M, González H, Ho C, Hsiao JK, Masliah E, Masterman D, Okonkwo O, Ryan L, Silverberg N, Fleisher A, Montine T, Kaye JA, Silbert LC, Schneider LS, Pawluczyk S, Becerra M, Brewer J, Heidebrink JL, Knopman D, Villanueva-Meyer J, Doody RS, Kass JS, Stern Y, Honig LS, Mintz A, Ances B, Mintun MA, Geldmacher D, Love MN, Grossman H, Goldstein MA, Shah RC, Lamar M, Duara R, Greig-Custo MT, Albert M, Onyike C, Smith A, Sadowski M, Wisniewski T, Shulman M, Doraiswamy PM, Petrella JR, James O, Karlawish JH, Wolk DA, Smith CD, Jicha GA, El Khouli R, Lopez OL, Porsteinsson AP, Thai G, Pierce A, Kelley B, Nguyen T, Womack K, Levey AI, Lah JJ, Burns JM, Swerdlow RH, Brooks WM, Silverman DHS, Kremen S, Graff-Radford NR, Farlow MR, van Dyck CH, Mecca AP, Chertkow H, Vaitekunis S, Black S, Stefanovic B, Heyn C, Hsiung G-YR, Sossi V, Finger E, Pasternak S, Rachinsky I, Grant I, Rogalski E, Mesulam M-M, Pomara N, Hernando R, Sarrael A, Rosen HJ, Miller BL, Perry D, Turner RS, Sperling RA, Johnson KA, Marshall GA, Yesavage J, Taylor JL, Chao S, Belden CM, Atri A, Spann BM, Killiany R, Stern R, Mez J, Obisesan TO, Ntekim OE, Lerner A, Ogrocki P, Tatsuoka C, Fletcher E, Maillard P, Olichney J, DeCarli C, Bates V, Capote H, Borrie M, Lee T-Y, Bartha R, Johnson S, Asthana S, Carlsson CM, Perrin A, Scharre DW, Kataki M, Tarawneh R, Hart D, Zimmerman EA, Celmins D, Miller DD, Koleva H, Shim H, Williamson JD, Craft S, Cleveland J, Ott BR, Drake J, Tremont G, Sabbagh M, Ritter A, Mintzer J, Masdeu J, Shi J, Newhouse P, Potkin S, Salloway S, Malloy P, Correia S, Kittur S, Pearlson GD, Blank K, Flashman LA, Seltzer M, Lee A, Relkin N, Chiang G, Alzheimer’s Disease Neuroimaging Initiative (2021) Higher CSF sTNFR1-related proteins associate with better prognosis in very early Alzheimer’s disease. Nat Commun 12:4001. https://doi.org/10.1038/s41467-021-24220-7
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National institute on aging-alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Hyman BT, Trojanowski JQ (1997) Editorial on consensus recommendations for the postmortem diagnosis of alzheimer disease from the national institute on aging and the reagan institute working group on diagnostic criteria for the neuropathological assessment of alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097. https://doi.org/10.1097/00005072-199710000-00002
Ishiki A, Okamura N, Furukawa K, Furumoto S, Harada R, Tomita N, Hiraoka K, Watanuki S, Ishikawa Y, Tago T, Funaki Y, Iwata R, Tashiro M, Yanai K, Kudo Y, Arai H (2015) Longitudinal assessment of tau pathology in patients with alzheimer’s disease using [18F]THK-5117 positron emission tomography. PLoS ONE 10:e0140311–e0140311. https://doi.org/10.1371/journal.pone.0140311
Jack CR Jr, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, Petersen RC (2013) Brain β-amyloid load approaches a plateau. Neurology 80:890–896. https://doi.org/10.1212/WNL.0b013e3182840bbe
Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Botha H, Graff-Radford J, Jones DT, Ferman TJ, Boeve BF, Kantarci K, Vemuri P, Mielke MM, Whitwell J, Josephs K, Schwarz CG, Senjem ML, Gunter JL, Petersen RC (2020) Predicting future rates of tau accumulation on PET. Brain 143:3136–3150. https://doi.org/10.1093/brain/awaa248
Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci 14:198. https://doi.org/10.3389/fncel.2020.00198
Kamphuis W, Middeldorp J, Kooijman L, Sluijs JA, Kooi E-J, Moeton M, Freriks M, Mizee MR, Hol EM (2014) Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol Aging 35:492–510. https://doi.org/10.1016/j.neurobiolaging.2013.09.035
Kapasi A, DeCarli C, Schneider JA (2017) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol (Berl) 134:171–186. https://doi.org/10.1007/s00401-017-1717-7
Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM (2015) Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology 85:535–542. https://doi.org/10.1212/WNL.0000000000001831
Kempthorne L, Yoon H, Madore C, Smith S, Wszolek ZK, Rademakers R, Kim J, Butovsky O, Dickson DW (2020) Loss of homeostatic microglial phenotype in CSF1R-related Leukoencephalopathy. Acta Neuropathol Commun 8:72–72. https://doi.org/10.1186/s40478-020-00947-0
Kim G-W, Park S-E, Park K, Jeong G-W (2021) White matter connectivity and gray matter volume changes following donepezil treatment in patients with mild cognitive impairment: a preliminary study using probabilistic tractography. Front Aging Neurosci 12:506. https://doi.org/10.3389/fnagi.2020.604940
Kneynsberg A, Combs B, Christensen K, Morfini G, Kanaan NM (2017) Axonal degeneration in tauopathies: disease relevance and underlying mechanisms. Front Neurosci 11:572–572. https://doi.org/10.3389/fnins.2017.00572
Kopeikina KJ, Hyman BT, Spires-Jones TL (2012) Soluble forms of tau are toxic in Alzheimer’s disease. Transl Neurosci 3:223–233. https://doi.org/10.2478/s13380-012-0032-y
Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues J-F, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P, the European Alzheimer’s Disease Initiative Investigators (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet, 41:1094–1099. https://doi.org/10.1038/ng.439
Latimer CS, Burke BT, Liachko NF, Currey HN, Kilgore MD, Gibbons LE, Henriksen J, Darvas M, Domoto-Reilly K, Jayadev S, Grabowski TJ, Crane PK, Larson EB, Kraemer BC, Bird TD, Keene CD (2019) Resistance and resilience to Alzheimer’s disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol Commun 7:91. https://doi.org/10.1186/s40478-019-0743-1
Latimer CS, Keene CD, Flanagan ME, Hemmy LS, Lim KO, White LR, Montine KS, Montine TJ (2017) Resistance to alzheimer disease neuropathologic changes and apparent cognitive resilience in the nun and honolulu-asia aging studies. J Neuropathol Exp Neurol 76:458–466. https://doi.org/10.1093/jnen/nlx030
Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Meltzer CC, Schimmel K, Tsopelas ND, DeKosky ST, Price JC (2005) Simplified quantification of pittsburgh compound B amyloid Imaging PET studies: a comparative analysis. J Nucl Med 46:1959
Low A, Mak E, Malpetti M, Passamonti L, Nicastro N, Stefaniak JD, Savulich G, Chouliaras L, Su L, Rowe JB, Markus HS, O’Brien JT (2021) In vivo neuroinflammation and cerebral small vessel disease in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Amp Psychiatry 92:45. https://doi.org/10.1136/jnnp-2020-323894
Lue L-F, Brachova L, Civin WH, Rogers J (1996) Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol 55:1083–1088. https://doi.org/10.1097/00005072-199655100-00008
Malpetti M, Kievit RA, Passamonti L, Jones PS, Tsvetanov KA, Rittman T, Mak E, Nicastro N, Bevan-Jones WR, Su L, Hong YT, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB (2020) Microglial activation and tau burden predict cognitive decline in Alzheimer’s disease. Brain 143:1588–1602. https://doi.org/10.1093/brain/awaa088
McAleese KE, Miah M, Graham S, Hadfield GM, Walker L, Johnson M, Colloby SJ, Thomas AJ, DeCarli C, Koss D, Attems J (2021) Frontal white matter lesions in Alzheimer’s disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol (Berl). https://doi.org/10.1007/s00401-021-02376-2
Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC (2006) [11C]PIB in a nondemented population. Neurology 67:446. https://doi.org/10.1212/01.wnl.0000228230.26044.a4
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G van, Berg L, participating CERAD neuropathologists (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Neurology, 41:479. https://doi.org/10.1212/WNL.41.4.479
Naj AC, Schellenberg GD, Alzheimer’s Disease Genetics Consortium (ADGC) 2017 Genomic variants, genes, and pathways of Alzheimer’s disease: an overview. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet, 174:5-26. https://doi.org/10.1002/ajmg.b.32499
Nasrabady SE, Rizvi B, Goldman JE, Brickman AM (2018) White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun 6:22. https://doi.org/10.1186/s40478-018-0515-3
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Tredici KD, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381. https://doi.org/10.1097/NEN.0b013e31825018f7
Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 68:1–14. https://doi.org/10.1097/NEN.0b013e3181919a48
Nordengen K, Kirsebom B-E, Henjum K, Selnes P, Gísladóttir B, Wettergreen M, Torsetnes SB, Grøntvedt GR, Waterloo KK, Aarsland D, Nilsson LNG, Fladby T (2019) Glial activation and inflammation along the Alzheimer’s disease continuum. J Neuroinflamm 16:46. https://doi.org/10.1186/s12974-019-1399-2
Oeckl P, Halbgebauer S, Anderl-Straub S, Steinacker P, Huss AM, Neugebauer H, von Arnim CAF, Diehl-Schmid J, Grimmer T, Kornhuber J, Lewczuk P, Danek A, Consortium for Frontotemporal Lobar Degeneration German, Ludolph AC, Otto M (2019) Glial fibrillary acidic protein in serum is increased in alzheimer’s disease and correlates with cognitive impairment. J Alzheimers Dis, 67:481–488. https://doi.org/10.3233/JAD-180325
Pantoni L, Garcia JH (1997) Cognitive impairment and cellular/vascular changes in the cerebral white mattera. Ann N Y Acad Sci 826:92–102. https://doi.org/10.1111/j.1749-6632.1997.tb48463.x
Perez-Nievas BG, Stein TD, Tai H-C, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Fernandez-Carballo L, de Munain EL, Perez J, Marquie M, Serrano-Pozo A, Frosch MP, Lowe V, Parisi JE, Petersen RC, Ikonomovic MD, López OL, Klunk W, Hyman BT, Gómez-Isla T (2013) Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain J Neurol 136:2510–2526. https://doi.org/10.1093/brain/awt171
Prins ND, Scheltens P (2015) White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 11:157–165. https://doi.org/10.1038/nrneurol.2015.10
Raj D, Yin Z, Breur M, Doorduin J, Holtman IR, Olah M, Mantingh-Otter IJ, Van Dam D, De Deyn PP, den Dunnen W, Eggen BJL, Amor S, Boddeke E (2017) Increased white matter inflammation in aging- and alzheimer’s disease brain. Front Mol Neurosci 10:206. https://doi.org/10.3389/fnmol.2017.00206
Raz N, Yang Y, Dahle CL, Land S (2012) Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta 1822:361–369. https://doi.org/10.1016/j.bbadis.2011.08.007
Reas ET (2017) Amyloid and tau pathology in normal cognitive aging. J Neurosci Off J Soc Neurosci 37:7561–7563. https://doi.org/10.1523/JNEUROSCI.1388-17.2017
Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR (2005) Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Dev Orig Aging Brain Blood Vessels 26:341–347. https://doi.org/10.1016/j.neurobiolaging.2004.06.019
Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX, Lee VM-Y, Kawas CH, Trojanowski JQ (2018) Non-Alzheimer’s contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol (Berl) 136:377–388. https://doi.org/10.1007/s00401-018-1872-5
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL (2007) Imaging β-amyloid burden in aging and dementia. Neurology 68:1718. https://doi.org/10.1212/01.wnl.0000261919.22630.ea
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481. https://doi.org/10.1126/science.1113694
Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis JAD 18:691–701. https://doi.org/10.3233/JAD-2009-1227
Sekar S, McDonald J, Cuyugan L, Aldrich J, Kurdoglu A, Adkins J, Serrano G, Beach TG, Craig DW, Valla J, Reiman EM, Liang WS (2015) Alzheimer’s disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol Aging 36:583–591. https://doi.org/10.1016/j.neurobiolaging.2014.09.027
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189–a006189. https://doi.org/10.1101/cshperspect.a006189
Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, Hyman BT (2011) Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol 179:1373–1384. https://doi.org/10.1016/j.ajpath.2011.05.047
Shanbhag NM, Evans MD, Mao W, Nana AL, Seeley WW, Adame A, Rissman RA, Masliah E, Mucke L (2019) Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol Commun 7:77. https://doi.org/10.1186/s40478-019-0723-5
Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, Savva G, Brayne C, Wharton SB (2010) Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging 31:578–590. https://doi.org/10.1016/j.neurobiolaging.2008.05.015
Simpson JE, Ince PG, Shaw PJ, Heath PR, Raman R, Garwood CJ, Gelsthorpe C, Baxter L, Forster G, Matthews FE, Brayne C, Wharton SB (2011) Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer’s pathology and APOE genotype. Neurobiol Aging 32:1795–1807. https://doi.org/10.1016/j.neurobiolaging.2011.04.013
Singh A, Allen D, Fracassi A, Tumurbaatar B, Natarajan C, Scaduto P, Woltjer R, Kayed R, Limon A, Krishnan B, Taglialatela G (2020) Functional integrity of synapses in the central nervous system of cognitively intact individuals with high alzheimer’s disease neuropathology is associated with absence of synaptic tau oligomers. J Alzheimers Dis 78:1661–1678. https://doi.org/10.3233/JAD-200716
Sobue A, Komine O, Hara Y, Endo F, Mizoguchi H, Watanabe S, Murayama S, Saito T, Saido TC, Sahara N, Higuchi M, Ogi T, Yamanaka K (2021) Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathol Commun 9:1. https://doi.org/10.1186/s40478-020-01099-x
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SWM, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. https://doi.org/10.1016/j.cell.2007.10.036
Terry RD (2000) Cell death or synaptic loss in alzheimer disease. J Neuropathol Exp Neurol 59:1118–1119. https://doi.org/10.1093/jnen/59.12.1118
Thal D, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Tredici K, Braak E, Braak H (2000) Sequence of A b-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. https://doi.org/10.1093/jnen/59.8.733
Tzioras M, Daniels MJD, King D, Popovic K, Holloway RK, Stevenson AJ, Tulloch J, Kandasamy J, Sokol D, Latta C, Rose J, Smith C, Miron VE, Henstridge C, McColl BW, Spires-Jones TL (2019) Altered synaptic ingestion by human microglia in Alzheimer’s disease. bioRxiv 795930. https://doi.org/10.1101/795930
Uematsu M, Nakamura A, Ebashi M, Hirokawa K, Takahashi R, Uchihara T (2018) Brainstem tau pathology in Alzheimer’s disease is characterized by increase of three repeat tau and independent of amyloid β. Acta Neuropathol Commun 6:1. https://doi.org/10.1186/s40478-017-0501-1
Verkhratsky A, Lazareva N, Semyanov A (2022) Glial decline and loss of homeostatic support rather than inflammation defines cognitive aging. Neural Regen Res 17:565–566. https://doi.org/10.4103/1673-5374.320979
Vogt L et al (1990) Laminar distribution of neuron degeneration in posterior cingulate cortex in Alzheimer’s disease. Acta Neuropathol 80(6):581–589
Vonsattel JPG, Del Amaya MP, Keller CE (2008) Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol (Berl) 115:509–532. https://doi.org/10.1007/s00401-007-0311-9
Vonsattel JPG, Myers RH, Tessa Hedley-Whyte E, Ropper AH, Bird ED, Richardson EP Jr (1991) Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 30:637–649. https://doi.org/10.1002/ana.410300503
Walker DG (2020) Defining activation states of microglia in human brain tissue: an unresolved issue for Alzheimer’s disease. Neuroimmunol Neuroinflamm 7:194–214. https://doi.org/10.20517/2347-8659.2020.09
Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun 9:1228. https://doi.org/10.1038/s41467-018-03566-5
Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC (2009) The alzheimer’s disease centers’ uniform data set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 23:91–101. https://doi.org/10.1097/WAD.0b013e318191c7dd
Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Holland D, Rongve A, Børte S, Winsvold BS, Drange OK, Martinsen AE, Skogholt AH, Willer C, Bråthen G, Bosnes I, Nielsen JB, Fritsche LG, Thomas LF, Pedersen LM, Gabrielsen ME, Johnsen MB, Meisingset TW, Zhou W, Proitsi P, Hodges A, Dobson R, Velayudhan L, Heilbron K, Auton A, Agee M, Aslibekyan S, Babalola E, Bell RK, Bielenberg J, Bryc K, Bullis E, Cameron B, Coker D, Partida GC, Dhamija D, Das S, Elson SL, Filshtein T, Fletez-Brant K, Fontanillas P, Freyman W, Gandhi PM, Hicks B, Hinds DA, Huber KE, Jewett EM, Jiang Y, Kleinman A, Kukar K, Lane V, Lin K-H, Lowe M, Luff MK, McCreight JC, McIntyre MH, McManus KF, Micheletti SJ, Moreno ME, Mountain JL, Mozaffari SV, Nandakumar P, Noblin ES, O’Connell J, Petrakovitz AA, Poznik GD, Schumacher M, Shastri AJ, Shelton JF, Shi J, Shringarpure S, Tian C, Tran V, Tung JY, Wang X, Wang W, Weldon CH, Wilton P, Sealock JM, Davis LK, Pedersen NL, Reynolds CA, Karlsson IK, Magnusson S, Stefansson H, Thordardottir S, Jonsson PV, Snaedal J, Zettergren A, Skoog I, Kern S, Waern M, Zetterberg H, Blennow K, Stordal E, Hveem K, Zwart J-A, Athanasiu L, Selnes P, Saltvedt I, Sando SB, Ulstein I, Djurovic S, Fladby T, Aarsland D, Selbæk G, Ripke S, Stefansson K, Andreassen OA, Posthuma D, 23andMe Research Team 2021 A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat Genet, 53:1276–1282, https://doi.org/10.1038/s41588-021-00921-z
Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA (2013) Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81:314–321. https://doi.org/10.1212/WNL.0b013e31829c5e8a
Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H (2017) Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140:1900–1913. https://doi.org/10.1093/brain/awx113
Acknowledgements
We are grateful to the study subjects and the Massachusetts ADRC Brain Bank for providing the tissue samples. We thank Lindsey Smith, Anastasie Mate, Leon Munting, Romain Perbet, Clara Munoz Castro, Emir Turkes and Alexandra Melloni for assisting with the processing of brain tissue samples and development of AI models used in this study. We also thank Karen Duff for her useful comments and suggestions.
Funding
Matthew P. Frosch received research funding from NIH Grants Nos. AG005134 and AG061206. Teresa Gómez-Isla received research funding from NIH Grants Nos. AG005134, AG036694, AG061206, AG06242 and AG069192, Cure Alzheimer’s Fund and MassCATs. Teresa Gómez-Isla participated as speaker in an Eli Lilly and Company sponsored educational symposium and served in an Eli Lilly Data Monitoring Committee.The current work was funded in part by Cure Alzheimer’s Fund and MassCATS grants and NIH P30AG06242 and R56AG069192 grants.
Author information
Authors and Affiliations
Contributions
RNT participated in study design, carried out immunostaining and image analysis, contributed to data analysis and interpretation, and drafted the manuscript. MSVM and OB participated in data acquisition. DD, TC and AG prepared the tissue blocks and sections from cases. MPF carried out the neuropathologic examination of cases, and participated in the study design, analysis and interpretation of data. TGI conceived the study and participated in its design and coordination, analysis and interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Informed consent was obtained from all individual participants included in the study and according to institutional procedures for autopsy consents for post-mortem tissue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Taddei, R.N., Sanchez-Mico, M.V., Bonnar, O. et al. Changes in glial cell phenotypes precede overt neurofibrillary tangle formation, correlate with markers of cortical cell damage, and predict cognitive status of individuals at Braak III-IV stages. acta neuropathol commun 10, 72 (2022). https://doi.org/10.1186/s40478-022-01370-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-022-01370-3