Abstract
Background
Anorexia nervosa (AN) is associated with abnormalities that may increase the risk of future cardiovascular disease. This study assessed the cardiovascular health of individuals who recovered from AN during adolescence by conducting wave power analysis.
Methods
Former AN patients discharged from the Royal Children’s and Monash Children’s Hospitals (N = 17) in Melbourne, Australia underwent ultrasound imaging of the right carotid artery. Wave power analysis was conducted to assess biomechanical interactions of the cardiovascular system. Patient measures were compared to healthy controls (N = 51).
Results
Eighty-eight percent of the former AN patients and controls were female, aged approximately 25 years, with a healthy body mass index. Mean carotid flow and pulsatility index were not different between groups. Carotid arterial strain and distensibility were lower, and the wave speed and beta stiffness index higher in the former AN patients. Characteristic impedance was not different nor were the forward and backward wave amplitudes. However, wave reflection indices (ratios of backward-to-forward compression wave area, and wave-related effect on pressure and hydraulic power) were 12–18% lower in the former AN patients (p < 0.05).
Conclusions
Increased carotid artery stiffness and reduced wave reflection are evident in young adults who recovered from adolescent AN. This may relate to an adaptive process that helps to maintain or restore flow and characteristic impedance despite increased vessel stiffness, with this warranting future investigation.
Plain English summary
Anorexia nervosa (AN) is an eating disorder which may cause permanent changes in the heart and blood vessels. Blood flow properties can provide information on the health of a patient’s heart and blood vessels. In this study of young adults who recovered from adolescent AN, blood flow analysis revealed altered properties compared to controls who had never experienced an eating disorder. These alterations may help to maintain or restore blood flow despite unhealthy changes in the blood vessels themselves. Further investigation is needed to better understand how the heart and blood vessels change during and after AN to guide treatments and ongoing care. Regular assessment of the heart and blood vessels after AN recovery could identify and monitor possible health risks early.
Similar content being viewed by others
Background
Anorexia nervosa (AN) is associated with cardiovascular complications including decreased cardiac output, electrophysiological abnormalities, and altered blood vessel properties [1,2,3,4]. AN is the third most prevalent chronic disease in adolescent girls and is the leading cause of mortality among psychiatric disorders [5, 6], with up to 30% of deaths attributed to cardiovascular complications [7].
Existing research suggests cardiovascular risk may extend beyond a period of malnutrition and subsequent recovery, persisting into adulthood and later life. There are reports of early onset of cardiovascular disease and an increased incidence of heart disease among other populations who survived a period of malnutrition during famine or war [8,9,10,11]. Yet, the cardiovascular health of AN patients is not typically assessed following discharge from clinical services and their long-term prognosis is unknown.
Arterial wave reflection is a key contributor to ventricular afterload and has been shown to predict cardiovascular mortality [12, 13]. Wave analysis can therefore provide valuable insight into the biomechanical interactions between the heart and blood vessels. Wave intensity or wave power analysis [14, 15] is one of the most well established and widely used wave analysis techniques for investigating forward and backward waves. This technique can reveal information on cardiac function and vascular interaction by studying haemodynamic signals from a single vascular site, such as the carotid artery. The indices derived from wave analysis have been shown to provide both additional physiological insights and risk information beyond that of traditional risk factors [16,17,18,19,20,21,22,23].
Wave intensity analysis has not been performed in the AN population, yet cardiovascular complications are a major concern. We hypothesised that individuals with a history of AN would demonstrate abnormalities in carotid wave patterns compared to healthy control subjects given the increased cardiovascular risk that has been identified in malnourished populations. The aim of this study was to extend our previous work investigating the long-term cardiovascular risk following AN recovery [24], by assessing biomechanical cardiovascular interactions in former patients using wave analysis techniques. This can assist early identification and treatment of cardiovascular abnormalities.
Methods
Study design and sample
Using a cross-sectional study approach, we recruited a group of young adults (N = 17) who were treated for adolescent AN (Diagnostic and Statistical Manual of Mental Disorders classification) [25] and clinically discharged from the Royal Children’s Hospital or Monash Children’s Hospital Eating Disorder Service, Melbourne between June 2008 and July 2016. Patients were eligible for inclusion if they had been discharged from the Eating Disorder Service five or more years ago, were aged 18 years and over, exhibited an Eating Disorder Examination Global Score within 1SD of community norms (i.e. they no longer exhibited features of an eating disorder), and possessed a body mass index (BMI) ≥ 18.5 kg/m2. Healthy age- and sex-matched controls (N = 51) were drawn from the Victorian Infant Collaborative Study (VICS) [26] control cohort, with a ratio of 3:1.
A sample size of 68 participants (17 former AN and 51 control) would enable differences of at least 0.7SD to be detected with 80% power using a two-sided type I error of 5%.
Study procedures
Participants laid supine with the head turned 45° to the left while an ultrasound of the right carotid artery was performed with a GE Vivid ultrasound machine (Vivid i BT06, 10–15 MHz linear array probe; GE Healthcare). Arterial diameter, pressure, and flow velocity waveforms were obtained from Doppler and M-mode images.
A diameter waveform (\(D\)) was obtained via semi-automated segmentation of the upper and lower vessel walls from the M-mode images. A pressure waveform (\(P\)) was then obtained by calibrating the diameter waveform to central mean and diastolic pressures [27]. Central blood pressure was estimated from peripheral measurement of the brachial blood pressure in the non-dominant arm using in-built functions in the SphygmoCor XCEL system (Atcor Medical, Sydney, Australia). Velocity waveforms were obtained by semi-automated segmentation of the upper and lower envelopes of the Doppler spectrum, with mean velocity (obtained as the intensity-weighted spectral mean) used for analysis [27]. After applying a velocity correction factor, as described by Kowalski and colleagues [27], volumetric blood flow waveforms (\(Q\)) were calculated as the product of velocity (\({U}_{mean}\)) and arterial cross-sectional area (\(A= \pi {D}^{2}/4\)):\(Q= {U}_{mean}A\)
Carotid artery flow is a vital contributor to cerebral haemodynamics due to the carotid arteries carrying over 80% of the cerebral blood supply [28].
Pulsatility index was calculated from the ratio of velocities, with this being a measure of vascular resistance distal to the carotid artery: \(Pulsatility \space Index= \frac{{U}_{max}-{U}_{min}}{{U}_{mean}}\)
Measures of wall mechanics were also calculated from diameter, area, and pressure parameters as follows.
where \(\rho\) is blood density (1.06 g/cm3).
Characteristic impedance (\({Z}_{c}\)) was calculated via the slope of the early \(P\)-\(Q\) relation [29, 30]. These measures indicate arterial stiffness and thereby the degree of vascular aging [9].
Time-corrected wave power (wp) was calculated from pressure and flow as wp = (d \(P/dt)(\) d \(Q/dt)\) [15]; this quantity is similar to wave intensity, which uses velocity rather than flow, but unlike wave intensity is conserved at junctions. Forward ( +) and backward (–) components of wave power were calculated as \({\text{w}}{p}_{\pm }=\pm \left(dP/dt\pm {Z}_{c}dQ/dt\right)/\left(4{Z}_{c}\right)\). Pressure and flow were likewise separated into components via \({P}_{\pm }=\left(P\pm {Z}_{c}Q\right)/2\) and \({{\text{Q}}}_{\pm }=\left(Q\pm P/{Z}_{c}\right)/2\). Finally, hydraulic pressure power was calculated as \(\Pi =PQ\) and was also separated into forward and backward components via \({\Pi }_{\pm }={P}_{\pm }{Q}_{\pm }\) [15].
The carotid wave intensity signal generally exhibits four main waves [31]: an initial pressure- and flow-increasing forward compression wave (FCW) arising from blood acceleration following aortic valve opening; a pressure-increasing flow-decelerating backward compression wave (BCW) arising from wave reflection in the distal vascular bed; a late systolic pressure- and flow-decreasing forward decompression wave (FDW) associated with flow deceleration prior to valve closure, and a mid-systolic forward decompression wave (FDWx) thought to arise from negative re-reflection of the BCW when this backward-running wave encounters the brachiocephalic trunk and/or aorta. The amplitude of each wave was quantified via its peak wave power (PWP), area, pressure effect (Δ \(P\)), and hydraulic power effect (ΔΠ). Wave reflection was quantified via the BCW-FCW area, Δ \(P\), and ΔΠ ratios [31].
Statistical analysis
General descriptive statistics of the former AN patients and controls were calculated. Body surface area was calculated using the DuBois formula [32]. Clinical data relating to the severity and duration of malnutrition obtained at the time of diagnosis were also summarised for the former AN patients. Mean carotid flow was indexed for height. Mean and standard deviation (SD) were reported for normally distributed data, or the median and interquartile range (IQR) for non-normally distributed data. Comparisons were made between former patients and controls using the Wilcoxon test with group as the variable of interest. For each analysis output, p-values less than or equal to 0.05 were considered statistically significant. All statistical analyses were performed using R software (version 4.0.1, R Foundation, Vienna, Austria).
Results
Population
Descriptive statistics of the former AN patients (N = 17) and healthy controls (N = 51) are presented in Table 1. The former AN patients and controls had an average age of 25 years and similar BMI and body surface areas. The BMI of both groups was within the healthy range indicating healthy body fatness [28]. Most (88%) of the former AN patients were female, all of whom had regular menstrual periods. The time interval since discharge from their respective eating disorder services ranged from 5–10 years with a mean (SD) interval of 7.4 (2.0) years.
At the time of diagnosis the former AN patients had a mean (SD) age of 15.8 (1.7) years and BMI of 16.1 (1.9) kg/m2. This indicates a moderately malnourished cohort [25]. The patients had lost an average of 25% (ranging from 10–37%) of their body weight over 5–24 months.
Wave power analysis
There was a trend towards higher mean carotid flow in the former AN patients (p = 0.071) that was also present when indexed for height (p = 0.083, Table 2), but the magnitude of the difference for indexed carotid flow was small (2%). There was some evidence of a small (~ 10%) difference in pulsatility index (p = 0.081). There was stronger evidence of a difference in carotid arterial stiffness (indicative of vascular aging), with the former AN patients exhibiting lower strain (p = 0.028), lower distensibility (p = 0.067), higher Bramwell-Hill wave speed (p = 0.061), and a higher Beta stiffness index (p = 0.038). There was no evidence of a difference in the characteristic impedance (\({Z}_{c}\)) between groups (p = 0.817).
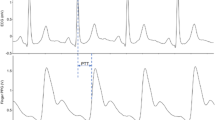
There was no evidence of a difference in the amplitudes of FCW, BCW, FDW or FDWx between former AN patients and controls (Table 3). However, former AN patients exhibited 12–18% lower BCW-FCW area, pressure, and power ratios (p = 0.032, 0.029, and 0.009, respectively). Figure 1 provides a representative example of components of the pressure, flow, and hydraulic power waveforms of a former AN patient; while Fig. 2 shows an example of the wave power analysis.
Discussion
In this first study of carotid wave analysis of former AN patients we have observed comparable blood flow and characteristic impedance, but higher carotid arterial stiffness. Forward and backward wave amplitudes were no different, but lower wave ratios suggested lesser wave reflection. The differences support our hypothesis that individuals with a history of AN would exhibit abnormalities in wave patterns compared to healthy controls.
Our data suggests that any reduction in mean cerebral flow (assessed by carotid flow in this study) during malnutrition was not sustained long-term in the former AN patients. This differs from a prior study that reported persistently reduced regional cerebral blood flow four years post treatment for adolescent AN [33]. That study measured cerebral flow via single positron emission computed tomography, whereas we measured carotid flow via ultrasound. Furthermore, our assessment time point was an additional 1–6 years beyond recovery; allowing potentially more time for cardiovascular normalisation. Further work would be needed to establish whether the differing findings arise from differences in methodology, patient characteristics, length of follow-up or a combination of these.
Lower BCW-FCW area, pressure, and power ratios signify lower cerebral wave reflection in the former AN patients. Bleasdale and colleagues suggested that less wave reflection may indicate reduced cerebral vasomotor tone [34]. A decrease in cerebral tone was identified during acute hypercapnia in a single population, and therefore it is unclear whether differences in baseline wave reflection in two groups may be accounted for by such differences in cerebral tone. Our data could suggest that differences in baseline tone exist between former AN patients and controls rather than arise from the period of malnutrition. In addition, less wave reflection promotes flow and could have contributed to the maintenance or normalisation of cerebral flow in former AN patients over time.
Reduced cerebral wave reflection has also been observed in healthy adults in response to acute inflammation [35]. Although there is evidence of elevated inflammatory markers in AN patients at the time of malnourishment [36], these typically normalise throughout recovery and the possible cardiovascular implications post recovery are unknown. Schroeder and colleagues suggested that lower wave reflection may increase the brain’s vulnerability to pulsatile haemodynamics [35]. Another study found lower carotid reflection index, pulsatility index, and mean velocity in older versus younger adults [37]. Our data may therefore imply that the former AN patients have a more ‘aged’ carotid/cerebral arterial network (consistent with increased carotid stiffness). However, our group has recently shown that although wave reflection promotes transmission of pulsatile pressure, it impedes pulsatile power transmission, to distal vascular beds [38]. Whether the lower wave reflection seen in former AN patients is potentially beneficial or detrimental is therefore unclear.
Our data revealed no difference in the FCW or FDW amplitude, which suggest systolic and diastolic function of the left ventricle are normal in the former AN patients [39]. Previous studies, using standard echocardiography and tissue Doppler imaging, have reported no diastolic abnormalities in AN patients at the time of malnourishment [40]; with this being supportive of our wave intensity findings. Furthermore, higher FCW intensity has been associated with cognitive decline in mid- to late-life [23]. Our observation of no difference in FCW between groups therefore implies recovered AN haemodynamics may not confer greater risk of cognitive decline in later life.
Lastly, measures of wall mechanics are consistent with previous findings in this population [24] in which the former AN patients had stiffer carotid vessels. However, the lack of change in characteristic impedance (\({Z}_{c}\)) indicates that the pressure-flow relationship is maintained despite increased carotid stiffness. Whilst this could be explained by an increase in the vessel size [15], we previously found no differences in the diameter of the carotid artery between former AN patients and controls [24], and therefore more detailed investigation of this finding is needed in future studies.
As our findings are specific to young adults who received specialist treatment and recovered from AN during adolescence, these may have limited generalizability to other populations. In addition, clinical data collected at the time of diagnosis indicate our AN population had not experienced the greatest degree of illness severity or chronicity. Our results may therefore reflect more favourable long-term outcomes than patients who had been severely malnourished or the period of malnutrition extended beyond two years. Furthermore, this study was not powered to detect sex differences in wave intensity and males and females may be affected differently. Further investigation in other populations who experienced malnutrition at different stages of growth or development and for varying durations, or recurrent episodic periods of malnutrition, would provide valuable insights into long-term cardiovascular risk from AN. Independent assessment of male and female cohorts may also be useful to identify sex-dependent cardiovascular risk, especially given the number of males afflicted is increasing [41].
This study draws attention to ongoing cardiovascular abnormalities in former AN patients. Regular assessment of blood pressure to test both the hearts pumping capacity and arterial stiffness, preferably alongside direct assessment of atrial stiffness via tonometry or ultrasound, could assist early identification of premature vascular aging or cardiovascular disease. This could allow intervention to optimise the duration and quality of a patients life. Ultimately, future research aimed at understanding the mechanisms underlying altered haemodynamics in AN and monitoring cardiovascular normalisation and maintenance (using both standard measures of blood pressure and heart rate as well as assessing arterial stiffness) will be pivotal to optimising treatment regimes and patient outcomes.
Conclusions
In conclusion, although ventricular function does not appear to be affected in the long-term in individuals with a history of adolescent AN, accelerated carotid vascular aging and altered cerebral vasomotor tone exist. When combined with our previous findings of altered large artery stiffness and abnormalities in endothelial function and autonomic control [24], the evidence suggests a period of significant malnutrition during adolescence due to AN has cardiovascular consequences which last into adulthood. Former AN patients may experience adaptive processes, involving reduced wave reflection, that help to maintain cerebral flow. This draws attention to the need for regular assessment of cardiovascular health in former AN patients.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AN:
-
Anorexia nervosa
- BCW:
-
Backward compression wave
- BMI:
-
Body mass index
- FCW:
-
Forward compression wave
- FDW:
-
Forward decompression wave
- FDWx:
-
Mid-systolic forward decompression wave
- PWP:
-
Peak wave power
- VICS:
-
Victorian Infant Collaborative Study
References
Spaulding-Barclay M, Stern J, Mehler PS. Cardiac changes in anorexia nervosa. Cardiol Young. 2016;26:623–8. https://doi.org/10.1017/S104795111500267X.
Sachs KV, Ben H, Philip SM, Mehler K, Krantz MJ. Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord. 2016;49:238–48. https://doi.org/10.1002/eat.22481.
Olivares JL, Vázquez M, Fleta J, Moreno LA, Pérez-González JM, Bueno M. Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Peds. 2005;164:383–6.
Escudero CA, Potts JE, Lam P-Y, De Souza AM. Doppler echocardiography assessment of aortic stiffness in female adolescents with anorexia nervosa. J Am Soc Echo. 2018;31:784–90. https://doi.org/10.1016/j.echo.2018.01.003.
Moskowitz L, Weiselberg E. Anorexia nervosa/atypical anorexia nervosa. Curr Probl Pediatr Adolesc Health Care. 2017;47:70–84. https://doi.org/10.1016/j.cppeds.2017.02.003.
Meczekalski B. Long-term consequences of anorexia nervosa. Maturitas. 2018;81:116. https://doi.org/10.1016/j.maturitas.2015.02.050.
Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psych Repor. 2012;14:406–14. https://doi.org/10.1007/s11920-012-0282-y.
Roseboom T, Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Hum Dev. 2006;82:485–91. https://doi.org/10.1016/j.earlhumdev.2006.07.001.
Rotar O, Moguchaia E, Boyarinova M, Kolesova E, Khromova N, Freylikhman O, et al. Seventy years after the siege of Leningrad: Does early life famine still affect cardiovascular risk and aging? J Hypertens. 2015;33:1772–9. https://doi.org/10.1089/bio.2015.0018.
Schnitker MA, Mattman PE, Bliss TL. A clinical study of malnutrition in Japanese prisoners of war. Ann Intern Med. 1951;35:69–96. https://doi.org/10.7326/0003-4819-35-1-69.
Magowska AM. The changing face of hunger: from fasting to the concept of atherogenesis. Adv Physiol Educ. 2020;44:734–40. https://doi.org/10.1152/advan.00048.2020.
Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NR, et al. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo-Scandinavian Cardiac Outcome Trial). J Am Coll Cardiol. 2010;56:24–30. https://doi.org/10.1016/j.jacc.2010.03.030.
Wang K-L, Cheng H-M, Sung S-H, Chuang S-Y, Li C-H, Spurgeon HA, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertens. 2010;55:799–805. https://doi.org/10.1161/HYPERTENSIONAHA.109.139964.
Parker KH, Jones CJ. Forward and backward running waves in the arteries: analysis using the method of characteristics. J Biomech Eng. 1990;112:322–6. https://doi.org/10.1115/1.2891191.
Mynard JP, Smolich JJ. Novel wave power analysis linking pressureflow waves, wave potential and the forward and backward components of hydraulic power. Am J Physiol Heart Circ. 2016;310:H1026–38. https://doi.org/10.1152/ajpheart.00954.2015.
Weber T, O’Rourke M, Lassnig E, Porodko M, Ammer M, Rammer M, et al. Pulse waveform characteristics predict cardiovascular events and mortality in patients undergoing coronary angiography. J Hypertens. 2010;28:797–805. https://doi.org/10.1097/hjh.0b013e328336c8e9.
Weber T, O’Rourke MF, Lassnig E, Porodko M, Ammer M, Rammer M, et al. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertens. 2012;60:534–41. https://doi.org/10.1161/hypertensionaha.112.194571.
Davies JE, Lacy P, Tillin T, Collier D, Cruickshank JK, Francis DP, et al. Excess pressure integral predicts cardiovascular events independent of other risk factors in the conduit artery functional evaluation substudy of Anglo-Scandinavian Cardiac Outcomes Trial. Hypertens. 2014;64:60–8. https://doi.org/10.1161/hypertensionaha.113.02838.
Zamani P, Jacobs DR Jr, Segers P, Duprez DA, Brumback L, Kronmal RA, et al. Reflection magnitude as a predictor of mortality: the Multi-Ethnic Study of Atherosclerosis. Hypertens. 2014;64:958–64. https://doi.org/10.1161/hypertensionaha.114.03855.
Zamani P, Lilly SM, Segers P, Jacobs JRDR, Bluemke DA, Duprez DA, et al. Pulsatile load components, resistive load and incident heart failure: the multi-ethnic study of atherosclerosis (MESA). J Card Fail. 2016;22:988–95. https://doi.org/10.1016/j.cardfail.2016.04.011.
Narayan O, Davies JE, Hughes AD, Dart AM, Parker KH, Reid C, et al. Central aortic reservoir-wave analysis improves prediction of cardiovascular events in elderly hypertensives. Hypertens. 2015;65:629–35. https://doi.org/10.1161/hypertensionaha.114.04824.
Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (Part 2): clinical and therapeutic considerations. J Cardiovasc Transl Res. 2017;10:261–74. https://doi.org/10.1007/s12265-017-9736-2.
Chiesa ST, Masi S, Shipley MJ, Ellins EA, Fraser AG, Hughes AD, et al. Carotid artery wave intensity in mid- to late-life predicts cognitive decline: the Whitehall II study. Eur Heart J. 2019;40:2300–9. https://doi.org/10.1093/eurheartj/ehz189.
Springall GAC, Caughey M, Zannino D, Kyprianou K, Mynard JP, Rudolph S, et al. Long-term cardiovascular consequences of adolescent anorexia nervosa. Pediatr Res. 2023. https://doi.org/10.1038/s41390-023-02521-5.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Cheong JLY, Wark JD, Cheung MM, Irving L, Burnett AC, Lee KJ, et al. Impact of extreme prematurity or extreme low birth weight on young adult health and well-being: the Victorian Infant Collaborative Study (VICS) 1991–1992 Longitudinal Cohort study protocol. BMJ Open. 2019;9: e030345. https://doi.org/10.1136/bmjopen-2019-030345.
Kowalski R, Beare R, Willemet M, Alastruey J, Smolich JJ, Cheung MMH, et al. Robust and practical non-invasive estimation of local arterial wave speed and mean blood velocity waveforms. Physiol Meas. 2017;38:2081. https://doi.org/10.1088/1361-6579/aa8de3.
Centres for Disease Control and Prevention. Healthy Weight, Nutrition, and Physical Activity. Updated June 3, 2022. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html
Dujardin J-PL, Stone DN. Characteristic impedance of the proximal aorta determined in the time and frequency domain: a comparison. Med Biol Eng Comput 1981;19:565–568. doi:https://doi.org/10.1007/bf02442770
Li JKJ. Time domain resolution of forward and reflected waves in the aorta. IEEE Trans Biomed Eng. 1986;33:783–5. https://doi.org/10.1109/TBME.1986.325903.
Mynard JP, Kondiboyina A, Kowalski R, Cheung MMH, Smolich JJ. Measurement, analysis and interpretation of pressure/flow waves in blood vessels. Front Physiol. 2020;11:1–26. https://doi.org/10.3389/fphys.2020.01085.
DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;5:863–71. https://doi.org/10.1001/archinte.1916.00080130010002.
Frampton I, Watkins B, Gordon I, Lask B. Do abnormalities in regional cerebral blood flow in anorexia nervosa resolve after weight restoration? Eur Eat Disord Review. 2011;19:55–8. https://doi.org/10.1002/erv.1047.
Bleasdale RA, Mumford CE, Campbell RI, Fraser AG, Jones CJH, Frenneaux MP. Wave intensity analysis from the common carotid artery: a new noninvasive index of cerebral vasomotor tone. Heart Vessels. 2003;18:202–6. https://doi.org/10.1007/s00380-003-0711-2.
Schroeder EC, Lefferts WK, Hilgenkamp TIM, Fernhall B. Acute systemic inflammation reduces both carotid and aortic wave reflection in healthy adults. Physiol Rep. 2019;7:e14203–e14203. https://doi.org/10.14814/phy2.14203.
Solmi M, Veronese N, Favaro A, Santonastaso P, Manzato E, Sergi G, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psych Endo. 2015;51:237–52. https://doi.org/10.1016/j.psyneuen.2014.09.031.
Lefferts W, DeBlois J, Augustine J, Keller A, Heffernan K. Age, sex, and the vascular contributors to cerebral pulsatility and pulsatile damping. J Appl Physiol. 2020;129:1092–101. https://doi.org/10.1152/japplphysiol.00500.2020.
Kondiboyina A, Smolich JJ, Cheung MMH, Mynard JP. Conduit arterial wave reflection promotes pressure transmission, but impedes hydraulic energy transmission, to the microvasculature. Am J Physiol Heart Circ. 2020;319:H66–75. https://doi.org/10.1152/ajpheart.00733.2019.
Ohte N, Narita H, Sugawara M, Niki K, Okada T, Harada A, et al. Clinical usefulness of carotid arterial wave intensity in assessing left ventricular systolic and early diastolic performance. Heart Vessels. 2003;18:107–11. https://doi.org/10.1007/s00380-003-0700-5.
Galetta F, Franzoni F, Cupisti A, Morelli E, Santoro G, Pentimone F. Early detection of cardiac dysfunction in patients with anorexia nervosa by tissue Doppler imaging. Int J Cardiol. 2005;101:33–7. https://doi.org/10.1016/j.ijcard.2004.03.006.
Mitchison D, Hay P, Slewa-Younan S, Mond J. The changing demographic profile of eating disorder behaviors in the community. BMC Public Health. 2014;14:943–9. https://doi.org/10.1186/1471-2458-14-943.
Acknowledgements
The authors would like to thank Stephanie Campbell and Claire May, Clinical Nurses at the RCH Eating Disorder Service, for compiling data within the Eating Disorder clinic database. We wish to acknowledge Jacinta Coleman, Kypros Kyprianou, and Michelle Caughey for facilitating governance approval and patient recruitment at the Monash Health site.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: GS is in receipt of the Australian Commonwealth Government Research Training Program Scholarship (RTPS). JC was supported by the NHMRC project grant (1104300), Medical Research Future Fund of Australia Career Development Fellowship (1141354). The Heart Research Group receives financial support in part from Royal Children’s Hospital Foundation, Big W and the Victorian Government’s Operational Infrastructure Support Program to the Murdoch Children’s Research Institute.
Author information
Authors and Affiliations
Contributions
GS was responsible for study conception and design. In addition, GS conducted the carotid ultrasound, collated and analysed all results, and completed the original manuscript draft. GS had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. MC and JM were involved in conception and design of the study, data interpretations and critical review of the manuscript. MY was also involved in study conception and design and review of the manuscript. GG and GS conducted wave power analysis of ultrasound images. DZ assisted with statistical analysis and reporting of all measures. JC was responsible for contribution of control cohort data. All authors edited and provided feedback on the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received ethics approval from the Royal Children’s Hospital Research Ethics and Governance Committee (HREC 2019.119), Monash Health Governance Committee (RES-20-471X), and complied with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all participants. All data collected through this study is stored and reported in deidentified format.
Consent for publication
Not applicable.
Competing interests
The author(s) declared no potential competing interests regarding the research, authorship and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Springall, G.A.C., Goldsmith, G., Zannino, D. et al. Carotid wave analysis in young adults with a history of adolescent anorexia nervosa: a case control study. J Eat Disord 12, 21 (2024). https://doi.org/10.1186/s40337-023-00963-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40337-023-00963-0