Abstract
Background
Weight gain and nutritional rehabilitation are essential first steps to achieve medical stabilization in anorexia nervosa, and frequent resistance to weight gain requires patients to consume high kilocalorie loads. Adaptive hypometabolism is common when patients begin treatment, and rebound hypermetabolism is suspected to be a significant barrier to weight gain. The aim of this review was to summarize existing data describing metabolic changes in anorexia nervosa during weight restoration. The reported findings challenge current hypotheses of weight gain resistance and highlight key areas for future research.
Methods
Using scoping review guidelines, three databases were searched for studies investigating metabolic changes in anorexia nervosa before and after renourishment. Two reviewers systematically screened the titles and abstracts of 447 articles, and full-text versions of 106 studies were assessed for eligibility. A total of 36 studies were included for review. Data regarding the study description, sample population (including age, weight, BMI, duration of treatment, and caloric intake), and metabolic variable descriptions were extracted.
Results
Female patients with anorexia nervosa from studies across 13 countries were included. Across the studies, average BMI increased from 13.7 kg/m2 at admission to 17.57 kg/m2. Patients presented to treatment with clinically reduced energy expenditure levels. After varying levels of nutritional rehabilitation and weight restoration, measured energy expenditure increased significantly in 76% of the studies. Energy expenditure values at the second timepoint increased to the standard range for normal weight female teenagers and adults. Despite these increases, the studies do not indicate the presence of a hypermetabolic state during renourishment. Additionally, all studies including both measured and predicted energy expenditure reported that predicted energy expenditure overestimated measured values.
Conclusion
This study provides a detailed evaluation of the literature investigating energy expenditure and metabolic rate in patients with anorexia nervosa before and following a period of renourishment. The findings from this review identify important gaps in the current beliefs of energy expenditure in anorexia nervosa and highlight a need for further exploration of metabolic alterations during weight restoration.
Plain English Summary
Nutritional rehabilitation and weight restoration are two primary goals of anorexia nervosa treatment that pose significant physiological and psychological challenges for patients. Patients often require high caloric loads to continue an adequate weight gain trajectory, but the underlying cause of weight gain resistance remains unknown. We completed a scoping review of research into energy expenditure and metabolic rate during treatment. Our search identified 447 relevant articles from academic databases, and 106 were deemed eligible after screening. We extracted data, including sample characteristics, kilocalorie intake, energy expenditure, and treatment information, from 36 studies. When individuals arrived for treatment, their energy expenditure was lower than that of individuals without an eating disorder due to the prolonged state of nutrient deprivation. After varying amounts of time and kilocalorie intake, most studies reported significant increases in energy expenditure. However, energy expenditure after a period of renourishment did not indicate an overactive metabolism (i.e., “hypermetabolism”). Funders should consider supporting exploration of additional factors that may be functioning as barriers to weight gain during treatment, in pursuit of making treatment more efficient and long-lasting. Additionally, future research describing metabolism in anorexia nervosa should provide more consistent methodologies, robust statical testing, and comprehensive reporting of dietary intake.
Similar content being viewed by others
Introduction
Background
Anorexia nervosa (AN), a severe psychiatric disorder characterized by extreme weight dysregulation, carries one of the highest mortality rates of all psychiatric illnesses [1, 2]. Affecting individuals of all ages, ancestries, and genders, AN has an estimated lifetime prevalence of up to 4% in females and 0.3% in males [3]. Within the AN diagnosis, patients may be classified as restricting or binge eating/purging type, and nearly 75% of patients with AN report a comorbid lifetime mood or anxiety disorder [2, 4]. Somatic symptoms associated with AN, although varying with severity and stage of illness, affect nearly every organ system in the body due to starvation or binge eating and/or purging behaviors [5]. AN presentation involves a complex interplay between biological, psychological, genetic and environmental factors, and remediating the consequences of severe caloric restriction are only one component of the current treatment paradigm [6].
To achieve medical stabilization, weight gain and nutritional rehabilitation (also referred to as clinical refeeding, renourishment, or weight restoration) are the first and essential goals of treatment. The process of therapeutic renourishment can be both physically and psychologically uncomfortable and relapse is high [7, 8]—highlighting the need for novel, safe, and effective therapeutic approaches. Full recovery is more common in adolescents than in adults [9], and on average, adult patients require 5–6 years of treatment until achieving remission [10]. Throughout the course of the illness, it is common for both adolescent and adult patients to undergo several cycles of therapeutic renourishment followed by loss of restored weight before achieving lasting recovery. One potential explanation for the lack of efficacy of current renourishment strategies is the need to consume a sustained high kilocalorie (kcal) diet, which is psychologically (due to fear of weight gain) and physically (due to discomfort, pain, and gastrointestinal complications) challenging to patients and can lead to premature discontinuation of treatment and jeopardize long-term recovery [11, 12]. As metabolic output forms the basis for caloric intake prescription, meticulous monitoring of the metabolic profile in individuals receiving treatment for AN is crucial for achieving successful renourishment. Research to advance the nutritional component of AN treatment protocols, however, lacks an updated and comprehensive characterization of metabolic changes in patients with AN.
Metabolic adaptations in anorexia nervosa
Metabolism is a series of chemical reactions by which calories consumed are converted (catabolized) into usable energy for healthy body function. Metabolic rate is often measured by indirect calorimetry and predicted using calculations including the Harris-Benedict and Schebendach equations [13]. Previous work has highlighted that these equations may not accurately predict metabolic rate in AN [14], as classical energy expenditure estimation based on age, height, weight, and sex fails to consider the metabolic profile in a disease state [15]. For AN, therapeutic renourishment plans are developed on the foundation of metabolic requirements and tailored to each individual patient. The current standard of care varies widely across treatment facilities, with some recommendations starting at 10 kilocalorie (kcal)/kg/day with increases of 5 kcal/kg/day [16] and others starting at 30–40 kcal/kg/day with increases up to 70–100 kcal/kg/day to promote faster weight gain [17]. Recent studies have highlighted the efficacy of higher-calorie refeeding, both in restoring medical stability more quickly and reducing overall hospital charges per participant [18]. Importantly, patients with AN in a chronic state of starvation can experience significant decreases in metabolic rate to compensate for the lack of energy intake [19]. These hypometabolic states are typically attributed to reduced protein turnover (i.e., reduced renewal and replacement of protein) and ATP supply pathways, providing the body with significant energy savings [20].
Adaptive hypometabolism prior to renourishment is common and well-documented [21, 22]. Less clear is whether rebound hypermetabolism (either from a hypo- or normo-metabolic baseline) occurs as commonly during nutritional rehabilitation. When weight gain stalls during inpatient or residential treatment, two common hypotheses are typically considered: (i) the patient is using exercise or other methods to inhibit weight gain, or (ii) the patient’s metabolic rate has shifted to a hypermetabolic state [23]. Hypermetabolic states are characterized by increased physiological responses including heart rate, blood pressure, body temperature, and protein and lipid catabolism, leading to excessive resting energy expenditure [24]. Hypermetabolism has been well described in circumstances of both chronic and acute stress (e.g., trauma [25], severe burn injury [26], and COVID-19 [27]), so it is conceivable that physiological stress from AN progression, in addition to other common AN complications such as chronically elevated cortisol [28] and difficulty sleeping [29], may also contribute to increased cellular metabolism during treatment. Anecdotal reports posit that increases in metabolic rate may be an important barrier to weight gain and weight maintenance in AN [23], and limited evidence reports a shift towards hypermetabolism during AN treatment [30, 31]. Proposed explanations for accelerated energy expenditure during nutritional rehabilitation include the occurrence of night sweats, irregular and/or elevated heart rate, and nervous system dysfunction [23].
As nutritional rehabilitation programs are centered around metabolic rate estimations, it is essential for providers to have an accurate depiction of the metabolic setting in each individual patient. Caloric initiation standards are based on basal metabolic rate (BMR); however, BMR is infrequently remeasured once renourishment begins, leaving dietetic teams to increase caloric load based on careful observation of weight gain, food intake, and physical activity on the unit [14]. Additionally, it is likely that studies investigating metabolism during AN treatment employ equations that were not developed for application to low body weight individuals [32]. These challenges highlight the need for comprehensive documentation of metabolic changes before, during, and after renourishment, rather than relying on a cross-sectional metabolic assessment upon hospital admission.
In an effort to consolidate and evaluate existing knowledge about metabolic changes during renourishment in individuals with AN, we conducted a scoping review of energy expenditure (EE) during nutritional rehabilitation. We anticipated finding consistent reports of moderate increases in energy expenditure after renourishment and aimed to aggregate existing knowledge on the commonly referred to “hypermetabolic state” that occurs during renourishment.
Methods
Search strategy
A scoping review search was performed in June and July 2023 through Covidence using PRISMA guidelines. Searches were conducted in PubMed, SCOPUS, and the Cumulated Index in Nursing and Allied Health Literature (CINHAL). Search terms included (“Energy Metabolism” [Mesh] OR “Energy Expenditure” OR “energy expenditures” OR “hypermetabolism” OR “hypermetabolic” OR “metabolic rate” OR “basal metabolism” OR “metabolic rates” OR “energy metabolism”) AND (“Anorexia Nervosa” [Mesh] OR “anorexia nervosa”). Medical Subject Headings (i.e., MeSH) assigned to files in PubMed were used to retrieve all records on the relevant subject (metabolism and AN) in a subjective manner, regardless of the vocabulary utilized by the author.
Inclusion and exclusion criteria
Studies and articles (including peer reviewed conference abstracts) published between 1980 and 2022 that investigated metabolic changes in patients with AN before (T1) and after (or during) renourishment (T2) were eligible for analysis. Relevant studies that reported any metabolic variable outcome, including resting energy expenditure (REE), total energy expenditure (TEE), basal metabolic rate (BMR), and resting metabolic rate (RMR) and recorded at least two time points (e.g., admission and discharge from treatment unit, baseline and follow-up from treatment, independent groups of active cases and recovered patients, etc.) were included. The following exclusion criteria were applied: (1) cross-sectional studies reporting only one metabolic timepoint; (2) case studies; (3) qualitative studies; (4) animal studies; (5) studies of individuals with other eating disorders; (6) presentations, dissertations, theses, book chapters, and other technical documentation.
Study selection and data extraction
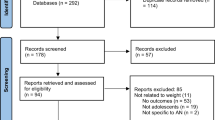
A flowchart was created to document general article screening progress in accordance with PRISMA guidelines (Fig. 1). K.R. and A.S. used Covidence to systematically assess articles using titles, abstracts, and full text copies. After the initial search, 448 studies were imported for screening, one of which was a duplicate. Of the 447 studies screened, 334 studies were deemed irrelevant based on title and/or abstract screening according to inclusion and exclusion criteria. The remaining 106 full-text studies were assessed for eligibility, and 70 were excluded due to study design (n = 55) or outcomes (n = 15) not of relevance for this review. A total of 36 studies were included (Fig. 1).
A Covidence-generated template was used to extract the following information: (1) Author and year of publication; (2) country where the study was conducted; (3) aim of the study; (4) inclusion and exclusion criteria; (5) population characteristics (including age, weight, BMI, duration of treatment, and caloric intake when available); (6) metabolic variable descriptions, numerical results, and correction factors. All study participants met diagnostic criteria for AN according to the DSM-III, DSM-IV, or DSM-5, depending on time of publication. Means and standard deviations were evaluated for each numeric variable when available. Metabolic variables (REE, TEE, RMR, and BMR) were standardized to kcal/day, and the longest duration from follow-up reported in each study was recorded for this review to maintain consistency throughout data extraction. Means of clinical characteristics and percent change in EE values from baseline to follow-up were calculated.
RESULTS
Study characteristics
The included studies evaluated female patients with AN in Australia (n = 2), Brazil (n = 1), Canada (n = 2), Czech Republic (n = 2), France (n = 4), Germany (n = 3), Israel (n = 2), Italy (n = 4), Japan (n = 2), Spain (n = 4), Sweden (n = 1), Switzerland (n = 1), and USA (n = 8) (Table 1). Studies were published between 1984 and 2020. A non-eating disorder (non-ED) control group was included in 55% of studies (n = 20). In addition to healthy female controls, three studies included “refed”, “rehabilitated”, or “recovered” patients with AN [33,34,35]; one study included a comparison group of weight-recovered females with an acute episode of AN in the last 10 years [36]; and one study included an additional comparison of “malnourished, dying patients” with other diseases [37]. Four of the studies [34, 35, 38, 39] recruited from outpatient treatment facilities, whereas the majority of included patients with AN were receiving inpatient treatment. All studies included only female participants across both AN and control groups, and only data on patients with AN were extracted.
The studies reported varying renourishment protocols, and energy intake ranged from 753 [40] to 3264 [41] kcal at baseline (T1) and 1658 [42] to 3600 [43] kcal at follow-up (T2). With the exception of Pettersson 2016 (-19.67% change in kcal) [41] and Pauly 2000 (0.00% percent change in kcal) [42], studies reported a higher kcal intake at the second timepoint (ranging from + 11.82% [44] to + 217.76% [45]). When the first measurement was obtained, the study participants had an average BMI of 13.7 kg/m2. At the T2 measurement and an average of 67 days of renourishment, the patients gained significant weight, had an average BMI of 17.57 kg/m2. Of note, the mean BMI at T2 falls outside of the recommended range for BMI (18.5–25 kg/m2).
Measured energy expenditure
Measured energy expenditure is reported in Table 2. The energy expenditure measurement with the longest duration from baseline was extracted as the second timepoint (T2). Of the included studies, 76% (n = 28) reported statistically significant increases in energy expenditure from baseline (T1) to follow-up (T2) timepoints. In contrast, five studies reported no significant differences [36, 46,47,48]. An additional four studies did not report numerical summaries of relevant statistical tests [43, 49,50,51]. On average, the studies reported a 24.99% change in EE from baseline to follow-up. Cuerda Compés 2005 reported the smallest percent change of 5.18% [52], and the EE values reported in Obarzanek 1994 showed the largest percent change of 62.80% [53].
Seven of the included studies adjusted the measured EE for body weight, lean body mass, or fat-free mass. In four of those studies [33, 38, 54, 55], the EE remained significantly lower at T1 than T2 even after the adjustment. In contrast, the remaining three studies [13, 56, 57] reported that the statistically significant difference diminished after accounting for body weight, lean body mass, or fat-free mass (FFM). One study showed that the ratio of REE to FFM significantly correlated with energy intake, anxiety, abdominal pain, and depressive mood [45]. The ratio also increased significantly with physical activity and cigarette smoking. A positive correlation between serum leptin levels and EE was reported in studies by Haas et al. [58] and Polito et al. [59]. However, no statistically significant correlation was found in a study published a few years earlier by Haas [44].
Twelve of the included studies [37, 38, 43,44,45, 51, 53, 55, 60,61,62,63] used multiple EE values across the renourishment period. Three of the studies [38, 44, 61] included EE measurements at the beginning, middle, and end of treatment, with no significant evidence of a hypermetabolic period during the middle of treatment compared to discharge. Furthermore, Haas 2005 reported that discharge REEs were still lower in AN than their non-ED controls [44]. Similarly, Obarzanek 1994 reported significant increases in RMR when patients were restudied during early refeeding [53]; RMR further increased during late refeeding to levels comparable to healthy volunteers but reverted to values lower than those of controls when the patients finished the target weight-stabilization. Vaisman 1991 and Vaisman 2004 reported gradual increases in REE (across either body weight percentages [63] or treatment duration [55]) persisted until stabilizing near discharge (8–10 weeks). Despite these increases, however, the mean REE failed to reach the “normal” range (90–110% of predicted REE) during hospitalization [55].
In contrast to gradual increases over time, Schebendach 1997, Rigaud 2000, and Pichard 1996 reported that the early weeks of refeeding (i.e., weeks 0–2) are the critical period for statistically significant increases in EE, as higher energy expenditure does not seem to persist in longer-term weight stable patients [37, 51, 62]. Additionally, Van Wymelbeke 2004 reported that the initial increase in REE during the first week of treatment represented 31% of the total REE increase over 2.5 months of refeeding [45]. Importantly, Krahn 1993 and Moukaddem 1997 reported EE results with detailed energy intake information [43, 57]. As patients increased their energy intake to 3600 kcal/day (by 300 kcal/day increments starting at 1200 kcal/day at baseline), Krahn 1993 reported that observed differences in REE ranged from 467 to 1049 kcal/day, highlighting large variability in metabolic recovery [43]. Moukaddem 1997 reported an 8% increase in REE after 1 week of refeeding, with slight variations in estimations based on 300 kcal or 700 kcal experimental loads [57].
Predicted energy expenditure
Predicted energy expenditure results across two timepoints were extracted if raw values were reported (i.e., not only as percentages of measured EE; Table 3). For consistency within this review, only predictions using the Harris-Benedict equation (n = 6) (most widely used [64]) or Bioelectrical Impedance Analysis (BIA; n = 1) were extracted. Five of the studies reported statistically significant changes between predicted EE values at baseline and follow up [13, 36, 44, 52, 60], and all studies that included raw predicted EE values reported the finding that predicted values overestimated measured EE in patients with AN. On average, the studies reported a 11.65% change in EE from baseline to follow-up, over 50% less than the average percent change in measured EE. Agüera 2015 reported the smallest percent change of 2.07% in patients with restricting AN [60], and DosReis 2020 reported the largest percent change of 53.36% [35]. Interestingly, Konrad 2007 reported that measured values of EE were 84% below values predicted with the Harris-Benedict Eq. [38]. These findings are consistent with prior literature suggesting that REE is likely to be higher than predicted during refeeding [13] due to the failure of predictive equations to adequately account for altered body composition and/or hormonal status in females with AN.
Discussion
Summary of findings
A comprehensive search revealed a complex relationship between metabolic trajectories and the renourishment process in AN, and the included studies reported consistently lower energy expenditure at the initiation of renourishment, compared with values reported in non-eating disorder controls. All studies highlighted subsequent increases in energy expenditure after differing durations of refeeding, with varying levels of statistical significance. The consistent report of moderate increases was not accompanied by reproducible reports of EE-associated clinical variables (e.g., BMI, FFM, or hormones) or a standard duration of refeeding needed to elicit these changes. Additionally, the studies, despite moderate increases, do not indicate evidence of energy expenditure that would indicate the presence of a hypermetabolic state, or energy expenditure > 110% of predicted REE [65]. This consensus definition of a hypermetabolic threshold relies heavily on studies of cancer cachexia and burn injuries, and the reliability of applying this numerical value in AN remains questionable due to frequent inaccuracies in predicted REE. Metabolic rates of the patients with AN at the second timepoint (either discharge from treatment, after a short period of refeeding, or recovered individuals) throughout all included studies fall within the normal range (total energy expenditure of 1910–2140 kcal/day for healthy female teenagers [66] and 1572–3687 kcal/day in healthy adults [67]). In summary, weight gain resistance in AN treatment does not seem to be fully explained by measures of metabolic rate and/or energy expenditure. Although there are increases in expenditure after initiation of renourishment, the data suggests that these increases are simply a normalization of expenditure out of the hypometabolic disease state and do not indicate hypermetabolism-driven weight gain resistance.
Limitations of extant literature
Although metabolic adaptations before and after treatment were described, the existing data do not support formal conclusions about metabolic changes that may occur during refeeding. Only 33% of included studies (n = 12) reported more than two measurements, and many studies did not take the second energy expenditure measurement during the highest energy intake period but rather once the energy intake requirement declined prior to discharge. Moreover, the current literature does not report sufficient data to characterize differences between restricting and binge eating/purging AN subtypes, although one study reported BMR to be higher in the restricting subtype [60]. It is also likely that different measurement techniques for assessing and/or predicting metabolic rate may lead to vastly different estimates, creating an additional layer of difficulty when developing literature-based nutritional rehabilitation programs. Most included studies did not report effect sizes, making the associated clinical relevance hard to interpret. Additionally, metabolic changes in other eating disorders and/or other populations of patients with AN, such as males and/or patients presenting in larger bodies (i.e., atypical AN [68]), are not well described. This warrants future investigation, as males are typically considered to report higher variations in metabolic activity [69] and individuals with higher weights may have significantly different metabolic rates compared to lean individuals [70,71,72].
Additionally, there is large variability in both length of stay and weight change among the included studies. Three studies with both short (7 days) [42, 57] and long (up to 11 months) [39] length of stay in the unit reported less than 1 unit change in BMI, which complicates the interpretation of these findings. Small weight changes may reflect changes in hydration status more than compartmental changes to body composition. When REE results from these reports are not considered, however, there is still no evidence of hypermetabolic spikes at discharge in the remaining studies. Variations in length of stay, weight change, and comparison groups highlight the need for more consistent characterization of metabolic changes during recovery.
Increasing scientific rigor
To gain a deeper understanding of the mechanistic basis of increases in energy expenditure in patients with AN during renourishment, it is imperative that the field fosters greater scientific rigor by seeking consistency across design, measurement, and analysis. The included studies discuss multiple hypotheses about why this hypermetabolic phenomenon may occur—such as shifts to bone formation [73], the continuation of protein synthesis in a resting state instead of just after meals [53], dietary induced thermogenesis spikes with greater amount and frequency of food [50]—but explanations for such a prompt rebound to or beyond typical metabolic rates remains elusive, leaving practitioners to adjust energy intake during this period without properly informed evidence-based guidance. Additionally, growing evidence from genetic studies uncovering both psychiatric and metabolic genetic underpinnings to AN [74] adds urgency to designing and conducting rigorous studies to more deeply understand metabolism in individuals with AN, both as a predisposing trait and additionally to understand changes in metabolism over time. As genetics explain ~ 40% of the variance in resting metabolic rate [75] and AN has twin-based heritability estimates of 50–60% [76], investigating the overlap between genetically influenced metabolic traits and AN phenotypes may provide meaningful insight.
Determining whether the purported hypermetabolic phase actually occurs is of considerable clinical importance. Tolerating increasingly high caloric prescriptions is physically and psychologically difficult for patients and can lead to marked gastrointestinal and emotional distress. Moreover, preventing the loss of therapeutically restored weight after discharge depends on accurate prediction of energy requirements to maintain or gain weight. If patients are not gaining weight with a prescribed caloric level and there is no evidence of hypermetabolism, then other reasons for the failure to gain weight must be explored.
Of relevance for refeeding, the primary role—among many—of the intestine is to harvest calories from the diet to sustain the body’s energy requirements. Prolonged caloric restriction can lead to a dysfunctional gut and potentially reduced calorie harvest from the diet [77]. Indeed, nutrient deprivation in individuals with AN could impact gut function and ultimately lead to a global reduction in the absorptive capacity of the gut, which may be misinterpreted as hypermetabolism during renourishment. Stated another way, although patients are being fed increasing numbers of calories, their guts may be unable to harvest and utilize those calories for weight restoration. Although renourishment, weight gain, and weight maintenance are major hurdles for recovery, scant information exists about the absorptive capacity of the gut, and its corresponding relationship with metabolism, in patients with AN. The very limited number of studies of intestinal epithelial alterations in AN reported disturbances in tissue architecture and a decrease in intestinal permeability [78, 79], and it is logical to posit that the microbial ecosystem inhabiting the gastrointestinal tract may be an additional barrier [32, 77]. As the body of research progresses, studies with consistent metabolic tracking may lead to investigation of the existence of a dysfunctional intestine in patients with AN, which may have significant implications for developing more effective and enduring renourishment strategies.
Recommendations
The clinical experience of hypermetabolism, albeit lacking robust empirical evidence, has a direct impact on renourishment protocols implemented by the clinical care team. If a patient is not making expected weight gain, all possible reasons should be explored in order to make informed clinical decisions. Although fear of weight gain may indeed lead patients to engage in treatment-interfering behaviors that inhibit weight gain, it should never automatically be assumed that this is the cause of a weight stall. Careful characterization of energy expenditure throughout the duration of treatment provides the best data for informed decision making among providers. Based on this review, considerable research is needed to enrich our understanding of metabolic factors and changes that are relevant to AN etiology, progression, treatment, and remission. As a start, we recommend the following:
-
I.
Future research should harmonize methodologies and standardize reporting of results. Robust studies should include detailed dietary data (i.e., energy intake), subtype categorizations, and information about the duration of illness. In addition to significance testing, effect sizes should be reported as well as metrics that capture the extent to which results are clinically meaningful.
-
II.
If physiological measurement via indirect calorimetry, doubly labeled water, or other methods is not available, studies utilizing predictive equations for energy expenditure should include subtype, body composition measures (fat free mass and fat mass through dual X-ray absorptiometry or bioelectrical impedance analysis [80]), and duration of illness in the equations for better predictive accuracy [15]. Recent findings from Bou Khalil et al. report that if FFM and FM measurements are not available, the Schebendach equation [81] will have the highest agreement with REE measured by indirect calorimetry [15]. Authors employing these methods should highlight shortcomings of these equations; for example, Cuerda 2007 reported that measured REE values did increase throughout hospitalization but still remained 10% lower than predicted values [13]. Treatment teams in the United States are frequently faced with the challenge of discharge prior to weight stabilization despite recent evidence that BMI at the end of treatment is a direct predictor of relapse [82]. In a climate of insurance thresholds that are based on percentages—such as discharge at 80% ideal body weight—it is imperative to acknowledge the implications of inaccurate expenditure predictions on weight stabilization and relapse risk. Although further research is needed to establish the most clinically relevant timepoints for energy expenditure assessment, we recommend energy expenditure estimations at admission, approximately two weeks into treatment, and prior to discharge to inform dietary prescriptions to support continued weight gain or weight maintenance. Although duration of inpatient stay is difficult to predict and varies due to many factors, a second measurement after a week or two of treatment will capture energy expenditure once fluid and electrolyte levels have stabilized. It is possible that persistent disruptions in homeostatic energy expenditure, in the presence of other indicators of active illness (such as lack of weight stabilization) may be important factors to consider prior to discharge at a certain percentage of ideal body weight.
-
III.
Funding for well-developed studies with a comprehensive characterization of energy expenditure over time, including multiple timepoints during renourishment, is imperative. Additionally, funding to investigate biological mechanisms that may be contributing to difficulties during treatment will greatly advance the field. Relevant biological avenues for exploration include: (i) the intestinal microbiota (including the development of microbiota directed complementary foods to be implemented during refeeding), (ii) genetics (with metabolic-, psychiatric- and nutri-genetic driven hypotheses), (iii) structural or physiological changes to the gut in AN, and (iv) other feeding and eating behaviors, such as time restricted eating, binge eating, and purging, that may play a role in the absorption or conversion of calories to tissue.
Conclusion
This scoping review provides an updated description of research examining variations in energy expenditure before and following a period of renourishment in patients with AN. After summarizing reports of measured and predicted EE, it is clear that patients currently ill with active AN present for treatment with slowed metabolic rates, and, after treatment, experience mild to moderate increases in metabolic rate. The commonly occurring and resolute resistance to weight gain, however, cannot be fully attributed to a hypermetabolic shift during renourishment. This review has highlighted many gaps for which further funding and research is essential, as there is an urgent need to explore metabolic abnormalities in AN. The reality faced by patients with AN is often a bleak one—with approximately one third of individuals with AN still not reaching recovery after 22 years of illness [83]. Decades of research into psychological and pharmacological interventions have left all those in the AN field—patients, caregivers, providers, and researchers alike—in need of more viable treatment options that provide a more promising future [84]. In a continued effort to shift the treatment paradigm, we propose that now is the time to finally understand the perplexing and remarkably resilient metabolic adaptions that occur in AN.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AN:
-
anorexia nervosa
- Kcal:
-
kilocalorie
- ATP:
-
adenosine triphosphate
- BMR:
-
basal metabolic rate
- EE:
-
energy expenditure
- REE:
-
resting energy expenditure
- TEE:
-
total energy expenditure
- RMR:
-
resting metabolic rate
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- ED:
-
eating disorder
- T1:
-
timepoint 1
- T2:
-
timepoint 2
- BMI:
-
body mass index
- FFM:
-
fat free mass
References
Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: shared genetic and environmental risk factors. Am J Psychiatry. 2000;157:469–71.
Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–21.
van Eeden AE, van Hoeken D, Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2021;34:515–24.
Fernandez-Aranda F, Pinheiro AP, Tozzi F, Thornton LM, Fichter MM, Halmi KA, et al. Symptom profile of major depressive disorder in women with eating disorders. Aust N Z J Psychiatry. 2007;41:24–31.
Mehler PS, Brown C. Anorexia nervosa - medical complications. J Eat Disord. 2015;3:11.
Yager J. Managing patients with severe and enduring anorexia nervosa: when is enough. Enough? J Nerv Ment Dis. 2020;208:277–82.
Berends T, Boonstra N, van Elburg A. Relapse in anorexia nervosa: a systematic review and meta-analysis. Curr Opin Psychiatry. 2018;31:445–55.
Bulik CM, Berkman ND, Brownley KA, Sedway JA, Lohr KN. Anorexia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40:310–20.
Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2:1099–111.
Herzog W, Schellberg D, Deter HC. First recovery in anorexia nervosa patients in the long-term course: a discrete-time survival analysis. J Consult Clin Psychol. 1997;65:169–77.
Strandjord SE, Sieke EH, Richmond M, Rome ES. Avoidant/restrictive food intake disorder: illness and hospital course in patients hospitalized for nutritional insufficiency. J Adolesc Health. 2015;57:673–8.
Hay P, Touyz S. Treatment of patients with severe and enduring eating disorders. Curr Opin Psychiatry. 2015;28:473–7.
Cuerda C, Ruiz A, Velasco C, Bretón I, Camblor M, García-Peris P. How accurate are predictive formulas calculating energy expenditure in adolescent patients with anorexia nervosa? Clin Nutr. 2007;26:100–6.
Marra M, Polito A, De Filippo E, Cuzzolaro M, Ciarapica D, Contaldo F, et al. Are the general equations to predict BMR applicable to patients with anorexia nervosa? Eat Weight Disord. 2002;7:53–9.
Bou Khalil R, Sultan A, Seneque M, Richa S, Lefebvre P, Renard E, et al. Clinical correlates of measured and predicted resting energy expenditure in patients with anorexia nervosa: a retrospective cohort study. Nutrients. 2022;14:2727.
Stanga Z, Brunner A, Leuenberger M, Grimble RF, Shenkin A, Allison SP, et al. Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008;62:687–94.
Cuerda C, Vasiloglou MF, Arhip L. Nutritional management and outcomes in malnourished medical inpatients: anorexia nervosa. J Clin Med. 2019;8:1042.
Garber AK, Cheng J, Accurso EC, Adams SH, Buckelew SM, Kapphahn CJ, et al. Short-term outcomes of the study of refeeding to optimize inpatient gains for patients with anorexia nervosa: a multicenter randomized clinical trial. JAMA Pediatr. 2021;175:19–27.
Kosmiski L, Schmiege SJ, Mascolo M, Gaudiani J, Mehler PS. Chronic starvation secondary to anorexia nervosa is associated with an adaptive suppression of resting energy expenditure. J Clin Endocrinol Metab. 2014;99:908–14.
McCue MD. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol Part Mol Integr Physiol. 2010;156:1–18.
Bouten CV, van Marken Lichtenbelt WD, Westerterp KR. Body mass index and daily physical activity in anorexia nervosa. Med Sci Sports Exerc. 1996;28:967–73.
Delvenne V, Lotstra F, Goldman S, Biver F, De Maertelaer V, Appelboom-Fondu J, et al. Brain hypometabolism of glucose in anorexia nervosa: a PET scan study. Biol Psychiatry. 1995;37:161–9.
Marzola E, Nasser JA, Hashim SA, Shih P-AB, Kaye WH. Nutritional rehabilitation in anorexia nervosa: review of the literature and implications for treatment. BMC Psychiatry. 2013;13:290.
Newton LE, Heimburger DC. Critical illness. Handbook of clinical nutrition. Elsevier; 2006. pp. 487–502.
Williams FN, Jeschke MG, Chinkes DL, Suman OE, Branski LK, Herndon DN. Modulation of the hypermetabolic response to trauma: temperature, nutrition, and drugs. J Am Coll Surg. 2009;208:489–502.
Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9.
Yu P-J, Cassiere H, DeRosa S, Bocchieri K, Yar S, Hartman A. Hypermetabolism and coronavirus disease 2019. JPEN J Parenter Enter Nutr. 2020;44:1234–6.
Haase CG, Long AK, Gillooly JF. Energetics of stress: linking plasma cortisol levels to metabolic rate in mammals. Biol Lett. 2016;12:20150867.
Chapman JL, Comas M, Hoyos CM, Bartlett DJ, Grunstein RR, Gordon CJ. Is metabolic rate increased in insomnia disorder? A systematic review. Front Endocrinol (Lausanne). 2018;9:374.
Kaye WH, Gwirtsman HE, Obarzanek E, George T, Jimerson DC, Ebert MH. Caloric intake necessary for weight maintenance in anorexia nervosa: nonbulimics require greater caloric intake than bulimics. Am J Clin Nutr. 1986;44:435–43.
Walker J, Roberts SL, Halmi KA, Goldberg SC. Caloric requirements for weight gain in anorexia nervosa. Am J Clin Nutr. 1979;32:1396–400.
Haas V, Stengel A, Mähler A, Gerlach G, Lehmann C, Boschmann M, et al. Metabolic barriers to weight gain in patients with anorexia nervosa: a young adult case report. Front Psychiatry. 2018;9:199.
Scalfi L, Di Biase G, Coltorti A, Contaldo F. Bioimpedance analysis and resting energy expenditure in undernourished and refed anorectic patients. Eur J Clin Nutr. 1993;47:61–7.
Polito A, Fabbri A, Ferro-Luzzi A, Cuzzolaro M, Censi L, Ciarapica D, et al. Basal metabolic rate in anorexia nervosa: relation to body composition and leptin concentrations. Am J Clin Nutr. 2000;71:1495–502.
Dos Reis TO, de Magalhães Oliveira F, Kattah FM, Pena NF, Soares MMS, da Gama Torres HO. Body composition and energy expenditure in anorexia nervosa: preliminary data of outpatients with recovering and active disease. J Eat Disord. 2022;10:167.
Platte P, Pirke KM, Trimborn P, Pietsch K, Krieg JC, Fichter MM. Resting metabolic rate and total energy expenditure in acute and weight recovered patients with anorexia nervosa and in healthy young women. Int J Eat Disord. 1994;1:45–52.
Rigaud D, Hassid J, Meulemans A, Poupard AT, Boulier A. A paradoxical increase in resting energy expenditure in malnourished patients near death: the king penguin syndrome. Am J Clin Nutr. 2000;72:355–60.
Konrad KK, Carels RA, Garner DM. Metabolic and psychological changes during refeeding in anorexia nervosa. Eat Weight Disord. 2007;12:20–6.
Dragani B, Malatesta G, Di Ilio C, De Cristofaro P. Dynamic monitoring of restricted eating disorders by indirect calorimetry: a useful cognitive approach. Eat Weight Disord. 2006;11:e9–14.
Satoh Y, Shimizu T, Lee T, Nishizawa K, Iijima M, Yamashiro Y. Resting energy expenditure and plasma leptin levels in adolescent girls with anorexia nervosa. Int J Eat Disord. 2003;34:156–61.
Pettersson C, Tubic B, Svedlund A, Magnusson P, Ellegård L, Swolin-Eide D, et al. Description of an intensive nutrition therapy in hospitalized adolescents with anorexia nervosa. Eat Behav. 2016;21:172–8.
Pauly RP, Lear SA, Hastings FC, Birmingham CL. Resting energy expenditure and plasma leptin levels in anorexia nervosa during acute refeeding. Int J Eat Disord. 2000;28:231–4.
Krahn DD, Rock C, Dechert RE, Nairn KK, Hasse SA. Changes in resting energy expenditure and body composition in anorexia nervosa patients during refeeding. J Am Diet Assoc. 1993;93:434–8.
Haas V, Onur S, Paul T, Nutzinger DO, Bosy-Westphal A, Hauer M, et al. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am J Clin Nutr. 2005;81:889–96.
Van Wymelbeke V, Brondel L, Marcel Brun J, Rigaud D. Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. Am J Clin Nutr. 2004;80:1469–77.
Svobodová J, Haluzík M, Papezová H, Rosická M, Nedvídková J, Kotrlíková E, et al. [The effect of partial refeeding on serum levels of leptin and resting energy expenditure in female patients with anorexia nervosa]. Cas Lek Cesk. 1999;138:748–52.
Winter TA, O’Keefe SJ, Callanan M, Marks T. The effect of severe undernutrition and subsequent refeeding on whole-body metabolism and protein synthesis in human subjects. JPEN J Parenter Enter Nutr. 2005;29:221–8.
Soto-Célix M, Riego-Valledor A, Español-Armengol N, Miján-de-la-Torre A. Pp156-sun malnutrition is not accompanied by an adaptative resting hypometabolism in malnourished anorexia nervosa females: a prospective cohort study. Clin Nutr. 2013;32:S81.
Dempsey DT, Crosby LO, Pertschuk MJ, Feurer ID, Buzby GP, Mullen JL. Weight gain and nutritional efficacy in anorexia nervosa. Am J Clin Nutr. 1984;39:236–42.
Kochavi B, Mendelowitsch S, Enoch-Levy A, Yaroslavsky A, Toledano A, Modan-Moses D, et al. Resting energy expenditure in acutely ill and stabilized patients with anorexia nervosa and bulimia nervosa. Int J Eat Disord. 2020;53:1460–8.
Pichard C, Kyle UG, Slosman DO, Penalosa B. Energy expenditure in anorexia nervosa: can fat-free mass as measured by bioelectrical impedance predict energy expenditure in hospitalized patients? Clin Nutr. 1996;15:109–14.
Cuerda Compés MC, Ruiz Sancho A, Moreno Rengel C, Iriondo Martínez MT, Velasco Gimeno C, Bretón Lesmes I, et al. [Study of energy expenditure in anorexia nervosa: agreement between indirect calorimatry and several equations]. Nutr Hosp. 2005;20:371–7.
Obarzanek E, Lesem MD, Jimerson DC. Resting metabolic rate of anorexia nervosa patients during weight gain. Am J Clin Nutr. 1994;60:666–75.
Yoshida NM, Yoshiuchi K, Kumano H, Sasaki T, Kuboki T. Changes in heart rate with refeeding in anorexia nervosa: a pilot study. J Psychosom Res. 2006;61:571–5.
Vaisman N, Rossi MF, Corey M, Clarke R, Goldberg E, Pencharz PB. Effect of refeeding on the energy metabolism of adolescent girls who have anorexia nervosa. Eur J Clin Nutr. 1991;45:527–37.
Melchior JC, Rigaud D, Rozen R, Malon D, Apfelbaum M. Energy expenditure economy induced by decrease in lean body mass in anorexia nervosa. Eur J Clin Nutr. 1989;43:793–9.
Moukaddem M, Boulier A, Apfelbaum M, Rigaud D. Increase in diet-induced thermogenesis at the start of refeeding in severely malnourished anorexia nervosa patients. Am J Clin Nutr. 1997;66:133–40.
Haas VK, Gaskin KJ, Kohn MR, Clarke SD, Müller MJ. Different thermic effects of leptin in adolescent females with varying body fat content. Clin Nutr. 2010;29:639–45.
Polito A, Cuzzolaro M, Raguzzini A, Censi L, Ferro-Luzzi A. Body composition changes in anorexia nervosa. Eur J Clin Nutr. 1998;52:655–62.
Agüera Z, Romero X, Arcelus J, Sánchez I, Riesco N, Jiménez-Murcia S, et al. Changes in body composition in anorexia nervosa: predictors of recovery and treatment outcome. PLoS ONE. 2015;10:e0143012.
Onur S, Haas V, Bosy-Westphal A, Hauer M, Paul T, Nutzinger D, et al. L-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. Eur J Endocrinol. 2005;152:179–84.
Schebendach JE, Golden NH, Jacobson MS, Hertz S, Shenker IR. The metabolic responses to starvation and refeeding in adolescents with anorexia nervosa. Ann N Y Acad Sci. 1997;817:110–9.
Vaisman N, Hahn T, Karov Y, Sigler E, Barak Y, Barak V. Changes in cytokine production and impaired hematopoiesis in patients with anorexia nervosa: the effect of refeeding. Cytokine. 2004;26:255–61.
Luy SC, Dampil OA. Comparison of the Harris-Benedict equation, bioelectrical impedance analysis, and indirect calorimetry for measurement of basal metabolic rate among adult obese Filipino patients with prediabetes or type 2 diabetes Mellitus. JAFES. 2018;33:152–9.
Dev R, Hui D, Chisholm G, Delgado-Guay M, Dalal S, Del Fabbro E, et al. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J Cachexia Sarcopenia Muscle. 2015;6:95–8.
Wong WW. Energy expenditure of female adolescents. J Am Coll Nutr. 1994;13:332–7.
Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, et al. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99:71–8.
Vo M, Golden N. Medical complications and management of atypical anorexia nervosa. J Eat Disord. 2022;10:196.
Halsey LG, Careau V, Pontzer H, Ainslie PN, Andersen LF, Anderson LJ, et al. Variability in energy expenditure is much greater in males than females. J Hum Evol. 2022;171:103229.
Jéquier E. Energy metabolism in human obesity. Soz Praventivmed. 1989;34:58–62.
Hoffmans M, Pfeifer WA, Gundlach BL, Nijkrake HG, Oude Ophuis AJ, Hautvast JG. Resting metabolic rate in obese and normal weight women. Int J Obes. 1979;3:111–8.
Hosseini B, Mirzaei K, Maghbooli Z, Keshavarz SA, Hossein-Nezhad A. Compare the resting metabolic rate status in the healthy metabolically obese with the unhealthy metabolically obese participants. J Nutr Intermed Metab. 2016;6:48–53.
Sum M, Mayer L, Warren MP. Bone mineral density accrual determines energy expenditure with refeeding in anorexia nervosa and supersedes return of menses. J Osteoporos. 2011;2011:720328.
Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51:1207–14.
Bouchard C, Pérusse L, Dériaz O, Després JP, Tremblay A. Genetic influences on energy expenditure in humans. Crit Rev Food Sci Nutr. 1993;33:345–50.
Yilmaz Z, Hardaway JA, Bulik CM. Genetics and epigenetics of eating disorders. Adv Genomics Genet. 2015;5:131–50.
Basolo A, Hohenadel M, Ang QY, Piaggi P, Heinitz S, Walter M, et al. Effects of underfeeding and oral Vancomycin on gut microbiome and nutrient absorption in humans. Nat Med. 2020;26:589–98.
Takimoto Y, Yoshiuchi K, Shimodaira S, Akabayashi A. Diamine oxidase activity levels in anorexia nervosa. Int J Eat Disord. 2014;47:203–5.
Monteleone P, Carratù R, Cartenì M, Generoso M, Lamberti M, Magistris LD, et al. Intestinal permeability is decreased in anorexia nervosa. Mol Psychiatry. 2004;9:76–80.
Abbaspour A, Reed KK, Hübel C, Bulik-Sullivan EC, Tang Q, Bulik CM, et al. Comparison of dual-energy X-ray absorptiometry and bioelectrical impedance analysis in the assessment of body composition in women with anorexia nervosa upon admission and discharge from an inpatient specialist unit. Int J Environ Res Public Health. 2021;18:11388.
Schebendach J, Golden NH, Jacobson MS, Arden M, Pettei M, Hardoff D, et al. Indirect calorimetry in the nutritional management of eating disorders. Int J Eat Disord. 1995;17:59–66.
Frostad S, Rozakou-Soumalia N, Dârvariu Ş, Foruzesh B, Azkia H, Larsen MP, et al. BMI at discharge from treatment predicts relapse in anorexia nervosa: a systematic scoping review. J Pers Med. 2022;12:836.
Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, et al. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J Clin Psychiatry. 2017;78:184–9.
Touyz S, Bryant E, Dann KM, Polivy J, Le Grange D, Hay P, et al. What kind of illness is anorexia nervosa? Revisited: some preliminary thoughts to finding a cure. J Eat Disord. 2023;11:221.
Acknowledgements
The authors would like to thank Jamie Conklin from the UNC-Chapel Hill Health Sciences Library for providing valuable support during article screening.
Funding
CMB is supported by NIMH (R56MH129437; R01MH120170; R01MH124871; R01MH119084; R01MH118278; R01 MH124871) and Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864). IMC is supported by NIMH (R01MH105684) and the UNC CH Nutrition Obesity Research Center (NORC, P30-DK056350-21).
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
KKR: conceptualization, methodology, formal analysis, writing—original draft, review and editing. AES: methodology, formal analysis. AA: formal analysis, writing—original draft, review and editing. KSB: writing—review and editing. CMB: conceptualization, writing—review and editing. IMC: conceptualization, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
CMB reports: Pearson (author, royalty recipient). IMC previously served as a consultant for Salix Pharmaceuticals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reed, K.K., Silverman, A.E., Abbaspour, A. et al. Energy expenditure during nutritional rehabilitation: a scoping review to investigate hypermetabolism in individuals with anorexia nervosa. J Eat Disord 12, 63 (2024). https://doi.org/10.1186/s40337-024-01019-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40337-024-01019-7