Abstract
Mild traumatic brain injuries (mTBIs) are a prevalent form of injury that can result in persistent neurological impairments. Microglial activation has become increasingly recognized as a key process regulating the pathology of white matter in a wide range of brain injury and disease contexts. As white matter damage is known to be a major contributor to the impairments that follow mTBI, microglia have rightfully become a common target of investigation for the development of mTBI therapies and biomarkers. Recent work has demonstrated that the efficacy of microglial manipulation as a therapeutic intervention following injury or disease is highly time-sensitive, emphasizing the importance of advancing our understanding of the dynamics of post-mTBI microglial activation from onset to resolution. Current reporting of microglial activation in experimental studies of mTBI is non-standardized, which has limited our ability to identify concrete patterns of post-mTBI microglial activation over time. In this review, we examine preclinical studies of mTBI that report on microglial activation in white matter regions to summarize our current understanding of these patterns. Specifically, we summarize timecourses of post-mTBI microglial activation in white matter regions of the brain, identify factors that influence this activation, examine the temporal relationship between microglial activation and other post-mTBI assessments, and compare the relative sensitivities of various methods for detecting microglial activation. While the lack of replicated experimental conditions has limited the extent of conclusions that can confidently be drawn, we find that microglia are activated over a wide range of timecourses following mTBI and that microglial activation is a long-lasting outcome of mTBI that may resolve after most typical post-mTBI assessments, with the exception of those measuring oligodendrocyte lineage cell integrity. We identify several understudied parameters of post-mTBI microglial activation in white matter, such as the inclusion of female subjects. This review summarizes our current understanding of the progression of microglial activation in white matter structures following experimental mTBI and offers suggestions for important future research directions.
Similar content being viewed by others
Background
Estimates have placed the annual global incidence of mild traumatic brain injury (mTBI) at over 600 cases per 100 000 people [1, 2], positioning the condition as a leading global health concern. Post-mTBI symptoms vary between people, but often include headaches, nausea, dizziness, impaired memory, poor concentration, and emotional issues such as irritability or anxiety [3]. While 80 to 90% of people experience symptoms that resolve within 7 to 10 days, the remaining 10 to 20% of individuals experience persistent symptoms that do not resolve for months or even years after injury [4].
Despite the high prevalence of mTBIs, there are no known methods to actively accelerate mTBI recovery or mitigate post-mTBI outcomes [5]. Consequently, the current state of mTBI care is restricted to advising patients to gradually return to full activity based on the progression of their symptoms [5]. The absence of effective therapeutic interventions may largely be attributed to our poor understanding of the complex and multifarious pathological sequelae of mTBIs. The heterogeneous nature of both the injuries sustained and their subsequent outcomes increases the difficulty of studying mTBIs in humans.
White matter regions of the brain, which are structures that primarily function to relay information between different brain regions, are known to be particularly vulnerable to microstructural damage in mTBIs [6, 7]. White matter damage following mTBIs has been strongly associated with the persistence of neurological deficits [8]. Though the precise mechanisms by which this damage occurs and recovers are not completely understood, some contributing processes have been identified. Diffuse axonal injury is a prevalent form of white matter damage following head trauma that is initiated by the mechanical forces of the trauma and is characterized by widespread patterns of damaged axons interspersed among intact ones, typically in midline white matter tracts such as the corpus callosum and internal capsules [6, 9]. For mild brain injuries, those mechanical forces may cause axonal cytoskeleton disruption or imbalances of intra-axonal ion concentrations that primarily lead to swelling and eventual secondary axonal disconnection. White matter damage can also occur in the form of degeneration or loss of oligodendrocyte lineage cells, which can subsequently lead to the disruption or loss of myelin. This disruption may be initiated by a variety of mechanisms after head trauma, including glutamate excitotoxicity, oxidative stress, and inflammatory consequences of activated astrocytes and microglia, reviewed in [10].
Emerging research has demonstrated a wide variety of contexts by which microglia interact with white matter during non-pathological conditions, including myelin control [11] and developmental myelination [12]. Following disease or pathology, there are several mechanisms by which microglia can function to mediate white matter damage and degeneration, including excessive post-injury myelin phagocytosis [13], inhibition of potentially assistive infiltrating macrophages [14], aberrant activation of the complement system [15], and the release of cytokines that are toxic to oligodendrocytes [16] or otherwise highly inflammatory [10, 17]. Contrasting these detrimental consequences are a variety of protective and restorative microglial functions, including debris clearance and the release of factors supporting axonal [18] and tissue regeneration [19,20,21,22]. This range of microglial functions associated with white matter injury and repair may be reflective of the many subpopulations of microglial cell types that exist during pathological conditions [23,24,25,26]. The wide range of degenerative and regenerative functions that microglia exhibit in white matter following injury position these cells as critical subjects of investigation for improving our understanding of mTBI pathologies.
Despite microglial activation being an established consequence of mTBI, the wide heterogeneity of injury parameters reported in experimental mTBI studies, including varied injury timepoints, brain regions assessed, animal models, and injury models themselves among others, create difficulties in generalizing the timecourse of microglial activation.
Detection methods of microglial activation are highly variable in regard to both technique (e.g., immunohistochemistry, electron microscopy, protein assays, transcriptional analyses, or flow cytometry) and readout (e.g., changes in microglial densities, morphologies, clustering patterns, or the detection of markers that are either specific to activated microglia or are commonly upregulated during microglial activation). These marker genes are usually associated with inflammatory microglial functions and typically do not distinguish between parenchymal microglia and infiltrating blood-derived macrophages [27]. The functions of these genes are varied, context-dependent, and still under investigation; more comprehensive summaries on what is known about them are available in other reviews [28, 29]. Though immunohistochemical quantification of ionized calcium binding adaptor molecule 1 (Iba1) and cluster of differentiation 68 (CD68) are the most commonly observed detection systems in this systematic review, there is no standardized system for choosing the marker or set of markers most appropriate for a given research question. The relative strengths and limitations of any set of markers remain poorly understood.
Understanding the timecourse and nature of microglial activation and their dependence on various injury and subject factors may be a critical step towards effectively harnessing microglia as therapeutic targets and biomarkers of injury progression.
Currently, it is common practice for experimental mTBI studies to interpret the extent of microglial activation as benchmarks for injury severity. While it does appear to be the case that more severe injuries lead to increases in the intensity and duration of microglial activation, it is not known how the usage of microglial activation as a scale of severity compares to other indicators of injury progression and outcome.
Though animal subjects commonly used for experimental mTBI such as mice have substantially less white matter than humans, many of the relevant cellular and molecular effects following injury (including microglial activation) can be reproduced in animal models [30]. In this systematic review, we examine studies of experimental mTBI that have quantified any form of microglial activation (changes in shape, size, density, count, gene expression, or cytokine production in response to injury) in white matter regions of the brain to summarize our current understanding on how subject factors, injury factors, and interventions influence microglial activation in white matter after mTBI. As well, we compare the timecourse of microglial activation to common behavioural assessments and other pathological measures to evaluate the usage of microglial activation as an indicator of injury progression and symptom resolution.
Methods
This systematic review is built on a protocol that adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement [31].
Search strategy
On November 25th, 2020, we collected studies from Embase and PubMed using the search strategy in Table 1.
Information sources and eligibility criteria
Articles from November 25th, 2020 and earlier mentioning microglia, white matter regions, and mTBI were searched for using PubMed and Embase. We included original, English research articles with no restrictions on date of publication or animal models used. We defined mTBI to be any traumatic brain injury that was described as mild, not described as moderate or severe, and did not induce noticeable skull fractures or brain lesions. To be included, studies needed to meet all of the following criteria: examination of experimental (preclinical) mTBI, quantification of microglial activation within at least one white matter region of the brain, statistical comparisons of microglial activation between injury groups and appropriate controls, and clear experimental design with complete reporting.
The stages of study selection are outlined in a PRISMA flow diagram (Fig. 1). We used the risk of bias tool from the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) [32] to assess biases present in the included studies (Additional File 1). A summary of statistical methods used during the evaluation of microglial activation in the white matter for each study is provided in Additional File 2.
PRISMA flow diagram. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. https://doi.org/10.1371/journal.pmed1000097 [33]
Study management
Duplicate studies were removed using EndNote X8 (Clarivate Analytics, Philadelphia, PA). All remaining studies were independently screened by two of the authors for eligibility criteria using Covidence’s systematic review manager. Conflicting decisions on the inclusion status of studies were jointly discussed and resolved. Data extraction was conducted independently by two of the authors using a pre-defined Microsoft Excel form for each study. Conflicting elements of the data extraction were jointly discussed and resolved. Finished forms were merged into one spreadsheet for meta-analyses (Additional File 3).
Data items
The complete list of data categories extracted can be found in the heading of Additional File 3. Briefly, all studies were extracted for general information (first author, year of publication, conflicts of interest), injury parameters (including number of injuries, inter-injury intervals, injury model, injury force, anesthesia, analgesia, injury location), all included assessments of impairment or pathology (behavioural tests, transcriptional analyses, protein assays, imaging metrics, histological assessments, etc.), assessment details (time between latest injury and assessment day, assessment outcome), and the sex, age, weight, and subject strain of the main experimental and control groups. When multiple experimental conditions were present, information was only extracted for conditions associated with at least one measurement of microglial activation in white matter.
Aim and objectives
The aim of this review is to summarize our current understanding of the temporal patterns of microglial activation in white matter regions following experimental mild traumatic brain injury. To achieve this aim, we set out to address the following objectives:
-
1.
Summarize the reported timecourses of post-mTBI microglial activation in white matter regions
-
2.
Identify subject and injury factors that are associated with changes in those timecourses
-
3.
Infer and compare timecourses of microglial activation across different detection methods
-
4.
Determine whether other common post-mTBI assessments resolve before or after the resolution of microglial activation in white matter regions
-
5.
Summarize the effects of post-mTBI interventions on microglial activation in white matter regions
Synthesis
To summarize the timecourses of post-mTBI microglial activation in the white matter, all extracted data entries (Additional File 3) were filtered to those comparing microglial activation in a white matter region of the brain between experimental injury groups and sham controls at the same timepoints. Any entries from the same study with different microglial activation detection methods or white matter regions assessed that were otherwise identical were pooled together (Additional File 4). In cases where two entries being merged had different outcomes (i.e., one detection method showed significantly increased microglial activation in white matter relative to shams while the other showed no significant difference relative to shams), the merged entry was marked as there being a significant increase in microglial activation. This was the only way in which two entries could conflict, as there were no entries showing significant decreases of microglial activation relative to shams. We chose to merge entries to identify how long any white matter region in the brain showed any signs of microglial activation. Unmerged entries remain available in Additional File 3 for accessible integration with the results of future work. After resolving conflicts, all entries that were identical apart from the time at which microglial activation was measured were clustered into separate experimental groups. Studies were assigned numeric IDs based on the largest number of mTBIs examined by that study. Experimental subgroups within studies were distinguished with alphabetic suffixes. A plot of timelines was generated to display experimental subgroups on the y-axis and the timepoints at which they examined microglial activation on the x-axis.
To determine whether other common post-mTBI assessments resolved before or after the resolution of microglial activation in the white matter, the latest timepoint of microglial activation within an experimental group was compared to the latest timepoint of all other reported deficits of that experimental group within each study. The number of experimental groups for which each assessment resolved before, after, or inconclusively relative to the resolution of microglial activation in the white matter was tallied.
To identify subject and injury factors that were associated with differences in microglial activation in the white matter, all entries within a study that were identical apart from a single data item relating to subject factors or injury factors were tallied for whether or not the change in that factor led to an increase, decrease, or inconclusive change in the timecourse of microglial activation in the white matter. This process was repeated to track how microglial activation in the white matter varied across changing interventions and detection methods.
Meta-biases and confidence in cumulative evidence assessments
We did not adhere to pre-defined methods of assessing potential meta-biases or relative degrees of confidence in our summarized evidence, as we did not find these assessments to be relevant to our systematic review of animal experiments.
Results
Included studies
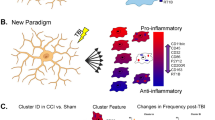
The initial search yielded 123 studies after deduplication. After screening, 30 articles were included in our quantitative synthesis. A brief summary of the parameters of the included studies is presented in Table 2. For studies containing more than one injury group or set of experimental conditions, those groups were separated into multiple rows in Table 2 and treated separately throughout the review. A summary of the distribution of major study parameters including sex, analgesic, white matter regions assessed, microglial activation detection methods, and animal models is shown in Fig. 2. An overview of each study’s contribution to Fig. 2 is available in Additional File 5.
Distributions of common parameters among included studies. A Sexes of included subjects. Both = male and female subjects examined separately; Mixed = male and female subjects pooled together. B Species of included subjects. C Injury model used. CI = controlled piston-driven impact; WD = weight drop; CH = Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA); BI = blast injury; FPI = fluid pulse injury; HIFU = high-intensity focused ultrasound; LIM = lateral impact model. C = closed-skull; O = open-skull. D Analgesia administered following mTBI procedure. E Regions in which microglial activation in the white matter was quantified. F Methods used to quantify microglial activation in the white matter. CD11b = cluster of differentiation 11b; CD68 = cluster of differentiation 68; EM = electron microscopy; H = histology; mRNA = messenger ribonucleic acid; qPCR = quantitative polymerase chain reaction
The majority of studies included male subjects only (23/30), whereas two studies included female subjects only. Four studies included both male and female subjects. Of those studies, two included separate male and female experimental groups (categorized as ‘Both’ in Fig. 2A), one included a single experimental group that pooled males and females together (categorized as ‘Mixed’), and one used both of the previously mentioned approaches (categorized as ‘Mixed and both’).
Most studies (22/30) used mouse models of mTBI. Some studies (7/30) used rat models, and one study used a ferret model (Fig. 2B).
Figure 2C shows the distribution of injury models across the included studies. The most commonly used injury model was some variation of closed-skull piston-driven controlled impact (CI [C]; 15 studies), followed by closed-head weight drop (WD [C]; 5 studies). The closed-head impact model of engineered rotational acceleration model (CH [C]) and open-skull piston-driven controlled impacts (CI [O]) were used in 3 studies each. The remaining injury models (blast injury (BI [C]), fluid pulse injury (FPI [O]), high-intensity focused ultrasound (HIFU [C])), and lateral impact model (LIM [C]) were present in one study each).
The usage of analgesic drugs was rarely reported. Buprenorphine and meloxicam were each used in 3 studies. Carprofen, cefazolin, and epinephrine were each used in one study each (Fig. 2D).
When assessing white matter regions for microglial activation, the corpus callosum was the most commonly sampled area (included in 22/30 studies). The optic tract was assessed in 6 studies, followed by the external capsule in 3 studies. The brachium of the superior colliculus, the fimbria, the internal capsule, and the olfactory nerve layer were included in 2 studies each. Remaining regions including the alveus, cingulum, optic nerve, pyramidal tract, striatum (structure containing both gray matter regions and white matter bundles (striatopallidal fibres / ‘pencils of Wilson’)), and subcortical white matter were assessed in one study each (Fig. 2E).
Iba1-based histology was used to detect microglial activation in 29/30 of the studies. CD68 was the next most commonly used marker, used in 6 studies. Detecting microglial activation with cluster of differentiation molecule 11B (CD11b) occurred in 2 studies. Quantification by electron microscopy was performed in one study. A breakdown of the specific quantification methods used, including colocalization-, morphology-, messenger ribonucleic acid (mRNA)-, area-, cell density-, and staining intensity-based approaches are shown in Fig. 2F.
All studies used isoflurane for anesthesia with the exception of one study, which used avertin.
Timecourses of post-mTBI white matter microglial activation
Post-mTBI microglial activation in the white matter was defined as any property of microglia, including marker expression, morphology, and distribution pattern, that were significantly higher in white matter regions of animals receiving mTBIs when compared to otherwise identical groups receiving sham surgeries. The assessment of any form of microglial activation in the white matter and its timing post-injury is plotted in Fig. 3. Studies were assigned numeric IDs based on the largest number of mTBIs examined. Studies which examined multiple injury conditions or subject parameters were further stratified into experimental groups denoted by an alphabetic suffix. Distinct experimental groups within individual studies are plotted separately. Parameters relevant to distinguishing experimental groups within individual studies are summarized in Table 2. For the studies contributing to Fig. 3, a detailed breakdown of all parameters, including the particular detection method used to detect microglial activation, is reported in Additional File 4.
Summary of reported timecourses of microglial activation across experimental groups of included studies. The x-axis represents days since their final injury (or days since injury in the case of single mTBI studies) on a logarithmic scale. Each row along the y-axis represents one set of experimental conditions (denoted by lettering from a to d from a particular study (denoted by identification numbers 01 to 30). Studies were arranged along the y-axis by the largest number of injuries (NOI) examined by any one of their experimental groups. Experimental groups within a study were arranged by NOI if possible or arranged randomly otherwise. For studies containing multiple experimental groups that differed by a parameter other than NOI, annotations containing the differing parameters are present. The number of injuries for each experimental group is listed in a colour coded column on the righthand side of the graph. Each circle marker represents a timepoint at which microglial activation was examined. Green circle markers indicate no detected change in microglial activation in the white matter relative to shams, while orange markers indicate significantly increased microglial activation in the white matter relative to shams. Depth = impact depth of controlled impactor during mTBI; ICI = ion channel inhibitors; III = inter-injury interval; MVM = mild ventriculomegaly; NOI = number of injuries; WT = wild-type
The ordering of experimental groups in Fig. 3 reveals a general trend of longer timepoints assessed for studies which examined a greater number of mTBIs. Assessments of microglial activation that showed no significant differences relative to shams appear to disproportionately occur in the lower half of Fig. 3, suggesting that a greater number of mTBIs may be associated with a longer timecourse of microglial activation.
The post-mTBI delay to microglial activation is the time taken between the injury and the onset of microglial activation. Though there is a delay to all post-mTBI microglial activation [34], only a handful of experimental studies have sampled enough early timepoints to observe it. A defined delay to post-mTBI microglial activation can be seen in groups 21a [35], 12a and 12d [36], 02b [37], 07a [38], and 16a [39] in the form of timecourses containing at least one early timepoint of no microglial activation (green dot in Fig. 3) preceding at least one timepoint of significantly increased microglial activation (orange dot in Fig. 3). In group 21a [35], a delay to activation between 1 and 5 days is observed for mice receiving a single piston-driven closed-skull controlled impact. In groups 12a and 12d [36], the delay to activation occurs between 2 and 7 days post-final-injury (dpfi). These groups represent two closed-head impact model of engineered rotational acceleration (CHIMERA) mTBIs delivered 1 day apart to 54-week-old wild-type mice and 24-week-old APP/PS1 mice, respectively. Group 02b [37], representing leukemia inhibitory factor (LIF) haplodeficient mice receiving a single controlled piston-driven impact, demonstrated delayed activation between 7 and 14 days post-injury. Group 07a [38], representing male mice given a single, CHIMERA injury, shows a delay to activation between 6 h and 1 day post-injury. Finally, group 16a [39], representing mice receiving 2 controlled piston-driven impacts separated by 1 day, show delayed activation occurring between 2 and 4 dpfi. Remaining plotted timecourses with increased microglial activation at the earliest timepoint measured suggest that those timepoints may be upper bounds for the associated groups’ delay to microglial activation. Alternatively, those groups may have sustained injuries that led to virtually no delay to microglial activation. The possibility of a microglial response occurring very shortly after injury is supported by the detection of microglial activation as early as 2 h post-final injury in groups 06a [40] and 27a [41], the former of which involved a single mTBI to mice. Other timecourses that show no microglial activation at any timepoint (groups 21a [35], 12b and 5c [36], 22a-c [42], 17a and 17c [43], 13b and 9b [44], and 01a [45]) are similarly ambiguous, as they may reflect injuries which do not induce microglial activation in the white matter, injuries with delays to microglial activation in the white matter longer than the latest sampled timepoint, or injuries where microglial activation in the white matter resolved prior to the first assessment timepoint. It is important to consider that activation timecourses for experimental groups receiving multiple injuries may be misleading to directly compare with activation reported in single mTBI studies, as it is unclear if the observed activation was initiated by the final mTBI or by any of the preceding mTBIs. Accounting for the possibility that the first mTBI initiated the activation in the previously mentioned groups would lead to the following ranges of delays in microglial activation: 2 to 8 days for 12a and 12d [36]; 7 to 14 days for 02b [37]; and 2 to 5 days for 16a [39]. The findings from studies 02 [37] and 12 [36] may, however be influenced to some extent by the abnormal conditions of the experimental groups they describe. Group 12a [36] contained APP/PS1 mice, group 12d [36] contained aged mice, and group 02b [37] contained LIF haplodeficient mice.
Intra-study differences are bolded for studies with multiple injury groups. M = mouse; R = rat; m = male; f = female; mix = males and females; NOI = number of injuries. WD = weight drop; CI = controlled piston-driven impact; FPI = fluid pulse injury; CH = Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA); BI = blast injury; HIFU = high-intensity focused ultrasound; LIM = lateral impact model. (C) and (O) suffices indicate closed-skull and open-skull injury models, respectively.
Several studies report microglial activation at relatively chronic phases of the injury, ranging from 2 weeks post-final-injury to 1 year post-final-injury. These studies include groups 29a ([46]; 20 weight drop injuries to mice spaced 1 to 3 days apart; activation lasting at least 90 dpfi), 21b and 21c ([35]; 5 controlled piston-driven impacts to mice spaced 1 or 2 days apart; activation lasting at least 70 dpfi), 19a ([47]; 4 fluid pulse injuries to rats spaced 7 days apart; activation lasting at least 90 dpfi), 17a-c ([43]; 2 weight drop injuries to rats spaced 1 day apart; activation lasting at least 90 dpfi), 23a ([48]; 5 controlled piston-driven impacts to mice spaced 2 days apart; activation lasting at least 270 dpfi), 18a ([49]; 3 weight drop injuries to mice; activation lasting at least 60 dpfi), 20a and 20b ([50]; 4 controlled piston-driven impacts given to mice; activation lasting at least 180 dpfi), 25a and 25b ([51]; 1 or 5 controlled piston-driven impacts spaced 2 days apart; activation lasting at least 360 dpfi), 26a ([52]; 5 controlled piston-driven impacts to mice spaced 2 days apart; activation lasting at between 90 and 180 dpfi), 08a ([53]; 1 controlled piston-driven impact to ferrets; activation lasting at least 112 dpfi), 09a and 09b ([54]; 1 controlled piston-driven impact to mice; activation lasting at least 30 dpfi), 16a ([39]; 2 controlled piston-driven impacts to mice spaced 1 day apart; activation lasting at least 49 dpfi), 30a ([55]; 30 controlled piston-driven impacts to mice spaced 1 to 3 days apart; activation lasting at least 365 dpfi), and 27a ([41]; 5 controlled piston-driven impacts spaced 1 day apart; activation lasting at least 42 dpfi). For the groups in which the final timepoint assessed showed increased microglial activation in the white matter, it is unknown whether or not that activation will eventually resolve.
A slight positive association was observed between number of injuries and the duration of microglial activation in Fig. 3. Using a similar approach to compare timecourses between different injury models produced results that were less clear. A variation of Fig. 3 in which studies were primarily sorted by injury model and secondarily sorted by number of injuries is provided in Additional File 6. As noted in Fig. 2, the closed-head form of the controlled piston-driven impact model (CI (C)) was the primary model of choice by a substantial margin with 15 studies (29 experiments) compared to 5 studies (8 experiments) using the second most popular injury model, closed-head form of the weight drop model (WD (C)). When attempting to examine how microglial activation may compare between the two most commonly used models, CI (C) and WD (C), there was high proportion of experimental groups that showed microglial activation at the latest examined timepoint (6/8 = 75% of WD (C) and 21/29 = 72% of CI (C)). As the end of microglial activation cannot be determined in these experimental groups meaningful comparisons between the models cannot be made.
Though the timecourses presented in Fig. 3 provide some information about the potential patterns of post-mTBI microglial activation in the white matter, such as the range of delays to activation and the positive association between the number of injuries and the duration of activation, they more strikingly reveal a highly inconsistent approach to sampling post-mTBI microglial activation. Critical areas of the timecourses which appear to be particularly lacking include the temporal resolution of assessing the acute stage, for which increased assessments may lead to a better understanding of the delays to microglial activation associated with a given injury model, as well as the upper limits of assessing the chronic stage, for which later assessments may provide insights into the permanence of the neuroinflammation being induced by the original injuries.
The influences of the annotated inter-study differences in Fig. 3 on outcomes of post-mTBI microglial activation in white matter regions is examined in the following sections of the review.
Subject and injury factors influencing post-mTBI microglial activation in the white matter
The effect of both age and Alzheimer’s status were examined in Cheng et al. [36]. Microglial activation in the white matter was measured in the optic tracts of 24-week-old and 54-week-old wild-type (WT) and APP/PS1 mice at 2 and 7 dpfi. Microglial activation in the white matter was absent on at both timepoints for the 24-week-old WT mice (ID 12c) but only present at 7 dpfi in the 54-week-old WT mice (ID 12d). Oddly, this age effect was reversed in APP/PS1 mice; microglial activation in the white matter was absent at both timepoints for 54-week-old APP/PS1 mice (ID 12b) but only present at 7 dpfi in 24-week-old APP/PS1 mice (ID 12a). The authors interpret the outcomes of the WT mice to be reflective of older animals having generally heightened microglial responses, while the outcomes of the young and old APP/PS1 respectively demonstrate a hypersensitivity of microglia around the initial time of amyloid-beta deposition and a hyposensitivity of microglia after prolonged inflammation. Further studies are required to determine if this finding occurred by chance or is indicative of a reproducible, biological phenomenon.
The effect of leukemia inhibitory factor (LIF) haplodeficiency was examined in Goodus et al. [37]. LIF is a cytokine that has been identified as playing a critical role in multiple aspects of the central nervous system’s response to injury, including neuroprotection, axonal regeneration, remyelination, and immune cell activation [56]. WT mice receiving an mTBI showed microglial activation in the corpus callosum at 2 dpfi, but no activation at 7 or 14 dpfi (ID 02a), while LIF± mice showed activation at 14 dpfi, but no activation at 2 or 7 dpfi (ID 02b). In addition to the delayed microglial response, the LIF± mice demonstrated more severe motor and sensory deficits following the mTBI than their WT counterparts. Whether the delayed response played a causative role in the exacerbated outcomes remains to be determined. The effect of the number of mTBIs on the timecourse of microglial activation in the white matter was examined in 5 studies, but conclusive answers were only found in 2 studies. In Bolton Hall et al. [35], mice receiving 5 mTBIs with an inter-injury interval of 1 day (ID 21b) or 5 mTBIs with an inter-injury interval of 2 days (ID 21c), but not 1 mTBI (ID 21a), showed increased microglial activation in the corpus callosum at 1 dpfi. In Fehily et al. [43], increased microglial activation in the corpus callosum was observed at 90 dpfi in mice receiving 2 mTBI (ID 17b), but not in mice receiving 1 mTBI (ID 17a) or 3 mTBIs (ID 17c). No interpretations of the increased activation observed following 2 injuries but not 3 injuries were provided.
Subject and injury factor comparisons were made between other experimental groups, but microglial activation in these cases were only assessed at timepoints that did not yield any conclusive information. This could have been due to the presence of microglial activation not being observed at any timepoint measured for both groups, persisting beyond the latest timepoint measured for both groups, or resolving between two intervals that overlapped between the two groups. In addition to the comparisons previously stated, these comparisons can be seen in Table 3.
The aforementioned findings collectively suggest that post-mTBI outcomes of microglial activation in white matter regions, and possibly many other post-mTBI outcomes by extension, are complex, non-linear, and dependent on the specific combination subject details and injury conditions. That complexity provides further evidence that the approach of intentionally replicating specific experimental conditions by future studies may accelerate our understanding of the overall pathology of mTBIs at a much faster rate than the approach of testing novel experimental conditions and attempting to integrate the subsequent findings with the existing literature.
Comparisons of methods for the detection and analysis of microglial activation
Though the detection of microglial activation is a common assessment following experimental mTBI, a considerable variety of methods were employed across the studies. There were 5 included studies which quantified microglial activation in the white matter in the same injury models using more than one approach. The summaries of these inter-approach comparisons are provided in Table 4.
In Haber et al. [60], the corpus callosum of mice given mTBIs showed increased immunoreactivity for CD68, but not Iba1, at 2 dpfi. In Haber et al. [61], increased Iba1 immunoreactivity in the corpus callosum was observed on 2 and 4 dpfi, while increased CD68 immunoreactivity was only observed at 2 days post-injury. In Robinson et al. [62], both Iba1 and CD68 were observed to have increased immunoreactivity in the fimbria of mTBI mice at 1 and 7 dpfi, which did not provide any conclusive evidence towards their orders of occurrence. Future work employing higher temporal resolution is required to determine the relative ordering of Iba1- and CD68-based activation.
In Schwerin et al. [53], the subcortical white matter of ferrets given one mTBI were measured for percentage area labeled of Iba1+ cells and for the number of Iba1+ cell clusters at 1, 7, 28, and 112 dpfi. Increased percentage area labeled was observed at 7 and 112 dpfi (the only reported instance of bimodal microglial activation across all included studies), while increased cell clustering was observed at 28 and 112 dpfi. In Yu et al. [41], CD11b+ cells in the corpus callosum of mice given 5 mTBIs were quantified by percentage area labeled and by the number of cells exhibiting non-resting morphologies. Activation by morphology and activation by area labeled were detected at 1, 7, and 42 dpfi, but only morphological analysis revealed significant activation at 2 h post injury.
These findings highlight both the complexity of microglial dynamics following mTBI as well as the impact of choice in analytical method on the determination of microglial activation. Understanding whether the relative sensitivities of the different approaches are injury-specific or have a consistent pattern that has yet to be identified may require a more formal investigation than can be provided by a meta-analysis of the current literature.
Persistence of microglial activation relative to other deficits and injury markers
We tallied the number of times all reported behavioural and pathological metrics of mTBI resolved before, after, or at inconclusive timepoints relative to the resolution of microglial activation in white matter regions (abbreviated as B:A:I; Fig. 4). The complete breakdown of the experimental groups contributing to Fig. 4 are provided in the “Assessment tallies” tab of Additional File 7. Brief descriptions of the included assessments are provided in the “Summary of assessments” tab of Additional File 7. Generally, studies made use of behavioural, histological, protein measurement, and mRNA measurement techniques to measure outcomes in learning, memory, motor coordination, axonal and neuronal degeneration, microglial and astrocytic activation, and regulation of inflammatory cytokines. Tallies from studies which only assessed microglial activation at immediate/early-acute timepoints (less than 7 days) were considered inconclusive to avoid misinterpreting cases in which microglial activation was yet to occur for cases in which it had rapidly resolved or would never begin. These studies included Eyolfson et al. ([42]; Study ID 2; activation measured at 5 dpfi) and Gatson et al. ([45]; Study ID 10; activation measured at 3 dpfi). Additionally, experimental groups with additional factors that may not be reflective of the general recovery process following mTBI were excluded. The excluded experimental groups were 12a and 12b (Cheng et al. [36]; animals were APP/PS1 mutants), 01b (Gatson et al. [45]; mice were treated with resveratrol), 02b (Goodus et al. [37]; mice had LIF± genotype), 20b (Maynard et al. [50]; animals were Sarm1-KO mutants), 18 and 19 (Mouzon et al. [57] and Mouzon et al. [51]; animals were hTau mutants), 14b (Namjoshi et al. [63]; animals were treated with androgenic–anabolic steroids), and 10b (Tu et al, [59]; animals had mild ventriculomegaly).
Contextualizing microglial activation in white matter relative to other post-mTBI assessments. ‘Before’ and ‘after’ tallies refer to when an assessment resolved relative to the resolution of microglial activation. Instances where limited temporal range or resolution of the assessment obscured the relative orders of resolution were tallied as ‘Inconclusive’. Specific assessment descriptions are provided in Additional File 7. 5-HT1B = 5-hydroxytryptamine receptor 1B; 8-OHdG = 8-hydroxy-2’-deoxyguanosine; AD = axial diffusivity; ADC = apparent diffusion coefficient; APP = amyloid precursor protein; Arg-1 = arginase-1; BDA = biotinylated dextran amine; CD11b = cluster of differentiation molecule 11b; CD206 = cluster of differentiation 206; CD40 = cluster of differentiation 40; CD68 = cluster of differentiation 68; CD86 = cluster of differentiation 86; CNPase = 2’,3’-Cyclic-nucleotide 3’-phosphodiesterase; CV = cresyl violet; DAPI = 4’,6-diamidino-2-phenylindole; FA = fractional anisotropy; FJC = Fluoro-Jade C; GFAP = glial fibrillary acidic protein; IL-1β = interleukin 1 beta; IL-12 = interleukin 12; IL-6 = interleukin 6; iNOS = inducible nitric oxide synthase; Ka = axial kurtosis; Kr = radial kurtosis; LFB = luxol fast blue; MBP = myelin basic protein; MD = mean diffusivity; MK = mean kurtosis; MOG = myelin oligodendrocyte glycoprotein; mRNA = messenger ribonucleic acid; MTR = magnetization transfer ratio; NF200 = neurofilament protein; NF-L = neurofilament light protein; NG2 = neural/glial antigen 2; Nrf2 = NF-E2 DNA binding protein; Olig2 = oligodendrocyte transcription factor; PDGFRα = Platelet-derived growth factor receptor α; PLP = proteolipid protein 1; p-STAT3 = phospho-signal transducer and activator of transcription 3; p-tau = phospho-tau; qPCR = quantitative polymerase chain reaction; RA = relative anisotropy; RD = radial kurtosis; RNA = ribonucleic acid; TNF-α = tumor necrosis factor alpha; TNFR1 = tumor necrosis factor receptor 1; TNFR2 = tumor necrosis factor receptor 2; t-tau = total tau
Histological measures (Fig. 4) were the most commonly reported outcomes of mTBI among the included studies. Many studies suggested that both Iba1+ gray matter activation (Fig. 4A; B:A:I: = 11:0:13; experimental groups 21b-c, 17b, 05a, 06a, 08a, 15a-b, 09a, 09c and 16a contribute to the before tallies) and brain-wide glial fibrillary acidic protein (GFAP)+ astrocytic activation (B:A:I: = 5:0:14; experimental groups 17b, 07a, 15a-b, 27a contribute to the before tallies) persist for less time than microglial activation in the white matter.
A large proportion of the markers associated with oligodendrocyte lineage cells showed abnormal levels persisting after the resolution of microglial activation in the white matter. The oligodendrocyte lineage markers in Fig. 4A include anti-adenomatous polyposis coli clone CC1 (B:A:I: = 0:1:0), 2’,3’-Cyclic-nucleotide 3’-phosphodiesterase (CNPase; B:A:I: = 0:1:0), myelin basic protein (MBP; B:A:I: = 1:1:0), myelin oligodendrocyte glycoprotein (MOG; B:A:I: = 1:0:0), neural/glial antigen 2 (NG2; B:A:I: = 1:0:0), oligodendrocyte transcription factor (Olig2; B:A:I: = 0:1:0), platelet-derived growth factor receptor α (PDGFRα; B:A:I: = 0:1:0), proteolipid protein 1 (PLP; B:A:I: = 0:1:0), and Rip (B:A:I: = 0:1:0). These assessments primarily came from experimental groups 02a (male and female mice receiving a single, closed-skull, controlled piston-driven impacts) and 04a (male mice receiving a single, open-skull, controlled piston-driven impact).
All protein levels (Fig. 4B), magnetic resonance imaging metrics (Fig. 4C), and mRNA levels (Fig. 4D) measured in post-mTBI brain across the included studies had either returned to sham levels before the resolution of microglial activation in the white matter or had persisted at least as long as its latest detection. Targets of protein and mRNA analyses were sparsely distributed across studies and predominantly focused on markers of microglia, macrophages, and inflammation. No transcripts were measured by more than one study.
Of the electron microscopy metrics (Fig. 4E), the number of oligodendrocytes (B:A:I: = 0:1:0), myelin integrity (B:A:I: = 0:1:0), and the average G-ratio of axons within the corpus callosum (B:A:I: = 0:1:0) were the only measures to have any occurrences of resolving later than microglial activation in the white matter. Though there is only a single tally for each of these measures, these findings support the possibility of prolonged post-mTBI oligodendrocyte lineage cell impairment following seen in Fig. 4A.
Most behavioural deficits following mTBI resolved prior to the resolution of microglial activation in the white matter or at inconclusive timepoints (Fig. 4F). The only exception was the open field test (B:A:I: = 3:2:7), which may better temporally correlate with microglial activation in the white matter than the other tallied tests. The two ‘after’ tallies for the open field test came from the experimental groups 15a and 15b [58], which included male mice receiving one or two closed-skull, controlled piston-driven impacts, respectively.
Remaining assessments (Fig. 4G) included measures of oxygen consumption rate, in situ end labeling (ISEL), flow cytometry counts of various immune cells in the brain parenchyma, electrophysiological recordings (conduction speeds of the corpus callosum), brain swelling, and serum corticosterone levels. ISEL (B:A:I: = 0:1:0), electrophysiology (B:A:I: = 0:1:0), and serum corticosterone (B:A:I: = 1:1:1) were the only metrics of these to have any occurrences of resolving later than microglial activation in the white matter. Similar to most other assessments tallied, more work employing these techniques is required before their order of resolution relative to the resolution of microglial activation in the white matter can be confidently determined.
These findings appear to collectively demonstrate that while microglial activation in white matter regions may be one of the most persistent effects of mTBI, it is likely outlasted by changes in the structure and expression of oligodendrocyte lineage cells.
Effects of interventions on microglial activation in the white matter and post-mTBI outcomes
Two of the included studies examined the effects of therapeutic interventions on microglial activation in the white matter and other post-mTBI outcomes.
In Gatson et al., 2013 [45], mice were treated with placebos (ID 01a) or 100 mg/kg resveratrol (ID 01b) at 5 min and 12 h following mTBI. Resveratrol (3,4’,5-trihydroxystilbene) is a plant-derived phenol and phytoalexin that has been suggested to have beneficial anti-inflammatory and immunomodulatory effects [64]. Unlike mice receiving placebos, the mice that were treated with resveratrol post-mTBI displayed no microglial activation in the corpus callosum, and no increases in hippocampal levels of the pro-inflammatory cytokines IL-6 or IL-12 at 3 dpfi. Whether the intervention delayed, prevented, or accelerated the resolution of microglial activation is unknown. Additionally, the absence of behavioural testing and measures of brain tissue integrity at later timepoints make it unclear if the altered microglial response was associated with a better post-mTBI outcome.
In Namjoshi et al. [63], male mice were treated with either a vehicle (group 14a) or a combination of androgenic–anabolic steroids (AAS; group 14b) including testosterone, nandrolone, and 17α-methyltestosterone for 8 weeks starting at the age of 8 weeks. Mice received 2 CHIMERA mTBIs spaced 1 day apart at the 7th week of their AAS or vehicle treatment, and assessments were carried out over the following week. Compared to vehicle-treated mice, the mice treated with the AAS combination showed no changes in behavioural performance but did have increased axonal damage and magnitude of microglial activation. However, as both the vehicle and AAS-treated groups showed increased microglial activation relative to shams at the only time of tissue collection (7 days dpfi), there was no conclusive difference in the timecourse of microglial activation observed between the two groups.
These studies indicate that the usage of interventions may be highly effective in altering the persistence of microglial activation in white matter regions following mTBI. Further studies will be required to determine how the direction of manipulating microglial activation and the timing of those manipulations interact to influence neurological recovery and brain repair.
Discussion
In this systematic review, we summarized the current state of literature regarding the timecourse of microglial activation following experimental mTBI. Despite the known significance of microglia in regulating white matter damage and repair following injury, there is insufficient information in the literature to confidently identify any robust patterns of post-mTBI microglial activation in the white matter. Post-mTBI microglial activation in the white matter reported in the included studies revealed a wide range of possible timecourses that remain challenging to explain given the limited number of studies for any particular set of experimental parameters. A single injury could not trigger any noticeable increase in microglial activation in some cases (e.g., ID 13b [44]) but initiated microglial activation persisting for over a year in others (e.g., ID 25a [51]). As well, the possibility of multimodal activation, as seen in a subgroup of Schwerin et al., 2018 [53] may be commonly overlooked by low sampling frequencies following mTBI.
One unknown relationship raised by the comparisons of the timelines observed in Fig. 3 and Figure S1 of Additional File 6 is the possibility that different injury models may produce different activation durations. In this review, any further investigation into this question was hindered by the small number of studies using injury models other than the CI (C) model as well as the high proportion of experimental groups for which the end of microglial activation was never determined.
Initial steps have been made towards understanding how different subject and injury factors can influence the course of mTBI. Among the studies included in this review, microglial activation in the white matter has been demonstrably altered by varying age [36], genotype [36, 37], number of injuries [35, 43], and therapeutic intervention [45, 63]. No conclusive determinations were made on the influence of inter-injury interval or subject sex on microglial activation in the white matter. The general trend of excluding female subjects from experimental mTBI research is problematic, particularly given the substantial body of evidence suggesting that males and female experience different outcomes following head injury [65,66,67,68,69]. There is an urgent need to increase research on the sex differences of the pathological mechanisms underlying mTBIs. The effects of other common mTBI parameters, such as injury location or the dosage and choice of anesthesia and analgesia were not examined by any of the included studies. Understanding how these parameters influence the course of post-mTBI recovery may allow for improved mTBI treatment plans in a clinical setting. The effect of inter-injury interval, which was only investigated by one study in this review ([35] – ID 21b vs. 21c) is especially important for groups with a high risk of repeat mTBI, including military personnel, children, the elderly, survivors of domestic violence, and athletes, as post-mTBI returns to full activity are currently entirely dependent on symptom resolution rather than any biological marker of resolution in brain vulnerability.
The limitations of symptom-based guidelines for mTBI patients returning to high-risk activity are emphasized by the majority of detectable behavioural deficits in experimental groups resolving prior to the end of microglial activation in the white matter. A patient with persisting neuroinflammation that sustains a subsequent mTBI may experience longer lasting and more serious impairment as a consequence [70]. Though the behavioural methods tallied in this review are not perfectly representative of the post-mTBI deficits that humans experience, the possibility of incomplete brain recovery persisting beyond the apparent resolution of symptoms for any patient of mTBI may be a valid generalization.
Though the mechanistic investigations of microglia are not typically conducted in studies related to mTBI, works from other neuropathological contexts have made progress in identifying specific details by which microglia can influence behavioural performance, protein and transcriptional levels, and microstructural brain changes during development, homeostasis, and pathology. Some examples include the capability of microglia to shape neural circuitry during development using the complement signaling pathway [71], that microglia can repair myelin following injury in a neuronal activity-dependent mechanism [72], that overexpression of the eukaryotic translation initiation factor 4E (eIF4E) in microglia causes autism-like behaviours in male mice only [73], that microglial activity detectable by positron emission tomography is a strong correlate of white matter structural integrity in patients of multiple sclerosis [74], and that microglia can contribute to Alzheimer’s Disease pathology by regulating tau phosphorylation [75].
While the observation that microglial activation persisted beyond the resolution of most other mTBI assessments in Fig. 4 may appear to indicate that these cells do not causally contribute to those impairments and deficits, substantial evidence from previous work demonstrating that microglia play critical roles in both the injury and repair components of neuropathological conditions (the concept of ‘good’ and ‘bad’ microglia in mTBI are reviewed in detail elsewhere [76]) suggest that this is not necessarily be true. It is instead more likely that activated microglia contributed to the development and resolution of some, if not all, of the assessments included in Fig. 4. For assessments that hypothetically progress completely independently of any microglial influence, the value of identifying their order of resolution relative to microglial activation in white matter regions is primarily restricted to identifying which assessments may be the most effective mTBI biomarkers.
Oligodendrocyte lineage marker disruption was found to persist after the resolution of microglial activation. Given the substantial body of evidence suggesting that white matter impairment is one of the strongest correlates of impairment following mTBI [6,7,8, 77], we interpret this finding as support for the possibility that microglia contribute to prolonged impairment following mTBI by mediating lasting damage to white matter structures of the brain. This damage may be caused during periods of overactivation but persist well after microglia have returned to homeostatic levels. Though microglia also function to repair white matter pathology following injury, the incomplete recovery of oligodendrocyte lineage cells to a homeostatic transcriptional signature could be indicative of those repair processes ending prematurely. This persistent white matter damage may be too subtle to detect through traditional experimental behavioural tests but may compound after additional injuries into more serious impairment. In the clinical context, this may be analogous to slight alterations in cognition or behaviour that are not easily observed by current techniques in symptom assessment.
The usage of more sophisticated methods of characterizing microglial activation is of high importance. Though the broader field of microglial research has moved on to investigating microglial activation as a multi-dimensional phenomenon, the mTBI studies featured in this review have all focused on relatively simple activation models. Some included studies have moved beyond the exclusive usage of markers like Iba1 or CD68 and have instead focused on the M1/M2 schema, which proposes a spectrum of activation along two major microglial phenotypes: “classical” activation of pro-inflammatory (M1) microglia and “alternative” activation of anti-inflammatory (M2) microglia. This model has since been considered an oversimplified system that may hinder our understanding of microglial biology more than it helps [78], resulting in recent decline in usage.
Currently, microglia are considered to execute their vast range of functions by adopting a similarly vast range of subtypes and reaction states, typically characterized through single-cell RNA sequencing. A recent review by Stratoulias et al. [79] provides a highly detailed overview of several major microglial subtypes reported as of 2019 alongside many of the key points of debate regarding the definition and identification of novel subtypes.
Disease-associated microglia (DAM) [80], a population with downregulated C-X3-C motif chemokine receptor 1 (Cx3cr1), cluster of differentiation 33 (CD33), and transmembrane protein 119 (Tmem119), were found to significantly slow the progression of a mouse model of AD.
Inflammatory-responsive microglia, characterized by upregulated levels of galectin 3 (Lgals3), cystatin F (Cst7), C–C motif chemokine ligand 4 (Ccl4), C–C motif chemokine ligand 3 (Ccl3), and interleukin 1 beta (Il1b) [81], were identified as potential drivers of age-related neuroinflammation.
Activated response microglia (ARMs), characterized by overexpression of MHC type II, Dickkopf-related protein 2 (Dkk2), hematopoietic growth factor inducible neurokinin-1 type (Gpnmb), and secreted phosphoprotein 1 (Spp1) [82], were identified as potential mediators of both age-, sex-, and genetic-based heightened risk for AD.
One microglial phenotype that is especially relevant to the subject of white matter damage is the integrin alpha X (CD11c) + microglia that were potentially first identified in a 1994 multiple sclerosis pathology study by Ulvestad et al. [83]. These cells are now recognized to play major roles in the regulation of myelin across developmental, homeostatic, and pathological contexts (described in detail by a 2020 review from Benmamar-Badel et al. [84]).
Though any of these phenotypes may contribute to mTBI pathology, there were notably no studies in this review which attempted to deeply characterize the microglial diversity associated with their preclinical models of mTBI. Future studies which take care to consider the role of microglia through the lens of highly diverse subtypes and activation states may greatly improve our understanding of how findings from different preclinical models of brain injury or other neuropathological conditions relate to each other and to the clinical mTBI setting.
In the context of detecting neuroinflammation in vivo as a biomarker of post-mTBI recovery, the current gold standard is the positron emission tomography (PET) of translocator protein (TSPO), which becomes highly upregulated in microglia following activation [85]. However, the invasiveness and exposure to radiation required for TSPO PET imaging may limit its usage in clinical contexts as a biomarker for mild brain injuries. Advancements in magnetic resonance imaging (MRI) techniques may eventually alternatively permit the accurate, whole-brain characterization of morphologically activated microglia in live subjects in a substantially less invasive way [86, 87].
Studies that pair these sophisticated techniques of detecting microglial activation with experimental designs that prioritize increased temporal resolution may begin to identify practical post-mTBI therapeutic windows to target microglia. The value in understanding the timing of microglial activation can be clearly seen in such studies as Willis et al. [88], in which microglial depletion and subsequent repopulation with a drug at the moment of injury had great therapeutic value, but the same treatment administered with a delay after injury had no beneficial effect. Similarly, in Brody et al. [89], constant microglial depletion starting 7 days prior to the injury and ending 21 days after the injury showed no therapeutic value.
In addition to improving the temporal resolution with which microglial activation is assessed, future studies should take increased care during the selection of markers used to identify activated microglia. Many markers commonly used to detect activated microglia are found on resting microglia as well. Some markers thought to be specific to activated forms of microglia include major histocompatibility complex II (MHC II) [90], translocator protein (TSPO) [91], restin-like alpha (FIZZ1) [92], and matrix metalloproteinase-9 (MMP9) [93]. Alternative approaches to using activation-specific markers include assessing the relative ratios of common microglial markers to identify activated signatures or to characterize activated morphologies among cells identified through individual common microglial markers [29].
Another concern associated with marker selection is the lack of discrimination between innate microglial cells of the central nervous system and peripheral macrophages which are capable of infiltrating the brain parenchyma upon injury [94, 95]. Markers which cannot distinguish between peripheral macrophages and microglia when used alone include CX3CR1, Iba1, cluster of differentiation 45 (CD45), epidermal growth factor-like module-containing mucin-like hormone receptor-like 1 (F4/80), and CD11b [96]. Some markers that are currently considered to be specific to microglia include transmembrane protein (TMEM119), P2Y purinoceptor 12 (P2RY12), and Sal-like protein 1 (SALL1) [97].
The work in Brody et al. [89] also highlights how the absence of microglial activation itself may not be unconditionally therapeutic; it is likely the specific types of activation blocked and the timing of those blocks that are critical in providing reversing and minimizing the damage that can follow an mTBI. In the context of interventions, we are still far from understanding mechanistic links between compounds affecting microglial activation and how they improve the outcomes of mTBIs. This gap must be addressed in order to develop clinically promising candidate therapies that are specific, selective, and effective.
Ultimately, the discordance of experimental paradigms, the lack of temporal range and resolution of data collection, and the limited numbers of markers employed for detecting microglial activation have greatly hindered our understanding of the underlying temporal patterns of microglial activation in white matter following experimental mTBI. Consequently, a cohesive model describing the timecourse of microglial activation following mTBI remains a goal for future syntheses. For future studies to accelerate our understanding of these patterns, we strongly encourage researchers using experimental mTBI models to consider replicating injury parameters from previously published studies in addition to increasing the range and resolution of microglial activation measurements in white matter regions.
Though a variety of these parameters may be beneficial for identifying broad patterns of microglial activation that are more likely to have translational value, it is clear that the current field of the role of microglia in experimental mTBI is spread thin. Improving the normalization of data collection in future studies will be an invaluable approach to improving our understanding of the role of microglia and the mechanisms underlying mTBI as a whole at a faster pace. One of the challenges of such normalization that is likely responsible for the discordance of the current literature is that the selection of precise injury model parameters, assessment timepoints, and markers is a largely arbitrary process. We do not have specific recommendations for any of those parameters, but for future experimental studies examining microglia in the context of post-mTBI white matter damage, we suggest three experimental design choices which may contribute to a more harmonized body of literature from which meaningful interpretations can more easily be drawn from: 1) when establishing a new model of mTBI or using a model for which the post-mTBI delay to microglial activation or duration of microglial activation are unknown, examine microglial activation in as many early acute (~ 1–7 days [98,99,100]) and chronic (> 14–60 days [98,99,100]) timepoints as possible; 2) when selecting experimental parameters, assessment techniques, and assessment timepoints for a new study, consider matching or including the choices from previous studies; 3) when characterizing microglial activation, use specific markers of microglial activation, use multiple markers, and use single cell ribonucleic acid (RNA)-sequencing or other rigorous transcriptional characterization approaches when possible.
Limitations
As a consequence of including studies that may have been statistically underpowered (see Additional File 2), this review has a risk of misinterpreting instances where one group has a lower magnitude but longer lasting period of microglial activation relative to another group as a situation in which that second group has the longer lasting activation. This review also has a risk of mischaracterizing the relative order of the resolution of different post-mTBI changes which used assessment methods of different sensitivities.
The analyses performed in this review relied on multiple assumptions and simplifications that limit the strength of their conclusions. When summarizing timecourses of microglial activation in the white matter, assessments within experimental groups involving different white matter regions or detection methods of microglial activation were pooled together. As well, the individual timecourses were not separated by detection method or white matter region. These simplifications allowed the timecourses observed across all studies to be directly compared but may lead to misleading interpretations when comparing timecourses that were generated from white matter regions or detection methods that have substantially different sensitivities to microglial activation in the white matter. Additionally, the merging of entries may have concealed evidence of differences in specific microglial subtypes generated by different experimental conditions. At this time, we believe that the lack of harmony across the included studies mitigates much of the potential consequence of this decision by precluding the possibility of any confident speculations of specific microglial subtypes from being generated.
All post-mTBI outcomes, including microglial activation, were treated as having unimodal timecourses of activation. This could lead to mischaracterizing timecourses of microglial activation in the white matter or of other assessments as having resolved prematurely. No analyses in this study accounted for differing impact locations between studies.
Another limitation of this review is that we did not restrict our search or inclusion criteria to only those studies which used detection methods specific to microglial activation. As the field of experimental mTBI moves towards more sophisticated and rigorous approaches to characterizing microglial activation, sufficient literature will become available with which such a review can be conducted.
During the preparation of the results in this review, there was no difference in weighting for each of the experimental groups in a multi-group study (e.g., the 4 groups present in [57]) or a single experimental group from a single-group study (e.g., the 1 group present in [46]). Consequently, our findings have a bias towards multi-group studies, where a greater number of experimental groups results in a greater degree of bias.
Conclusion
The summaries presented in this systematic review highlight several areas of post-mTBI microglial activation in the white matter research that require additional investigation. Some remaining questions about microglial activation are presented in Box 1. Increased efforts to replicate experimental conditions and verify existing data are needed to confidently interpret the timecourse of microglial activation following mTBI. Future studies applying higher temporal and transcriptional resolution will be critical towards understanding the natural role of microglia in the course of post-mTBI resolution and novel strategies to manipulate microglia in ways that can facilitate complete and improved post-mTBI recovery.
Availability of data and materials
All data in this systematic review was obtained from the included articles. All data generated or analysed during this systematic review are included in this published article and its supplementary information files.
Abbreviations
- 5-HT1B:
-
5-Hydroxytryptamine receptor 1B
- 8-OHdG:
-
8-Hydroxy-2’-deoxyguanosine
- AAS:
-
Androgenic–anabolic steroids
- APP:
-
Amyloid precursor protein
- Arg-1:
-
Arginase-1
- ARMs:
-
Activated response microglia
- B:A:I:
-
Ratio of tallies for mTBI assessments resolving before, after, or at inconclusive timepoints relative to the resolution of microglial activation in white matter regions
- BDA:
-
Biotinylated dextran amine
- BI:
-
Blast injury
- [C]:
-
Suffix indicating closed-skull injury
- Ccl3:
-
C–C motif chemokine ligand 3
- Ccl4:
-
C–C motif chemokine ligand 4
- CD11c:
-
Integrin alpha X
- CD206:
-
Cluster of differentiation 206
- CD33:
-
Cluster of differentiation 33
- CD40:
-
Cluster of differentiation 40
- CD45:
-
Cluster of differentiation 45
- CD68:
-
Cluster of differentiation 68
- CH:
-
Closed-head impact model of engineered rotational acceleration
- CHIMERA:
-
Closed-head impact model of engineered rotational acceleration
- CI:
-
Piston-driven controlled impact
- CNPase:
-
2’,3’-Cyclic-nucleotide 3’-phosphodiesterase
- Cst7:
-
Cystatin F
- CV:
-
Cresyl violet
- CX3CR1:
-
C-X3-C motif chemokine receptor 1
- DAM:
-
Disease-associated microglia
- DAPI:
-
4’,6-Diamidino-2-phenylindole
- Dkk2:
-
Dickkopf-related protein 2
- dpfi:
-
Days post-final-injury
- eIF4E:
-
Eukaryotic translation initiation factor 4E
- EM:
-
Electron microscopy
- F4/80:
-
Epidermal growth factor-like module-containing mucin-like hormone receptor-like 1
- FJC:
-
Fluoro-Jade C
- FIZZ1:
-
Restin-like alpha
- FPI:
-
Fluid pulse injury
- GFAP:
-
Glial fibrillary acidic protein
- Gpnmb:
-
Hematopoietic growth factor inducible neurokinin-1 type
- HIFU:
-
High-intensity focused ultrasound
- Iba1:
-
Ionized calcium binding adaptor molecule 1
- ICI:
-
Ion channel inhibitors
- IL-12:
-
Interleukin 12
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin 6
- iNOS:
-
Inducible nitric oxide synthase
- ISEL:
-
In situ end labeling
- LFB:
-
Luxol fast blue
- Lgals3:
-
Galectin 3
- LIF:
-
Leukemia inhibitory factor
- LIM:
-
Lateral impact model
- MBP:
-
Myelin basic protein
- MHC II:
-
Major histocompatibility complex II
- MMP9:
-
Matrix metalloproteinase-9
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MRI:
-
Magnetic resonance imaging
- mRNA:
-
Messenger ribonucleic acid
- mTBI:
-
Mild traumatic brain injury
- NF200:
-
Neurofilament protein 200
- NF-L:
-
Neurofilament light protein
- NG2:
-
Neural/glial antigen 2
- Nrf2:
-
Neurofilament 2
- NOI:
-
Number of injuries
- [O]:
-
Suffix indicating open-skull injury
- Olig2:
-
Oligodendrocyte transcription factor
- P2RY12:
-
P2Y purinoceptor 12
- PDGFRα:
-
Platelet-derived growth factor receptor α
- PET:
-
Positron emission tomography
- PRISMA-P:
-
Preferred reporting items for systematic review and meta-analysis protocols
- PLP:
-
Proteolipid protein 1
- p-STAT3:
-
Phospho-signal transducer and activator of transcription 3
- p-tau:
-
Phospho-tau
- qPCR:
-
Quantitative polymerase chain reaction
- RNA:
-
Ribonucleic acid
- SALL1:
-
Sal-like protein 1
- Sarm1-KO:
-
Sterile alpha and toll-interleukin receptor motif containing 1
- Spp1:
-
Secreted phosphoprotein 1
- SYRCLE:
-
Systematic review centre for laboratory animal experimentation
- TMEM119:
-
Transmembrane protein 119
- TNF-α:
-
Tumor necrosis factor alpha
- TNFR1:
-
Tumor necrosis factor receptor 1
- TNFR2:
-
Tumor necrosis factor receptor 2
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
- TSPO:
-
Translocator protein
- t-tau:
-
Total-tau
- WD:
-
Weight drop
- WT:
-
Wild-type
References
Holm L, Cassidy JD, Carroll LJ, Borg J (2005) Neurotrauma task force on mild traumatic brain injury of the WHO collaborating centre. Summary of the WHO collaborating centre for neurotrauma task force on mild traumatic brain injury. J Rehabil Med 37:137–141
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, et al. (2018) Estimating the global incidence of traumatic brain injury. J Neurosurg, 1–18
Cole WR, Bailie JM (2020) Neurocognitive and psychiatric symptoms following mild traumatic brain injury. In: Laskowitz D, Grant G (eds). Translational Research in Traumatic Brain Injury [Internet]. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016 [cited 2020 Nov 3]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK326715/
Quinn DK, Mayer AR, Master CL, Fann JR (2018) Prolonged postconcussive symptoms. Am J Psychiatry 175:103–111
Silverberg ND, Duhaime A-C, Iaccarino MA (2019) Mild traumatic brain injury in 2019–2020. JAMA
Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM (2016) White matter involvement after TBI: clues to axon and myelin repair capacity. Exp Neurol 275(Pt 3):328–333
Filley CM, Kelly JP (2018) White matter and cognition in traumatic brain injury. J Alzheimers Dis 65:345–362
Hellstrøm T, Westlye LT, Kaufmann T, Trung Doan N, Søberg HL, Sigurdardottir S et al (2017) White matter microstructure is associated with functional, cognitive and emotional symptoms 12 months after mild traumatic brain injury. Sci Rep 7:13795
Johnson VE, Stewart W, Smith DH (2013) Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43
Shi H, Hu X, Leak RK, Shi Y, An C, Suenaga J et al (2015) Demyelination as a rational therapeutic target for ischemic or traumatic brain injury. Exp Neurol 272:17–25
Nemes-Baran AD, White DR, DeSilva TM (2020) Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep 32:108047
Hughes AN, Appel B (2020) Microglia phagocytose myelin sheaths to modify developmental myelination. Nat Neurosci, 1–12
Zrzavy T, Machado-Santos J, Christine S, Baumgartner C, Weiner HL, Butovsky O et al (2018) Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol 28:791–805
Plemel JR, Stratton JA, Michaels NJ, Rawji KS, Zhang E, Sinha S et al (2020) Microglia response following acute demyelination is heterogeneous and limits infiltrating macrophage dispersion. Sci Adv 6:eaay6324
Zhang L-Y, Pan J, Mamtilahun M, Zhu Y, Wang L, Venkatesh A et al (2020) Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 10:74–90
Merrill JE (1991) Effects of interleukin-1 and tumor necrosis factor-alpha on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci 13:130–137
McDonough A, Lee RV, Weinstein JR (2017) Microglial interferon signaling and white matter. Neurochem Res 42:2625–2638
Tanaka T, Ueno M, Yamashita T (2009) Engulfment of Axon Debris by Microglia requires p38 MAPK activity. J Biol Chem 284:21626–21636
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci Soc Neurosci 27:2596–2605
Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X et al (2013) Microglia/Macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab 33:1864–1874
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y et al (2015) HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA 112:2853–2858
Lee J, Hamanaka G, Lo EH, Arai K (2019) Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther 25:1290–1298
Sato-Hashimoto M, Nozu T, Toriba R, Horikoshi A, Akaike M, Kawamoto K, et al. (2019) Microglial SIRPα regulates the emergence of CD11c+ microglia and demyelination damage in white matter. Bergles DE, Zoghbi HY, Calabresi PA, Molofsky A, (eds). eLife. eLife Sciences Publications, Ltd, 8: e42025
Staszewski O, Hagemeyer N (2019) Unique microglia expression profile in developing white matter. BMC Res Notes 12:367
van der Poel M, Ulas T, Mizee MR, Hsiao C-C, Miedema SSM, Adelia et al (2019) Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis-associated changes. Nat Commun 10:1139
Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T et al (2016) TMEM119 marks a subset of microglia in the human brain. Neuropathology 36:39–49
Korzhevskii DE, Kirik OV (2016) Brain Microglia and Microglial markers. Neurosci Behav Physi 46:284–290
Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci [Internet]. Frontiers; 2020 [cited 2021 Feb 15];14. Available from: https://doi.org/10.3389/fncel.2020.00198/full
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14:128–142
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6:e1000097
Jassam YN, Izzy S, Whalen M, McGavern DB, Khoury JE (2017) Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95:1246–1265
Bolton Hall AN, Joseph B, Brelsfoard JM, Saatman KE (2016) Repeated closed head injury in mice results in sustained motor and memory deficits and chronic cellular changes. PLoS ONE 11:e0159442
Cheng WH, Stukas S, Martens KM, Namjoshi DR, Button EB, Wilkinson A et al (2018) Age at injury and genotype modify acute inflammatory and neurofilament-light responses to mild CHIMERA traumatic brain injury in wild-type and APP/PS1 mice. Exp Neurol 301:26–38
Goodus MT, Kerr NA, Talwar R, Buziashvili D, Fragale JEC, Pang KCH et al (2016) Leukemia inhibitory factor haplodeficiency desynchronizes glial reactivity and exacerbates damage and functional deficits after a concussive brain injury. J Neurotrauma 33:1522–1534
Namjoshi DR, Cheng WH, Bashir A, Wilkinson A, Stukas S, Martens KM et al (2017) Defining the biomechanical and biological threshold of murine mild traumatic brain injury using CHIMERA (Closed Head Impact Model of Engineered Rotational Acceleration). Exp Neurol 292:80–91
Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K et al (2011) Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 70:551–567
McCabe JT, Moratz C, Liu Y, Burton E, Morgan A, Budinich C et al (2014) Application of high-intensity focused ultrasound to the study of mild traumatic brain injury. Ultrasound Med Biol 40:965–978
Yu F, Shukla DK, Armstrong RC, Marion CM, Radomski KL, Selwyn RG et al (2017) Repetitive model of mild traumatic brain injury produces cortical abnormalities detectable by magnetic resonance diffusion imaging, histopathology, and behavior. J Neurotrauma 34:1364–1381
Eyolfson E, Carr T, Khan A, Wright DK, Mychasiuk R, Lohman AW (2020) Repetitive mild traumatic brain injuries in mice during adolescence cause sexually dimorphic behavioral deficits and neuroinflammatory dynamics. J Neurotrauma
Fehily B, Bartlett CA, Lydiard S, Archer M, Milbourn H, Majimbi M et al (2019) Differential responses to increasing numbers of mild traumatic brain injury in a rodent closed-head injury model. J Neurochem 149:660–678
Fidan E, Lewis J, Kline AE, Garman RH, Alexander H, Cheng JP et al (2016) Repetitive mild traumatic brain injury in the developing brain: effects on long-term functional outcome and neuropathology. J Neurotrauma 33:641–651
Gatson JW, Liu M-M, Abdelfattah K, Wigginton JG, Smith S, Wolf S et al (2013) Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg 74:470–475
Angoa-Pérez M, Zagorac B, Anneken JH, Briggs DI, Winters AD, Greenberg JM et al (2020) Repetitive, mild traumatic brain injury results in a progressive white matter pathology, cognitive deterioration, and a transient gut microbiota dysbiosis. Sci Rep 10:8949
Brooks DM, Patel SA, Wohlgehagen ED, Semmens EO, Pearce A, Sorich EA et al (2017) Multiple mild traumatic brain injury in the rat produces persistent pathological alterations in the brain. Exp Neurol 297:62–72
Ferguson S, Mouzon B, Paris D, Aponte D, Abdullah L, Stewart W et al (2017) Acute or delayed treatment with anatabine improves spatial memory and reduces pathological sequelae at late time-points after repetitive mild traumatic brain injury. J Neurotrauma 34:1676–1691
Maynard ME, Underwood EL, Redell JB, Zhao J, Kobori N, Hood KN et al (2019) Carnosic acid improves outcome after repetitive mild traumatic brain injury. J Neurotrauma 36:2147–2152
Maynard ME, Redell JB, Zhao J, Hood KN, Vita SM, Kobori N et al (2020) Sarm1 loss reduces axonal damage and improves cognitive outcome after repetitive mild closed head injury. Exp Neurol 327:113207
Mouzon B, Bachmeier C, Ojo J, Acker C, Ferguson S, Crynen G et al (2019) Chronic white matter degeneration, but no tau pathology at one-year post-repetitive mild traumatic brain injury in a tau transgenic model. J Neurotrauma 36:576–588
Ojo JO, Bachmeier C, Mouzon BC, Tzekov R, Mullan M, Davies H et al (2015) Ultrastructural changes in the white and gray matter of mice at chronic time points after repeated concussive head injury. J Neuropathol Exp Neurol 74:1012–1035
Schwerin SC, Chatterjee M, Imam-Fulani AO, Radomski KL, Hutchinson EB, Pierpaoli CM et al (2018) Progression of histopathological and behavioral abnormalities following mild traumatic brain injury in the male ferret. J Neuro Res 96:556–572
Sherman M, Liu M-M, Birnbaum S, Wolf SE, Minei JP, Gatson JW (2016) Adult obese mice suffer from chronic secondary brain injury after mild TBI. J Neuroinflammation 13:171
Winston CN, Noël A, Neustadtl A, Parsadanian M, Barton DJ, Chellappa D et al (2016) Dendritic spine loss and chronic white matter inflammation in a mouse model of highly repetitive head trauma. Am J Pathol 186:552–567
Slaets H, Hendriks JJA, Stinissen P, Kilpatrick TJ, Hellings N (2010) Therapeutic potential of LIF in multiple sclerosis. Trends Mol Med 16:493–500
Mouzon B, Saltiel N, Ferguson S, Ojo J, Lungmus C, Lynch C et al (2018) Impact of age on acute post-TBI neuropathology in mice expressing humanized tau: a Chronic Effects of Neurotrauma Consortium Study. Brain Inj England 32:1285–1294
Semple BD, Sadjadi R, Carlson J, Chen Y, Xu D, Ferriero DM et al (2016) Long-term anesthetic-dependent hypoactivity after repetitive mild traumatic brain injuries in adolescent mice. Dev Neurosci 38:220–238
Tu T-W, Lescher JD, Williams RA, Jikaria N, Turtzo LC, Frank JA (2017) Abnormal injury response in spontaneous mild ventriculomegaly wistar rat brains: a pathological correlation study of diffusion tensor and magnetization transfer imaging in mild traumatic brain injury. J Neurotrauma 34:248–256
Haber M, AbdelBaki SG, Grin’kina NM, Irizarry R, Ershova A, Orsi S, et al. (2013) Minocycline plus N-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp Neurol, 249:169–177
Haber M, James J, Kim J, Sangobowale M, Irizarry R, Ho J et al (2018) Minocycline plus N-acteylcysteine induces remyelination, synergistically protects oligodendrocytes and modifies neuroinflammation in a rat model of mild traumatic brain injury. J Cereb Blood Flow Metab 38:1312–1326
Robinson S, Berglass JB, Denson JL, Berkner J, Anstine CV, Winer JL et al (2017) Microstructural and microglial changes after repetitive mild traumatic brain injury in mice: Microstructure in Repetitive Mild Brain Injury. J Neurosci Res 95:1025–1035
Namjoshi DR, Cheng WH, Carr M, Martens KM, Zareyan S, Wilkinson A et al (2016) Chronic exposure to androgenic-anabolic steroids exacerbates axonal injury and microgliosis in the CHIMERA mouse model of repetitive concussion. PLoS ONE 11:e0146540
Dahan S, Segal Y, Shoenfeld Y (2017) Dietary factors in rheumatic autoimmune diseases: a recipe for therapy? Nat Rev Rheumatol 13:348–358
Villapol S, Loane DJ, Burns MP (2017) Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65:1423–1438
Mollayeva T, Mollayeva S, Colantonio A (2018) Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol 14:711–722
Gupte RP, Brooks WM, Vukas RR, Pierce JD, Harris JL (2019) Sex differences in traumatic brain injury: What We Know and What We Should Know. J Neurotrauma 36:3063–3091
Tucker LB, Velosky AG, Fu AH, McCabe JT (2019) Chronic neurobehavioral sex differences in a murine model of repetitive concussive brain injury. Front Neurol [Internet]. Frontiers; [cited 2021 Apr 21];10. Available from: https://doi.org/10.3389/fneur.2019.00509/full
Levin HS, Temkin NR, Barber J, Nelson LD, Robertson C, Brennan J, et al. (2021) Association of sex and age with mild traumatic brain injury-related symptoms: a TRACK-TBI study. JAMA Netw Open 4: e213046.
Greco T, Ferguson L, Giza C, Prins ML (2019) Mechanisms underlying vulnerabilities after repeat mild traumatic brain injuries. Exp Neurol 317:206–213
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R et al (2012) Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74:691–705
Ronzano R, Roux T, Thetiot M, Aigrot MS, Richard L, Lejeune FX et al (2021) Microglia-neuron interaction at nodes of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nat Commun 12:5219
Xu Z-X, Kim GH, Tan J-W, Riso AE, Sun Y, Xu EY et al (2020) Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat Commun 11:1797
Rissanen E, Tuisku J, Vahlberg T, Sucksdorff M, Paavilainen T, Parkkola R et al. (2018) Microglial activation, white matter tract damage, and disability in MS. Neurol Neuroimmunol Neuroinflamm, 5: e443
Li Y, Liu L, Barger SW, Griffin WST (2003) Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK Pathway. J Neurosci 23:1605–1611
Loane DJ, Kumar A (2016) Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol 275:316–327
Braun M, Vaibhav K, Saad NM, Fatima S, Vender JR, Baban B et al. (2017) White matter damage after traumatic brain injury: a role for damage associated molecular patterns. Biochimica et Biophysica Acta (BBA) - Molecul Basis Disease. 1863: 2614–2626
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19:987–991
Stratoulias V, Venero JL, Tremblay M, Joseph B (2019) Microglial subtypes: diversity within the microglial community. EMBO J 38:e101997
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK et al (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell Elsevier 169:1276-1290.e17
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A et al (2019) Single-Cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50:253-271.e6
Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I et al (2019) The major risk factors for alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep 27:1293-1306.e6
Ulvestad E, Williams K, Mørk S, Antel J, Nyland H (1994) Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol 53:492–501
Benmamar-Badel A, Owens T, Wlodarczyk A (2020) Protective microglial subset in development, aging, and disease: lessons from transcriptomic studies. Front Immunol 11:430
Werry EL, Bright FM, Piguet O, Ittner LM, Halliday GM, Hodges JR, et al. (2019) Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. Int J Mol Sci [Internet]. [cited 2021 May 20];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6650818/
Yi SY, Barnett BR, Torres-Velázquez M, Zhang Y, Hurley SA, Rowley PA, et al. (2019) Detecting microglial density with quantitative multi-compartment diffusion MRI. Front Neurosci [Internet]. Frontiers; [cited 2021 Apr 13];13. Available from: https://doi.org/10.3389/fnins.2019.00081/full
Gazdzinski LM, Mellerup M, Wang T, Adel SAA, Lerch JP, Sled JG et al (2020) White matter changes caused by mild traumatic brain injury in mice evaluated using neurite orientation dispersion and density imaging. J Neurotrauma 37:1818–1828
Willis EF, MacDonald KPA, Nguyen QH, Garrido AL, Gillespie ER, Harley SBR et al (2020) Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell Elsevier 180:833-846.e16
Bennett RE, Brody DL (2014) Acute reduction of microglia does not alter axonal injury in a mouse model of repetitive concussive traumatic brain injury. J Neurotrauma 31:1647–1663
Lebedeva T, Dustin ML, Sykulev Y (2005) ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol 17:251–258
Pannell M, Economopoulos V, Wilson TC, Kersemans V, Isenegger PG, Larkin JR et al (2020) Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia 68:280–297
Pauleau A-L, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ (2004) Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol 172:7565–7573
Könnecke H, Bechmann I (2013) The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol 2013:914104
Russo MV, Latour LL, McGavern DB (2018) Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol 19:442–452
Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505:223–228
Amici SA, Dong J, Guerau-de-Arellano M (2017) Molecular mechanisms modulating the phenotype of macrophages and microglia. Front Immunol 8:1520
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G et al (2014) Identification of a unique TGF-β dependent molecular and functional signature in microglia. Nat Neurosci 17:131–143
Yang Z, Wang P, Morgan D, Bruijnzeel AW, Lin D, Pan J et al (2015) Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Sci Rep 5:11178
Witcher KG, Bray CE, Dziabis JE, McKim DB, Benner BN, Rowe RK et al (2018) Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation. Glia 66:2719–2736
Izzy S, Liu Q, Fang Z, Lule S, Wu L, Chung JY et al (2019) Time-dependent changes in microglia transcriptional networks following traumatic brain injury. Front Cell Neurosci 13:307
Hernandez A, Tan C, Plattner F, Logsdon AF, Pozo K, Yousuf MA et al (2018) Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes. Mol Brain 11:64
Bennett RE, Mac Donald CL, Brody DL (2012) Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci Lett 513:160–165
Acknowledgements
Not applicable.
Funding
This work was supported by an award from The Scottish Rite Charitable Foundation of Canada.
Author information
Authors and Affiliations
Contributions
PSV, NS, and ALW conceived the manuscript’s outline. PSV and NS did the literature search and screening. PSV wrote the first draft of the manuscript. ALW, NS and LNH significantly contributed to the manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing financial interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: SYRCLE Risk of Bias Tool. Risk of bias assessment for included studies.
Additional file 2
: Statistical tests used to evaluate microglial activation. File containing the statistical tests used by each study to evaluate microglial activation as well as a summary of if and how power analyses were included.
Additional file 3
: Full extractions. Complete table of data items extracted from included studies.
Additional file 4
: Pooled extractions for microglial activation timecourses. Data items relevant to timecourses of microglial activation in white matter regions.
Additional file 5
: Summary of major study parameters. File containing the parameters of each study that contributed to Figure 2.
Additional file 6
: Supplementary Figure 1: Timecourses arranged by injury model. A variation of Figure 3 in which timecourses are arranged by injury model rather than number of injuries.
Additional file 7
: Tallies to contextualize microglial activation. Tab 1: Evaluations of the relative order of the resolution of microglial activation in the white matter compared to the resolution of other included assessments for included studies. Tab 2: Descriptions of included assessments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Velayudhan, P.S., Schwab, N., Hazrati, LN. et al. Temporal patterns of microglial activation in white matter following experimental mild traumatic brain injury: a systematic literature review. acta neuropathol commun 9, 197 (2021). https://doi.org/10.1186/s40478-021-01297-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-021-01297-1