Abstract
Background and objective
Endometriosis and adenomyosis are two common diseases that impair women’s health, and dienogest is one of the pharmacologic treatments which is the first-line therapeutic option for patients with pelvic pain and individuals who have no desire for immediate pregnancy. The goal of this study was to summarize the current evidence of adverse events associated with dienogest as well as the prevalence of these adverse events during treatment with dienogest.
Methods
Several databases (PubMed, Embase, Cochrane Central and Clinicaltrials.gov, etc.) and the US FDA Adverse Event Reporting System (FAERS) Public Dashboard were searched on May 31, 2023, using the topic words alongside free words of dienogest and “adverse reaction”. Studies were incorporated into this research if they reported or assessed safety issues or adverse reactions of dienogest during the period of endometriosis treatment or adenomyosis therapy. The extracted information comprised trial design, dienogest and control group demographics, as well as reported side effects.
Results
This systematic review comprehended 39 publications in total. The mean age of patients in the included studies was 34.43 years. The follow-up duration varied from 3 to 60 months. Most adverse reactions were common and not serious, and the most common adverse reactions during dienogest medication were abnormal uterine bleeding (55%, 95% CI 37–73%), amenorrhea (17%, 95% CI 2–42%) and swelling (13%, 95% CI 3–28%). Uncommon adverse reactions included dysmenorrhea (0.2%, n = 1), dyspepsia (0.4%, n = 1), and (lower) abdominal pain (1%, 95% CI 0–3%), urticaria (1%, 95% CI 0–3%) and peritonitis (1%, n = 1). Serious adverse reactions including decreased lumbar spine Bone Mineral Density (BMD), depression, peritonitis and so on have been reported. Heterogeneity assessment revealed that patient number and study design are influencing factors to adverse reaction prevalence. Moreover, abdominal pain, diarrhea, nausea and vomiting, back pain and anemia are side effects reported both in the FAERS database and in the systematic review.
Conclusions
Dienogest’s most frequent side effects were not severe. Dienogest is generally safe for treating endometriosis and adenomyosis. Nevertheless, people should be aware of serious adverse reactions, such as decreased lumbar spine BMD and hemorrhagic shock.
Similar content being viewed by others
Introduction
Endometriosis and adenomyosis, two diseases that frequently impair women’s health, share extremely similar pathophysiologies, the tissues of which both originate from the intracavity endometrium. Endometriosis is a prevalent, often life-affecting condition that occurs in most women during adolescence [1]. Globally, 5–10% of women of reproductive age are affected by endometriosis [2]. It is a chronic, inflammatory, gynecologic disease characterized by the presence of endometrial-like tissue outside the uterus and can evolve to include symptoms and conditions encompassing multiple systems [1, 3]. This complex disease has a considerable impact on the quality of life of affected individuals and has no cure [4]. It is associated with a large disability in daily living, causing socioeconomic deterioration and burden [5]. A systematic review revealed that endometriosis and adenomyosis were even associated with reduced pregnancy and live birth rates, and increased miscarriage in women [6]. The declining birth rate is not only a patient issue but also a serious national issue. Population aging and shrinkage overlap each other and significantly impact society at large through health issues, becoming a looming demographic challenge starting from Asia [7]. Infertility and pain are the major concerns of endometriosis. In regard to infertility, the available therapeutic options are surgical approaches; and pharmacological therapies are most often suggested for endometriosis-associated pain [8].
Surgical, pharmacologic and nonpharmacologic approaches for treating patients with endometriosis are recommended by guidelines and the literature [1, 3–4, 9], and pharmacologic treatment is the first-line therapeutic option for patients with pelvic pain and no desire for immediate pregnancy [10]. Progestins, combined oral contraceptives (COCs), gonadotropin-releasing hormone agonists (GnRHa), and the levonorgestrel-releasing intrauterine system (LNG-IUS) are suggested as pharmacological therapies [8]. Moreover, oral contraceptives, the LNG-IUS, and Dienogest (DNG) should be used as first-line medical options for pain and heavy menstrual bleeding caused by endometriosis and adenomyosis [1, 4, 9] according to guidelines and expert opinions [9, 11].
DNG 2 mg is an effective and tolerable alternative to surgical intervention for the long-term management of endometriosis and is comparable to COCs for the relief of endometriosis-associated pelvic pain and health-related quality of life (QoL) [12] and is better than GnRH-a after surgery for endometriosis [13]. DNG, a fourth-generation progestin, has long been used as a progestogen in combination with ethinylestradiol as an oral contraceptive [14]. In January 2008, DNG 2 mg was first launched in Japan as a new drug for treating endometriosis and has since been approved in many countries and regions around the world. In 2009, DNG received approval for the treatment of endometriosis in the European Union [11].
The reduction in endometriotic lesions and pain symptoms and improvement in the QoL are significantly greater in women taking dienogest than women taking continuous COC [15]. In areas where it is marketed, progestin DNG appears to be superior to COCs for treating adenomyosis [16]. A larger evidence base supports the use of dienogest therapy compared with GnRHa as first-line medical therapy [17]. Long-term treatment with DNG 2 mg has been shown to decrease recurrent endometrioma size, which may indicate an additional benefit of its use in medical treatment [18]. Moreover, both of these treatment regimens (GnRHa and COC) are associated with suboptimal safety and tolerability [19–20], which limits their long-term use. In clinical trials, 2 mg of DNG once daily was reported to be generally safe and well tolerated for the treatment of endometriosis [11].
Convincing safety and tolerability data, in combination with proven efficacy, represent key characteristics when choosing an optimal therapy for long-term use in endometriosis [21]. An observational study of 157 patients with endometriosis evaluated the efficacy and long-term safety (up to 108 months) of DNG treatment, and the study revealed that long-term therapy was associated with greater rates of side effects, such as headache, weight gain and libido reduction [22]; however, the sample size was small, especially the maximum duration of observation (only 1 patient). To our knowledge, most clinical studies have been limited to small retrospective and prospective studies, and few large-scale clinical studies have evaluated the safety of DNG. Strowitzki [21] described the safety profile of dienogest in the European population, but was limited to 4 clinical trials with less homogeneity (a pilot study of high doses of 20 mg/day, a phase II dose-ranging study, and a phase III trial) involving 332 women. There are currently no large population-based safety studies conducted with DNG.
Thus, the goal of this systematic review and meta-analysis was to compile the most recent data on adverse events of DNG and how frequently they were reported while using it.
Methods
Data sources and search strategy
The following electronic bibliographic databases were searched: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials, ChiCTR, SinoMed, CNKI, and VIP from conception to May 2023. We conducted a search in the abovementioned databases using the following topic words and free words related to our review theme: (“Dienogest” OR “visanne” OR “STS-557”) AND (“case report” OR “adverse reaction” [e.g., adverse drug reaction]). A search was conducted for any relevant research in the references and linked review articles, letters, and protocols. Synonyms of search terms suggested by the search engines were used. The detailed search algorithms are available in Electronic Supplementary Material 1.
To further document the adverse effects of DNG, information was also retrieved from the FAERS Public Dashboard [23], a spontaneous reporting system. ‘Dienogest’ and ‘visanne’ were used as the search terms and were restricted to the “primary suspect” (PS) drug. SAS software (version 9.4) was utilized to mine the DNG adverse event data that were accessible from 2010 Q1 to 2023 Q2 on the FAERS Public Dashboard.
Symptoms of AEs were coded by using the Medical Dictionary for Regulatory Activities (MedDRA 26.0) with preferred terms (PTs) and the system organ class (SOC), an internationally standardized, clinically validated terminology [24]. The FDA does not demand proof of a drug’s causal connection to an incident, and it should be stressed. Additionally, FAERS data cannot be used to produce incidence, risk assessment, or risk rating.
Inclusion and exclusion criteria
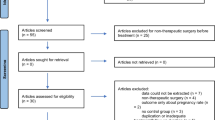
The studies included in this meta-analysis were the following types of studies: (1) human studies; (2) studies that specifically considered DNG as the exposure; and (3) studies that specifically indicated adverse reactions or safety outcomes during DNG use. Studies were excluded if they were (1) animal studies, systematic reviews, or meta-analyses; (2) used DNG in combination with oral contraceptives; (3) lacked safety or side effect information about DNG; or (4) were conducted on males. The case reports and case studies that contained information that was not applicable for calculating pooled data were included in the systematic review but excluded from the meta-analysis. The preferred reporting items for systematic reviews and meta-analysis (PRISMA) diagram of the systematic literature review process are displayed in Fig. 1.
Data extraction
The articles that were identified through the search were preserved in EndNote X8 (Thomson Reuters, New York, USA), a citation manager. The first author went over the titles and abstracts of the remaining articles after the duplicate articles were eliminated, retrieving particular terms for exclusion (e.g., words such as review, meta-analysis, systematic review, and animal were searched). The remaining titles and abstracts were examined afterwards. The first author created the abstraction form for the extraction process, and the coauthor reviewed it. The first and second authors separately retrieved data of research design, location, patient demographics, DNG dose regimens and the control group dose regimen, time since first take, types of adverse events and their relevance, and potential bias in each study. Reactions are coded by a dataset from the MedDRA, and they were analyzed at the preferred term (PT) level of the hierarchy (i.e., MedDRA Level 4 descriptions). We used standardized MedDRA terminology to categorize each AE during the extraction process. The FAERS files were downloaded, and the following details were retrieved from the FAERS files: demographic characteristics, suspected and concomitant drugs, the reporting source, the indication and the PT name and the SOC name of the reported adverse reactions.
In this study, serious side effects were defined as “any untoward medical occurrence that at any dose results in death; is life-threatening; requires inpatient hospitalization or prolongation of existing hospitalization; or results in persistent or significant disability or incapacity”. The serious adverse reactions reported by the included studies were also incorporated. Furthermore, adverse reactions are classified as very common, common (or frequent), uncommon (or infrequent), rare, and very rare at frequencies greater than or equal to 1/10, less than 1/10 to higher than (or equal to) 1/100, less than 1/100 to higher than (or equal to) 1/1000, less than 1/1000 to higher than (or equal to) 1/10,000, and less than 1/10,000, in that order.
Risk of bias
Depending on the kind of included studies, various methods were utilized to assess the ri-sk of bias. The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials (CCRAB-RCT) [25] and Jadad’s quality scales, methodological index for nonrandomized studies (MINORS) [26] and Newcastle-Ottawa Quality Assessment Scale (NOS) [27] were used separately to evaluate clinical trials, nonrandomized experimental studies, cohort studies, and observational studies, respectively. For Jadad’s quality scales, studies with a total score of 4–7 were classified as high-quality studies, and those with a score of 1–3 were classified as low-quality studies. For the MINORS scale, 9–12 points were considered to indicate moderate-quality literature, and scores below 12 points were not included in the meta-analysis. For the NOS, articles with 6 or more stars were considered to be of higher quality and were included in the study. According to the risk of bias summary of Review Manager Software (version 5.3; The Nordic Cochrane Collaboration, Copenhagen), the following biases were analyzed: selection, detection, attrition, reporting and unknown sources of potential bias. The results of the risk of bias evaluation are provided in Electronic Supplementary Material 2. All 10 clinical trials were of high quality. There were 4 nonrandomized controlled trials scoring 12, all of which were included. A total of 16 cohort studies and observational studies received 6 stars or more.
Statistical methods
Inclusion in this meta-analysis was restricted to studies that reported the prevalence of adverse reactions. The statistical analysis was performed by the main author (LRR). All the statistical analyses of the meta-analysis were performed using Stata software version 15.1 (StataCorp LP). We pooled the categorical variables as rates with 95% confidence intervals (CIs). A random effect model was used under the assumption that the data came from varied populations with different distributions. The magnitude of heterogeneity was assessed by I2 statistic. Meta-analyses, heterogeneity testing, and bias risk assessment were also performed. I2 values less than 25% suggested minimal heterogeneity, while I2 values greater than 75% showed significant heterogeneity [28]. And the I2 value between 25% and 50% displayed moderate heterogeneity. A sensitivity analysis was also conducted, in which one study was omitted and the other was analyzed to estimate whether the results could have been markedly affected by a single study. Additionally, a funnel plot was used to assess the presence of publication bias. Moreover, a subgroup analysis or a meta-regression model was utilized when necessarily accounted for a major heterogeneity.
The signal monitoring procedure was used to summarize the rates of adverse effects of DNG using SAS version 9.4. For the statistical characterization of the cohorts, we utilized the reporting odds ratio (ROR) [29] and the proportional reporting ratio (PRR) [30] score, established measures of disproportionality in pharmacovigilance, in our analysis. The proportional imbalance method based on a four-cell table (Table 1) was used for signal mining in this study. According to the ROR method, the number of reports (n) was ≥ 3, and the 95% CI of the ROR value was > 1. Moreover, according to the PRR method, an adverse event was generated if n ≥ 3, PRR ≥ 2, and χ2 ≥ 4 [31]. A valid signal must meet the conditions of both the ROR and PRR methods.
Results
Study characteristics
A comprehensive search of the literature revealed 957 articles that were not redundant. Thirty-nine publications were included in the systematic review based on the inclusion and exclusion criteria. Nine studies [18, 32,33,34,35,36,37,38,39] were not included in the meta-analysis because of a lack of accurate adverse reaction report (ADR) data or a small sample size (n ≦ 30). Of the remaining 30 articles [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68], 10 were clinical trials, 4 were nonrandomized experimental studies, and 16 were cohort studies and observational studies. The quality of the included articles varied (Table 2 displays a summary of the quality of studies). The 39 articles were carried out in America (USA 1), Europe (Italy 4, Germany 3, Russia 1, “Italy and Ukraine” 1, multicenter 2), Asia (Korea 6, Japan 9, China 7, India 1, Turkey 1, multicenter 1) and Africa (Egypt 2). The studies that were considered had a mean age of 34.43 years. A dosage regimen consisting of DNG 2 mg tablet was used in 24 studies [12, 40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. A dose regimen of DNG (1 mg tablet twice) was used in four studies [63,64,65,66]. Two doses of Dienogest (0.5 mg tablet) were used in one study [67], while at least one dose was used in another study [68]. The duration of the study ranged from 3 to 60 months. The papers that make up this meta-analysis, in our opinion, cover a global population that is receiving treatment for endometriosis or adenomyosis using clinically appropriate DNG regimens. The duration of the included studies and the total number of patients (n = 6748) in this meta-analysis were adequate to capture the infrequent, or even rare side effects of dienogest.
Results of the meta-analyses
The statistical results of each selected study were converted into effect sizes and combined in the meta-analyses. The very common adverse reactions observed during the use of DNG were as follows: abnormal uterine bleeding (55%; 95% CI 37–73%; n = 25, I2 = 98.27%), amenorrhea (17%; 95% CI 2–42%; n = 5, I2 = 97.78%) and swelling (13%; 95% CI 3–28%; n = 4, I2 = 86.93%). A detailed summary of the pooled frequency of adverse reactions is shown in Table 3. Obviously, the prevalence of some adverse reactions presented considerable heterogeneity between studies. Moreover, several side effects, such as nausea/vomiting, dizziness, decreased libido, and malaise, showed low or moderate heterogeneity. The forest plots of the meta-analyses and sensitivity analysis results are shown in Figs. 2 and 3. Only one adverse effect (nausea/vomiting) was discussed in the text due to space limitations. For the detailed heterogeneity assessment and sensitivity analysis, including forest plots and funnel plots, of the remaining adverse reactions, please refer to the supplemental data (Electronic Supplementary Material 3).
Safety of dienogest in users
In this study, we found that the adverse effects of DNG can be categorized as reproductive system and breast disorders, gastrointestinal disorders, skin and subcutaneous tissue disorders, nervous system disorders, psychiatric disorders, etc. The majority of adverse actions were mild and frequent. Uncommon adverse reactions identified included dysmenorrhea (0.2%; n = 1) [43], dyspepsia (0.4%; n = 1) [43], abdominal pain (1%; 95% CI 0–3%), urticaria (1%; 95% CI 0–3%) and peritonitis (1%; n = 1) [63], while the reported serious adverse reactions included a decrease in lumbar spine BMD [45], depression [54, 57], peritonitis [63], vomiting [68], asthenia, dysmenorrhea [68] and serious abnormal uterine bleeding. The serious adverse events reported by Cho BS [43] during DNG medication were dermal cyst, salivary gland calculus, fibrocystic breast disease, postprocedural infection, and thyroid cancer, but causality with Visanne® was considered unlikely. Momoeda M also suggested that the serious adverse events (ulcerative colitis, colonic polyps, and splenic injury) that occurred in his study were not causally related to DNG [64].
The adverse effects of dienogest were found involving multiple SOCs, with the most common being reproductive system and breast disorders (9 PTs), psychiatric disorders (6 PTs) and investigations (4 PTs). The top 10 most frequently reported adverse events were abnormal uterine bleeding (n = 25), headache (n = 23), breast discomfort (n = 22), weight gain (n = 17), hot flashes (n = 16), mood disorders (n = 15), nausea (n = 12), acne (n = 9), vaginal dryness (n = 6), alopecia (n = 6), decreased libido (n = 6), and insomnia (n = 6). Hot flashes are common endocrine side effects with an incidence of 9% (95% CI 5–15%; n = 16, I2 = 73.77%). Nervous system disorders included headache (8%; 95% CI 6–11%; n = 23, I2 = 73.77%). The investigations included weight gain (7%; 95% CI 5–9%; n = 17, I2 = 76.03%) and decreased bone density (5%; 95% CI 3–8%; n = 2). Other common adverse reactions were breast discomfort (7%; 95% CI 4–10%; n = 22, I2 = 88.79%), decreased libido (7%; 95% CI 5–9%; n = 6, I2 = 0%), mood disorders (7%; 95% CI 3–11%; n = 15, I2 = 90.17%), and fatigue (7%; 95% CI 1–15%; n = 3, I2 = 74.99%).
Results of heterogeneity assessment and sensitivity analysis
Heterogeneity assessment of each adverse reaction revealed that some adverse reactions presented minor or moderate heterogeneity (I2 ≤ 50%, or 50%<I2 ≤ 75%), while some demonstrated considerable heterogeneity (I2 ≥ 75%). Among the included studies, one conducted by Cho BS [43] led to considerable heterogeneity in several adverse reactions. The heterogeneity decreased after this particular study was removed in the following adverse reactions: abnormal uterine bleeding, breast tenderness, nausea/vomiting, alopecia, headache, dizziness, mood disorders, insomnia and sleep disorder. Additionally, two studies (Kim et al. [48] and Yang et al. [60]) contributed largely to the heterogeneity of an adverse reaction, namely weight gain. Moreover, one study (Harada et al. [63]) greatly affected the heterogeneity of hot flushes.
In addition, a meta-regression model and subgroup analysis were both carried out for adverse reactions with considerable heterogeneity. The meta-regression model for each adverse reaction with great heterogeneity revealed that the following factors had an impact: patient number, year and study design. Furthermore, subgroup analysis elucidated that study design, observation period, and patient number were influencing factors of outcomes’ heterogeneity. In detail, the heterogeneity of breast tenderness was reduced by dividing the included studies into several groups according to study design (the heterogeneity in the RCT group was lower, I2 = 76.42%). The heterogeneity of weight gain was also reduced by dividing the included studies into several groups according to the study design (the heterogeneity in the RCT group was lower, I2 = 20.75%) and observation period (the heterogeneity in the 6-month observation group was lower, I2 = 47.16%). For more detailed information, please see Table 4.
Descriptive data from the US FDA adverse events reporting system (FAERS) database
A search of the FAERS database revealed 5 reports of adverse effects of DNG limited to the “primary suspect”; 4 were from healthcare professionals, and 1 was from a consumer. The indications, drug combinations, pt_names and soc_names in these five reports are shown in Table 5. These 5 cases were reported from different countries (Japan, Italy, Germany, and India); 3 were reported in 2020, 1 in 2012, and 1 in 2017. The ROR and PRR methods were used for calculations. Two positive SOC signals were screened for vascular disorders and gastrointestinal disorders, respectively (Table 6). Abdominal pain, diarrhea, nausea and vomiting are gastrointestinal side effects reported not only in the FAERS database but also in systematic reviews and included in the specification. In addition, both the FAERS database and the systematic review reported back pain and anemia. Arthralgia, pulmonary embolism, hypertension, osteoma, papillary thyroid cancer and thyroid calcification were found in the FAERS database; however, these conditions were not found during the systematic review. Bleeding-related adverse events, such as shock haemorrhagic, haemorrhage and haemorrhagic stroke, were found in two cases which were treated with DNG combined with warfarin and aspirin.
Discussion
In this analysis, we were able to identify 39 publications with a certain standard of reporting quality describing the safety of DNG in patients with endometriosis or adenomyosis and providing specific recommendations for adverse drug reactions from different authors, which would allow direct transfer into clinical practice. According to the results of the present study, the common medication dose regimen used for DNG is 2 mg/d, and most adverse reactions related to DNG are common and not serious. This large sample size of the entire population was used for the side effect study. Compared with those of the dienogest drug package insert, most of the side effects were included, and the reliability of this research was confirmed to some extent.
Strowitzki et al. [21] reported that the most common AEs were headache (9%), breast discomfort (5.4%), depressed mood (5.1%), and acne (5.1%), and these AEs were also reported in the present study, with prevalences of 8%, 7%, 7%, and 2%, respectively. The incidences of “hypoestrogenic” ADRs in this pooled population treated with dienogest were as follows: vaginal dryness (6%), loss of libido (7%), and hot flushes (9%). The current study is more comprehensive, and the relevant information on side effects is richer. Another recent observational study [22] reported that menstrual changes (22.9%) were the most common side effect of DNG, which is consistent with the results of the present study. Other adverse reactions reported in the Maiorana A’ study included headache (17.2%), weight gain (17.8%) and loss of libido (32.7%). All of these side effects were reported in the present study, and the incidences of these adverse reactions were 8%, 7% and 7%, respectively.
According to the meta-analysis, all the included studies reported adverse reactions involving the reproductive system and breast disorders. Abnormal uterine bleeding was the most frequently reported ADR [69]. During the process of dienogest treatment, 55% of the patients in this study experienced changes in bleeding patterns, such as vaginal bleeding, vaginal spotting, abnormal uterine bleeding, metrorrhagia and irregular menstruation. Changes in bleeding patterns varied from patient to patient. Even in the same patient, bleeding patterns differed between the first and the second treatment with Dienogest [70]. A healthy, nulliparous 37-year-old woman developed hypovolemic shock during the re-administration of DNG therapy, but she had only spotty bleeding intermittently during her first medication [70]. A large prospective cohort study [43] revealed that with increasing treatment duration, the number of patients with favorable bleeding patterns (such as amenorrhoea, infrequent bleeding, and normal/acceptable bleeding) increased, whereas the number of patients with unfavorable bleeding patterns (such as frequent bleeding, irregular bleeding, and prolonged bleeding) decreased. Prolonged use of dienogest is beneficial for reducing abnormal uterine bleeding.
The pooled estimate of breast tenderness due to DNG in this study was 7% (95% CI 0.04–0.10, I2 88.79%). Progesterone may increase breast discomfort, and the risk of new-onset breast tenderness triples with estrogen plus progestin (RR 3.07) and doubles with estrogen alone (RR 2.15) compared to that with placebo [71]. Crandall CJ’s research indicated that breast tenderness was associated with a greater risk of breast cancer [71]. However, Schindler AE et al. suggested that high-dose progestin (dienogest, 20 mg/d) could reduce the size of the mammary gland and promote the regression of mastopathic changes [72]. However, the impact of dienogest on the mammary gland needs to be further investigated.
The average amenorrhea rate was 17% in this study. The rate of amenorrhea steadily increased as the duration of treatment with dienogest prolonged [49]. In another large prospective cohort study, amenorrhea was observed in 29.63% of patients treated with DNG at 3 months and increased to 53.20% at more than 12 months [43]. The causes of amenorrhea can be categorized as outflow tract abnormalities, primary ovarian insufficiency, hypothalamic or pituitary disorders (such as medication), other endocrine gland disorders, sequelae of chronic disease, physiologic disorders, or induced symptoms [73]. Medication can cause hyperprolactinemia, resulting in hypothalamic or pituitary disorders [73]. The author speculated that DNG may also cause amenorrhea by affecting the hypothalamic-pituitary axis. Swelling is the last of the three most common adverse reactions. A national survey of umbilical endometriosis conducted in Japan revealed that swelling in the umbilicus is a frequent symptom in patients [74]. Two patients with primary spontaneous inguinal endometriosis presented with painful swelling in the right groin area [75]. In addition, adenomyosis can also cause severe swelling of the uterus [76]. On this basis, swelling may be a clinical symptom of the disease and may have little or nothing to do with dienogest.
A decrease in lumbar spine BMD is a serious adverse reaction [45]. In a study in which patients were taking DNG for 18 months, 20% of the patients had a BMD (Z-zone) below the expected range for their age in the lumbar spine or femoral neck (Park SY) [53]. After 3 years of treatment with dienogest, the BMD decreased significantly at both the lumbar spine (−4.4%) and femur neck (−3.6%) compared to the baseline in nearly 80% of the patients [77]. In the first 12 months, DNG treatment led to a transient decrease in BMD compared to that in patients treated with the LNG-IUS, but after 24 months of treatment, the rate of bone loss was comparable between these two groups [78]. Compared with that at baseline, the BMD at the lumbar spine significantly decreased after the first 6 months (−2.2%) and 1 year (−2.7%) of DNG treatment in 75% of patients [79]. A prospective cohort study of 52 reproductive aged women showed that the BMD at the lumbar spine significantly decreased after the first 6 months of treatment in both the COC after GnRH agonist (−3.5%) and DNG (−2.3%) groups [80]. However, one study [61] showed that dienogest treatment for up to 52 weeks had no significant detrimental effect on lumbar BMD compared with 28 weeks of medication, with mean relative changes from baseline of–0.4492% (52 weeks) and −0.8558% (28 weeks). In adolescents with suspected endometriosis, treating with 2 mg DNG for 52 weeks decreased lumbar BMD [45]. Long-term DNG treatment has an adverse effect on BMD, which may limit its long term use, particularly for young women and adolescents who have not reached maximum bone density. However, the extent of its impact on BMD is no greater than that of GnRH agonist or LNG-IUS [78,79,80]. Moreover, age-related decreases in BMD are also influential factors [78].
In addition to systematically evaluating the adverse effects of dienogest through the literature review, this study also searched for real-world adverse effects reported by patients and healthcare professionals through the FAERS database. Vascular disorders and gastrointestinal disorders were 2 positive SOC signals detected. The SOC name “gastrointestinal disorders” contained PT terms such as “abdominal pain upper”, “diarrhea”, “nausea” and “vomiting”, which were all identified by Bayesian analysis. The SOC name “vascular disorders” included PT terms, including “hypertension”, “shock hemorrhagic” and “hemorrhage”, but this SOC was not identified in the systematic review. The patient who experienced hemorrhagic shock was a 45-year-old woman with adenomyosis. She was administered dienogest after 6 months of gonadotropin-releasing hormone analog treatment and had been receiving warfarin and aspirin [81]. Warfarin is a widely used anticoagulant with a narrow therapeutic range [82], and aspirin is an antiplatelet agent for a variety of thromboembolic diseases [83]. Both of these drugs can increase bleeding events [84,85,86]. Hence, care must be taken for patients receiving dienogest therapy, especially for patients receiving anticoagulants or antiplatelet agents simultaneously. In addition, patients who revert to the dienogest after taking dydrogesterone [70] or GnRH-a [81] must be closely monitored and educated to prevent critical events such as hemorrhagic shock.
A limitation of this article is that the incidence of several adverse effects, such as abnormal uterine bleeding and depression, may be influenced by disease symptoms. Changes in the menstrual cycle and progressive dysmenorrhea are the main symptoms of adenomyosis [87]. The Visanne Post-approval Observational Study (VIPOS) is a large real-world study performed examining the safety of dienogest and other treatments for endometriosis management in routine clinical practice [88]. The VIPOS study described women’s experiences with endometriosis in the real world and suggested that one of the most frequently reported endometriosis-associated symptoms was heavy/irregular bleeding (50.8%). Additionally, 55.6% of the women with endometriosis reported feeling “down”, depressed, or hopeless [89]. Of the 30 included trials, seven used DNG for the treatment of adenomyosis, and the rest used DNG for the treatment of endometriosis. Therefore, the statistical incidence of abnormal uterine bleeding and mood disorders may be due in part to preexisting symptoms of the disease. However, this is an objective limitation, which means that researchers are challenged to identify adverse reactions.
Another limitation is the high heterogeneity of the pooled statistics from the meta-analysis. A possible explanation is that the included studies were conducted in different countries around the world. Differences in dosing regimens, duration of treatment, and study design also contributed to study heterogeneity. The third limitation is that most of the included studies were designed to evaluate the efficacy of dienogest in the treatment of endometriosis and adenomyosis. Thus, the statistical analysis of the included studies may have had less power to detect a significant difference between the medication group and the nonusers. This limitation was overcome by pooling the data from each study and the meta-analysis of adverse effects, regardless of the differences in comparators. Furthermore, the association between AEs and DNG reported in the observational studies included in this meta-analysis does not imply a causal relationship between DNG and AEs.
The findings of this systematic review and meta-analysis offer extensive safety information on DNG and have several clinical implications. Initially, serious adverse reactions (such as a decrease in lumbar spine BMD, peritonitis and severe vomiting) to DNG were not common, and common adverse reactions were not serious (such as abnormal uterine bleeding, amenorrhea and swelling). Therefore, long-term use of DNG, if effective, should be encouraged in people with endometriosis or adenomyosis. Secondly, the literature review revealed multiple uncommon (dysmenorrhea, dyspepsia and (lower) abdominal pain, urticaria and peritonitis) or serious adverse events (depression, asthenia and serious abnormal uterine bleeding). This can be used as a hypothesis for further observational studies or systematic reviews and meta-analyses to prove whether the incidence is associated with the use of DNG. Thirdly, this study not only summarized and analyzed the adverse reactions to dienogest reported in the literature but also reviewed the publicly available FAERS database, which provides real-world safety information about DNG.
Conclusion
These pooled analyses from 30 clinical trials of DNG 2 mg represent a contribution to evidence-based medicine for endometriosis and adenomyosis, providing outcomes of potential relevance to daily practice. This systematic review and meta-analysis revealed that most prevalence adverse reactions of DNG, like abnormal uterine bleeding (55%, 95% CI 37%~73%), amenorrhea (17%, 95% CI 2%~42%), and swelling (13%, 95% CI 3%~28%), were common and not serious. The serious adverse reactions identified included a decrease in lumbar spine BMD, depression, peritonitis, vomiting, asthenia, dysmenorrhea and severe abnormal uterine bleeding. Abdominal pain, diarrhea, nausea and vomiting are gastrointestinal side effects reported not only in the FAERS database but also in the systematic review and included in the specification. DNG is generally safe for treating endometriosis and adenomyosis, but serious adverse reactions (lumbar spine BMD decrease and hemorrhagic shock) should be aware.
Abbreviations
- FAERS:
-
the US FDA Adverse Event Reporting System
- BMD:
-
Bone Mineral Density
- COCs:
-
combined oral contraceptives
- GnRHa:
-
gonadotropin-releasing hormone agonists
- LNG-IUS:
-
the levonorgestrel-releasing intrauterine system
- DNG:
-
Dienogest
- QoL:
-
quality of life
- CENTRAL:
-
the Cochrane Central Register of Controlled Trials
- PS:
-
primary suspect
- MedDRA:
-
the Medical Dictionary for Regulatory Activities
- PTs:
-
preferred terms
- PT:
-
preferred term
- SOC:
-
the system organ class
- PRISMA:
-
The preferred reporting items for systematic reviews and meta-analysis
- CCRAB-RCT:
-
The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials
- MINORS:
-
Jadad’s quality scales, methodological index for nonrandomized studies
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- ROR:
-
reporting odds ratio
- PRR:
-
proportional reporting ratio
- ADR:
-
adverse reaction report
- RCT:
-
Randomized controlled trial
- VIPOS:
-
Visanne Post-approval Observational Study
References
Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022;379:e070750. https://doi.org/10.1136/bmj-2022-070750.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–52.
Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis. ESHRE Endometriosis Guideline Group. Hum Reprod Open. 2022;2022(2):hoac009.
Allaire C, Bedaiwy MA, Yong PJ. Diagnosis and management of endometriosis. CMAJ. 2023;195(10):E363–71. https://doi.org/10.1503/cmaj.220637.
Wang PH, Yang ST, Chang WH, et al. Endometriosis: part I. Basic concept. Taiwan J Obstet Gynecol. 2022;61(6):927–34. https://doi.org/10.1016/j.tjog.2022.08.002.
Vercellini P, Viganò P, Bandini V, et al. Association of endometriosis and adenomyosis with pregnancy and infertility. Fertil Steril. 2023;119(5):727–40. https://doi.org/10.1016/j.fertnstert.2023.03.018.
Nakatani H. Ageing and shrinking population: the looming demographic challenges of super-aged and super-low fertility society starting from Asia. Glob Health Med. 2023;5(5):257–63. https://doi.org/10.35772/ghm.2023.01057.
Kalaitzopoulos DR, Samartzis N, Kolovos GN, et al. Treatment of endometriosis: a review with comparison of 8 guidelines. BMC Womens Health. 2021;21(1):397. https://doi.org/10.1186/s12905-021-01545-5.
Dason ES, Maxim M, Sanders A, et al. Guideline 437: diagnosis and management of adenomyosis. J Obstet Gynaecol Can. 2023;45(6):417–e4291. https://doi.org/10.1016/j.jogc.2023.04.008.
Chapron C, Marcellin L, Borghese B, et al. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–82.
Murji A, Biberoğlu K, Leng J, et al. Use of dienogest in endometriosis: a narrative literature review and expert commentary. Curr Med Res Opin. 2020;36(5):895–907. Epub 2020 Mar 31.
El Taha L, Abu Musa A, Khalifeh D, et al. Efficacy of dienogest vs combined oral contraceptive on pain associated with endometriosis: randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2021;Dec:267:205–12.
Tang M, Yang W, Zhang H. Comparison of the efficacy of dienogest and GnRH-a after endometriosis surgery. Comp Study BMC Womens Health. 2023;23(1):85.
Foster RH, Wilde MI, Dienogest. Drugs. 1998;56(5):825–33, discussion 834-5.
Piacenti I, Viscardi MF, Masciullo L, et al. Dienogest versus continuous oral levonorgestrel/EE in patients with endometriosis: what’s the best choice? Gynecol Endocrinol. 2021;37(5):471–5. Epub 2021 Mar 2.
Cope AG, Ainsworth AJ, Stewart EA. Current and future medical therapies for Adenomyosis. Semin Reprod Med. 2020;38(2–03):151–6. https://doi.org/10.1055/s-0040-1719016.
Kim MR, Chapron C, Römer T, et al. Clinical diagnosis and early medical management for endometriosis: Consensus from Asian Expert Group. Healthcare (Basel). 2022;10(12):2515. https://doi.org/10.3390/healthcare10122515.
Uludag SZ, Demirtas E, Sahin Y, et al. Dienogest reduces endometrioma volume and endometriosis-related pain symptoms. J Obstet Gynaecol. 2021;41(8):1246–51. https://doi.org/10.1080/01443615.2020.1867962.
Mohamad NV, Ima-Nirwana S, Chin KY. The skeletal effects of Gonadotropin-releasing hormone antagonists: a concise review. Endocr Metab Immune Disord Drug Targets. 2021;21(10):1713–20. https://doi.org/10.2174/1871530321666201216164410.
Burchardt NA, Eliassen AH, Shafrir AL, et al. Oral contraceptive use by formulation and breast cancer risk by subtype in the nurses’ Health Study II: a prospective cohort study. Am J Obstet Gynecol. 2022;226(6):821.e1–e26.
Strowitzki T, Faustmann T, Gerlinger C, et al. Safety and tolerability of dienogest in endometriosis: pooled analysis from the European clinical study program. Int J Womens Health. 2015;7:393–401.
Maiorana A, Maranto M, Restivo V, et al. Evaluation of long-term efficacy and safety of dienogest in patients with chronic cyclic pelvic pain associated with endometriosis. Arch Gynecol Obstet. 2023. https://doi.org/10.1007/s00404-023-07271-7.
Kumar A. The newly available FAERS public dashboard: implications for health care professionals. Hosp Pharm. 2019;54(2):75–7.
Combi C, Zorzi M, Pozzani G, et al. From narrative descriptions to MedDRA: automagically encoding adverse drug reactions. J Biomed Inf. 2018;84:184–99. https://doi.org/10.1016/j.jbi.2018.07.001. Epub 2018 Jul 4.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–23.
Moore N, Hall G, Sturkenboom M, et al. Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: the example of sertindole. Pharmacoepidemiol Drug Saf. 2003;12(4):271–81.
Sakaeda T, Tamon A, Kadoyama K, et al. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803.
Jeong SH, Lee D, Kim SK, et al. Symptom-alleviating effect and adverse effect of dienogest in Korean women with endometriosis. Gynecol Endocrinol. 2018;34(11):970–4. https://doi.org/10.1080/09513590.2018.1469610.
Köhler G, Faustmann TA, Gerlinger C, et al. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of dienogest daily for endometriosis. Int J Gynaecol Obstet. 2010;108(1):21–5. https://doi.org/10.1016/j.ijgo.2009.08.020.
Krakhotkin DV, Silkina MN, Chernylovskyi VA, et al. The dienogest-related cystitis in women with endometriosis: a prospective, controlled, comparative study. J Obstet Gynaecol. 2022;42(6):2492–7. Epub 2022 Jun 2.
Lang J, Yu Q, Zhang S, et al. Dienogest for Treatment of endometriosis in Chinese women: a placebo-controlled, randomized, double-blind phase 3 study. J Womens Health (Larchmt). 2018;27(2):148–55. https://doi.org/10.1089/jwh.2017.6399. Epub 2017 Oct 30.
Luisi S, Parazzini F, Angioni S, et al. Dienogest treatment improves quality of life in women with endometriosis. J Endometr Pelvic Pain Disorders. 2015;7(4):124–8. https://doi.org/10.5301/je.5000232.
Miao J, Lu J, Tang J, et al. Long-term treatment of dienogest with symptomatic adenomyosis: retrospective analysis of efficacy and safety in clinical practice. Gynecol Endocrinol. 2022;38(8):656–60. https://doi.org/10.1080/09513590.2022.2098948.
Hirata T, Izumi G, Takamura M. Efficacy of dienogest in the treatment of symptomatic adenomyosis: a pilot study. Gynecol Endocrinol. 2014;30(10):726–9. https://doi.org/10.3109/09513590.2014.926882.
Takagi H, Sakamoto J, Sasagawa T. Long-term treatment of endometriosis with dienogest for up to five years. Clin Exp Obstet Gynecol. 2019;46(3):398–402.
Abdou AM, Ammar IMM, Alnemr AAA, et al. Dienogest versus Leuprolide acetate for recurrent pelvic pain following laparoscopic treatment of endometriosis. J Obstet Gynaecol India. 2018;68(4):306–13.
Ceccaroni M, Clarizia R, Liverani S, et al. Dienogest vs GnRH agonists as postoperative therapy after laparoscopic eradication of deep infiltrating endometriosis with bowel and parametrial surgery: a randomized controlled trial. Gynecol Endocrinol. 2021;37(10):930–3.
Chandra A, Rho AM, Jeong K, et al. Clinical experience of long-term use of dienogest after surgery for ovarian endometrioma. Obstet Gynecol Sci. 2018;61(1):111–7.
Cho BS, Roh JW, Park J, et al. Safety and effectiveness of dienogest (Visanne®) for treatment of endometriosis: a large prospective cohort study. Reprod Sci. 2020;27(3):905–15.
Del Forno S, Mabrouk M, Arena A, et al. Dienogest or Norethindrone acetate for the treatment of ovarian endometriomas: can we avoid surgery? Eur J Obstet Gynecol Reprod Biol. 2019;238:120–4.
Ebert AD, Dong L, Merz M, et al. Dienogest 2 mg Daily in the treatment of adolescents with clinically suspected endometriosis: the VISanne Study to Assess Safety in ADOlescents. J Pediatr Adolesc Gynecol. 2017;30(5):560–7.
Hassanin AI, Youssef AA, Yousef AM, et al. Comparison of dienogest versus combined oral contraceptive pills in the treatment of women with adenomyosis: a randomized clinical trial. Int J Gynaecol Obstet. 2021;154(2):263–9.
Ji M, Yuan M, Jiao X, et al. A cohort study of the efficacy of the dienogest and the gonadotropin-releasing hormone agonist in women with adenomyosis and dysmenorrhea. Gynecol Endocrinol. 2022;38(2):164–9.
Kim SA, Um MJ, Kim HK, et al. Study of dienogest for dysmenorrhea and pelvic pain associated with endometriosis. Obstet Gynecol Sci. 2016;59(6):506–11.
Lee JH, Song JY, Yi KW, et al. Effectiveness of Dienogest for treatment of recurrent endometriosis: multicenter data. Reprod Sci. 2018;25(10):1515–22.
Maiorana A, Incandela D, Parazzini F, et al. Efficacy of dienogest in improving pain in women with endometriosis: a 12-month single-center experience. Arch Gynecol Obstet. 2017;296(3):429–33.
Malik R, Mann MK. Role of Dienogest in endometriosis in young women. J Obstet Gynaecol India. 2021;71(5):522–9.
Ota Y, Andou M, Yanai S, et al. Long-term administration of dienogest reduces recurrence after excision of endometrioma. J Endometr Pelvic Pain Disorders. 2015;7(2):63–7.
Park SY, Kim SH, Chae HD, et al. Efficacy and safety of dienogest in patients with endometriosis: a single-center observational study over 12 months. Clin Exp Reprod Med. 2016;43(4):215–20.
Petraglia F, Hornung D, Seitz C, et al. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2012;285(1):167–73.
Römer T. Long-term treatment of endometriosis with dienogest: retrospective analysis of efficacy and safety in clinical practice. Arch Gynecol Obstet. 2018;298(4):747–53.
Strowitzki T, Faustmann T, Gerlinger C, et al. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):193–8.
Strowitzki T, Marr J, Gerlinger C, et al. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633–41.
Takaesu Y, Nishi H, Kojima J, et al. Dienogest compared with gonadotropin-releasing hormone agonist after conservative surgery for endometriosis. J Obstet Gynaecol Res. 2016;42(9):1152–8.
Xu B, Li HJ, Jia WL, et al. Clinical study of dienogest in the treatment of refractory endometriosis-associated pain. Zhonghua Fu Chan Ke Za Zhi. 2021;56(3):178–84.
Yang S, Liu Y, Wen J et al. Clinical efficacy of Dienogest versus Levonorgestrel-releasing intrauterine system for adenomyosis. Evid Based Complement Alternat Med. 2022:1995472.
Yu Q, Zhang S, Li H, et al. Dienogest for treatment of endometriosis in women: a 28-Week, Open-Label, extension study. J Womens Health (Larchmt). 2019;28(2):170–7.
Wang QM, Fu XW, Zhu Y. Clinical efficacy analysis and security evaluation of dienogest in the treatment of adenomyosis. Chin J Practical Gynecol Obstet. 2022;38(1):105–7.
Harada T, Momoeda M, Taketani Y, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis-a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91(3):675–81.
Momoeda M, Harada T, Terakawa N, et al. Long-term use of dienogest for the treatment of endometriosis. J Obstet Gynaecol Res. 2009;35(6):1069–76.
Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2017;108(4):673–8.
Osuga Y, Watanabe M, Hagino A. Long-term use of dienogest in the treatment of painful symptoms in adenomyosis. J Obstet Gynaecol Res. 2017;43(9):1441–8.
Osuga Y, Hayashi K, Kanda S. Long-term use of dienogest for the treatment of primary and secondary dysmenorrhea. J Obstet Gynaecol Res. 2020;46(4):606–17.
Techatraisak K, Hestiantoro A, Soon R, et al. Impact of long-term dienogest therapy on quality of life in Asian women with endometriosis: the prospective non-interventional study ENVISIOeN. Reproductive Sci. 2022;29(4):1157–69.
Kobayashi H, Efficacy. Adverse events, and challenges of Dienogest in the management of symptomatic adenomyosis: a comparison with different hormonal treatments. Gynecol Obstet Invest. 2023;88(2):71–80.
Nishino K, Hayashi K, Chaya J, et al. Effective salvage of acute massive uterine bleeding using intrauterine balloon tamponade in a uterine adenomyosis patient on dienogest. J Obstet Gynaecol Res. 2013;39(3):738–41.
Crandall CJ, Aragaki AK, Cauley JA, et al. Breast tenderness and breast cancer risk in the estrogen plus progestin and estrogen-alone women’s health initiative clinical trials. Breast Cancer Res Treat. 2012;132(1):275–85.
Schindler AE, Henkel A, Christensen B, et al. Dienogest and the breast. Gynecol Endocrinol. 2009;25(7):472–4.
Klein DA, Paradise SL, Reeder RM. Amenorrhea: a systematic approach to diagnosis and management. Am Fam Physician. 2019;100(1):39–48.
Hirata T, Koga K, Kitade M, et al. A National Survey of Umbilical endometriosis in Japan. J Minim Invasive Gynecol. 2020;27(1):80–7.
Chou CW, Lai PT, Huang CC, et al. Primary spontaneous inguinal endometriosis: two cases with emphasis on the diagnostic approach. Taiwan J Obstet Gynecol. 2023;62(3):474–9.
Ono N, Asano R, Nagai K, et al. Evaluating the safety of dienogest in women with adenomyosis: a retrospective analysis. J Obstet Gynaecol Res. 2021;47(4):1433–40.
Kim SE, Lim HH, Lee DY, et al. The long-term effect of Dienogest on bone mineral density after surgical treatment of endometrioma. Reprod Sci. 2021;28(5):1556–62. https://doi.org/10.1007/s43032-020-00453-7.
Ota I, Taniguchi F, Ota Y, et al. A controlled clinical trial comparing potent progestins, LNG-IUS and dienogest, for the treatment of women with adenomyosis. Reprod Med Biol. 2021;20(4):427–34. https://doi.org/10.1002/rmb2.12408.
Seo JW, Lee DY, Yoon BK et al. Effects of long-term postoperative dienogest use for treatment of endometriosis on bone mineral density. Eur J Obstet Gynecol Reprod Biol 2017;212:9–12. https://doi.org/10.1016/j.ejogrb.2017.03.011
Seo JW, Lee DY, Kim SE et al. Comparison of long-term use of combined oral contraceptive after gonadotropin-releasing hormone agonist plus add-back therapy versus dienogest to prevent recurrence of ovarian endometrioma after surgery. Eur J Obstet Gynecol Reprod Biol. 2019;236:53–7. https://doi.org/10.1016/j.ejogrb.2019.02.032
Takamura M, Koga K, Harada M et al. A case of hemorrhagic shock occurred during dienogest therapy for uterine adenomyosis. Case Rep J Obstet Gynaecol Res. 2020. https://doi.org/10.1111/jog.14519. Online ahead of print.
Anzabi Zadeh S, Street WN, Thomas BW. Optimizing warfarin dosing using deep reinforcement learning. J Biomed Inf. 2023;137:104267. https://doi.org/10.1016/j.jbi.2022.104267
Patrono C. Fifty years with aspirin and platelets. Br J Pharmacol. 2023;180(1):25–43. https://doi.org/10.1111/bph.15966.
Cloud GC, Williamson JD, Thao LTP, et al. Low-dose aspirin and the risk of stroke and intracerebral bleeding in healthy older people: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(7):e2325803. https://doi.org/10.1001/jamanetworkopen.
Stanifer JW, Pokorney SD, Chertow GM, et al. Apixaban versus Warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141(17):1384–92. https://doi.org/10.1161/CIRCULATIONAHA.119.044059.
Isobe T, Matsui K, Ishioka K, et al. Recurrent hemorrhagic shock from hemorrhagic cystitis due to neurogenic bladder. IJU Case Rep. 2021;4(6):379–81. https://doi.org/10.1002/iju5.12354.
Etrusco A, Barra F, Chiantera V, et al. Current medical therapy for adenomyosis: from bench to bedside. Drugs. 2023;83(17):1595–611. https://doi.org/10.1007/s40265-023-01957-7.
Heinemann K, Imthurn B, Marions L, et al. Safety of Dienogest and other hormonal treatments for endometriosis in Real-World Clinical Practice (VIPOS): a large noninterventional study. Adv Ther. 2020;37(5):2528–37.
Becker K, Heinemann K, Imthurn B, et al. Real world data on symptomology and diagnostic approaches of 27,840 women living with endometriosis. Sci Rep. 2021;11(1):20404. https://doi.org/10.1038/s41598-021-99681-3.
Funding
Clinical Pharmacy Research Fund Project of China International Medical Foundation (Project No. Z-2021-46-2101-2023).
Ethics declarations
Ethics approval and consent to participate
Not applicable. This study involved nonhuman subject related research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, RR., Xi, Q., Tao, L. et al. A systematic review and Bayesian analysis of the adverse effects of dienogest. BMC Pharmacol Toxicol 25, 43 (2024). https://doi.org/10.1186/s40360-024-00767-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-024-00767-1