Abstract
Background
Migration enables organisms to access resources in separate regions that have predictable but asynchronous spatiotemporal variability in habitat quality. The classical migration syndrome is defined by key traits including directionally persistent long-distance movements during which maintenance activities are suppressed. But recently, seasonal round-trip movements have frequently been considered to constitute migration irrespective of the traits required to meet this movement type, conflating common outcomes with common traits required for a mechanistic understanding of long-distance movements. We aimed to test whether a cetacean ceases foraging during so-called migratory movements, conforming to a trait that defines classical migration.
Methods
We used location and dive data collected by satellite tags deployed on beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea population, which undertake long-distance directed movements between summer and winter areas. To identify phases of directionally persistent travel, behavioural states (area-restricted search, ARS; or Transit) were decoded using a hidden-Markov model, based on step length and turning angle. Established dive profiles were then used as a proxy for foraging, to test the hypothesis that belugas cease foraging during these long-distance transiting movements, i.e., they suppress maintenance activities.
Results
Belugas principally made directed horizontal movements when moving between summer and winter residency areas, remaining in a Transit state for an average of 75.4% (range = 58.5–87.2%) of the time. All individuals, however, exhibited persistent foraging during Transit movements (75.8% of hours decoded as the Transit state had ≥ 1 foraging dive). These data indicate that belugas actively search for and/or respond to resources during these long-distance movements that are typically called a migration.
Conclusions
The long-distance movements of belugas do not conform to the traits defining the classical migration syndrome, but instead have characteristics of both migratory and nomadic behaviour, which may prove adaptive in the face of unpredictable environmental change. Such patterns are likely present in other cetaceans that have been labeled as migratory. Examination of not only horizontal movement state, but also the vertical behaviour of aquatic animals during directed movements is essential for identifying whether a species exhibits traits of the classical migration syndrome or another long-distance movement strategy, enabling improved ecological inference.
Similar content being viewed by others
Background
Long-distance movement strategies have evolved in response to spatiotemporal fluctuations in environmental conditions and variation in the selective pressures exerted on an animal throughout ontogeny [1,2,3]. Predictable and asynchronous spatiotemporal variability in habitat quality between two or more regions favour migration [2]. Moving between two or more consistent and distinct home ranges, often over a seasonal cycle, can be highly adaptive if it enables greater exploitation of resources at successive sites of predictable quality than at intermediate sites or remaining at one site year-round [3,4,5]. Unpredictable spatiotemporal variability in resources that are patchily distributed over large areas instead favours nomadism [6], whereby individuals benefit from searching for and responding to resources, resulting in unpredictable trajectories among individuals or among years for an individual [3].
Diverse taxa across the animal kingdom have been shown to exhibit a consistent suite of traits associated with migratory movements, termed the migratory syndrome [5]. Based on the definition of Kennedy ([7], p. 8): “Migratory behavior is persistent and straightened-out movement effected by the animal’s own locomotory exertions or by its active embarkation upon a vehicle. It depends on some temporary inhibition of station keeping responses, but promotes their eventual disinhibition and recurrence”. We refer to this definition as “classical migration”. Therefore, a key behavioural trait of the migration syndrome is that during an animal’s persistent and straightened out-movements, maintenance activities required for growth and reproduction are suppressed, and consequently a truly migrating animal is undistracted by local resources such as food and mates that would normally evoke a response [5, 7]. Migrants may undertake brief ‘stopovers’ at predictable sites between summer and winter residency areas primarily for recovery or energy accumulation on route to their destination [8,9,10], but during directed movements in the migratory phase the animal is undistracted and ceases feeding [2, 5]. Numerous experimental and observational studies on terrestrial species such as insects and birds have found strong support for the migratory syndrome (see [5, 7, 11] for examples). Over the past two decades, however, seasonal round-trip movements between discreet residency areas have often been considered to constitute migration irrespective of the suite of traits required to meet the classical definition of this movement type [12,13,14]. Established in seminal literature [2, 7, 15], focusing on the common behavioural and physiological traits underlying long-distance movements, rather than their ecological outcomes is key to providing a mechanistic understanding of the causes and consequences of movement strategies [16,17,18], i.e., what makes an individual migratory versus non-migratory [7]. Furthermore, understanding whether a species undertakes classical migration (i.e., displays multiple traits of the migration syndrome) or exhibits characteristics of other long-distance movement strategies such as nomadism is critical as these strategies differ in their success depending on spatiotemporal predictability of resources [3, 4], their stability under environmental change, and how measures should best be applied for conservation management [19,20,21].
Due to difficulties in observing animals during their long-distance movements, the behavioural traits of the classical migration syndrome have been difficult to test for many species until the proliferation of animal telemetry [22, 23]. Animal telemetry, electronic tags deployed on animals that transmit data to receivers (typically satellites or acoustic receivers), has enabled analysis of movement trajectories in two-dimensional space and has revealed irregular horizontal movements in species traditionally considered as migratory ([6] and references therein). But directionally persistent horizontal movement tracks between seasonal residency areas, classified through various state-space models applied to movement data, are frequently referred to as migrations (e.g., [24,25,26,27]). Marine animals move in a three-dimensional environment, yet in labelling movement phases as migrations, studies rarely examine vertical movements that are essential for testing whether an animal conforms to the traits of the migration syndrome, i.e., straightened out and undistracted movements with maintenance activities suppressed. This is especially notable in endothermic marine megafauna, for which recent studies have shown that deep, vertical excursions, including foraging behaviour, can occur during directionally persistent horizontal movement tracks [28, 29].
The infraorder Cetacea includes species that exhibit some of the longest distance movements in the animal kingdom, often referred to as migrations, as well as species that are considered nomads and residents [30,31,32]. Many populations exhibit directionally persistent and seasonal low to high latitude long-distance movements, indicative of migrations, linked to spatiotemporal variation in foraging opportunities and pressures related to predation and temperature requirements of calves [1, 33, 34]. But recent studies have revealed complexities among cetacean movements. Some species exhibit partial migrations depending on age, sex, and reproductive status [30, 35]. Aseasonal movements provide evidence of the importance of moulting as a major driver behind long-distance movements [27]. Non-migratory populations or portions of populations exist within species that otherwise are considered to migrate [32, 36, 37]. Cetaceans can use predictable stopover sites [10, 38], but evidence of exploratory behaviour and supplemental feeding during what are traditionally considered as migratory movements [39,40,41] raises questions about whether these movements conform to the migration syndrome (i.e., classical migration).
The current study aims to test the assumption that perceived migratory movements in a highly mobile cetacean are undistracted and maintenance activities are suppressed, following the traits defining classical migration. By examining movements in three-dimensions using location and dive data collected by long-term satellite tags deployed on beluga whales (Delphinapterus leucas) from the wide-ranging Eastern Beaufort Sea (EBS) population, which undertake > 2000 km long distance movements between summer and winter areas [42, 43], we tested the hypothesis that beluga whales cease feeding during so-called migratory movements using established dive profiles as a proxy for foraging. Our intention is to reiterate the importance of the mechanisms that drive classical migration, rather than purely defining the outcome of a movement type.
Materials and methods
Data collection
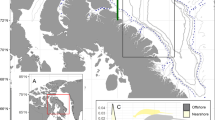
Belugas from the EBS population were tagged at Hendrickson Island, Northwest Territories, Canada (Fig. 1) in July 2018 and 2019, as part of a project co-developed with the Inuvialuit; details on tagging methods can be found in Storrie et al. [44]. For this study we used data from six male belugas tagged in 2018 equipped with SPLASH10-F-238 tags (Wildlife Computers Ltd., Redmond, WA, United States), as these belugas transmitted data for 161–357 days covering long-distance movements between seasonal residency areas in fall (n = 6) and spring (n = 3). Tags sampled depth (± 0.5 m) at 1 s intervals and transmitted the data as hour-long time series messages of depths subsampled at 75 s intervals. Tags also collected Fastloc-GPS locations (hereafter GPS), and Argos locations were estimated each time a transmission was made. A range of tag programming setups were used to inform on the relationship between the settings and tag longevity for future studies (see [44] for details of tag programming). In brief, tags were programmed with higher transmission limits and to collect GPS locations (at 7–30 min intervals) every day from July through September (400–1040 transmissions per day) and switched to have lower transmission limits with GPS locations collected every 7th day from October/November onwards (175–740 transmissions per day). Three of the belugas were double tagged with a MiniPAT (pop-up archival tag) (Wildlife Computers Ltd., Redmond, WA, United States), which archived and then transmitted depth time series data after release from the animal. MiniPAT data were used to fill in missing depth data from the SPLASH10-F-238 tags (Table 1).
Location processing
Strict filtering of the location data and continuous-time correlated random walk (CTCRW) models [45] were used to estimate locations over hourly intervals with the greatest accuracy possible. GPS locations calculated using < 5 satellites and/or with a residual value > 35 were removed [46], and remaining GPS locations were assigned semi-major- and semi-minor axis errors of 50 m and an ellipse orientation of 0°. Argos locations that had a semi-major axis error > 3 km were removed. Argos locations were then filtered by speed (5 m/s) and turning angle (15° and 25°, unless spikes were < 2500 and 5000 m, respectively) [47] using the fit_ssm function in the foieGras package v0.7-6 [48] in R v 4.1.2 [49]. Further Argos locations were removed if they occurred within 10 min of a GPS location. Argos and GPS locations were then combined, and locations were removed if they had a semi-major axis error > 500 m and occurred within 20 min of a location with a semi-major axis error of ≤ 500 m. Tracks were then split into segments when there were gaps > 6 h, and track segments were removed if they contained < 4 locations and/or covered < 3 h. After filtering the location data, remaining locations within each segment occurred 43.8 min apart on average. CTCRW models were fit to each track segment using the fit_ssm function [48] and locations predicted at hourly intervals. Diagnostic plots were checked and all CTCRW-modeled tracks were viewed to confirm movement trajectories had been modeled appropriately (i.e., did not contain a modeled location that would require unrealistic speeds or turning angles between high accuracy locations in the raw filtered data).
Modelling movement state
A hidden Markov model was fit using the fit_hmm function in the momentuHMM v1.5.4 [50, 51] package in R, to decode behavioural states at each hourly CTCRW-modeled location. Step length (modeled with a gamma distribution) and turning angle (modeled with a Von Mises distribution) were used in a two-state model; area-restricted search (ARS) (slow and tortuous movements) or Transit (fast and directed movements). A two-state model was selected to allow for simple biological interpretation between movement states typically associated with foraging, resting, or social behaviour (ARS) vs. migrating or commuting behaviour (Transit) [24,25,26, 39, 52]. Each track segment was assigned a unique ID and models were fit using all track segments for all individuals. A histogram of step lengths was plotted to inform on initial parameters in the first model, m1, following recommendations by Michelot et al. [53]. These were set at means of 1 and 5 km, and standard deviations of 2 and 3 km for ARS and Transit, respectively. Initial parameters for turning angle in m1 were set at a concentration of 1 for ARS (i.e., high frequency of turning angles) and 13 for Transit (persistence in direction). A second model, m2, was fit using the parameter estimates from m1 as the initial parameters. To check whether different initial parameters resulted in convergence at different parameter estimates, two additional models, m3 and m4, were fit with initial parameters for step length and turning angle that were less and greater than the parameter estimates from m1, respectively. A further model, m5, was fit with extreme values for the step length initial parameters, using the minimum step length as the mean for the ARS state and the maximum step length as the mean for the Transit state. Full details on parameters can be found in Supplementary Material 1. The model with extreme initial parameters, m5, converged at biologically meaningless parameter estimates and transition probabilities (ARS mean step length = 0.006 km/hr, transition probability from Transit to ARS = 0.000), so was rejected. All other models converged at identical parameter estimates and transition probabilities, so m1 was selected (Supplementary Material 1). The stateProbs function in momentuHMM was used to calculate the probability of each location being in either of the states; state-decoded locations with a probability of < 0.9 of being ARS or Transit were labeled as Uncertain and excluded from the analyses.
Distinguishing long-distance movements vs. seasonal residency phases
EBS belugas undertake some of the longest distance movements among this species, that are often referred to as migrations [44, 54, 55]. Overwintering at lower latitudes in the Bering Sea, EBS belugas move northeastwards in spring to form summer congregations in estuaries and feed in deep offshore areas, before travelling west in fall [42,43,44]. To test our hypothesis related to migration, we first needed to isolate the arena in which belugas undertake long-distance movements by removing known seasonal residency regions where shorter directed movements between local resource patches can occur. This was achieved by creating 50% utilization distrbution (UD) polygons from the locations which had been state-decoded as ARS, separately for the six months centered on summer (April-September, bandwidth = 75 km) and winter (October-March, bandwidth = 100 km), using the adehabitatHR v0.4.19 package [56] in R (see details in Supplementary Material 2). Polygons were extended to the nearest land barrier to prevent short-term movements by belugas outside of the 50% UDs but within establshed seasonal residency areas [42, 43] being defined as occurring during the long-distance movement phase. State-decoded locations which occurred within these polygons were excluded from analyses and all remaining locations are hereafter referred to as occurring during the long-distance movement phase.
Testing whether maintenance activities are suppressed during long-distance movements
EBS beluga dives were previously characterized by time and depth metrics, classified into types, and likely functions identified for each type [44]. Four dive types had time-depth structures that indicated they were used principally for foraging: Deep Benthic, Deep Pelagic V, Deep Pelagic W, and Intermediate Benthic. These dives are hereafter referred to as foraging dives. The designation of foraging dives was based on the depths of these dives (median max. depth for these dive types ranged from 50.5 to 576.0 m), as well as other components of their time-depth structure such as descent and ascent rates (see [44] for full details). Alternative functions for deep dives can relate to species’ responses to predators at shallow depths, whereby they may socialize [57] or sleep [58] at greater depths. Killer whales (Orcinus orca), the only fully aquatic predator of belugas, are increasingly moving north of the Bering Strait [59], but given their relative scarcity and the high frequency of deep dives by belugas (e.g., 16,531/90,211 dives analysed in [44] were to depths > 101 m), we considered that predator avoidance is unlikely the primary function of these four dive types. These four foraging dives are also unlikely to represent an optimal transiting depth to avoid surface wave drag, the impacts of which apply at depths less than three times an animal’s body diameter [60] (equivalent to a depth of ~ 3 m in belugas). Deep skewed-shaped dives, where an animal uses its buoyancy to passively drift through the water column, may be used by marine mammals in sleeping, resting or facilitating digestion [61, 62]. The one skew-shaped dive that was identified in EBS belugas [44] was proposed to have foraging as its most likely function, but this was excluded from the current analyses due to uncertainty around whether drifting through the water column could represent another function. The total number of foraging dives was calculated for each hour and assigned to each state-decoded location based on the time stamp. Hours were removed if they did not contain complete dive data, i.e., if an hour of depth time series data was followed by an hour of missing data and a dive crossed the two hours it could not be characterized, so the depth time series data from that dive was removed and the hour in which it started classified as incomplete (see [44] for details). To test whether belugas cease foraging during long-distance movements typically labeled as migratory, we calculated the total number of foraging dives that started during each hourly location that was state-decoded as Transit, and compared this to the number of dives that occurred during locations that were state-decoded as ARS for reference. This was calculated separately for each of the two periods of long-distance movements; Fall and Spring.
Results
Long-distance movements
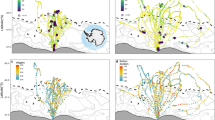
Tagged belugas initiated their westward movements between mid-August and early September but there was variability in the routes taken (Fig. 1) and distances travelled (2498–6030 km, Table 1). Three belugas took similar routes over the deep waters of the Arctic Basin towards the Chukchi Plateau (LC2018#1, LC2018#3 and LC2018#8). One beluga went north before heading west (LC2018#4), and two belugas headed west via a more southern route along the Beaufort Slope (LC2018#2 and LC2018#6). Two belugas spent several weeks around the Chukchi Plateau and Mendeleev Ridge (LC2018#3 and LC2018#8), whereas one beluga passed through this region and went 700 km further west (LC2018#1). All belugas entered the winter residency area in the Chukchi Sea between mid-October and mid-November. The three belugas that transmitted data into their Spring long-distance movements took more direct routes towards the summer residency area in April and May, travelling distances of 832–1079 km (Table 1), past the northwest coast of Alaska and along the Beaufort Slope.
Horizontal movement behavioural states
A total of 20,014 locations estimated at hourly intervals from the CTCRW models were available for decoding states using the HMM. Parameter estimates indicated locations decoded as the ARS state were characterized by slow speeds (mean step = 2.09 km, sd = 1.37 km, Fig. 2a) and frequent directional changes (angle concentration = 1.22, Fig. 2b), whereas Transit locations had higher speeds (mean step = 4.39 km, sd = 1.15 km, Fig. 2a) and fewer directional changes (angle concentration = 10.97, Fig. 2b). After removal of state-decoded locations with a probability < 0.9 of being in either state, 16,620 locations remained. A total of 5,373 of these locations contained complete dive data, and of these, 1,593 were recorded during the long-distance movement phase (1,224 in Fall, and 369 in Spring), enabling an assessment of whether foraging dives occurred during perceived migratory movements (Table 1). Belugas recorded an average of 75.4% (range = 58.5–87.2%) of the time in a Transit state during the long-distance movement phase (Table 1), compared with 39.1% (range = 27.6–45.5%) during summer and winter residency phases.
Foraging behaviour during perceived migratory movements
While belugas were in the long-distance movement phase, foraging dives were recorded during the majority of hours decoded as Transit in both Fall and Spring (80.7% and 63.3% of hours with ≥ 1 dive, respectively, Fig. 3). Foraging dives were frequent while belugas were in the Transit state during Fall (e.g., 59.2% of hours with ≥ 2 dives) and comparable to the number of dives during ARS movements (67.4% of hours with ≥ 2 dives), whereas during spring belugas tended to make fewer dives during Transit than ARS movements (e.g., 23.7 vs. 64.6% of hours with ≥ 2 dives).
Percentage of hours where a given number of foraging dives [44] were recorded for the locations decoded as Transit or ARS while belugas were in the long-distance movement phase in (a) Fall and (b) Spring
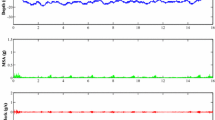
Representative examples of belugas making foraging dives whilst in a Transit state during fall can be seen in Fig. 4, identifying this population actively searches for and/or responds to resources during long-distance movements between summer and winter residency areas. From 1st September at 18:00 to 2nd September at 13:00 (19 h), beluga LC2018#3 was in a Transit state over a distance of 78 km and recorded 18 dives to between 240 and 840 m (Fig. 4a), with 3 h of data where no dives were made. From 13th September at 16:00 to 22:00 (6 h) beluga LC2018#3 was in an ARS state over a distance of 10 km; it recorded 9 dives to between 160 and 710 m, but also remained at depths ≤ 12.5 m over a two-hour period (Fig. 4b). It then entered an Uncertain state for four hours, recording 5 dives to > 290 m, before entering a Transit state over 40 km between 03:00 and 12:00 (9 h) on 14th September, undertaking 24 dives to depths > 100 m (Fig. 4b). Beluga LC2018#1 was in a transit state on 6th September (Fig. 4c); between 00:00 and 19:00 (19 h) it traveled 75 km and recorded 23 dives to between 170 and 710 m. Foraging dive frequency was comparable during transit movements and ARS movements (Fig. 4d). The above examples each had 4–8 h of dive data missing during which additional foraging dives may have been made. Foraging dives were made during the Transit state whilst belugas were in the long-distance movement phase during both fall and spring; further examples of state-decoded tracks and corresponding dive profiles can be found in Supplementary Material 3.
State-decoded locations recorded by six belugas between July 2018 and June 2019. The long-distance movement phase analysed includes all locations not bounded by the summer or winter residency area polygons. ‘Uncertain*’ locations (probability of < 0.9 of being in either the ARS or Transit state), when present, shown in zoomed inset panels only. Zoomed inset panels a-d show examples of state-decoded beluga tracks, with the corresponding dive profiles colour-coded by state shown in the lower panels. (a) beluga LC2018#3, 1st -2nd Sep 2018, (b) beluga LC2018#3, 13th -14th Sep 2018, (c) beluga LC2018#1, 6th Sep 2018 (d) beluga LC2018#8, 6th Sep 2018. Black bars above dive profiles denote periods with missing depth data. All times are given in UTC. Zoomed inset panels only show the track of the individual beluga referred to for that period. Seafloor depth is denoted by brown shading when within the range of depths shown on the y-axis
Discussion
Migration is defined as a movement syndrome with distinct characteristics (i.e., predictable, directional and undistracted movements [5, 7]) that is shared among diverse species [64]. Here we demonstrate that a highly mobile marine predator, beluga whales from the EBS population, undertake predominantly directionally persistent horizontal movements (i.e., Transit state) between discrete summer and winter residency areas, typically associated with migratory behaviour [24, 26, 65]. But whilst doing so, belugas persistently exhibit deep vertical excursions characteristic of foraging and/or exploratory behaviour; more akin to a nomadic strategy whereby animals search for and respond to resources during long-distance movements [6]. These data highlight that long-distance movements and an assigned transient state derived only from horizontal movement data should not be assumed to represent classical migration, where movements are undistracted and maintenance activities are suppressed [5, 7, 11]. Classifying these movements as migration ultimately underestimates the behavioural ecology of a given species. It would appear that the long-distance movements of beluga whales include traits of both migration and nomadism, but conform wholly to the classical views of neither [2,3,4, 6], which may be more ubiquitous across aquatic predators than previously thought.
Migration and nomadism both allow species to exploit spatially heterogeneous resources, but whilst seasonal predictability in the spatial distribution of resources favors migration, spatiotemporal unpredictability of resources favors nomadism [3, 6]. Consequently, these two movement strategies require different behavioural and physiological traits to access resources [5]. Migrants require memory mechanisms (through genetics, experience, or communication) to ensure that they arrive at and/or depart from a site to coincide with known resource abundance or deterioration [3, 66]. Nomads use exploratory behaviour and respond to local conditions to locate patchily distributed and unpredictable resources [3, 6]. Migrants also rely more on prior deposition of energy reserves, primarily as lipids, and morphological adaptations to minimize the cost of movement than nomads due to the greater distances travelled and the longer non-feeding periods that must be endured [5].
Persistent foraging/exploratory behaviour by EBS belugas during their long-distance movements coupled with differences in routes taken among individuals could suggest that belugas adopt a nomadic strategy after departing a seasonal residency area, searching for and responding to resources while moving in the general direction of their next seasonal residency area. Irregularly fluctuating resources over large geographic areas lead to unpredictable and spatially variable nomadic movement patterns among individuals and within an individual across years [3, 4]. Reconciling components of both migratory and nomadic strategies over a seasonal cycle within an individual requires separate predictable and unpredictable features over the range of EBS belugas. In certain situations, sea ice can be a predictable feature, constraining beluga distribution south of the Bering Strait in winter [42] while land-fast sea ice prevents movements into estuaries and the Arctic Archipelago until summer [67,68,69]. In contrast, interannual variability in sea ice concentration and the position of the ice edge throughout the Arctic Basin and Beaufort Slope [70, 71], where belugas undertake their long-distance movements during fall and spring, are less predictable. These regions, however, can be highly productive if somewhat ephemeral, dependent on sea ice concentration, upwelling storms, and daily light cycles, and may support abundant foraging opportunities by cetaceans [72, 73]. Given a temporal mismatch between optimal conditions in summer and winter residency areas; the flexibility in movement strategies among beluga populations (residents [74, 75] vs. migrants [43, 76]); and their ability to detect prey over large distances [77], EBS belugas may benefit from switching to a nomadic strategy (exploring and responding to resources, [6]) in this unpredictable intermediate region rather than moving directly between summer and winter areas.
Recent studies have acknowledged a continuum of movement strategies between the traditional resident/nomadic/migratory trichotomy [4, 6, 78, 79], and foraging behaviour during directional movements that have traditionally been labeled as migrations is not uncommon. Notably, several bird and bat species exhibit a ‘fly-and-forage’ strategy, where individuals frequently switch between feeding and flying during long-distance movements towards the next residency area [80, 81]. This represents an alternative strategy to classical migration, either opportunistically to minimize prolonged stopovers [80], or an adaptation to reduce the amount of energy that must be deposited prior to migration [82]. EBS belugas have a thick blubber layer (10–15 cm) prior to their fall migration and a thinner layer (~ 5 cm) following their spring migration [83], indicating some deposition of energy reserves prior to long-distance movements, but their persistent foraging behaviour while transiting has similarities to the ‘fly-and-forage’ strategy. This suggests that the continuum of long-distance movement strategies found in birds (fly-and-forage, stopovers, non-stop classical migration, [14]) may also be present in cetaceans.
Foraging behaviour during traditionally assumed migratory movements between core residency areas may be more common in cetaceans than is currently acknowledged. For instance, whilst female and immature sperm whales (Physeter macrocephalus) remain at subtropical latitudes year-round and exhibit nomadic behaviour [84], males undertake long-distance seasonal movements to higher latitudes [30, 85]. But partially digested prey in the stomachs of males caught during spring and fall [86] suggest they feed during these movements. Equally, killer whales (Orcinus orca) tagged in the Southern Ocean make aseasonal long-distance movements to lower latitudes likely to facilitate moulting [27], during which they recorded dives (mean depth = 37.5 m, individual maxima = 182–380 m) at depths unlikely to represent optimal transiting [60]. Given the findings of the present study, we suggest a closer examination of dive behaviour during cetaceans’ traditionally inferred migratory movements is required to identify whether foraging has been underestimated at these times.
Foraging during directed movements would seem less likely for the mysticetes, which are typically larger than odontocetes and so have lower costs of transport [87, 88], can undertake long fasting periods [89], but rely on thresholds of prey density for energetically efficient foraging [90, 91]. Evans & Bearhop [14] recently drew from terminology used to organize reproductive strategies to propose a framework organizing migratory strategies along a continuum from capital migration (no feeding during long-distance movements, conforming to classical migration) to income migration (regular feeding during long-distance movements). The cetaceans generally fit well into this framework; the mysticetes are typically capital breeders (use stored energy during reproduction) and the odontocetes are income breeders (feed during reproduction) [92]. Given that these reproductive strategies depend on an animal’s ability to store energy and that equivalent size mysticetes and odontocetes have vastly different seasonal energy reserve requirements [89], it is unsurprising that many of the mysticetes are considered migratory [32], whereas the odontocetes include more species which are year-round resident, nomadic, or partially migratory (e.g., [84, 85, 93, 94]). But the traditional view of mysticetes undertaking a “feast or famine” approach over an annual cycle (i.e., classical migration); foraging intensively at high latitudes during summer to build up energy reserves to fuel their long-distance movements and reproduction during other times of the year, has recently been brought into question by evidence of a continuum of movement strategies within this group [32]. For instance, humpback whales (Megaptera novaeangliae) in the southwest Pacific have been observed feeding outside of their summer residency areas [95], and may remain in an ARS state for > 30 days in regions along their migration route [39]. Blue whales (Balaenoptera musculus) and fin whales (Balaenoptera physalus) tagged in the North Atlantic frequently switched between transiting and ARS movements during their migrations [52, 65]. Differences in the location and frequency of ARS movements among individuals in both species [52, 65], and the use of ARS movements by blue whales progressing northwards [52], are more akin to nomadic and march-and-browse strategies, respectively, than migrations with predictable stopover sites [4, 14, 65]. Furthermore, whilst other studies on mysticetes have shown little or no ARS behaviour along migration routes [96], it is typically assumed that foraging does not occur during a transiting (directed) state, which may not be the case.
Recent studies on the Arctic cetaceans have revealed shifts in long-distance movement strategies associated with changes in sea ice: narwhals and belugas delay their fall migrations as the ice-free season lengthens [55, 97] and bowhead whales have been identified overwintering in their typical summer ground [98]. The success of shifts in the timing of long-distance movements will largely depend on whether the cues for departure from a site are concomitant with suitable environmental conditions at the destination site [99, 100]. EBS belugas tagged in the 1990s and 2000s were reported to make deep dives during their long-distance movements [101], but quantifying foraging during this period was limited by unknown time-depth structures of dives and horizontal movement state (ARS vs. Transit). These historical data, however, suggest foraging behaviour during more recent long-distance movements is unlikely a response associated with changing environmental conditions in the region [102]. We note that the current study only included adult males and EBS belugas segregate by age, sex, and reproductive status [54]. A nearshore long-distance movement route has been observed by Inuvialuit in fall: “when you stand at Shingle Point and look out, you can see whale blows as far as the eye can see, heading toward Alaska. Thousands of them.” (Dennis Arey, Aklavik Hunters and Trappers Committee, pers. comm. to S. MacPhee, October 2022). This route was not taken by belugas in the present study, which went offshore shortly after being tagged, so it is unknown whether the findings of the present study can be applied to the entire population. But, the diversity of movement strategies among beluga populations [43, 74, 75] and the strategy in EBS belugas which includes elements of both migration and nomadism, suggests high plasticity in movement strategies in this species that may provide greater resilience in the face of environmental change when compared with obligate migrants or residents [20, 21]. Strong social bonds and shared movement cultures, as found among belugas [103, 104], are common in nomadic populations [6], whereby individuals in a social group can share information on suitable habitats and foraging success [84]. We posit that with the increasing unpredictability of environmental conditions in the Arctic [105], beluga whales will exhibit more nomadic movements and possibly less predictable summer and winter ranges, which may lead to distinct movement cultures and reproductive isolation among social groups [103, 106].
Conclusion
Beluga whales from the Eastern Beaufort Sea population exhibit persistent vertical foraging behaviour during horizontal directionally persistent long-distance movements, which contradicts a key trait of the classical migration syndrome, where movements are undistracted and maintenance activities are suppressed. The long-distance movements of belugas have characteristics of both migratory and nomadic behaviour; strategies which rely on opposite ends of a continuum of resource predictability from high to low, respectively. The diversity and flexibility in beluga long-distance movement strategies may prove highly adaptive in the face of unpredictable environmental change.
Long-distance movements in cetaceans are frequently referred to as migrations, but foraging behaviour during these movements may be more common than is currently acknowledged. Labeling the continuum of long-distance movements in cetaceans as migrations potentially conflates common outcomes with common traits. We are not proposing that all long-distance movements in cetaceans include persistent foraging behaviour, but suggest that identifying whether a species exhibits traits of the classical migration syndrome or another long-distance movement strategy such as nomadism is essential for improved ecological inference. This will be critical in the face of increasing unpredictability of the spatiotemporal distribution of resources associated with climate change.
Data Availability
The datasets used herein are co-owned by the Department of Fisheries and Oceans Canada and the Inuvialuit (represented by the Game Council, and the Fisheries Joint Management Committee). The collaboration honours the UN Declaration on the Rights of Indigenous Peoples Act and Inuvialuit data sovereignty as they co-lead the program and co-manage this beluga population. The datasets presented in this article are available upon reasonable request. Requests should be directed to LL (Lisa.Loseto@dfo-mpo.gc.ca) and will be reviewed by the Inuvialuit Game Council and Fisheries and Oceans Canada.
Abbreviations
- EBS:
-
Eastern Beaufort Sea
- ARS:
-
Area-restricted search
References
Fokkema W, van der Jeugd HP, Lameris TK, Dokter AM, Ebbinge BS, de Roos AM, et al. Ontogenetic niche shifts as a driver of seasonal migration. Oecologia. 2020;193(2):285–97.
Dingle H, Drake AV. What is Migration? Bioscience. 2007;57(2):113–21.
Mueller T, Fagan WF. Search and navigation in dynamic environments - from individual behaviors to population distributions. Oikos. 2008;117:654–64.
Jonzén N, Knudsen E, Holt RD, Sæther B. Uncertainty and predictability: the niches of migrants and nomads. Animal Migration: a synthesis. Oxford University Press; 2011. 91–109.
Dingle H. Animal migration: is there a common migratory syndrome? J Ornithol. 2006;147:212–20.
Teitelbaum CS, Mueller T. Beyond Migration: causes and consequences of Nomadic Animal movements. Trends Ecol Evol. 2019;34(6):569–81.
Kennedy JS. Migration, behavioural and ecological. In: Rankin MA, editor. Migration: mechanisms and adaptive significance. Contributions in Marine Science (Supplement Vol 27); 1985. p. 5–26.
Monteith KL, Hayes MM, Kauffman MJ, Copeland HE, Sawyer H. Functional attributes of ungulate migration: landscape features facilitate movement and access to forage. Ecol Appl. 2018;28(8):2153–64.
Schmaljohann H, Eikenaar C, Sapir N. Understanding the ecological and evolutionary function of stopover in migrating birds. Biol Rev. 2022;97(4):1231–52.
Abrahms B, Hazen EL, Aikens EO, Savoca MS, Goldbogen JA, Bograd SJ, et al. Memory and resource tracking drive blue whale migrations. PNAS. 2019;116(12):5582–7.
Dingle H. Migration: the biology of life on the move. Oxford University Press; 1996.
Berger J. The last Mile: how to sustain Long-Distance Migration in Mammals. Conserv Biol. 2004;18(2):320–31.
Soriano-Redondo A, Gutiérrez JS, Hodgson D, Bearhop S. Migrant birds and mammals live faster than residents. Nat Commun. 2020;11(1).
Evans SR, Bearhop S. Variation in movement strategies: Capital versus income migration. J Anim Ecol. 2022;91(10):1961–74.
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, et al. A movement ecology paradigm for unifying organismal movement research. PNAS. 2008;105(49):19052–9.
Bauer S, Klaassen M. Mechanistic models of animal migration behaviour - their diversity, structure and use. J Anim Ecol. 2013;82(3):498–508.
Chapman JW, Reynolds DR, Wilson K. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol Lett. 2015;18(3):287–302.
Risely A, Klaassen M, Hoye BJ. Migratory animals feel the cost of getting sick: a meta-analysis across species. J Anim Ecol. 2018;87(1):301–14.
Runge CA, Martin TG, Possingham HP, Willis SG, Fuller RA. Conserving mobile species. Front Ecol Environ. 2014;12(7):395–402.
Robinson RA, Crick HQP, Learmonth JA, Maclean IMD, Thomas CD, Bairlein F, et al. Travelling through a warming world: climate change and migratory species. Endanger Species Res. 2009;7(2):87–99.
Gilroy JJ, Gill JA, Butchart SHM, Jones VR, Franco AMA. Migratory diversity predicts population declines in birds. Ecol Lett. 2016;19(3):308–17.
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science. 2015;348(6240):1255642.
Kays R, Crofoot MC, Jetz W, Wikelski M. Terrestrial animal tracking as an eye on life and planet. Science. 2015;348(6240):aaa2478.
Bailey H, Mate BR, Palacios DM, Irvine L, Bograd SJ, Costa DP. Behavioural estimation of blue whale movements in the Northeast Pacific from state-space model analysis of satellite tracks. Endanger Species Res. 2010;10(1):93–106.
Mackay AI, Bailleul F, Carroll EL, Andrews-Goff V, Scott Baker C, Bannister J et al. Satellite derived offshore migratory movements of southern right whales (Eubalaena australis) from australian and New Zealand wintering grounds. PLoS ONE. 2020;15(5).
Riekkola L, Andrews-Goff V, Friedlaender A, Zerbini AN, Constantine R. Longer migration not necessarily the costliest strategy for migrating humback whales. Aquat Conserv. 2020;30(5):937–48.
Pitman RL, Durban JW, Joyce T, Fearnbach H, Panigada S, Lauriano G. Skin in the game: epidermal molt as a driver of long-distance migration in whales. Mar Mamm Sci. 2020;36(2):565–94.
Planque Y, Huon M, Caurant F, Pinaud D, Vincent C. Comparing the horizontal and vertical approaches used to identify foraging areas of two diving marine predators. Mar Biol. 2020;167(2).
Bestley S, Jonsen ID, Hindell MA, Harcourt RG, Gales NJ. Taking animal tracking to new depths: synthesizing horizontal - vertical movement relationships for four marine predators. Ecology. 2015;96(2):417–27.
Mizroch SA, Rice DW. Ocean nomads: distribution and movements of sperm whales in the North Pacific shown by whaling data and Discovery marks. Mar Mamm Sci. 2013;29(2):E136–65.
Servidio A, Pérez-Gil E, Pérez-Gil M, Cañadas A, Hammond PS, Martín V. Site fidelity and movement patterns of short-finned pilot whales within the Canary Islands: evidence for resident and transient populations. Aquat Conserv. 2019;29:227–41.
Geijer CKA, Notarbartolo di Sciara G, Panigada S. Mysticete migration revisited: are Mediterranean fin whales an anomaly? Mamm Rev. 2016;46(4):284–96.
Corkeron PJ, Connor RC. Why do baleen whales migrate? Mar Mamm Sci. 1999;15(4):1228–45.
Clapham P. Why do baleen whales migrate? A response to Corkeron and Connor. Mar Mamm Sci. 2001;17(2):432–6.
Gowan TA, Ortega-Ortiz JG, Hostetler JA, Hamilton PK, Knowlton AR, Jackson KA, et al. Temporal and demographic variation in partial migration of the North Atlantic right whale. Sci Rep. 2019;9(1):353.
Pomilla C, Amaral AR, Collins T, Minton G, Findlay K, Leslie MS, et al. The world’s most isolated and distinct whale population? Humpback whales of the arabian sea. PLoS ONE. 2014;9(12):e114162.
Aschettino JM, Engelhaupt DT, Engelhaupt AG, DiMatteo A, Pusser T, Richlen MF et al. Satellite Telemetry reveals spatial overlap between Vessel High-Traffic Areas and Humpback Whales (Megaptera novaeangliae) Near the Mouth of the Chesapeake Bay. Front Mar Sci. 2020;7.
Shuert C, Hussey NE, Marcoux M, Heide-Jørgensen MP, Dietz R, Auger-Méthé M. Divergent migration routes reveal contrasting energy-minimization strategies to deal with differing resource predictability. Mov Ecol. 2023;11(31).
Andrews-Goff V, Bestley S, Gales NJ, Laverick SM, Paton D, Polanowski AM, et al. Humpback whale migrations to Antarctic summer foraging grounds through the southwest Pacific Ocean. Sci Rep. 2018;8(1):1–14.
Bedriñana-Romano L, Zerbini AN, Andriolo A, Danilewicz D, Sucunza F. Individual and joint estimation of humpback whale migratory patterns and their environmental drivers in the Southwest Atlantic Ocean. Sci Rep. 2022;12(1):7487.
Stockin KA, Burgess EA. Opportunistic feeding of an adult Humpback Whale (Megaptera novaeangliae) migrating along the Coast of Southeastern Queensland, Australia. Aquat Mamm. 2005;31(1):120–3.
Citta JJ, Richard P, Lowry LF, O’Corry-Crowe G, Marcoux M, Suydam R, et al. Satellite telemetry reveals population specific winter ranges of beluga whales in the Bering Sea. Mar Mamm Sci. 2017;33(1):236–50.
Hauser DDW, Laidre KL, Suydam RS, Richard PR. Population-specific home ranges and migration timing of Pacific Arctic beluga whales (Delphinapterus leucas). Polar Biol. 2014;37(8):1171–83.
Storrie L, Hussey NE, MacPhee SA, O’Corry-Crowe G, Iacozza J, Barber DG, et al. Year-round dive characteristics of male Beluga Whales from the Eastern Beaufort Sea Population Indicate Seasonal shifts in foraging strategies. Front Mar Sci. 2022;8:715412.
Johnson DS, London JM, Lea MA, Durban JW. Continuous-time correlated random walk model for animal telemetry data. Ecology. 2008;89(5):1208–15.
Dujon AM, Lindstrom RT, Hays GC. The accuracy of Fastloc-GPS locations and implications for animal tracking. Methods Ecol Evol. 2014;5(11):1162–9.
Freitas C, Lydersen C, Fedak MA, Kovacs KM. A simple new algorithm to filter marine mammal Argos locations. Mar Mamm Sci. 2008;24(2):315–25.
Jonsen I, Patterson T, foieGras. Fit Continuous-Time State-Space and Latent Variable Models for Quality Control of Argos Satellite (and Other) Telemetry Data and for Estimating Movement Behaviour. R package version 0.7-6 [Internet]. 2021 [cited 2022 Jun 26]. Available from: https://cran.r-project.org/web/packages/foieGras/index.html.
R Core Team. R: A language and environment for statistical computing. [Internet]. Vienna, Austria; 2021 [cited 2022 Jun 20]. Available from: https://www.R-project.org.
McClintock BT, Michelot T. momentuHMM: maximum likelihood analysis of animal movement behavior using multivariate hidden Markov models. R package version 1.5.4 [Internet]. 2021 [cited 2022 Jun 22]. Available from: https://cran.r-project.org/web/packages/momentuHMM/index.html.
McClintock BT, Michelot T, momentuHMM. R package for generalized hidden Markov models of animal movement. Methods Ecol Evol. 2018;9(6):1518–30.
Lydersen C, Vacquié-Garcia J, Heide-Jørgensen MP, Øien N, Guinet C, Kovacs KM. Autumn movements of fin whales (Balaenoptera physalus) from Svalbard, Norway, revealed by satellite tracking. Sci Rep. 2020;10(1).
Michelot T, Langrock R. A short guide to choosing initial parameter values for the estimation in moveHMM [Internet]. 2022 [cited 2022 Sep 12]. Available from: https://cran.rstudio.org/web/packages/moveHMM/vignettes/moveHMM-starting-values.pdf.
Loseto LL, Richard P, Stern GA, Orr J, Ferguson SH. Segregation of Beaufort Sea beluga whales during the open-water season. Can J Zool. 2006;84(12):1743–51.
Hauser DDW, Laidre KL, Stafford KM, Stern HL, Suydam RS, Richard PR. Decadal shifts in autumn migration timing by Pacific Arctic beluga whales are related to delayed annual sea ice formation. Glob Chang Biol. 2017;23(6):2206–17.
Calenge C. adehabitatHR: home range estimation. R package version 0.4.19. [Internet]. 2021 [cited 2022 Jul 8]. Available from: https://cran.r-project.org/web/packages/adehabitatHR/index.html.
Aguilar de Soto N, Madsen PT, Tyack P, Arranz P, Marrero J, Fais A et al. No shallow talk: cryptic strategy in the vocal communication of Blainville’s beaked whales. Mar Mamm Sci. 2012;28(2).
Kendall-Bar JM, Williams TM, Mukherji R, Lozano DA, Pitman JK, Holser RR et al. Brain activity of diving seals reveals short sleep cycles at depth. Science. 2023;380:260–5.
Willoughby AL, Ferguson MC, Stimmelmayr R, Brower AA. Bowhead whale (Balaena mysticetus) carcasses documented during the 2019 aerial surveys in the eastern Chukchi and western Beaufort seas: a follow-up to evidence of bowhead whale and killer whale (Orcinus orca) co-occurrence during 2009–2018. Polar Biol. 2022;45(12):1723–8.
Hertel H. Structure, form and movement. New York: Reinhold Publishing Co; 1966.
Crocker DE, Le Boeuf BJ, Costa DP. Drift diving in female northern elephant seals: implications for food processing. Can J Zool. 1997;75(1):27–39.
Blackwell SB, Tervo OM, Lemming NE, Quakenbush LT, Heide-Jørgensen MP. Drift dives in a Bowhead Whale (Balaena mysticetus). Aquat Mamm. 2022;48(6):656–60.
Hijmans RJ, Karney C, Williams E, Vennes C, geosphere. Spherical Trigonometry. R package version 1.5–14 [Internet]. 2021 [cited 2022 Jul 12]. Available from: https://cran.r-project.org/web/packages/geosphere/index.html.
Abrahms B, Seidel DP, Dougherty E, Hazen EL, Bograd SJ, Wilson AM et al. Suite of simple metrics reveals common movement syndromes across vertebrate taxa. Mov Ecol. 2017;5(1).
Silva MA, Prieto R, Jonsen I, Baumgartner MF, Santos RS. North Atlantic Blue and Fin Whales suspend their Spring Migration to Forage in Middle Latitudes: building up Energy Reserves for the Journey? PLoS ONE. 2013;8(10):e76507.
Fagan WF, Lewis MA, Auger-Méthé M, Avgar T, Benhamou S, Breed G, et al. Spatial memory and animal movement. Ecol Lett. 2013;16(10):1316–29.
Galley RJ, Else BGT, Howell SEL, Lukovich JV, Barber DG. Landfast sea ice conditions in the Canadian Arctic: 1983–2009. Arctic. 2012;65(2):133–44.
Hornby CA, Hoover C, Iacozza J, Barber DG, Loseto LL. Spring conditions and habitat use of beluga whales (Delphinapterus leucas) during arrival to the Mackenzie River Estuary. Polar Biol. 2016;39(12):2319–34.
Norton P, Harwood LA. Distribution, abundance, and behaviour of white whales in the Mackenzie estuary. Environmental Studies Revolving Funds Report No. 036. Ottawa, ON; 1986.
Goessling HF, Tietsche S, Day JJ, Hawkins E, Jung T. Predictability of the Arctic sea ice edge. Geophys Res Lett. 2016;43(4):1642–50.
Babb DG, Landy JC, Lukovich JV, Haas C, Hendricks S, Barber DG et al. The 2017 reversal of the Beaufort Gyre: can Dynamic Thickening of a Seasonal Ice Cover during a reversal limit summer ice melt in the Beaufort Sea? J Geophys Res Oceans. 2020;125(12).
Storrie L, Hussey NE, MacPhee SA, O’Corry-Crowe G, Iacozza J, Barber DG, et al. Empirically testing the influence of light regime on diel activity patterns in a marine predator reveals complex interacting factors shaping behaviour. Funct Ecol. 2022;36(11):2727–41.
Lin P, Pickart RS, Stafford KM, Moore GWK, Torres DJ, Bahr F, et al. Seasonal variation of the Beaufort shelfbreak jet and its relationship to Arctic cetacean occurrence. J Geophys Res Oceans. 2016;121(12):8434–54.
Hobbs RC, Laidre KL, Vos DJ, Mahoney BA, Eagleton M. Movements and area use of belugas, Delphinapterus leucas, in a subarctic alaskan estuary. Arctic. 2005;58(4):331–40.
Vacquié-Garcia J, Lydersen C, Ims RA, Kovacs KM. Habitats and movement patterns of white whales Delphinapterus leucas in Svalbard, Norway in a changing climate. Mov Ecol. 2018;6(1):1–12.
Smith AJ, Higdon JW, Richard P, Orr J, Bernhardt W, Ferguson SH. Beluga whale summer habitat associations in the Nelson River estuary, western Hudson Bay, Canada. PLoS ONE. 2017;12(8):e0181045.
Zahn MJ, Laidre KL, Stilz P, Rasmussen MH, Koblitz JC. Vertical sonar beam width and scanning behavior of wild belugas (Delphinapterus leucas) in West Greenland. PLoS ONE. 2021;16(9 September):e0257054.
Chapman BB, Brönmark C, Nilsson J, Hansson LA. The ecology and evolution of partial migration. Oikos. 2011;120(12):1764–75.
Newton I. Advances in the study of irruptive migration. Ardea. 2006;94(3):433–60.
Strandberg R, Alerstam T. The strategy of fly-and-forage migration, illustrated for the osprey (Pandion haliaetus). Behav Ecol Sociobiol. 2007;61(12):1865–75.
Šuba J, Petersons G, Rydell J. Fly-and-forage strategy in the bat pipistrellus nathusii during autumn migration. Acta Chiropt. 2012;14(2):379–85.
Dias MP, Granadeiro JP, Catry P. Do seabirds differ from other Migrants in their travel arrangements? On Route strategies of cory’s shearwater during its Trans-Equatorial Journey. PLoS ONE. 2012;7(11):e49376.
Ostertag SK, Loseto LL, Snow K, Lam J, Hynes K, Gillman DV. “That’s how we know they’re healthy”: the inclusion of traditional ecological knowledge in beluga health monitoring in the Inuvialuit Settlement Region. Arct Sci. 2018;1–29.
Whitehead H. Consensus movements by groups of sperm whales. Mar Mamm Sci. 2016;32(4):1402–15.
Lefort KJ, Hussey NE, Jones JM, Johnson KF, Ferguson SH. Satellite-tracked sperm whale migrates from the canadian Arctic to the subtropical western North Atlantic. Mar Mamm Sci. 2022;1–7.
Best PB. Food and feeding of sperm whales Physeter macrocephalus off the West Coast of South Africa. S Afr J Mar Sci. 1999;(21):393–413.
Williams TM. The evolution of cost efficient swimming in marine mammals: limits to energetic optimization. Philosophical Trans Royal Soc B: Biol Sci. 1999;354(1380):193–201.
Slater GJ, Goldbogen JA, Pyenson ND. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proceedings of the Royal Society B: Biological Sciences. 2017;284(1855).
Irvine LG, Thums M, Hanson CE, McMahon CR, Hindell MA. Quantifying the energy stores of capital breeding humpback whales and income breeding sperm whales using historical whaling records. R Soc Open Sci. 2017;4(3).
Piatt JF, Methven DA. Threshold foraging behavior of baleen whales. Mar Ecol Prog Ser. 1992;84:205–10.
Hazen EL, Friedlaender AS, Goldbogen JA. Blue whales (Balaenoptera musculus) optimize foraging efficiency by balancing oxygen use and energy gain as a function of prey density. Sci Adv. 2015;1(9):e1500469.
Oftedal OT. Lactation in Whales and Dolphins: evidence of divergence between baleen-and toothed-species. Vol. 2, J Mammary Gland Biol Neoplasia. 1997.
Oremus M, Poole MM, Albertson GR, Baker CS. Pelagic or insular? Genetic differentiation of rough-toothed dolphins in the Society Islands, French Polynesia. J Exp Mar Biol Ecol. 2012;432–433:37–46.
Abecassis M, Polovina J, Baird RW, Copeland A, Drazen JC, Domokos R et al. Characterizing a foraging hotspot for short-finned pilot whales and blainville’s beaked whales located off the west side of Hawai’i island by using tagging and oceanographic data. PLoS ONE. 2015;10(11).
Pirotta V, Owen K, Donnelly D, Brasier MJ, Harcourt R. First evidence of bubble-net feeding and the formation of ‘super-groups’ by the east australian population of humpback whales during their southward migration. Aquat Conserv. 2021;31(9):2412–9.
Modest M, Irvine L, Andrews-Goff V, Gough W, Johnston D, Nowacek D et al. First description of migratory behavior of humpback whales from an Antarctic feeding ground to a tropical calving ground. Anim Biotelem. 2021;9(1).
Shuert CR, Marcoux M, Hussey NE, Peter Heide-Jørgensen M, Dietz R, Auger-M Eth EM. Decadal migration phenology of a long-lived Arctic icon keeps pace with climate change. Proceedings of the National Academy of Sciences. 2022;119(45):e2121092119.
Insley SJ, Halliday WD, Mouy X, Diogou N. Bowhead whales overwinter in the Amundsen Gulf and Eastern Beaufort Sea. R Soc Open Sci. 2021;8(4):202268.
Post E, Forchhammer MC. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Trans Royal Soc B: Biol Sci. 2008;363(1501):2369–75.
Møller AP, Rubolini D, Lehikoinen E. Populations of migratory bird species that did not show a phenological response to climate change are declining. PNAS. 2008;105(42):16195–200.
Hauser DDW, Laidre KL, Parker-Stetter SL, Horne JK, Suydam RS, Richard PR. Regional diving behavior of Pacific Arctic beluga whales Delphinapterus leucas and possible associations with prey. Mar Ecol Prog Ser. 2015;541:245–64.
Huntington HP, Danielson SL, Wiese FK, Baker M, Boveng P, Citta JJ, et al. Evidence suggests potential transformation of the Pacific Arctic ecosystem is underway. Nat Clim Chang. 2020;10(4):342–8.
O’Corry-Crowe G, Suydam R, Quakenbush L, Potgieter B, Harwood L, Litovka D et al. Migratory culture, population structure and stock identity in North Pacific beluga whales (Delphinapterus leucas). PLoS ONE. 2018;13(3).
O’Corry-Crowe G, Suydam R, Quakenbush L, Smith TG, Lydersen C, Kovacs KM et al. Group structure and kinship in beluga whale societies. Sci Rep. 2020;10(1).
Overland JE. Rare events in the Arctic. Clim Change. 2021;168.
Whitehead H. Gene–culture coevolution in whales and dolphins. Proc Natl Acad Sci U S A. 2017;114(30):7814–21.
Acknowledgements
We acknowledge our partners the Inuvialuit Game Council and Fisheries Joint Management Committee for their work in supporting the EBS beluga tagging program, as well as members of the Tagging Advisory Group. Special thanks to members of the field crew for their work in data collection, advice on project delivery, and discussions on beluga ecology: John Noksana Sr., Joseph Felix Jr., Raymond Ettagiak, James Pokiak and Mikkel Panaktalok (Tuktoyaktuk), Lawrence Kaglik and Linley Day (Inuvik), Dennis Arey and Dwayne Benoit (Aklavik), Patrick Akhiatak (Ulukhaktok), Norman Anikina (Sachs Harbour), Émilie Couture (Granby Zoo/Canadian Wildlife Health Cooperative) and Benjamin Lamglait (Canadian Wildlife Health Cooperative), Greg Elias (Aurora Research Institute), and Jeremy Hansen (Fisheries and Oceans Canada, Inuvik). Thank you to Laura Murray (Fisheries and Oceans Canada), Emily Way-Nee (Joint Secretariat), Jimmy Kalinek (Only Way Outfitting) and James Keevik for key logistical support.
Funding
Funding was provided by Crown-Indigenous Relations and Northern Affairs Canada (Beaufort Regional Strategic Environmental Assessment), Fisheries and Oceans Canada (Intergovernmental Strategy, Strategic Program for Ecosystem-based Research and Advice, National Conservation Plan), the Fisheries Joint Management Committee (Tarium Niryuitait Marine Protected Area Funds, Anguniaqvia niqiqyuam Marine Protected Area Funds, FJMC Core Funds), and Natural Resources Canada (Polar Continental Shelf Program). PhD stipend for LS was provided by ArcticNet and the University of Manitoba Graduate Fellowship. The following approvals were required for whale capture and handling procedures: DFO License to Fish for Scientific Purposes (S-18/19-3020-YK), Freshwater Institute Animal Care Committee permit (FWI-ACC-2018-24), and Letter(s) of Support from Inuvialuit Hunters and Trappers Committees.
Author information
Authors and Affiliations
Contributions
LS performed all data analyses, wrote the manuscript, and created all figures and tables. LS, NH and LL conceptualized the study. LL and SM led on program implementation and secured the funding and partnerships. LL, NH, SM, and GO’C-C planned the field programs. LS, LL, NH, SM, and GO’C-C collected the data. NH, LL and ES were involved in critical discussions of the manuscript with LS throughout the process. All authors read, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Storrie, L., Loseto, L.L., Sutherland, E.L. et al. Do beluga whales truly migrate? Testing a key trait of the classical migration syndrome. Mov Ecol 11, 53 (2023). https://doi.org/10.1186/s40462-023-00416-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40462-023-00416-y