Abstract
Background
Bipolar disorder (BD) is a severe psychiatric disorder characterized by changes in mood that alternate between (hypo) mania or depression and mixed states, often associated with functional impairment and cognitive dysfunction. But little is known about biomarkers that contribute to the development and sustainment of cognitive deficits. The aim of this study was to review the association between neurocognition and biomarkers across different mood states.
Method
Search databases were Web of Science, Scopus and PubMed. A systematic review was carried out following the PRISMA guidelines. Risk of bias was assessed with the Newcastle–Ottawa Scale. Studies were selected that focused on the correlation between neuroimaging, physiological, genetic or peripheral biomarkers and cognition in at least two phases of BD: depression, (hypo)mania, euthymia or mixed. PROSPERO Registration No.: CRD42023410782.
Results
A total of 1824 references were screened, identifying 1023 published articles, of which 336 were considered eligible. Only 16 provided information on the association between biomarkers and cognition in the different affective states of BD. The included studies found: (i) Differences in levels of total cholesterol and C reactive protein depending on mood state; (ii) There is no association found between cognition and peripheral biomarkers; (iii) Neuroimaging biomarkers highlighted hypoactivation of frontal areas as distinctive of acute state of BD; (iv) A deactivation failure has been reported in the ventromedial prefrontal cortex (vmPFC), potentially serving as a trait marker of BD.
Conclusion
Only a few recent articles have investigated biomarker-cognition associations in BD mood phases. Our findings underline that there appear to be central regions involved in BD that are observed in all mood states. However, there appear to be underlying mechanisms of cognitive dysfunction that may vary across different mood states in BD. This review highlights the importance of standardizing the data and the assessment of cognition, as well as the need for biomarkers to help prevent acute symptomatic phases of the disease, and the associated functional and cognitive impairment.
Similar content being viewed by others
Background
Cognition in bipolar disorder
Bipolar disorder (BD) is a chronic psychiatry disease characterized by the recurrence of acute mood episodes with euphoric, depressive or mixed clinical features (Carvalho et al. 2020). Cognitive alterations negatively affect the disease course, the functional outcome in mood disorders, particularly in BD (Burdick et al. 2010; Mora et al. 2013; Torrent et al. 2012). Previous literature shows that BD patients’ present impairment in most cognitive domains (processing speed, declarative memory, executive function and attention) compared to healthy controls (HCs) (Li et al. 2020; Sanches et al. 2015). Despite this cognitive impairment being also present during remission phases (Bourne et al. 2013; Chen et al. 2023), there is considerable heterogeneity among patients with BD. This heterogeneity ranges from patients with intact cognition and performance comparable to HCs, to patients with significant global cognitive impairment (Burdick et al. 2014; Ehrlich et al. 2022), suggesting that there are different subgroups in function of cognitive performance. Studies examining the prevalence of cognitive impairment in BD report inconsistent results. A recent study examines the prevalence of cognitive impairment in a cohort of euthymic patients and estimates that 35% of patients experienced clinically significant cognitive deficits (Tsapekos et al. 2021).
In recent years, most studies have focused on euthymic patients’ cognition, while fewer compare it across both the acute and euthymic phases of BD. Research suggest it worsens during manic or depressive acute episodes (Kurtz and Gerraty 2009). For example, executive function (EFs) problems may arise early and tends to be exacerbated during depression and after manic episodes, suggesting it may be considered as a marker of the disease state (Elshahawi et al. 2011; López-Jaramillo et al. 2010). Supporting this, studies show worse cognitive performance in BD patients compared to HCs, with depression impacting working memory more than hypomania (Schouws et al. 2020) and manic episodes causing the most significant impairment across various cognitive domains (Vrabie et al. 2015). However, there are conflicting findings, with one study not detecting cognitive differences between depressive, manic and euthymic BD states (Martínez-Arán et al. 2004). This highlights the need for further research to understand how BD’s different phases influence cognitive function.
Accordingly, one of the main objectives in the management of psychiatric disorders would be to prevent or limit any cognitive deterioration by studying the factors involved in neurocognitive performance (Martínez-Arán and Vieta 2015), including both objective and subjective measures to asses’ cognition from a longitudinal perspective (Sanguinetti Czepielewski et al. 2023). Holistic and comprehensive treatment of BD requires both subjective and objective cognitive measures (Bonnín et al. 2024). Furthermore, there are no clinically available treatments with direct pro-cognitive efficacy in mood disorders (Miskowiak et al. 2017) and there is little understanding of the reasons why some patients with BD develop significant cognitive deficits, while others remain cognitively intact during the different affective phases of the illness.
Factors influencing cognition in BD
Cognition can also be affected by various factors, such as clinical symptoms, age of onset, the incidence of psychosis and pharmacological treatments (Uluyol et al. 2020). The effect of medications on cognition has been a subject of debate, with some studies suggesting cognitive deficits during prolonged lithium treatment (Wingo et al. 2009), while others report no significant effects (Burdick et al. 2020). Similarly, antipsychotic medication has also been linked to poorer cognitive performance (Cullen et al. 2016). Additionally, a higher estimated Intelligence Quotient (IQ) before the onset of the illness is associated with a slower cognitive decline as people age (Tsapekos et al. 2021), and better cognitive performance in late adolescence was associated with a lower risk of BD (Hiyoshi et al. 2017).
BD has a high prevalence of psychiatric comorbidities. Research suggests that these comorbid conditions, diagnosed in over half of adult BD patients during their lifetime (Loftus et al. 2020), may contribute to cognitive impairment by influencing neurobiological pathways involved in mood regulation and cognitive function. Individuals with BD and substance use disorder (SUD) may have greater cognitive impairment compared to individuals with BD without SUD comorbidity (Gogia et al. 2022). Other studies suggest a high prevalence of overweight and obesity among patients with BD (Afzal et al. 2021). Obesity-related conditions are associated with systemic inflammation and insulin resistance, which have been linked to alterations in brain structure and function, particularly in regions involved in cognitive processes (Schmitt and Gaspar 2023). Furthermore, there is evidence of a possible negative effect of overweight on cognitive function (Restrepo Moreno et al. 2017; Yim et al. 2012), with an observed effect of higher body mass index (BMI) on lower cortical thickness (McWhinney et al. 2023). Therefore, addressing these comorbidities may be essential for improving cognitive outcomes in individuals with BD.
Association between biomarkers and cognitive dysfunction in BD
Understanding the physiological and biological mechanisms underlying cognitive impairment in BD remains a challenge (Strawbridge et al. 2021). Identification of biomarkers has become a promising tool to guide diagnosis, predict clinical status, help understand the pathophysiology of mental disorders and inform treatment strategies. While current diagnostic criteria for mental disorders are based solely on clinical features and behavioral observations, with no substantial biological validation (Brückl et al. 2020), Frey et al. (2013) summarized BD-related biomarkers from genetic, peripheral, and neuroimaging biomarkers. In addition, ‘omics' technologies, have contributed to the rapid discovery of many potential biomarkers (García-Gutiérrez et al. 2020). Recent years have seen an increase in the number of studies focusing on the neural correlates of BD (Muneer 2020), however few studies have addressed whether there is an association between biological mechanisms and cognitive dysfunction in the different affective states of BD. Peripheral biomarkers, including different classes of cytokines such as IL-6 and tumor necrosis factor alpha (TNF-α), markers of oxidative stress, and markers of the innate immune system, have also been of interest to understand the basis physiological and consequences of BD (Strawbridge et al. 2021). However, whether blood-based biomarkers are a reliable source for assessing changes within the brain is an ongoing debate. In a recent review, Chaves-Filho et al. (2024) describe important findings in the different phases of BD. For example, they highlight changes in glutamate levels in regions such as the dorsolateral prefrontal cortex (DLPFC) or the anterior cingulate cortex (ACC) during manic and depressive phases. Similarly, they describe an increased systemic proinflammatory response, with hypomanic or manic episodes associated with higher serum levels of IL-8 and TNF- α compared to early depressive episodes.
Inflammatory markers and neuroimaging findings suggest potential associations with cognitive dysfunction. An association has been observed between the inflammatory state measured by C-reactive Protein (CRP) and cytokine levels in peripheral blood and cognitive impairment in patients with schizophrenia and BD (Misiak et al. 2018; Uluyol et al. 2020). Furthermore, patients with first-episode BD exhibited worse EFs and higher tumor necrosis factor receptor 1 (TNFR1) levels than HCs (Chen et al. 2020). Hence, there appears to be an association between inflammatory processes and executive dysfunction. It has also been observed that peripheral Brain Derived Neurotrophic Factor (BDNF) levels may contribute to cognitive deficits in patients with BD (Petersen et al. 2021).
Neuroimaging has revolutionized the diagnosis and treatment of neurological and psychiatric disorders, offering insights into biomarkers treatment response and personalized therapies (Yen et al. 2023). Some standard neuroimaging techniques used in cognitive neuroscience research include functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI) and electroencephalography (EEG). They provide new opportunities to investigate functional and structural connectivity, mapping brain networks, decoding cognitive processes, and identifying neurological disease biomarkers (Yen et al. 2023). The abnormalities in various brain networks, such as the default mode network (DMN) or the cognitive control network (CEN), are likely to different neural circuits that intertwine to form the phenotype of BD. Thus, BD is associated with alterations in both frontal and posterior structures of the DMN, primarily in the prefrontal cortex (PFC), posterior cingulated and inferior parietal regions (Bi et al. 2022). A longitudinal neuroimaging study demonstrated changes in prefrontal regions across mood states in subjects with BD. BD patients in manic phase exhibited increased connectivity with the right middle frontal gyrus compared to HCs, whereas in depressed BD subject’s connectivity was increase with the right medial frontal gyrus and left middle frontal gyrus (Cerullo et al. 2012). Another study showed different brain activity patterns depending on cognitive impairment, those with poorer cognitive performance exhibit lower activity in regions associated with CEN and higher activity DMN, whereas cognitively preserved patients show minimal hypoactivity compared to HCs (Zarp Petersen et al. 2022).
Another prominent focus of attention on “hot cognition” has been emotional processing in patients with BD. These studies suggest that the neural circuits involved in emotion processing and regulation are altered, with a primary role attributed to the amygdale (Wu et al. 2024). Additional longitudinal studies collected in a review have reinforced the notion that chronic cortical abnormalities exist within the frontal networks regulating emotion in BD (Chaves-Filho et al. 2024).
Finally, the use of machine learning techniques has emerged as a promising tool to distinguish between BD and similar conditions, allowing for more personalized treatment based on measurable markers (Colombo et al. 2022). Advanced semi-supervised machine learning techniques can aid in the detection of inflammation subgroups based on accessible peripheral blood biomarkers or use machine learning algorithms (Alexandros Lalousis et al. 2023) and neuropsychological measures to identify patients with BD (Wu et al. 2016). Thus, these algorithms are ideal for assessing multifactorial disorders and estimating the probability of specific outcomes at the individual level, as suggested by the literature.
Mood states in BD
The state of euthymia or remission is defined as the absence of criteria for major mood episodes according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or low ratings on mood questionnaires such as the Hamilton Depression Rating Scale (HDRS) and the Young Mania Rating Scale (YMRS) (Wang et al. 2015). Depression is characterized by a mood bias toward negative affect and loss of interest, while mania or hypomania is characterized by elevated, expansive, or irritable mood (Wu et al. 2024). Similarly, we find that approximately 40% of patients with BD, experience mixed episodes, defined as a manic state with depressive features, or manic symptoms in a patient with bipolar depression (Castle 2014).
In the literature we found that there seem to be differences and abnormal activation depending on mood (Brady et al. 2017; Keener and Phillips 2007; Sundaresh et al. 2018; Wu et al. 2024). Furthermore, despite an observed link between cognitive impairment in BD and neuroinflammation mechanisms (Bauer et al. 2014) and brain activity (Zarp Petersen et al. 2022), little is known about the biological events that underlie the cognitive deficits observed during the acute and euthymic phases of BD.
In this way, we hypothesize that there is an association between specific biomarkers and cognitive performance in different mood states of BD. This association may vary depending on the type of biomarker and the specific mood state.
Specifically, we expect to observe distinct patterns of association between biomarkers and cognition in depressive (BDD), manic (BDM), euthymic (BDE), and mixed (BDX) mood states.
The aim of this systematic review was to synthesize studies in the literature that evaluated the association between biomarkers and cognition in patients with BD according to affective state, since, to date, no study has systematically included these three factors.
Methods
Systematic search strategy
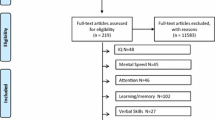
The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 30th March, 2023 (Registration No.: CRD42023410782). Following the PRISMA guidelines (Preferred Reporting Items for Systematic reviews and Meta-analyses; Page et al. 2021), a systematic review was carried out of studies investigating biomarkers and cognition in the different mood states of BD (see Supplementary Material Appendix 1 for PRISMA Checklist). Searches of PubMed, SCOPUS and the Web of Science (WOS) were carried out for the past 10 years (from 2013), and we included studies of patients with BD in which data from at least two different mood states were compared. We started the search in August 2022 and concluded it in December 2022. To update the systematic review, a final search has been done to include those studies that could have been done during 2023. Figure 1 provides the methodological procedure followed was based on the PRISMA guidelines (Moher et al. 2009).
PRISMA flow diagram of the literature search and study selection. From: Babineau 2014. Product Review: Covidence (Systematic Review Software)
The syntax search [‘bipolar disorder’] AND [mood] AND [biomarker] AND [cogni*] in PubMed and its equivalents in the other databases were used. For the second search, studies from January 2023 to December 2023 have been included.
Note that the general term biomarker was used to include neuroimaging, physiological, genetic or peripheral markers.
Eligibility
Eligibility criteria were: (a) the study was carried out on adult patients (≥ 18 years of age); (b) they were diagnosed with BD according to the criteria of the International Classification of Diseases (CIE-10) or the DSM (DSM-IV to DSM-5); (c) the study included a comparison of at least two phases of BD (mania, depression, mixed state or euthymia); (d) that includes a neurocognitive assessment (subjective and objective cognitive measures) and its association with a biomarker; (e) published from 2013 to December 2023 (e) longitudinal or cross-sectional studies and (f) it was written in English, Spanish or French.
The exclusion criteria were: (a) if the patients had a history of psychosis; (b) if the article was a family study, systematic review, book chapter, case report or a meta-analysis; (c) non-access articles.
Data extraction
Two authors (CRLA and APR) performed the review independently using the Covidence program and any disagreements on study selection were resolved by a third person (JPR). Covidence is a web-based collaboration software platform that streamlines the production of systematic and other literature reviews (Babineau 2014).
Data synthesis
The characteristics of the extracted studies are divided into two different tables. Table 1 gathers the studies that have examined peripheral biomarkers and cognition in different mood states of BD. Table 2 lists the various neuroimaging studies. The following characteristics were extracted from Tables 1 and 2: Author/year; Sample size; Sex; Mood state; Criteria to establish mood state; Biomarker; Cognitive assessment; Association between biomarker and cognition; Main Findings.
Quality assessment
A quality assessment was carried out by APR and MHF using the Newcastle–Ottawa Quality Assessment Scale (Wells et al. 2000), rating each study in Table S2 (see Supplementary Material Appendix 2).
Results
A total of 1824 articles were recovered for screening, of which 803 duplicates were removed and 687 were excluded as they did not deal with BD, while 4 articles were included as a result of the Snowballing effect (Wohlin 2014). Subsequent to review of titles and abstracts, 687 records were discarded and the full manuscripts of 336 studies were examined in detail. Of the articles included, only 16 explored an association between biomarkers and cognition in different affective states, most of which demonstrated a correlation between the cognitive functions evaluated and the different alterations during the mood phases of the disorder.
Cognitive and biomarkers findings across affective state
Studies included markers from serum or plasma and neuroimaging. The studies were grouped into the following cognitive domains according to the cognitive tasks used used: "attention", "executive functions", "memory (working memory and verbal memory)", "IQ" "self-reported cognitive" and "Cognitive Screening Test ".
Fourteen studies used a combination of neuroimaging and neurocognitive assessments to investigate the affective states in BD (Alonso-Lana et al. 2019; Estudillo-Guerra et al. 2020; Gao et al. 2023; Kopf et al. 2023; Lai et al. 2018; Magioncalda et al. 2016; Magioncalda et al. 2015; Martino et al. 2016; Mikawa et al. 2015; Nishimura et al. 2015; Pomarol-Clotet et al. 2015; Rive et al. 2016; Velasques et al. 2013; Yang et al. 2020).
One study investigates attention using quantitative EEG parameters (qEEG), two studies employ resting-state functional magnetic resonance imaging (rs-fMRI), one study uses magnetic resonance spectroscopy (MRS), one study uses DTI, and there are five fMRI studies, among which one employs a technique to examine typology (connectome), along with two studies using the proton near-infrared spectroscopy (NIRS), one using functional near-infrared spectroscopy (f-NIRS) and one study performed Brain perfusion single-photon emission computed tomography (SPECT). Two studies used peripheral markers (Guidara et al. 2021; Idemoto et al. 2021). In these two studies, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), high sensitivity C-reactive protein (hs-CRP), low-density lipoprotein cholesterol (LDL-C), and serum Glial cell line-derived neurotrophic factor (GDNF) are used as measures of peripheral biomarkers.
Four studies used a longitudinal design (Alonso-Lana et al. 2019; Estudillo-Guerra et al. 2020; Kopf et al. 2023; Nishimura et al. 2015) and twelve were cross-sectional studies (Gao et al. 2023; Guidara et al. 2021; Idemoto et al. 2021; Lai et al. 2018; Magioncalda et al. 2016; Magioncalda et al. 2015; Martino et al. 2016; Mikawa et al. 2015; Pomarol-Clotet et al. 2015; Rive et al. 2016; Velasques et al. 2013; Yang et al. 2020).
Attention
Four studies carry out a neurocognitive evaluation of attention (Magioncalda et al. 2015, 2016; Martino et al. 2016; Velasques et al. 2013).
Three of them evaluate sustained attention with the continuous performance test (CPT; Magiocalda et al. 2016; Martino et al. 2016), where we find that BD patients showed lower number of total hits and higher number of total omission errors. On the one hand, BDM patients showed that structural changes in the cingulum were related to the deficits found at the attentional level. Furthermore, it was found that the perigenual anterior cingulate cortex (PACC) and posterior cingulate cortex (PCC) functional connectivity was decreased in BDM when compared to both HCs and BDD patients and the structural connectivity (SC) of the cingulum, especially its anterior part, was decreased in BDM when compared to HCs (Martino et al. 2016).
When microstructural abnormalities in the white matter (WM) were investigated using DTI neuroimaging technique, subgroups of BD patients showed different spatial patterns of WM alterations (Magioncalda et al. 2016). The BDE patients had minor and localized WM alterations in the midline structures, whereas the WM alterations were more diffuse in the BDM patients, affecting both midline and lateral structures, and there were stronger and more widespread WM alterations in BDD patients. In addition, these WM alterations were associated with attention deficits. Similarly, in another study these authors found differences in functional connectivity from the PACC to other regions in the posterior DMN between patients in manic or depressed episode and HCs, but no differences between the BD patient subgroups (Magioncalda et al. 2015).
Using qEEG, Velasques et al. (2013) found that BDM patients showed lower saccade latency than BDD patients or the HCs. In a prosaccadic attention task the BDM patients showed stronger gamma coherence in the frontal cortex than in the other groups (BDD and HCs).
Processing speed
Only one study evaluates processing speed (Estudillo-Guerra et al. 2020). Six months after an acute episode of mania, patients in euthymic state do not show differences in this cognitive sphere. At follow-up using SPECT technique, a decrease in perfusion was observed in the right middle temporal gyrus (MTG) and the right superior temporal gyrus (STG).
Executive functions
Seven studies explored EFs (Estudillo-Guerra et al. 2020; Lai et al. 2018; Magioncalda et al. 2016; Magioncalda et al. 2015; Mikawa et al. 2015; Nishimura et al. 2015; Rive et al. 2016). Three of them found no differences in performance between the groups (BD in different states and HCs) in the cognitive task (Mikawa et al. 2015; Nishimura et al. 2015; Rive et al. 2016).
Estudillo-Guerra et al. 2020 explored cognitive deficits in acute BDM patients and their subsequent evaluation after 6 months (euthymic state). This study evaluates cognitive functions using the Spanish version of the Screen for Cognitive Impairment in Psychiatry Scale (SCIP‐S). A subtest contains the Verbal Fluency Test (VFT) to evaluate EFs. A negative correlation between Brodmann area (BA) 25 and positive with BA 38 and 21 was found during a manic episode. At follow-up cognitive impairment in VFT correlated with changes increased perfusion in the bilateral ACC. Fluency prompted by letter showed a correlation with PACC and supragenual anterior cingulate cortex (SACC) (Magioncalda et al. 2015). By contrast, in another study with fMRI, there was increased activation in the dorsolateral prefrontal cortex (DLPFC) of BDD patients, and in the parietal cortex (PC) compared to the BDE patients (Rive et al. 2016). However, hypoactivation of the left DLPFC and of the left ventrolateral prefrontal cortex (VLPFC) during a VFT was found in patients with hypomanic symptoms, while this activation was less prominent in the DLPFC of BDD patients (Nishimura et al. 2015). In addition, this study followed hypomanic patients who showed significantly greater concentration changes of oxygenated hemoglobin (oxy-Hb) in the left DLPFC and frontopolar prefrontal cortex (FPPFC) when experiencing hypomanic symptoms compared to when they were absent. Similarly, the oxy-Hb levels induced by executive tasks were significantly lower in BDD than BDE patients (Mikawa et al. 2015). Finally, another study failed to find differences between the BD groups (Lai et al. 2018), showing a decrease in the N-acetylaspartate to creatine ratio (NAA/Cr) in the bilateral basal ganglia compared to the HCs. Nevertheless, the decrease in NAA/Cr ratios was negatively correlated with total errors and TMT-B uptake, but there was no correlation between the NAA/Cr in the right basal ganglia and the scores of WCST and TMT-B in acute-episode BD patients.
Memory
Working memory
Regarding working memory, four studies used an n-back paradigm (Alonso-Lana et al. 2019; Kopf et al. 2023; Pomarol-Clotet et al. 2015; Yang et al. 2020) and one used the SCIP-S subtest (Estudillo-Guerra et al. 2020). We found worse performance in BDM and BDD patients compared to HCs and BDE patients.
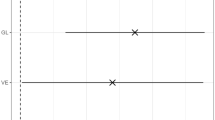
In a first study, the BDM group obtained worse results in the two versions of the n-back task compared to the BDD patients and HCs individuals (Pomarol-Clotet et al. 2015). However, when the cognitive load was increased (2-back version), the BDD patients also differed from the HCs. Surprisingly, the BDE patients did not differ from the HCs. There was reduced activation in the left and right dorsal PC and precuneus in BDM patients, and failure to de-activate the medial frontal cortex was evident in all BD groups.
In a longitudinal study using Brain Perfusion SPECT, patients were assessed during a manic episode and later, in a state of euthymia after about 12 months (Alonso-Lana et al. 2019). Similar to previous findings, BDM patients performed worse than HCs and BDE patients. Activation during the cognitive task showed weaker activation in the left DLPFC, PC, and bilateral superior precuneus in BDM patients, while the BDE group continued to exhibit failure in ventromedial prefrontal cortex (vmPFC) deactivation. During the working memory test of SCIP-S, manic episodes were associated with limited perfusion in the right orbitofrontal cortex (OFC), whereas no significant differences were observed during euthymia (Estudillo-Guerra et al. 2020).
Finally, functional neuroimaging data was used to provide an intuitive method to study fMRI-inferred neural efficiency in the whole brain, allowing interindividual differences related to the task to be predicted (connectome; Yang et al. 2020). An overall increase of the functional connectome was detected and there was a more homogeneous distribution in BDD patients. Interestingly, the maladaptive modulation of the functional connectome was associated with worse performance in working memory.
Verbal memory
Only one study assessed verbal memory (Estudillo-Guerra et al. 2020), with immediate verbal learning correlated to the temporal polar cortex. No significant correlation of manic episodes with delayed verbal learning was detected, although a significant correlation was seen in euthymic states.
Intellectual quotient
Concerning the IQ, HCs had a higher mean current IQ than the BDD and BDM patients but not the BDE patients (Pomarol-Clotet et al. 2015).
When the relationship between neurotrophic factors and cognition was studied in different mood phases of BD (Idemoto et al. 2021), no differences in plasma GDNF levels were evident between the affective states. Furthermore, no correlation was found between IQ and serum GDNF levels. However, after controlling for factors such as sex, age, BMI, estimated IQ, and diagnosis, serum GDNF levels in BD patients were lower in remission and depression states than HCs (this did not occur in patients in a manic or mixed state).
Cognitive screening test
Differences in the levels of oxysterols and CRP were analyzed in the distinct groups of BD, with lower cholesterol levels (Tchol, 24-OCH) reported in BDM patients relative to BDD patients and in patients with severe manic episode compared to those with moderate manic episode for 24-OCH levels (Guidara et al. 2021). By contrast, CRP levels were higher in BDM patients and in patients with severe manic episode compared to those with moderate manic episode. No correlations with the Montreal Cognitive Assessment (MoCA) were found.
Self-reported cognitive
A study utilizes a self-report measure (subjective cognition) to assess cognitive dysfunction with the Perceived Deficits Questionnaire (PDQ) and fNIRS (Gao et al. 2023). Despite finding differences in activation between patients in acute state and their remission state in the follow-up (BDM patients showed reduced network homogeneity compared to BDE), no association with cognition was found.
Discussion
This systematic review represents an effort to synthesize the reliable evidence available on the associations between biomarkers and cognition in different phases of BD. When we look at these associations, we found a total of 16 articles that have addressed this issue.
Acute mood episodes
Neuroimaging biomarker
Functional neuroimaging studies has highlighted the importance of modular and hierarchical brain networks for the functional integration of neural operations related to cognitive function (Park and Friston, 2013). Cognitive control and EFs are associated with activity in the PFC (Menon and D’Esposito 2022). Activation of the DLPFC, superior frontal gyrus, superior parietal lobule and precuneus are common neural correlates of working memory, EFs and attention (Friedman and Robbins 2022; Saldarini et al. 2022). Data from the n-back paradigm and fMRI studies suggest that there is a mood-state dependent hypoactivation in DLPFC and PC. In the included studies, during states of mania or depression there appears to be hypoactivation in the prefrontal and parietal cortex within the framework of a task that requires EFs or working memory (Bi et al. 2022; Brooks et al. 2015; Fleck et al. 2012; Penfold et al. 2015; Rodríguez-Cano et al. 2017; Takizawa et al. 2014). Although we found this hypoactivation also in the euthymic group (Saldarini et al. 2022), there could be less activation in frontal regions during the acute states of BD (Schumer et al. 2023). In fact, we found that moving from mania to euthymia was associated with an increase in activation in these areas (Strakowski et al. 2016). Interestingly, Peterson and colleagues (2021), found this hypoactivation in patients with poor cognitive performance, but after covarying for subsyndromal mood symptoms, it does not remain in DLPFC cluster in cognitively normal patients, which would imply that brain activity in the DLPFC region would be associated with cognitive performance, independently of sub-syndromic mood symptoms. Of note, functional neuroimaging research focused specifically on the mixed states of bipolar disorder is notably sparse (Wu et al. 2024).
However, a resting-state study observed reduced network homogeneity in the right inferior parietal lobe in patients with BDM compared to BDE (Gao et al. 2023). While this may serve as a potential biomarker for predicting mania remission status according to the authors, no correlations with cognitive tasks were found. This could be attributed to the nature of the task, as this region has been associated with language, social cognition, and other functions (Numssen et al. 2021).
On the other hand, DTI, a type of MRI used to visualize the WM tracts in the brain (Yen et al. 2023), has revealed WM abnormalities during all affective states of BD (Hu et al. 2020), being more prevalent in active phases of the disease. Thus, it seems that an acute mood state may be associated with acute state-dependent microstructural WM changes (Zanneti et al. 2009). BDD patients have the largest overall cluster size of WM alterations relative to BDE or BDM patients. Although no association between fractional anisotropy (FA) and antidepressants was evident in a meta-analysis (Favre et al. 2019), this could explain the difference between the alterations in the acute state, as other studies have found an association with treatment (Diego-Adeliño et al. 2014). Nevertheless, longitudinal studies would be better suited to identify and predict the effect of age, illness duration/severity and medication on WM microstructure in patients with BD (Favre et al. 2019).
Regarding MRS, different studies report conflicting results for the NAA/Cr ratio. Both an increase (Zhong et al. 2018) and a decrease in the NAA/Cr ratio were detected in the bilateral lenticular nucleus of BDD and BDM patients relative to the HCs (Frye et al. 2007). However, a correlation was found between the NAA/Cr ratio in the left basal ganglia in acute-episode BD patients and those with better EFs (Zhong et al. 2018). Similarly, other patterns of impaired functional connectivity have been proposed within dorsal attention networks that could differentiate mood states in BD, such as weaker connectivity in BDE patients and hyper-connectivity in BDM patients (Brady et al. 2017; Cerullo et al. 2012). A study using VFT found that BDD patients had weaker activation in both the right and left PFC than controls (Fu et al. 2018). In addition, patients show weaker activation for a second cognitive task (Tower of London test) in the bilateral DLPFC (Fu et al. 2018), although an increase in activity was described in the frontostriatal areas (Rive et al. 2016).
On the other hand, functional connectivity studies of the brain (functional connectome) show distinct patterns in specific neural networks in patients with different states of BD. An increase in the small world (functional connectome) was described in BDD patients, associated with worse performance in working memory (Yang et al. 2020). These results might reflect a compensatory effect to control excessive rumination in the DMN (Claeys et al. 2022) or compensatory activity required by patients in a more severe state of BD, as observed in other disorders (Xing et al. 2016).
Neuropsychological data support the differences found between patients in an acute state, who present worse cognitive performance (Ryan et al. 2012). Therefore, verbal memory, attention and EFs seem to be affected in manic states (Bourne et al. 2013; Kurtz and Gerraty 2009; Vrabie et al. 2015) and these deficits correlate with brain alterations (Benabarre et al. 2005; Pattanayak et al. 2012; Yamada et al. 2015). In contrast, deficits in working memory processing have also been consistently reported in euthymic patients (Thompson et al. 2007; Daglas et al. 2015) and no main effect of mood is found (Manelis et al. 2022). Therefore, differences have been seen in EFs and working memory in mania (Volkert et al. 2016), however finding differences between mania and euthymia may be due to a higher number of past manic episodes that were associated with poorer cognitive performance (Martínez-Arán et al. 2004) or the history of psychosis (Allen et al. 2010; Simonsen et al. 2011), which we have tried to consider in this review.
Peripheral biomarkers
Regarding peripheral biomarkers, we see that lower cholesterol levels (Fusar-Poli et al. 2020) were reported in BDM patients relative to BDD patients, as well as higher CRP levels (Ekinci and Ekinci 2020; Tsai et al. 2017). However, other studies failed to find differences between the depressive and manic state (Sundaresh et al. 2018). Some studies have pointed towards an inflammatory component in BD, and it was suggested that elevated CRP levels might rather be a state than a marker in this condition (Evers et al. 2019; Fernandes et al. 2016). Although we found no correlations between cognitive variables and markers of inflammation here, serum CRP expression was negatively correlated with performance scores of immediate memory, language and attention in BD patients when the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used (Bauer et al. 2014).
The serum GDNF levels in BD patients in a mixed state showed no significant difference from those in HCs. Although altered levels of GDNF were only found in BDD patients, an increase in serum GDNF relative to the activity of the immune system occurred in BDM and BDD patients (Tunca et al. 2014), and there was no difference between BDE patients and HCs (Rosa et al. 2006). Moreover, the estimated IQ values, verbal memory and EFs of the BD mixed group were significantly lower than those of HCs (Vreeker et al. 2016). We have seen that GDNF levels in BDD patients decrease relative to those of the HCs (Takebayashi et al. 2006; Zhang et al. 2010), whereas those levels in BD patients in a mixed or manic state were comparable to those of the HCs. Conversely, GDNF plasma levels were higher in BDE patients relative to BDM patients and HCs (Barbosa et al. 2011). Similarly, serum GDNF increases in bipolar patients during acute manic and depressive episodes (Rosa et al. 2006). There is no clear relationship between GDNF and mood states, although GDNF mRNA expression may be increased by antidepressants or lithium (De-Paula et al. 2016; Sousa et al. 2011). An association between peripheral levels of GDNF and cognitive function was found in patients with major depressive disorder (MDD; Liu et al. 2022; Zhang et al. 2014), which could suggest that GDNF is a biomarker for both BD and MDD in depressive states (Zinchuk et al. 2022).
Euthymic/remission states
Neuroimaging biomarker
In fMRI studies, de-activation failure has been reported in the vmPFC in BDD and BDM patients, persisting in remission (Fernández-Corcuera et al. 2013; Tian et al. 2020; Verdolini et al. 2023). This de-activation failure finding unique to BD may be core to the illness and akin to a trait mechanism not impacted by mood states.
A meta-analysis consistently found trait-related deficits in EFs and verbal memory in patients with BD (Bourne et al. 2013). Executive dysfunction was also evident in BDE patients in our systematic review and hence, EFs deficits in BD may persist across different mood states, both in acute episodes and the euthymic state (Bourne et al. 2013; Rosa et al. 2010; Volkert et al. 2016). However, we found here studies where the performance of euthymic patients is comparable to that of HCs, which could be due to the subtype of BD type I or II (Dittmann et al. 2008) or to cognitive heterogeneity within the sample. This highlights the need to differentiate subgroups by cognitive performance (Burdick et al. 2014). Concerning working memory, there are deficiencies in the manic or depressed state but not in euthymia. Indeed, most fMRI studies using an n-back paradigm suggested there were no significant differences in accuracy or reaction times between BDE patients and HCs (Cremaschi et al. 2013). However, elsewhere such deficits seem to persist during disease remission. (Oh et al. 2019; Srivastava et al. 2019; Volkert et al. 2016).
In euthymic state there seems to be parietal hypoactivation (Hajek et al. 2013) and normalization of DLPFC activation, which is mainly altered during manic episodes (Van der Schot et al. 2010).
Hypoactivation of the PFC in verbal fluency tasks has also been found (Yoshimura et al. 2014). Regarding MRS, during the euthymic state the NAA/Cr ratio in the bilateral lenticular nucleus was lower than in HCs (Kraguljac et al. 2012), although they did not exhibit changes in the NAA/Cr ratio in the temporal or parietal cortex (Brambilla et al. 2005).
In the context of cognitive performance, it is observed that BDE patients also achieve lower performance than HCs, and these differences seem to increase with task complexity (Volkert et al. 2016). Furthermore, despite dysfunction in brain circuits related to working memory in patients with BD, other intact systems may help overcome this deficiency (Cremaschi et al. 2013).
Peripheral biomarkers
Unlike what was found in the study included here, where the differences GDNF levels for patients in the euthymic state were only found after correction (Rosa et al. 2006), Barbosa et al. (2011) found higher GDNF levels in BDE compared to BDM patients. Other studies do not find differences in GDNF levels between euthymia and HCs (Tunca et al. 2014). The inconsistency of results could be due to type II error, and larger sample sized studies are needed. With growing evidence that inflammation contributes to cognitive impairment in several medical conditions, it is crucial to investigate this aspect in bipolar disorder. However, until now, the relationship between inflammatory markers and affective symptoms is not completely (Strawbridge et al. 2021).
Conclusions
Findings about state-specific anomalies across different studies are difficult to compare or interpret, in part because of differences in paradigms and technique used. Our findings highlight core regions involved in BD that are not only mood-specific, but also observed across mood states. Although individuals are clinically in remission, they still show abnormalities in brain connectivity, but a state-dependent topology appears to exist in BD and there appear to be underlying mechanisms of cognitive dysfunction that may be different in the different mood states of bipolar disorder.
Consequently, this systematic review highlights the need for greater consistency in the use of staging models in BD research to standardize the results and identify biomarkers. Monitoring patients and verifying the most significant biomarkers could prevent the onset of acute episodes, and the functional and cognitive deterioration this entails. Similarly, it could allow a more precise differential diagnosis and improve the patient’s quality of life. As such, it is important to create a framework incorporating genetics, neuroimaging and cognitive sciences in order to refine the classification of mental disorders. A multidimensional approach combining peripheral and neuroimaging biomarkers may provide a more comprehensive understanding of cognitive deficits across affective states in BD.
The results obtained in this review demonstrate the importance of considering BD with its different characteristics and shows the need for further longitudinal studies, as there are insufficient studies on hypo/mania, mixed states and clinical comparisons. Examining individuals in different affective states is crucial to identify mechanisms dependent on traits or the current state of symptoms (state), allowing the study of disease mechanisms to develop improved methods of diagnosis and treatment.
Limitations
This systematic review has several limitations. Firstly, the sample of patients that we found in most studies is small and this may be due to the difficulty of evaluating these patients in acute states.
Due to the heterogeneity in BD, we sought to be rigorous with the inclusion and exclusion criteria. For instance, as the impact of psychotic symptoms on cognition remains unclear, we excluded studies indicating patients had psychosis symptoms or if a history of psychosis was not included as a covariate. Conversely, most of the included studies do not seem to control this factor, potentially confounding the results.
Methodological heterogeneity within and between studies is an important limitation of the articles included in this review, as different modalities are used in neuroimaging studies.
The absence of consensus for defining euthymia and the definition of clinically significant impairment also imposes difficulties in both clinical practice and research. Although the duration of clinical remission has been associated with a significant improvement of residual symptoms, in this review, the range of duration to establish a euthymic state is from 2 to 6 months.
The effects of medication or comorbidities (obesity, SUD) are other factors to consider in the search for biomarkers and in neuropsychological assessments. An important limitation is also the cross-sectional nature of the studies available, along with the small samples. Furthermore, the longitudinal studies analyzed here experience significant loss of patients to follow-up, complicating interpretation.
Future directions
The following systematic review could serve to create interventions that combine cognitive rehabilitation with biological treatments. It would be interesting to consider subsyndromal conditions and the presence of residual mood symptoms, since they could have a negative impact on certain cognitive spheres and the cognitive deficit in the euthymic state could change after controlling for these factors (Tsitsipa et al. 2015). More powerful longitudinal studies that follow patients across mood cycles will be crucial to clarify the relationship between neurocognitive impairment and mood. When employing cross-sectional designs, potential confounding factors such as disease subtype, mood state, psychotropic medication use, illness duration, and comorbidities should be carefully considered, as they may influence neuroimaging measures.
Additionally, neuroimaging studies reveal problem areas such as DLPFC in patients with mental disorders. To improve cognition, we could use non-drug techniques such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) targeting those target areas (Hyde et al. 2022). Integrating different neuroimaging techniques such as DTI with other imaging modalities such as functional magnetic resonance imaging (fMRI) or EEG could equally provide a comprehensive understanding of how structural connectivity correlates with existing brain networks.
On another front, ongoing research delves into how neurocognitive functioning differs between BD patients with and without psychosis experiences (Glahn et al. 2007). It's imperative to clarifying the definition of the BD patient subgroup and ensuring homogeneous clinical samples, including unmedicated BD patients and patients experiencing a first episode of mania or hypomania.
It should be noted that it would be interesting for future reviews to include digital biomarkers, since in recent years there has been an increase in studies in this area. Digital biomarker represents a new approach aimed at measuring the human behavior by using smartphones. Preliminary results suggest that passive data collection could be used as a potential alternative to standard neuropsychological assessments (Nguyen et al. 2023).
Finally, the integration of advanced semi-supervised machine learning techniques could be a compelling approach to stratify subgroups in BD based on mood, complementing and addressing the heterogeneity often found in clinical practice.
Availability of data and materials
All data generated during this study are included in this published article (supplementary information files).
Abbreviations
- 24-OCH:
-
24-Hydroxycholesterol
- BD:
-
Bipolar disorder
- BDD:
-
Patients with bipolar disorder in a depressed state
- BDE:
-
Patients with bipolar disorder in a euthymic state
- BDM:
-
Patients with bipolar disorder in a manic state
- BDNF:
-
Brain-derived neuorotrophic factor
- BG:
-
Basal ganglia
- BMI:
-
Body mass index
- CEN:
-
Cognitive control network
- CPT:
-
Continuous performance test
- CRP:
-
C-reactive protein
- DLPFC:
-
Dorsolateral prefrontal cortex
- DMN:
-
Default mode network
- DSM:
-
Diagnostic and statistical manual of mental disorders
- DTI:
-
Diffusion tensor magnetic resonance imaging
- EEG:
-
Electroencephalogram
- EFs:
-
Executive functions
- FA:
-
Fractional anisotropy
- fMRI:
-
Functional magnetic resonance imaging
- fNIRS:
-
Functional near infrared spectroscopy
- FPPFC:
-
Frontopolar prefrontal cortex
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GDNF:
-
Glial cell-derived neurotrophic factor
- HCs:
-
Healthy subjects
- HDRS:
-
Hamilton depression rating scale
- IL-6:
-
Interleukin-6
- IL:
-
Interleukin
- IQ:
-
Intellectual quotient
- MD:
-
Mean diffusivity
- MDD:
-
Major depressive disorder
- MoCA:
-
Montreal cognitive assessment
- MRS:
-
Magnetic resonance spectroscopy
- MTG:
-
Middle temporal gyrus
- NAA/CR:
-
N-acetylaspartate to creatine ratio
- NIRS:
-
Near infrared spectroscopy
- OFC:
-
Orbitofrontal cortex
- Oxy-Hb:
-
Oxygenated hemoglobinOxyhemoglobin
- PACC:
-
Perigenual anterior cingulate cortex
- PC:
-
Parietal cortex
- PCC:
-
Posterior cingulate cortex
- PFC:
-
Prefrontal cortex
- qEEG:
-
Quantitative EEG parameters
- RD:
-
Radial diffusivity
- Rs-fMRI:
-
Resting State functional magnetic resonance imaging
- SACC:
-
Supragenual anterior cingulate cortex
- SC:
-
Structural connectivity
- SCIP-S:
-
Screen for cognitive impairment in psychiatry scale
- SPECT:
-
Single-photon emission computed tomography
- STG:
-
Superior temporal gyrus
- Tchol:
-
Total cholesterol
- TMT-B:
-
Trail making test part B
- TNF- α:
-
Tumor necrosis factor α
- TNFR1:
-
Tumor necrosis factor receptor 1
- TNRF1:
-
Tumor necrosis factor receptor 1
- VFT:
-
Verbal fluency test
- VLPFC:
-
Ventrolateral prefrontal cortex
- vmPFC:
-
Ventromedial prefrontal cortex
- WCST:
-
Wisconsin card sorting test
- WM:
-
White matter
- YMRS:
-
Young mania rating scale
References
Afzal M, Siddiqi N, Ahmad B, Afsheen N, Aslam F, Ali A, Ayesha R, Bryant M, Holt R, Khalid H, Ishaq K, Koly KN, Rajan S, Saba J, Tirbhowan N, Zavala GA. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol. 2021;12:769309. https://doi.org/10.3389/fendo.2021.769309.
Alexandros Lalousis P, Schmaal L, Wood SJ, Reniers LEPR, Cropley VL, Watson A, Pantelis C, Suckling J, Barnes NM, Pariante C, Jones PB, Joyce E, Barnes TRE, Lawrie SM, Husain N, Dazzan P, Deakin B, Shannon Weickert C, Upthegrove R. Inflammatory subgroups of schizophrenia and their association with brain structure: a semi-supervised machine learning examination of heterogeneity. Brain Behav Immun. 2023;113:166–75. https://doi.org/10.1016/j.bbi.2023.06.023.
Allen DN, Randall C, Bello D, Armstrong C, Frantom L, Cross C, Kinney J. Are working memory deficits in bipolar disorder markers for psychosis? Neuropsychology. 2010;24(2):244–54. https://doi.org/10.1037/a0018159.
Alonso-Lana S, Moro N, McKenna PJ, Sarró S, Romaguera A, Monté GC, Maristany T, Goikolea JM, Vieta E, Salvador R, Pomarol-Clotet E. Longitudinal brain functional changes between mania and euthymia in bipolar disorder. Bipolar Disord. 2019;5:449–57. https://doi.org/10.1111/bdi.12767.
Babineau J. Product review: covidence (systematic review software). J Can Health Libr Assoc Journal De L’association Des Bibliothèques De La Santé Du Canada. 2014;35(2):68–71. https://doi.org/10.5596/c14-016.
Barbosa IG, Huguet RB, Sousa LP, Abreu MN, Rocha NP, Bauer ME, Carvalho LA, Teixeira AL. Circulating levels of GDNF in bipolar disorder. Neurosci Lett. 2011;502(2):103–6. https://doi.org/10.1016/j.neulet.2011.07.031.
Bauer IE, Pascoe MC, Wollenhaupt-Aguiar B, Kapczinski F, Soares JC. Inflammatory mediators of cognitive impairment in bipolar disorder. J Psychiatr Res. 2014;56:18–27. https://doi.org/10.1016/j.jpsychires.2014.04.017.
Benabarre A, Vieta E, Martínez-Arán A, Garcia-Garcia M, Martín F, Lomeña F, Torrent C, Sánchez-Moreno J, Colom F, Reinares M, Brugue E, Valdés M. Neuropsychological disturbances and cerebral blood flow in bipolar disorder. Aust N Z J Psychiatry. 2005;39(4):227–34. https://doi.org/10.1080/j.1440-1614.2004.01558.x.
Bi B, Che D, Bai Y. Neural network of bipolar disorder: toward integration of neuroimaging and neurocircuit-based treatment strategies. Transl Psychiatry. 2022;12:143. https://doi.org/10.1038/s41398-022-01917-x.
Bonnín CM, Sánchez-Moreno J, Lima F, Roca X, Segú X, Montejo L, Solé B, Hidalgo-Mazzei D, Martin-Parra S, Martínez-Arán A, Vieta E, Torrent C, Rosa AR. Factors associated with the discrepancy between objective and subjective cognitive impairment in bipolar disorder. J Affect Disord. 2024;349:210–6. https://doi.org/10.1016/j.jad.2024.01.012.
Bourne C, Aydemir Ö, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh JT, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr Scand. 2013;128(3):149–62. https://doi.org/10.1111/acps.12133.
Brady RO Jr, Tandon N, Masters GA, Margolis A, Cohen BM, Keshavan M, Öngür D. Differential brain network activity across mood states in bipolar disorder. J Affect Disord. 2017;207:367–76. https://doi.org/10.1016/j.jad.2016.09.041.
Brambilla P, Stanley JA, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer D, Keshavan MS, Soares JC. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86(1):61–7. https://doi.org/10.1016/j.jad.2004.12.008.
Brooks JO 3rd, Vizueta N, Penfold C, Townsend JD, Bookheimer SY, Altshuler LL. Prefrontal hypoactivation during working memory in bipolar II depression. Psychol Med. 2015;45(8):1731–40. https://doi.org/10.1017/S0033291714002852.
Brückl TM, Spoormaker VI, Sämann PG, Brem AK, Henco L, Czamara D, Elbau I, Grandi NC, Jollans L, Kühnel A, Leuchs L, Pöhlchen D, Schneider M, Tontsch A, Keck ME, Schilbach L, Czisch M, Lucae S, Erhardt A, Binder EB. The biological classification of mental disorders (BeCOME) study: a protocol for an observational deep-phenotyping study for the identification of biological subtypes. BMC Psychiatry. 2020;20(1):213. https://doi.org/10.1186/s12888-020-02541-z.
Burdick KE, Goldberg JF, Harrow M. Neurocognitive dysfunction and psychosocial outcome in patients with bipolar I disorder at 15-year follow-up. Acta Psychiatr Scand. 2010;122(6):499–506. https://doi.org/10.1111/j.1600-0447.2010.01590.x.
Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, Malhotra AK. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol Med. 2014;44(14):3083–96. https://doi.org/10.1017/S0033291714000439.
Burdick KE, Millett CE, Russo M, Alda M, Alliey-Rodriguez N, Anand A, Balaraman Y, Berrettini W, Bertram H, Calabrese JR, Calkin C, Conroy C, Coryell W, DeModena A, Feeder S, Fisher C, Frazier N, Frye M, Gao K, Garnham J, Gershon ES, Glazer K, Goes FS, Goto T, Harrington GJ, Jakobsen P, Kamali M, Kelly M, Leckband S, Løberg EM, Lohoff FW, Maihofer AX, McCarthy MJ, McInnis M, Morken G, Nievergelt CM, Nurnberger J, Oedegaard KJ, Ortiz A, Ritchey M, Ryan K, Schinagle M, Schwebel C, Shaw M, Shilling P, Slaney C, Stapp E, Tarwater B, Zandi P, Kelsoe JR. The association between lithium use and neurocognitive performance in patients with bipolar disorder. Neuropsychopharmacology. 2020;45(10):1743–9. https://doi.org/10.1038/s41386-020-0683-2.
Carvalho AF, Firth J, Vieta E. Bipolar disorder. N Engl J Med. 2020;383(1):58–66. https://doi.org/10.1056/NEJMra1906193.
Castle DJ. Bipolar mixed states: still mixed up? Curr Opin Psychiatry. 2014;27(1):38–42. https://doi.org/10.1097/YCO.0000000000000029.
Cerullo MA, Fleck DE, Eliassen JC, Smith MS, DelBello MP, Adler CM, Strakowski SM. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord. 2012;14(2):175–84. https://doi.org/10.1111/j.1399-5618.2012.01002.x.
Chaves-Filho A, Eyres C, Blöbaum L, Landwehr A, Tremblay MÈ. The emerging neuroimmune hypothesis of bipolar disorder: an updated overview of neuroimmune and microglial findings. J Neurochem. 2024. https://doi.org/10.1111/jnc.16098.
Chen MH, Kao ZK, Chang WC, Tu PC, Hsu JW, Huang KL, Su TP, Li CT, Lin WC, Tsai SJ, Bai YM. Increased proinflammatory cytokines, executive dysfunction, and reduced gray matter volumes in first-episode bipolar disorder and major depressive disorder. J Affect Disord. 2020;274:825–31. https://doi.org/10.1016/j.jad.2020.05.158.
Chen MH, Wang L, Li H, Song H, Zhang X, Wang D. Altered intrinsic brain activity and cognitive impairment in euthymic, unmedicated individuals with bipolar disorder. Asian J Psychiatr. 2023;80:103386. https://doi.org/10.1016/j.ajp.2022.103386.
Claeys EHI, Mantingh T, Morrens M, Yalin N, Stokes PRA. Resting-state fMRI in depressive and (hypo)manic mood states in bipolar disorders: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2022;113:110465. https://doi.org/10.1016/j.pnpbp.2021.110465.
Colombo F, Calesella F, Mazza MG, Melloni EMT, Morelli MJ, Scotti GM, Benedetti F, Bollettini I, Vai B. Machine learning approaches for prediction of bipolar disorder based on biological, clinical and neuropsychological markers: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;135:104552. https://doi.org/10.1016/j.neubiorev.2022.104552.
Cremaschi L, Penzo B, Palazzo M, Dobrea C, Cristoffanini M, Dell’Osso B, Altamura AC. Assessing working memory via N-back task in euthymic bipolar I disorder patients: a review of functional magnetic resonance imaging studies. Neuropsychobiology. 2013;68(2):63–70. https://doi.org/10.1159/000352011.
Cullen B, Ward J, Graham NA, Deary IJ, Pell JP, Smith DJ, Evans JJ. Prevalence and correlates of cognitive impairment in euthymic adults with bipolar disorder: a systematic review. J Affect Disord. 2016;15(205):165–81. https://doi.org/10.1016/j.jad.2016.06.063.
Daglas R, Yücel M, Cotton S, Allott K, Hetrick S, Berk M. Cognitive impairment in first-episode mania: a systematic review of the evidence in the acute and remission phases of the illness. Int J Bipolar Disord. 2015;3:9. https://doi.org/10.1186/s40345-015-0024-2.
de Diego-Adeliño J, Pires P, Gómez-Ansón B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, Martín-Blanco A, Alvarez E, Pérez V, Portella MJ. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med. 2014;44(6):1171–82. https://doi.org/10.1017/S003329171300158X.
de Sousa RT, van de Bilt MT, Diniz BS, Ladeira RB, Portela LV, Souza DO, Forlenza OV, Gattaz WF, Machado-Vieira R. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494(1):54–6. https://doi.org/10.1016/j.neulet.2011.02.054.
De-Paula VJ, Gattaz WF, Forlenza OV. Long-term lithium treatment increases intracellular and extracellular brain-derived neurotrophic factor (BDNF) in cortical and hippocampal neurons at subtherapeutic concentrations. Bipolar Disord. 2016;18(8):692–5. https://doi.org/10.1111/bdi.12449.
Dittmann S, Hennig-Fast K, Gerber S, Seemüller F, Riedel M, Emanuel Severus W, Langosch J, Engel RR, Möller HJ, Grunze HC. Cognitive functioning in euthymic bipolar I and bipolar II patients. Bipolar Disord. 2008;10(8):877–87. https://doi.org/10.1111/j.1399-5618.2008.00640.x.
Ehrlich TJ, Ryan KA, Burdick KE, Langenecker SA, McInnis MG, Marshall DF. Cognitive subgroups and their longitudinal trajectories in bipolar disorder. Acta Psychiatr Scand. 2022;146(3):240–50. https://doi.org/10.1111/acps.13460.
Ekinci O, Ekinci A. Inflammatory parameters and blood lipid values across the different mood states in patients with bipolar disorder. Derg Klin Psikiyatri. 2020. https://doi.org/10.5505/kpd.2020.98216.
Elshahawi HH, Essawi H, Rabie MA, Mansour M, Beshry ZA, Mansour AN. Cognitive functions among euthymic bipolar I patients after a single manic episode versus recurrent episodes. J Affect Disord. 2011;130(1–2):180–91. https://doi.org/10.1016/j.jad.2010.10.027.
Estudillo-Guerra MA, Pacheco-Barrios K, Cardenas-Rojas A, Adame-Ocampo G, Camprodon JA, Morales-Quezada L, Gutiérrez-Mora D, Flores-Ramos M. Brain perfusion during manic episode and at 6-month follow-up period in bipolar disorder patients: correlation with cognitive functions. Brain Behav. 2020;10(6):e01615. https://doi.org/10.1002/brb3.1615.
Evers AK, Veeh J, McNeill R, Reif A, Kittel-Schneider S. C-reactive protein concentration in bipolar disorder: association with genetic variants. Int J Bipolar Disord. 2019;7(1):26. https://doi.org/10.1186/s40345-019-0162-z.
Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abé C, et al. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology. 2019;44(13):2285–93. https://doi.org/10.1038/s41386-019-0485-6.
Fernandes BS, Steiner J, Molendijk ML, Dodd S, Nardin P, Gonçalves CA, Jacka F, Köhler CA, Karmakar C, Carvalho AF, Berk M. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(12):1147–56. https://doi.org/10.1016/S2215-0366(16)30370-4.
Fernández-Corcuera P, Salvador R, Monté GC, Salvador Sarró S, Goikolea JM, Amann B, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148(2–3):170–8.
Fleck DE, Eliassen JC, Durling M, Lamy M, Adler CM, DelBello MP, Shear PK, Cerullo MA, Lee JH, Strakowski SM. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17(3):325–36. https://doi.org/10.1038/mp.2010.108.
Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, Leboyer M, Berk M, Malhi GS, Lopez-Jaramillo C, Taylor VH, Dodd S, Frangou S, Hall GB, Fernandes BS, Kauer-Sant’Anna M, Yatham LN, Kapczinski F, Young LT. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry. 2013;47(4):321–32. https://doi.org/10.1177/0004867413478217.
Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2022;47(1):72–89. https://doi.org/10.1038/s41386-021-01132-0.
Frye MA, Thomas MA, Yue K, Binesh N, Davanzo P, Ventura J, O’Neill J, Guze B, Curran JG, Mintz J. Reduced concentrations of N-acetylaspartate (NAA) and the NAA-creatine ratio in the basal ganglia in bipolar disorder: a study using 3-Tesla proton magnetic resonance spectroscopy. Psychiatry Res. 2007;154(3):259–65. https://doi.org/10.1016/j.pscychresns.2006.11.003.
Fu L, Xiang D, Xiao J, Yao L, Wang Y, Xiao L, Wang H, Wang G, Liu Z. Reduced prefrontal activation during the tower of London and verbal fluency task in patients with bipolar depression: a multi-channel NIRS study. Front Psychiatry. 2018;9:214. https://doi.org/10.3389/fpsyt.2018.00214.
Fusar-Poli L, Amerio A, Cimpoesu P, Natale A, Salvi V, Zappa G, Serafini G, Amore M, Aguglia E, Aguglia A. Lipid and glycemic profiles in patients with bipolar disorder: cholesterol levels are reduced in Mania. Medicina. 2020;57(1):28. https://doi.org/10.3390/medicina57010028.
Gao Y, Guo X, Wang S, Huang Z, Zhang B, Hong J, Zhong Y, Weng C, Wang H, Zha Y, Sun J, Lu L, Wang G. Frontoparietal network homogeneity as a biomarker for mania and remitted bipolar disorder and a predictor of early treatment response in bipolar mania patient. J Affect Disord. 2023;339:486–94. https://doi.org/10.1016/j.jad.2023.07.033.
García-Gutiérrez MS, Navarrete F, Sala F, Gasparyan A, Austrich-Olivares A, Manzanares J. Biomarkers in psychiatry: concept, definition, types and relevance to the clinical reality. Front Psychiatry. 2020;11:432. https://doi.org/10.3389/fpsyt.2020.00432.
Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–6. https://doi.org/10.1016/j.biopsych.2007.02.001.
Gogia M, Shah AQ, Kapczinski F, de Azevedo CT. The impact of substance use disorder comorbidity on cognition of individuals with bipolar disorder: a systematic review. Psychiatry Res. 2022;311:114525. https://doi.org/10.1016/j.psychres.2022.114525.
Guidara W, Messedi M, Maalej M, Naifar M, Khrouf W, Grayaa S, Maalej M, Bonnefont-Rousselot D, Lamari F, Ayadi F. Plasma oxysterols: altered level of plasma 24-hydroxycholesterol in patients with bipolar disorder. J Steroid Biochem Mol Biol. 2021;211:105902. https://doi.org/10.1016/j.jsbmb.2021.105902.
Hajek T, Alda M, Hajek E, Ivanoff J. Functional neuroanatomy of response inhibition in bipolar disorders–combined voxel based and cognitive performance meta-analysis. J Psychiatr Res. 2013;47(12):1955–66. https://doi.org/10.1016/j.jpsychires.2013.08.015.
Hiyoshi A, Sabet JA, Sjöqvist H, Melinder C, Brummer RJ, Montgomery S. Precursors in adolescence of adult-onset bipolar disorder. J Affect Disord. 2017;218:353–8. https://doi.org/10.1016/j.jad.2017.04.071.
Hu R, Stavish C, Leibenluft E, Linke JO. White matter microstructure in individuals with and at risk for bipolar disorder: evidence for an endophenotype from a voxel-based meta-analysis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;12:1104–13. https://doi.org/10.1016/j.bpsc.2020.06.007.
Hyde J, Carr H, Kelley N, Seneviratne R, Reed C, Parlatini V, Garner M, Solmi M, Rosson S, Cortese S, Brandt V. Efficacy of neurostimulation across mental disorders: systematic review and meta-analysis of 208 randomized controlled trials. Mol Psychiatry. 2022;27(6):2709–19. https://doi.org/10.1038/s41380-022-01524-8.
Idemoto K, Niitsu T, Hata T, Ishima T, Yoshida S, Hattori K, et al. Serum levels of glial cell line-derived neurotrophic factor as a biomarker for mood disorders and lithium response. Psychiatry Res. 2021;301:113967. https://doi.org/10.1016/j.psychres.2021.113967.
Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9(6):512–20. https://doi.org/10.1007/s11920-007-0070-2.
Kopf J, Glöckner S, Althen H, Cevada T, Schecklmann M, Dresler T, Kittel-Schneider S, Reif A. Neural responses to a working memory task in acute depressed and remitted phases in bipolar patients. Brain Sci. 2023;13(5):744. https://doi.org/10.3390/brainsci13050744.
Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC. Neurometabolites in schizophrenia and bipolar disorder—a systematic review and meta-analysis. Psychiatry Res. 2012;203(2–3):111–25. https://doi.org/10.1016/j.pscychresns.2012.02.003.
Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23(5):551–62. https://doi.org/10.1037/a0016277.
Lai S, Zhong S, Liao X, Wang Y, Huang J, Zhang S, Sun Y, Zhao H, Jia Y. Biochemical abnormalities in basal ganglia and executive dysfunction in acute- and euthymic-episode patients with bipolar disorder: a proton magnetic resonance spectroscopy study. J Affect Disord. 2018;225:108–16. https://doi.org/10.1016/j.jad.2017.07.036.
Li W, Zhou FC, Zhang L, Ng CH, Ungvari GS, Li J, Xiang YT. Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: a meta-analysis of comparative studies. J Affect Disord. 2020;274:652–61. https://doi.org/10.1016/j.jad.2020.04.051.
Liu X, Li P, Ma X, Zhang J, Sun X, Luo X, Zhang Y. Association between plasma levels of BDNF and GDNF and the diagnosis, treatment response in first-episode MDD. J Affect Disord. 2022;315:190–7. https://doi.org/10.1016/j.jad.2022.07.041.
Loftus J, Scott J, Vorspan F, Icick R, Henry C, Gard S, Kahn JP, Leboyer M, Bellivier F, Etain B. Psychiatric comorbidities in bipolar disorders: an examination of the prevalence and chronology of onset according to sex and bipolar subtype. J Affect Disord. 2020;267:258–63. https://doi.org/10.1016/j.jad.2020.02.035.
López-Jaramillo C, Lopera-Vásquez J, Gallo A, Ospina-Duque J, Bell V, Torrent C, Martínez-Arán A, Vieta E. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12(5):557–67. https://doi.org/10.1111/j.1399-5618.2010.00835.x.
Magioncalda P, Martino M, Conio B, Escelsior A, Piaggio N, Presta A, Marozzi V, Rocchi G, Anastasio L, Vassallo L, Ferri F, Huang Z, Roccatagliata L, Pardini M, Northoff G, Amore M. Functional connectivity and neuronal variability of resting state activity in bipolar disorder–reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp. 2015;36(2):666–82. https://doi.org/10.1002/hbm.22655.
Magioncalda P, Martino M, Conio B, Piaggio N, Teodorescu R, Escelsior A, Marozzi V, Rocchi G, Roccatagliata L, Northoff G, Inglese M, Amore M. Patterns of microstructural white matter abnormalities and their impact on cognitive dysfunction in the various phases of type I bipolar disorder. J Affect Disord. 2016;193:39–50. https://doi.org/10.1016/j.jad.2015.12.050.
Manelis A, Halchenko YO, Bonar L, et al. Working memory updating in individuals with bipolar and unipolar depression: fMRI study. Transl Psychiatry. 2022;12:441. https://doi.org/10.1038/s41398-022-02211-6.
Martinez-Arán A, Vieta E. Cognition as a target in schizophrenia, bipolar disorder and depression. Eur Neuropsychopharmacol. 2015;25(2):151–7. https://doi.org/10.1016/j.euroneuro.2015.01.007.
Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161(2):262–70. https://doi.org/10.1176/appi.ajp.161.2.262.
Martino M, Magioncalda P, Saiote C, Conio B, Escelsior A, Rocchi G, Piaggio N, Marozzi V, Huang Z, Ferri F, Amore M, Inglese M, Northoff G. Abnormal functional-structural cingulum connectivity in mania: combined functional magnetic resonance imaging-diffusion tensor imaging investigation in different phases of bipolar disorder. Acta Psychiatr Scand. 2016;134(4):339–49. https://doi.org/10.1111/acps.12596.
McWhinney SR, Abé C, Alda M, Benedetti F, Bøen E, Del Mar Bonnin C, Borgers T, Brosch K, Canales-Rodríguez EJ, Cannon DM, Dannlowski U, Diaz-Zuluaga AM, Dietze LMF, Elvsåshagen T, Eyler LT, Fullerton JM, Goikolea JM, Goltermann J, Grotegerd D, Haarman BCM, Hahn T, Howells FM, Ingvar M, Jahanshad N, Kircher TTJ, Krug A, Kuplicki RT, Landén M, Lemke H, Liberg B, Lopez-Jaramillo C, Malt UF, Martyn FM, Mazza E, McDonald C, McPhilemy G, Meier S, Meinert S, Meller T, Melloni EMT, Mitchell PB, Nabulsi L, Nenadic I, Opel N, Ophoff RA, Overs BJ, Pfarr JK, Pineda-Zapata JA, Pomarol-Clotet E, Raduà J, Repple J, Richter M, Ringwald KG, Roberts G, Ross A, Salvador R, Savitz J, Schmitt S, Schofield PR, Sim K, Stein DJ, Stein F, Temmingh HS, Thiel K, Thomopoulos SI, van Haren NEM, Vargas C, Vieta E, Vreeker A, Waltemate L, Yatham LN, Ching CRK, Andreassen OA, Thompson PM, Hajek T, ENIGMA Bipolar Disorder Working Group. Mega-analysis of association between obesity and cortical morphology in bipolar disorders: ENIGMA study in 2832 participants. Psychol Med. 2023;53(14):1–11. https://doi.org/10.1017/S0033291723000223.
Menon V, D’Esposito M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology. 2022;47(1):90–103. https://doi.org/10.1038/s41386-021-01152-w.
Mikawa W, Tsujii N, Akashi H, Adachi T, Kirime E, Shirakawa O. Left temporal activation associated with depression severity during a verbal fluency task in patients with bipolar disorder: a multichannel near-infrared spectroscopy study. J Affect Disord. 2015;1(173):193–200. https://doi.org/10.1016/j.jad.2014.10.051.
Misiak B, Stańczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res. 2018;192:16–29. https://doi.org/10.1016/j.schres.2017.04.015.
Miskowiak KW, Burdick KE, Martinez-Aran A, Bonnin CM, Bowie CR, Carvalho AF, Gallagher P, Lafer B, López-Jaramillo C, Sumiyoshi T, McIntyre RS, Schaffer A, Porter RJ, Torres IJ, Yatham LN, Young AH, Kessing LV, Vieta E. Methodological recommendations for cognition trials in bipolar disorder by the International society for bipolar disorders targeting cognition task force. Bipolar Disord. 2017;19(8):614–26. https://doi.org/10.1111/bdi.12534.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med. 2013;43(6):1187–96. https://doi.org/10.1017/S0033291712001948.
Muneer A. The discovery of clinically applicable biomarkers for bipolar disorder: a review of candidate and proteomic approaches. Chonnam Med J. 2020;56(3):166–79. https://doi.org/10.4068/cmj.2020.56.3.166.
Nguyen TM, Leow AD, Ajilore O. A review on smartphone keystroke dynamics as a digital biomarker for understanding neurocognitive functioning. Brain Sci. 2023;13(6):959. https://doi.org/10.3390/brainsci13060959.
Nishimura Y, Takahashi K, Ohtani T, Ikeda-Sugita R, Kasai K, Okazaki Y. Dorsolateral prefrontal hemodynamic responses during a verbal fluency task in hypomanic bipolar disorder. Bipolar Disord. 2015;17(2):172–83. https://doi.org/10.1111/bdi.12252.
Numssen O, Bzdok D, Hartwigsen G. Functional specialization within the inferior parietal lobes across cognitive domains. Elife. 2021;10:e63591. doi: 10.7554/eLife.63591.
Oh DH, Lee S, Kim SH, Ryu V, Cho HS. Low working memory capacity in euthymic bipolar I disorder: No relation to reappraisal on emotion regulation. J Affect Disord. 2019;252:174–81. https://doi.org/10.1016/j.jad.2019.04.042.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411.
Pattanayak RD, Sagar R, Mehta M. Neuropsychological performance in euthymic Indian patients with bipolar disorder type I: correlation between quality of life and global functioning. Psychiatry Clin Neurosci. 2012;66(7):553–63. https://doi.org/10.1111/j.1440-1819.2012.02400.x.
Penfold C, Vizueta N, Townsend JD, Bookheimer SY, Altshuler LL. Frontal lobe hypoactivation in medication-free adults with bipolar II depression during response inhibition. Psychiatry Res. 2015;231(3):202–9. https://doi.org/10.1016/j.pscychresns.2014.11.005.
Petersen NA, Nielsen MØ, Coello K, Stanislaus S, Melbye S, Kjærstad HL, Sletved KSO, McIntyre RS, Frikke-Smith R, Vinberg M, Kessing LV. Brain-derived neurotrophic factor levels in newly diagnosed patients with bipolar disorder, their unaffected first-degree relatives and healthy controls. Bjpsych Open. 2021;7(2):e55. https://doi.org/10.1192/bjo.2021.9.
Pomarol-Clotet E, Alonso-Lana S, Moro N, Sarró S, Bonnin MC, Goikolea JM, Fernández-Corcuera P, Amann BL, Romaguera A, Vieta E, Blanch J, McKenna PJ, Salvador R. Brain functional changes across the different phases of bipolar disorder. Br J Psychiatry. 2015;206(2):136–44. https://doi.org/10.1192/bjp.bp.114.152033.
Restrepo Moreno S, García Valencia J, Vargas C, López-Jaramillo C. Cognitive development in patients with bipolar disorder and metabolic syndrome. Rev Colomb Psiquiatr. 2019;48(3):149–55. https://doi.org/10.1016/j.rcp.2017.10.003.
Rive MM, Koeter MW, Veltman DJ, Schene AH, Ruhé HG. Visuospatial planning in unmedicated major depressive disorder and bipolar disorder: distinct and common neural correlates. Psychol Med. 2016;46(11):2313–28. https://doi.org/10.1017/S0033291716000933.
Rodríguez-Cano E, Alonso-Lana S, Sarró S, Fernández-Corcuera P, Goikolea JM, Vieta E, Maristany T, Salvador R, McKenna PJ, Pomarol-Clotet E. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017;19(5):386–95. https://doi.org/10.1111/bdi.12517.
Rosa AR, Frey BN, Andreazza AC, Ceresér KM, Cunha AB, Quevedo J, Santin A, Gottfried C, Gonçalves CA, Vieta E, Kapczinski F. Increased serum glial cell line-derived neurotrophic factor immunocontent during manic and depressive episodes in individuals with bipolar disorder. Neurosci Lett. 2006;407(2):146–50. https://doi.org/10.1016/j.neulet.2006.08.026.
Rosa AR, Reinares M, Michalak EE, Bonnin CM, Sole B, Franco C, Comes M, Torrent C, Kapczinski F, Vieta E. Functional impairment and disability across mood states in bipolar disorder. Value Health. 2010;13(8):984–8. https://doi.org/10.1111/j.1524-4733.2010.00768.x.
Ryan KA, Vederman AC, McFadden EM, Weldon AL, Kamali M, Langenecker SA, McInnis MG. Differential executive functioning performance by phase of bipolar disorder. Bipolar Disord. 2012;14:527–36.
Saldarini F, Gottlieb N, Stokes PRA. Neural correlates of working memory function in euthymic people with bipolar disorder compared to healthy controls: a systematic review and meta-analysis. J Affect Disord. 2022;297:610–22. https://doi.org/10.1016/j.jad.2021.10.084.
Sanches M, Bauer IE, Galvez JF, Zunta-Soares GB, Soares JC. The management of cognitive impairment in bipolar disorder: current status and perspectives. Am J Ther. 2015;22(6):477–86. https://doi.org/10.1097/MJT.0000000000000120.
Sanguinetti Czepielewski L, Kauer-Sant’Anna M, Kapczinski F, Bücker J. The relationship between objective and subjective cognitive performance to clinical features in bipolar disorder: a 6 years follow-up study. Eur J Psychiatry. 2023;37(1):63–6.
Schmitt LO, Gaspar JM. Obesity-induced brain neuroinflammatory and mitochondrial changes. Metabolites. 2023;13(1):86. https://doi.org/10.3390/metabo13010086.
Schouws SNTM, Korten N, Beekman ATF, Stek ML, Dols A. Does cognitive function in older bipolar patients depend on recurrent or current mood symptoms? Int J Geriatr Psychiatry. 2020;35(10):1163–70. https://doi.org/10.1002/gps.5352.
Schumer MC, Chase HW, Rozovsky R, Eickhoff SB, Phillips ML. Prefrontal, parietal, and limbic condition-dependent differences in bipolar disorder: a large-scale meta-analysis of functional neuroimaging studies. Mol Psychiatry. 2023;28(7):2826–38. https://doi.org/10.1038/s41380-023-01974-8.
Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Faerden A, Jónsdóttir H, Ringen PA, Opjordsmoen S, Melle I, Friis S, Andreassen OA. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37(1):73–83. https://doi.org/10.1093/schbul/sbp034.
Srivastava C, Bhardwaj A, Sharma M, Kumar S. Cognitive deficits in euthymic patients with bipolar disorder: state or trait marker? J Nerv Ment Dis. 2019;207(2):100–5. https://doi.org/10.1097/NMD.0000000000000920.
Strakowski SM, Fleck DE, Welge J, Eliassen JC, Norris M, Durling M, Komoroski RA, Chu WJ, Weber W, Dudley JA, Blom TJ, Stover A, Klein C, Strawn JR, DelBello MP, Lee JH, Adler CM. fMRI brain activation changes following treatment of a first bipolar manic episode. Bipolar Disord. 2016;18(6):490–501. https://doi.org/10.1111/bdi.12426.
Strawbridge R, Carter R, Saldarini F, Tsapekos D, Young AH. Inflammatory biomarkers and cognitive functioning in individuals with euthymic bipolar disorder: exploratory study. Bjpsych Open. 2021;7(4):e126. https://doi.org/10.1192/bjo.2021.966.
Sundaresh A, Rajkumar R, Krishnamoorthy R, Leboyer M, Negi V, Tamouza R. C-reactive protein in bipolar disorder in an indian clinical setting. J Clin Diagnostic Res. 2018. https://doi.org/10.7860/jcdr/2018/37177.12014.
Takebayashi M, Hisaoka K, Nishida A, Tsuchioka M, Miyoshi I, Kozuru T, Hikasa S, Okamoto Y, Shinno H, Morinobu S, Yamawaki S. Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int J Neuropsychopharmacol. 2006;9(5):607–12. https://doi.org/10.1017/S1461145705006085.
Takizawa R, Fukuda M, Kawasaki S, Kasai K, Mimura M, Pu S, Noda T, Niwa S, Okazaki Y. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85:498–507. https://doi.org/10.1016/j.neuroimage.2013.05.126.
Thompson JM, Gray JM, Hughes JH, Watson S, Young AH, Ferrier IN. Impaired working memory monitoring in euthymic bipolar patients. Bipolar Disord. 2007;9(5):478–89. https://doi.org/10.1111/j.1399-5618.2007.00470.x.
Tian F, Diao W, Yang X, Wang X, Roberts N, Feng C, Jia Z. Failure of activation of striatum during the performance of executive function tasks in adult patients with bipolar disorder. Psychol Med. 2020;50(4):653–65. https://doi.org/10.1017/S0033291719000473.
Torrent C, Martinez-Arán A, del Mar BC, Reinares M, Daban C, Solé B, Rosa AR, Tabarés-Seisdedos R, Popovic D, Salamero M, Vieta E. Long-term outcome of cognitive impairment in bipolar disorder. J Clin Psychiatry. 2012;73(7):e899-905. https://doi.org/10.4088/JCP.11m07471.
Tsai SY, Chung KH, Chen PH. Levels of interleukin-6 and high-sensitivity C-reactive protein reflecting mania severity in bipolar disorder. Bipolar Disord. 2017;19(8):708–9. https://doi.org/10.1111/bdi.12570.
Tsapekos D, Strawbridge R, Cella M, Wykes T, Young AH. Cognitive impairment in euthymic patients with bipolar disorder: prevalence estimation and model selection for predictors of cognitive performance. J Affect Disord. 2021;294:497–504. https://doi.org/10.1016/j.jad.2021.07.036.
Tsitsipa E, Fountoulakis KN. The neurocognitive functioning in bipolar disorder: a systematic review of data. Ann Gen Psychiatry. 2015;14:42. https://doi.org/10.1186/s12991-015-0081-z.
Tunca Z, Ozerdem A, Ceylan D, Yalçın Y, Can G, Resmi H, Akan P, Ergör G, Aydemir O, Cengisiz C, Kerim D. Alterations in BDNF (brain derived neurotrophic factor) and GDNF (glial cell line-derived neurotrophic factor) serum levels in bipolar disorder: the role of lithium. J Affect Disord. 2014;166:193–200. https://doi.org/10.1016/j.jad.2014.05.012.
Uluyol OB, Onur OS, Ekinci A, Guclu O. Relationship between serum uric acid levels and cognitive functions in bipolar disorder. Psychiatry Clin Psychopharmacol. 2020;30(2):165–74. https://doi.org/10.5455/pcp.20200321090156.
Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res. 2010;184(3):135–42. https://doi.org/10.1016/j.pscychresns.2010.07.009.
Velasques B, Bittencourt J, Diniz C, Teixeira S, Basile LF, Inácio Salles J, et al. Changes in saccadic eye movement (SEM) and quantitative EEG parameter in bipolar patients. J Affect Disord. 2013;145(3):378–85. https://doi.org/10.1016/j.jad.2012.04.049.
Verdolini N, Moreno-Ortega M, Salgado-Pineda P, Monté G, de Aragón AM, Dompablo M, McKenna PJ, Salvador R, Palomo T, Pomarol-Clotet E, Rodriguez-Jimenez R. Failure of deactivation in bipolar disorder during performance of an fMRI adapted version of the Stroop task. J Affect Disord. 2023;329:307–14. https://doi.org/10.1016/j.jad.2023.02.132.
Volkert J, Schiele MA, Kazmaier J, Glaser F, Zierhut KC, Kopf J, Kittel-Schneider S, Reif A. Cognitive deficits in bipolar disorder: from acute episode to remission. Eur Arch Psychiatry Clin Neurosci. 2016;266(3):225–37. https://doi.org/10.1007/s00406-015-0657-2.
Vrabie M, Marinescu V, Talaşman A, Tăutu O, Drima E, Micluţia I. Cognitive impairment in manic bipolar patients: important, understated, significant aspects. Ann Gen Psychiatry. 2015;14:41. https://doi.org/10.1186/s12991-015-0080-0.
Vreeker A, Boks MP, Abramovic L, Verkooijen S, van Bergen AH, Hillegers MH, et al. High educational performance is a distinctive feature of bipolar disorder: a study on cognition in bipolar disorder, schizophrenia patients, relatives and controls. Psychol Med. 2016;46(4):807–18. https://doi.org/10.1017/s0033291715002299.
Wang Z, Chen J, Zhang C, Gao K, Hong W, Xing M, Wu Z, Yuan C, Huang J, Peng D, Wang Y, Lu W, Yi Z, Yu X, Zhao J, Fang Y. Guidelines concordance of maintenance treatment in euthymic patients with bipolar disorder: data from the national bipolar mania pathway survey (BIPAS) in mainland China. J Affect Disord. 2015;182:101–5. https://doi.org/10.1016/j.jad.2015.04.028.
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Mar 2024.
Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ. Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry. 2009;70(11):1588–97. https://doi.org/10.4088/JCP.08r04972.
Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. ACM International Conference Proceeding Series. 2014. doi: https://doi.org/10.1145/2601248.2601268
Wu MJ, Passos IC, Bauer IE, Lavagnino L, Cao B, Zunta-Soares GB, Kapczinski F, Mwangi B, Soares JC. Individualized identification of euthymic bipolar disorder using the Cambridge Neuropsychological Test Automated Battery (CANTAB) and machine learning. J Affect Disord. 2016;192:219–25. https://doi.org/10.1016/j.jad.2015.12.053.
Wu YK, Su YA, Li L, Zhu LL, Li K, Li JT, Mitchell PB, Yan CG, Si TM. Brain functional changes across mood states in bipolar disorder: from a large-scale network perspective. Psychol Med. 2024;54(4):763–74. https://doi.org/10.1017/S0033291723002453.
Xing M, Tadayonnejad R, MacNamara A, Ajilore O, DiGangi J, Phan KL, Leow A, Klumpp H. Resting-state theta band connectivity and graph analysis in generalized social anxiety disorder. Neuroimage Clin. 2016;12(13):24–32. https://doi.org/10.1016/j.nicl.2016.11.009.
Yamada S, Takahashi S, Ukai S, Tsuji T, Iwatani J, Tsuda K, et al. Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: a tract-specific analysis study. J Affect Disord. 2015;174:542–8. https://doi.org/10.1016/j.jad.2014.12.022.
Yang J, Ouyang X, Tao H, Pu W, Fan Z, Zeng C, Huang X, Chen X, Liu J, Liu Z, Palaniyappan L. Connectomic signatures of working memory deficits in depression, mania, and euthymic states of bipolar disorder. J Affect Disord. 2020;274:190–8. https://doi.org/10.1016/j.jad.2020.05.058.
Yen C, Lin CL, Chiang MC. Exploring the frontiers of neuroimaging: a review of recent advances in understanding brain functioning and disorders. Life. 2023;13(7):1472. https://doi.org/10.3390/life13071472.
Yim CY, Soczynska JK, Kennedy SH, Woldeyohannes HO, Brietzke E, McIntyre RS. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur Psychiatry. 2012;27(3):223–8. https://doi.org/10.1016/j.eurpsy.2011.02.004.
Yoshimura Y, Okamoto Y, Onoda K, Okada G, Toki S, Yoshino A, Yamashita H, Yamawaki S. Psychosocial functioning is correlated with activation in the anterior cingulate cortex and left lateral prefrontal cortex during a verbal fluency task in euthymic bipolar disorder: a preliminary fMRI study. Psychiatry Clin Neurosci. 2014;68(3):188–96. https://doi.org/10.1111/pcn.12115.
Zanetti MV, Jackowski MP, Versace A, Almeida JR, Hassel S, Duran FL, Busatto GF, Kupfer DJ, Phillips ML. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259(6):316–28. https://doi.org/10.1007/s00406-009-0002-8.
Zarp Petersen J, Varo C, Skovsen CF, Ott CV, Kjaerstad HL, Vieta E, Harmer CJ, Knudsen GM, Kessing LV, Macoveanu J, Miskowiak KW. Neuronal underpinnings of cognitive impairment in bipolar disorder: a large data-driven functional magnetic resonance imaging study. Bipolar Disord. 2022;24(1):69–81. https://doi.org/10.1111/bdi.13100.
Zhang X, Zhang Z, Sha W, Xie C, Xi G, Zhou H, Zhang Y. Effect of treatment on serum glial cell line-derived neurotrophic factor in bipolar patients. J Affect Disord. 2010;126(1–2):326–9. https://doi.org/10.1016/j.jad.2010.03.003.
Zhang X, Ru B, Sha W, Xin W, Zhou H, Zhang Y. Performance on the Wisconsin card-sorting test and serum levels of glial cell line-derived neurotrophic factor in patients with major depressive disorder. Asia Pac Psychiatry. 2014;6(3):302–7. https://doi.org/10.1111/appy.12120.
Zhong S, Wang Y, Lai S, Liu T, Liao X, Chen G, Jia Y. Associations between executive function impairment and biochemical abnormalities in bipolar disorder with suicidal ideation. J Affect Disord. 2018;241:282–90. https://doi.org/10.1016/j.jad.2018.08.031.
Zinchuk MS, Guekht AB, Druzhkova TA, Gulyaeva NV, Shpak AA. Glial cell line-derived neurotrophic factor (GDNF) in blood serum and lacrimal fluid of patients with a current depressive episode. J Affect Disord. 2022;318:409–13. https://doi.org/10.1016/j.jad.2022.09.025.
Acknowledgements
Not applicable.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA. This work was also supported by the “Programa Operativo de Andalucía FEDER, Iniciativa Territorial Integrada ITI 2014-2020” Conserjería de Salud - Junta de Andalucía” (PI-0009-2017) and the “Conserjería de Salud y Familias - Junta de Andalucía” (PEMP-0008-2020).
Author information
Authors and Affiliations
Contributions
CRLA and APR performed the systematic literature search, the screening and the data extraction, and wrote the manuscript. MHF and APR carried out the quality assessment. CRLA, EB and JIRR critically reviewed the content of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Ramos, A., Romero-López-Alberca, C., Hidalgo-Figueroa, M. et al. A systematic review of the biomarkers associated with cognition and mood state in bipolar disorder. Int J Bipolar Disord 12, 18 (2024). https://doi.org/10.1186/s40345-024-00340-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40345-024-00340-z