Abstract
Background
Throughout their range, red deer are a well-studied species. In Italy, this species occupies two ecologically different ranges: the Alps and the Apennines. Although several studies have described the spatial behaviour of red deer in the Italian Alps, no data are available for the Apennine population.
Results
The spatial behaviours of 13 deer from the Northern Apennines range were analysed for the first time using GPS-GSM telemetry from 2011 to 2017. Red deer displayed two coexisting strategies, i.e., migratory and stationary. In our sample, females tended to migrate more than males. We found a high level of interindividual variability in the date of migration/return, while each migratory deer was very conservative during the study period. The migration ranges were on average 12 ± 4.2 km from the resident range. Both migratory and resident deer displayed high site fidelity. No switch from the migratory to stationary strategy was observed for any deer during the study period; however, the period could have been too short to detect any switch. At the management level, over 18 management cycles occurred during the study period, and a spatial mismatch was found between deer range and management units (districts) in 38.9% (7) of the cases. Merging the districts belonging to each province to obtain an area of approximately 1000 km2 would partially address such spatial mismatch, reducing its occurrence to 22%.
Conclusions
Despite the small sample size, these results can guide future management actions. However, an in-depth study with a larger sample size is required to better understand and manage the red deer Apennines population.
Similar content being viewed by others
Background
Ungulate spatial behaviour is a complex process shaped by climate, species life-history strategies, population density, and other factors, such as predation pressure and human disturbance (hunting, recreation; [12, 57, 69]. Thus, strategies displayed by individuals related to space use, as the result of different pressures, aim to maximize individual fitness under different ecological conditions. In this regard, migration plays a central role in use of space since it is thought to be mainly an adaptation to changing resource availability and quality [2, 34, 37], a response to competition when density increases [59] but see also [55] or a way to prevent predation on offspring without any clear relation to population density [4, 8]. However, according to Mysterud et al. [56] and several hypotheses, a combination of ecological triggers can explain the same migration pattern. Therefore, migration is a complex process, with behavioural plasticity making seasonal movements very flexible, further blurring the line between discrete categories of residency and migration [17].
The red deer (Cervus elaphus) is a well-studied species that exhibits differences in its use of space across its distributional range. In the northern hemisphere, where resource availability and climate are markedly seasonal, populations are partial migrants, including both migratory and resident individuals [55, 56], with a negative density dependence on the likelihood of migration [55]. Partial migration is known to occur in several other large herbivores (for a review see [7].
In Italy, red deer are found both in the Alps and in the Apennines, where they face very different environmental conditions mainly associated with climate and food quality, availability, and accessibility throughout the year. These conditions affect the species’ feeding habits, as suggested by tooth wear, which occurs much faster in Alpines than in Apennine for red deer of the same age class [25]. When compared to Alpine deer, Apennine red deer are generally similar in weight (eviscerated weight ± sd,adult, male: 130.4 ± 15.6 kg, female: 72.6 ± 10.0 kg—[5], adult, male: 140.71 ± 22.26, female: 73.68 ± 9.55 kg—Apollonio et al. [3]; for the Apennines and Trentino Alps, respectively) or heavier (adult, male: 110.87 ± 21.36 kg; female: 68.19 ± 10.93 kg, [35], Stelvio National Park, eastern sector).
During the last 20 years, in the northern region of the Apennines, the species distribution range and density have increased. Thus, its management has become increasingly important and mainly aimed at reducing conflicts between human activities and deer presence, namely, road accidents, damage to agricultural activity and impact on forest renovation. Management objectives are pursued by setting hunting quotas based upon population size, which is assessed through direct counts. Management is therefore particularly challenging when the target is moving, as in the case of a migratory population, because both the functional population unit (sensu [21] and the corresponding management area cannot be properly defined.
We present the first data on the spatial ecology of red deer from the northern Apennines. In the Alps, red deer have been found to behave as partially migrant (i.e., only part of the population migrates; [10, 11, 14, 50], while in the Apennines, this has never been investigated. Considering the high migration propensity shown by the species populations spanning environmental gradients across Europe Peter et al. [63], we expected that red deer in the Apennines would also exhibit partial migration.
Moreover, we assessed whether the space-use strategy displayed by the red deer affects their management in the study area. Since population-level management is difficult to achieve if individuals cross the administrative limits of management units, we also discussed (i) whether the current deer management approach is consistent with the population unit principle (i.e., the management unit should encompass a population unit and both resident and migratory ranges) and effective to meet management objectives and (ii) what possible solutions exist for improving the species management in the northern Italian Apennines.
Materials and methods
Study site
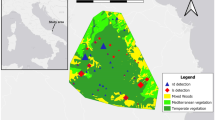
The study area is located in the Italian Northern Apennines within the provinces of Parma (PR), Modena (MO), and Reggio-Emilia (RE, Emilia Romagna region) to the north and the provinces of Lucca (LU) and Massa Carrara (MS, Tuscany region—Fig. 1) to the south.
The area is characterized by a continental climate, with dry hot summers and cold, snowy winters. Between October and March, snow cover occurs from 14.9 to 207.9 days, while snow depth varies between 1.5 and 104.3 cm (1961–1990; [24]. The abundance of snowfall is negatively correlated with altitude, and below 100 m asl., it becomes negligible.
The area is mainly hilly and mountainous, with elevations ranging from 265 (Langhirano, PR) to 2165 m (Monte Cimone, MO). Sixty percent of the study area is covered by forests dominated by beech wood (Fagus sylvatica) at higher altitudes and oak wood (Quercus sp.) at lower altitudes. Approximately 38% of the area is covered by agricultural pastures, arable land, and orchards (mainly olive and cherry groves). Urban areas are limited to the valleys where the road density is also higher; however, a few mountain villages are scattered at higher altitudes.
Five ungulate species are present in the area: in addition to red deer, roe deer (Capreolus capreolus), mouflon (Ovis aries, distributed mainly along the border with the Tuscany region, with small and isolated groups in the province of Reggio Emilia), fallow deer (Dama dama) and wild boar (Sus scrofa) are also present. Wolf (Canis lupus) and red fox (Vulpes vulpes) are the only predators of red deer in the area.
Deer population and management
The presence of red deer in the area is due to several reintroductions carried out mainly during the 1980s and the beginning of 2000, coupled with occasional escapes from enclosures [30, 51, 61]. Since early 2000, regional administrations (Tuscany and Emilia Romagna), which share the population, have monitored the presence of this species, and in 2011, the first distribution map was generated, with the population being quantified therein. In 2012, 3500–3900 deer were estimated to be present in the area (1.9–2.2 n/km2), and hunting was allowed for the first time [32]. Since 2012, the minimum population size has been quantified yearly through observation point counts in spring, carried out at dawn and dusk in agricultural areas and forest edges. To date, the red deer population has continued to increase and expand in distribution. The annual hunting quota varies between 19.9% and 34.6% of the minimum population size quantified in the spring.
In both Emilia Romagna and Tuscany, red deer management is carried out in wide management areas (ACATER) that encompass the deer population unit distribution range. Currently, there are three deer management ACATERs ranging from 3900 to 5840 km2 shared by Emilia Romagna and Tuscany [76].
Within such areas, annual population monitoring is carried out, and annual management plans are prepared and agreed upon by a management committee representing public and private stakeholders. A 5-year management plan is also prepared for each ACATER, which determines the main objectives for red deer management. Hunting is carried out in subunits within each ACATER (called “districts”) ranging from 170 to 432 km2, and the hunting plan is assigned per district. Each management plan specifies the target (red deer population estimate, culls, impacts on habitat and crops, road accidents, etc.), the methods (monitoring of both populations and impacts, hunting, crop protection, etc.) and the management objectives, which are primarily aimed at reducing deer impacts. Reducing these impacts is also achieved by setting appropriate annual hunting quotas (split into sex and age classes), which are spatially allocated among hunting units (districts) based on the results of the direct counts in each district. Thus, during the hunting season, red deer are expected to occur the same district in which they have been counted, allowing the quotas to be met.
The study site is in western ACATER (5840 km2, including the abovementioned regional administrations and four provinces, Fig. 1).
At the study site, the hunting season occurs from October until mid-March and from January to mid-March for males and females, respectively. Spring counts are carried out after the end of the hunting season (late March—late April). The period between the 16th of March of one year and the 15th of March of the following year is therefore considered the management time unit, hereafter referred to as the “management cycle”. Each management cycle falls during two calendar years, starting with the spring counts and ending with the implementation of the hunting plan, for which its quotas are based on the results of these counts.
Deer capture and tracking
Deer were captured within the Secchia River catchment area (Fig. 1) through telenarcosis and baited corrals traps. Telenarcosis was carried out at night, and animals were located using a spotlight mounted on a 4 × 4 vehicle. Darts were equipped with a radio transmitter (TeleDart® Transmitter Dart-TDS), and darted animals were located through a directional, four-element YAGI antenna and an ICOM-IC-R20 radio receiver. Sedation was induced by a veterinary doctor using a pharmacological mix of tiletamine hydrochloride, zolazepam hydrochloride and xylazine [42]. An antagonist of xylazine (atipamezole) was used to reverse sedation [54]. Two corral traps were set up in the valleys of Dolo (Costalta, Reggio Emilia) and Dragone rivers (Riccovolto, Modena, Fig. 1). Captured deer were sedated before handling using the same protocol as telenarcosis.
Captured deer were sexed, measured, and fitted with ear tags and GPS-GSM collars (Vectronic Aerospace—GPS PRO Light-2 model TARIC 8526 91 20) if older than one year of age. This device model has been used in different studies [39], as it allows data to be collected remotely using dedicated hardware (Vectronic Aerospace GSM-2 Ground Station) and provides the accuracy of the locations through the dilution of precision (DOP), a measure of the quality of satellite geometry, which is assigned to each location [47].
The collars were programmed to collect 4 locations per day (every 6 h) for the first 13 months of tracking and 2 locations (every 12 h) afterwards.
Only data from deer followed for at least one year were used for the analysis, and only locations obtained from the triangulation of at least four satellites (validation GPS-3D) were retained for the analysis [6, 39]. To assess pregnancy and the presence of fawns, tagged females were periodically located and observed as long as necessary to assess their status. Tagged deer were excluded from culling based on local regulations.
Data analysis
Home range estimation and site fidelity
Home ranges were calculated using continuous-time stochastic movement models and autocorrelated kernel density estimation (AKDE, [28, 29]. This estimator allows the calculation of rigorous HR estimations accounting for autocorrelation and irregular sampled fixes while providing an optimal weighting of the data, giving appropriate weight to time-isolated relocations corresponding to gaps in the data. It also accounts for the accuracy of each relocation using the corresponding DOP. AKDE however only works for stationary data,i.e., it is not suitable to describe dispersing, migrating, or shifting home ranges. Therefore, before computing 95% home ranges, we first detected stationary subsets of positions using variograms (see below).
We classified animals as “migrants” if they displayed regular movements between two or more discrete areas that, even at a short distance < 10–50 km, did not overlap [7], while “residents” were animals that displayed a continuous, overlapping use of the same range [1, 38, 52].
We calculated variograms and movement models, and estimated home range sizes using the ctmm package in R [18]. Deer residency was evaluated using variograms. For resident individuals, the variogram reached an asymptote at a timescale roughly corresponding to the home range crossing time. When semivariance did not reach an asymptote, as in the case of dispersers, individuals were not assumed to be residents [58] and therefore excluded from the analysis. Both variogram analysis [27] and model selection were used to confirm that there was evidence of range residency in the data and that it was therefore reasonable to perform the home-range estimation.
When the variogram showed spikes or regular patterns of variation, some level of migration or spatial instability was exhibited. In such cases, we used R software [68] to perform a cluster analysis (Package segclust2d, [62] on latitude and longitude to identify the locations that clustered in space and time. This analysis allows for segmentation of multivariate time series, obtaining clusters that correspond to different stationary phases. When all the locations were assigned to one cluster, the individual was considered resident; otherwise, it was considered migratory (i.e., locations were assigned to more than one cluster).
We also classified deer as “migrant” if their GPS relocations clustered in disjointed areas, as detected by visual inspection. Such areas were clearly identifiable on the map and used recursively by the same deer in different years for some time and represented stationary phases. Relocations falling in these clusters were used to calculate the home range, excluding positions pertaining to the migration route.
The parameters of the variogram and its shape produce different movement models whose SVFs (semivariance function, [27] fit is evaluated via maximum likelihood. Models were compared, and the best fitting model was selected using the Akaike information criterion (AIC, see [18] for a thorough description of the method). Once the appropriate model was selected, AKDE was used to calculate the home range [18].
For individuals who expressed migratory behaviour, the total home range was calculated as the sum of the disjoined home ranges, i.e., each temporary residency sub-area, without including the positions along the migration routes. Such areas were referred to as the “primary resident” and “migration” ranges hereafter, where the first was the area where an individual spent most of his time (> 50%) and the latter was the area where it moved for a shorter period. In migrants, the distance between the migration and primary seasonal ranges was calculated as the Euclidean distance between the centroids of the two ranges.
To quantify the site fidelity displayed by migrant deer in different years, AKDE home range pairwise overlap was calculated with the Bhattacharyya coefficient (BC, also called the Bhattacharyya Affinity, [9, 75], which has several advantages [75]. This coefficient varies between 0 (no overlap) and 1 (full overlap) and was calculated with the ctmm package, which also provides the CI. Given that the migration ranges were identified spatially and not temporally, one year corresponded to the migration event, i.e., the period spent in the migration (and the primary seasonal) range each time the primary seasonal range was left to move into the migration range and vice versa.
The range altitude for migrating deer was calculated as the average altitude value of the locations falling into each range (migration and primary seasonal). The altitude values were obtained from the regional topographic online database [70], whose metadata are available for consultation.
Deer movement in relation to management units
To verify whether current deer management units (both ACATER and districts) are spatially appropriate and effective, we assessed whether deer were present in the same district (the smaller management unit) during both the spring counts and the following hunting season.
For each management cycle, if the district where the individual deer were counted during annual monitoring proved to be the same as the district that accounted for at least 95% of its locations during the following hunting season, then the match between count and hunting districts was considered positive, and it was negative otherwise (mismatch between counting and hunting districts). Thus, if a deer during the hunting season visited the same district where it was counted during annual monitoring, then we considered the match between count and hunting district to be positive. For each management cycle, only deer positions collected during the concerned periods (counts and hunting, see “Deer population and management” section) were used. We also quantified the percentage overlap between deer home ranges and the districts and calculated the number of districts used by each deer. The analysis was carried out using the free source software QGIS [66].
Results
Deer GPS data, home ranges and space-use strategy
From August 2011 to January 2016, 23 deer were captured, 78% through telenarcosis and 22% with baited corral traps. Only 22 individuals were old enough to be fitted with radio collars. However, some collars (N = 4) stopped working after a very short period, and other animals died shortly after capture (N = 5). The cause of death was assessed only for two deer: one died because of preexisting pathologies, while the other, a male, showed signs of fight injuries.
Thirteen individuals, tracked for 12–55 months with an average of one location every 8.14 h, were considered for the analysis. The average dilution of precision (DOP see “Materials and Methods”) for the retained locations (N = 21,244) was 3.43 m (95th percentile 7.2 m); 87% of the locations used for the analysis had a DOP value < 5 m.
Six deer were identified as residents (46.2%, 4 males and 2 females), five as migrants (38.4%, 4 females and 1 male), and two as dispersers (15.4%, 1 male and 1 female, Table 1).
Migration started in spring (April, N = 2), summer (August, N = 2) and autumn (October, N = 1). Spring migrants were back in the primary seasonal range (i.e., where the deer spent most of their time > 50%, see “Methods”) in summer (August), while later migrants spent most of their time there between October and December.
Despite the high interindividual variability, the dates of migration did not vary as much for each deer in different years (Table 1). Migrant deer tracked for longer than one year showed little variation in departure dates (23.8 ± 8.9 and 3.5 ± 2.5 days, maximum and minimum average difference ± sd) among years. The return dates, on the other hand, were more variable (the maximum and minimum average differences were 58 ± 73.8 and 11.8 ± 14.1 days, respectively). Migratory deer spent on average (± sd) 263 days (± 26) in the primary seasonal range and 92 days (± 24) in the migration range.
Overall, migratory deer used larger home ranges than resident deer (35.06 km2 ± 15.55 and 18.73 km2 ± 11.33, respectively), although the difference was not significant (Welch two-sample t test, t = 2.08, df = 9.14, p value = 0.06). Migration and primary seasonal ranges were on average (± sd) 12.04 ± 4.2 km apart (Table 1).
Migrant deer tracked for more than one year (at least two years of data: N = 4 for the primary seasonal range, and N = 5 for the migration range) were very traditional in their range choice, using generally the same primary seasonal and migration ranges every year (BC = 0.89 CI 0.83–0.94 and BC = 0.78, CI 0.64–0.94, respectively).
Almost all the tracked deer used one discrete primary seasonal and migratory range, except one female, which used two disjoined subareas in both the primary and migratory ranges (Fig. 2).
The mean altitude (± sd) of the migratory ranges was quite variable (788.6 m asl ± 547.9; 338.6–1776.4 m asl, minimum and maximum, respectively) and not significantly different from that of the primary seasonal ranges (760.3 ± 296.1 m asl; 341.4–1169.6 m asl, minimum and maximum, respectively; Wilcoxon test, W = 14, p value = 0.5887).
Mismatch between deer range and management units
Migrant and resident deer used on average (± sd) 2.8 ± 0.98 and 2 ± 0.89 districts, respectively. The home ranges of both migrant and resident deer showed substantial overlap with only one district (mean overlap home range vs. district 73.18% vs. 91.91%, respectively), limiting the use of the other districts (min–max overlap: 0–30% for migrant; 0–18% for resident).
Analysing data collected over 6 years for 13 deer (some of them followed for more than one year), corresponding to a total of 18 management cycles, in 38.9% of the cases (7 over 18), a mismatch occurred between the districts (which are the operative management units) and the deer distribution (negative match; Fig. 3, Table 2). If provincial borders were considered instead, then the mismatch was reduced to 22% (4 over 18), while ACATER (Areale del Cervo dell’Appennino Tosco-Emiliano-Romagnolo) borders encompassed all but one (11,289) monitored deer.
Example of mismatch for deer 11,759 (diamond) and 11,760 (circle). During the hunting season, both deer were not present in the district they were counted. Open symbols: GPS positions during the annual counts; filled symbols: GPS positions during the hunting season. REDC2, MODC1, and REDC3: districts’names within Modena (MO) and Reggio Emilia (RE) provinces
Discussion
According to our data, red deer in the Apennines are partially migrant; migration dates vary among individuals but remain constant at the individual level across years. Females tend to migrate more than males and show fidelity to both the strategy adopted and the ranges used.
The partial migration strategy is described for ungulates [7] and documented in several European red deer populations [45] for a review), including northern countries (Norway, [53],Sweden, [43]. Partial migration has also been found to be a strategy for red deer in the Italian Alps (Tarvisio and Val Susa, [50],Stelvio National Park, [14], Paneveggio Natural Park, [11]. Both Alpine and Apennine populations also display range fidelity and no switching between strategies (but see [11].
The migration distances found in this study (12 ± 4.2 km) are comparable in magnitude to migration distances displayed by several alpine populations (Italian Alps: 8–10 km, [50],5.20 ± 3.25 km, [14],7.7 km, -only females, range 0.9–31 km, [11].
Although our results were limited by the small sample size, migration seemed to be displayed more by females than by males, as also found in the Italian Alps [50], in contrast to the results of Bocci et al. [11], who found 87% of stags and 49% of hinds were migrants (shifters, Italian north-eastern Alps). In fact, Peters et al. [63] found that the probability of migration was higher in males than in females. Under the forage maturation hypothesis, red deer, as mixed feeders, are expected to migrate, and under the body size constraint hypothesis, the propensity to migrate should be biased towards males [63]. However, the only migratory male in our study moved in late summer from west to east, keeping an average constant altitude in both the migratory (551 m a. s. l.) and the resident (582 m a. s. l.) ranges, thus possibly not driven by forage maturation. It is possible that the movements during late summer displayed by two individuals (the only migratory male and one female) were linked to the search for mating opportunities. Notably, these individuals reached two well-established mating territories in two different areas where the male remained until the end of the mating season while the female remained until mid-October. Female red deer have been reported to make substantial movements, including changing harems when in oestrus [73]. Female movements among mating areas have been observed in the red deer Cervus elaphus hispanicus in Doñana (Spain, [20], where rutting males can defend mating territories [19], a mating strategy—also recorded in our study site—that coexists with the defence of mobile harems described for red deer in northern Europe [22]. Movements to mate have also been reported for female roe deer Capreolus capreolus [49, 71], which are known to perform rut excursions (lasting from hours to days) that provide the opportunity for active mate choice. In the present study, movement to mating areas could have been carried out within a migration framework.
Two females left at the onset of spring, in April, to give birth away from the primary seasonal range (20 and 13 km, Table 1) and came back at the end of August, when the calves were able to follow the mother [22]. No altitudinal variation characterized these movements, and the timing of forage maturation was likely the same in both areas, which are characterized by very similar habitats. Therefore, it seems that the hypothesis of access to better quality resources for parturition and lactation was not supported [37]. On the other hand, altitudinal variations characterize the movements of female red deer in Stelvio National Park, where females move towards summer ranges between half April and half May (median May 2nd, min-max April 5th–June 7th, [14]. These movements are likely due to the search for plants at a relatively early stage of growth [14], which have greater palatability and nutritive value [40]. Migration towards wintering ranges was more variable over time (median November 10th, min-max August 22nd–December 26th), probably due to weather conditions, notably snowfall [14].
A later migration date was exhibited by a female, who started the migration in a period when rutting is generally over in the Apennines. The only possible explanation for this movement was the access to abundant, predictable, and heavily localized food resources (chestnut).
Hunting represents an important tool to regulate ungulate populations [16, 36, 46, 64, 65], and seasonal migration complicates harvest management. At the same time, hunting can affect migratory and sedentary deer differently, i.e., when the movement involves a migration from a protected area to hunting grounds, resulting in overexploitation of migrants [13, 43]. The efficacy of red deer management depends upon the capacity of managing a deer functional population unit to affect population dynamics [46] in accordance with management goals. A management unit’s size is therefore important and should encompass an entire population unit during both monitoring and hunting. This study shows that this principle is not fully accomplished by the district design in our study area. The evidence that deer use more than one district supports the need to increase the area of this operative hunting unit. Bocci et al. [11] proposed a circular area with a radius equal to the maximum distance covered by a deer during migration: an area of 1300 km2 would include 75% of the monitored deer. In our study, merging the districts belonging to each province to obtain an area of approximately 1000 km2 would reduce the mismatch highlighted in this study from 38.9% to 22%.
Conclusions
Although the small sample size did not allow for more in-depth analyses, the variability among dates of migration found in the present study suggests that migration in the Apennines might be triggered by several drivers and that any of the current hypotheses alone, including the forage maturation hypothesis, is unlikely to fully support all the observed migration movements. A cultural, learned component could play a role in the observed timing of migration and in the choice of the migratory area itself. In fact, migration in ungulates has been recognized as a learned behaviour, the result of knowledge acquired from experience and culturally transmitted information [44]. Evidence of migration as a learnt behaviour transmitted from mother to young has been reported in moose and white-tailed deer [60, 74], underlining the importance of the early social environment, rather than the physical environment, to determine behaviour in adulthood.
There was no switch from migration to sedentary behaviour across the study period. However, time scale is crucial to detecting any switching, and our temporal window was not large enough to observe any variations in factors sufficient to encourage switching. It has been demonstrated that migratory behaviour is characterized by flexibility in response to factors such as density [55], predation risk, and a combination of both to maximize lifetime reproductive success [26]. Switching behaviour has been reported in several species of large herbivores (but see [72], such as elk [26], roe deer [63], and red deer, where it is more pronounced in males than in females (23% and 1%, respectively, [63],in addition, red deer can also switch behaviour in response to winter severity [12].
Both migrants and resident red deer displayed site fidelity across the study period. Site fidelity is a behavioural adaptation whose fitness benefits have been explained by the advantage of already acquired knowledge of resource and security cover distributions, which can increase survival and reproductive success [15, 33].
This study highlights the need for management at a larger scale, comparable with the home range used by the species. An increase in district size, however, should not compromise the effectiveness of coordination among the administrations involved in red deer management. Solutions envisaged by other authors, such as coordination between management units [53], have already been applied within the ACATER, but as shown in the present study, they have not resolved the issue of mismatch. The hunting season in Italy is differentiated between males and females. The female hunting season is among the shortest in Europe, while the male one is comparable with that in most European countries [65]. Extending the hunting season as suggested by other authors [48] would not resolve the problems and would generate other issues. For example, extending the hunting season in spring would imply hunting during late pregnancy, when the young are still dependent on the mother and/or in the presence of antlerless deer [65, 67]. We found that by merging the districts within provincial borders, the mismatch was reduced.
Factors that could influence the spatial behaviour of deer (e.g., ecological and environmental barriers; [41] should also be identified to improve red deer management in the Apennines. The spatial distribution of roads in our study area (in particular, if they are at the bottom of a valley and flanked by a river) could also play a role in shaping the home ranges and their size. Further studies are thus required to clarify these important aspects of the red deer ecology in the Apennines, which could help in identifying more functional management units.
Our findings are limited by the small sample size, which was primarily due to deer capture difficulties. A pilot study carried out in the provinces of Modena [31] has already shown extremely low deer capture success with corral traps: in four winter seasons, one corral trap, active for 182 nights, captured only three individuals (0.02 deer per night). At the study site, hunting has been permitted since 2012. Since then, deer have changed their behaviour by becoming increasingly wary (as also documented in [23]. This decreased the effectiveness of telenarcosis as a capture method: nine individuals were captured in the two years before 2012, while only eight were captured in the four years after 2012.
Availability of data and materials
The datasets analysed during the current study are available in the MoveBank repository: https://www.movebank.org/cms/movebank-mainrepository.
References
Abrahms B, Seidel DP, Dougherty E, Hazen EL, Bograd SJ, Wilson AM, et al. Suite of simple metrics reveals common movement syndromes across vertebrate taxa. Mov Ecol. 2017;5(1):1–11.
Albon SD, Langvatn R. Plant phenology and the benefits of migration in a temperate ungulate. Oikos. 1992;65:502–13.
Apollonio M, Chirichella R, De Marinis AM, Bazzanella G, Brugnoli A, Ferraro E. et al. Camoscio, cervo e capriolo in Trentino. Rapporto su status e gestione. Quaderni dell’Associazione Cacciatori Trentini. 1. 2019
Barten NL, Bowyer RT, Jenkins KJ. Habitat use by female caribou: tradeoffs associated with parturition. J Wildl Manage. 2001;65:77–92.
Becciolini V, Bozzi R, Viliani M, Biffani S, Ponzetta MP. Body measurements from selective hunting: biometric features of red deer (Cervus elaphus) from Northern Apennine Italy. It J Anim Sci. 2016;15(3):461–72.
Becciolini V, Lanini F, Ponzetta M. Impact of capture and chemical immobilization on the spatial behaviour of red deer Cervus elaphus hinds. Wildlife Biol. 2019;1:1–8.
Berg JE, Hebblewhite M, St Clair CC, Merrill EH. Prevalence and mechanisms of partial migration in ungulates. Front Ecol Evol. 2019;7:325.
Bergerud AT, Ferguson R, Butler HE. Spring migration and dispersion of woodland caribou at calving. Anim Behav. 1990;39(2):360–8.
Bhattacharyya A. On a measure of divergence between two statistical populations defined by their probability distribution. Bull Cal Math Soc. 1943;35:99–109.
Bocci A, Monaco A, Brambilla P, Angelini I, Lovari S. Alternative strategies of space use of female red deer in a mountainous habitat. Ann Zool Fennici. 2010;47(1):57–66.
Bocci A, Angelini I, Brambilla P, Monaco A, Lovari S. Shifter and resident red deer: intrapopulation and intersexual behavioural diversities in a predator-free area. Wildl Res. 2012;39(7):573–82.
Bojarska K, Kurek K, Śnieżko S, Wierzbowska I, Król W, Zyśk-Gorczyńska E, Baś G, Widera E, Okarma H. Winter severity and anthropogenic factors affect spatial behaviour of red deer in the Carpathians. Mammal Res. 2020;65(4):815–23.
Bolger DT, Newmark WD, Morrison TA, Doak DF. The need for integrative approaches to understand and conserve migratory ungulates. Ecol Lett. 2008;11:63–77.
Bonardi A. Previsional models for management and conservation of Alpine fauna the red deer (Cervus elaphus) case in the Stelvio National Park. PhD thesis, XXII Course, Insubria University. 2009.
Bose S, Forrester TD, Brazeal JL, Sacks BN, Casady DS, Wittmer HU. Implications of fidelity and philopatry for the population structure of female black-tailed deer. Behav Ecol. 2017;28(4):983–90.
Brown TL, Decker DJ, Riley SJ, Enck JW, Lauber TB, Curtis PD, et al. The future of hunting as a mechanism to control white-tailed deer populations. Wildlife Soc Bull. 2000;28:797–807.
Cagnacci F, Focardi S, Heurich M, Stache A, Hewison AJM, Morellet N, et al. Partial migration in roe deer: migratory and resident tactics are end points of a behavioural gradient determined by ecological factors. Oikos. 2011;120:1790–802.
Calabrese JM, Fleming CH. Ctmm: an R package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol Evol. 2016;7(9):1124–32.
Carranza J, Alvarez F, Redondo T. Territoriality as a mating strategy in red deer. Anim Behav. 1990;40(1):79–88.
Carranza J, Valencia J. Red deer females collect on male clumps at mating areas. Behav Ecol. 1999;10(5):525–32.
Caughley G. Analysis of vertebrate populations. London, New York: Wiley-Interscience publication; 1977.
Clutton-Brock TH, Guinness FE, Albon SD. Red deer: behavior and ecology of two sexes. Chicago: University of Chicago press; 1982.
Cromsigt JP, Kuijper DP, Adam M, Beschta RL, Churski M, Eycott A, et al. Hunting for fear: innovating management of human–wildlife conflicts. J Appl Ecol. 2013;50:544–9.
De Bellis A, Pavan V, Levizzani V. Climatologia e variabilità interannuale della neve sull’Appennino Emiliano-Romagnolo. Quaderno Tecnico ARPA-SIM 19. 2010. https://cupdf.com/document/climatologia-e-variabilita-interannuale-della-neve-sullclimatologia-della.html?page=1.
De Marinis AM. Valutazione dell’età nei Cervidi tramite esame della dentatura. Guida pratica all’identificazione delle classi di età del Cervo. Manuali e Linee Guida ISPRA n. 90.2/2013. 2013. https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/valutazione-delleta-nei-cervidi-tramite-esame-della-dentatura.
Eggeman SL, Hebblewhite M, Bohm H, Whittington J, Merrill EH. Behavioural flexibility in migratory behaviour in a long-lived large herbivore. J Anim Ecol. 2016;85(3):785–97.
Fleming CH, Calabrese JM, Mueller T, Olson KA, Leimgruber P, Fagan WF. From fine-scale foraging to home ranges: a semivariance approach to identifying movement modes across spatiotemporal scales. Am Nat. 2014;183(5):154–67.
Fleming CH, Fagan WF, Mueller T, Olson KA, Leimgruber P, Calabrese JM. Rigorous home range estimation with movement data: a new autocorrelated kernel density estimator. Ecology. 2015;96(5):1182–8.
Fleming CH, Calabrese JM. A new kernel density estimator for accurate home-range and species-range area estimation. Methods Ecol Evol. 2017;8(5):571–9.
Fontana R, Lanzi A, Gianaroli M. Distribuzione e stima della consistenza del cervo (Cervus elaphus) nella provincia di Reggio Emilia. Atti Soc Nat Mat Modena. 2000;131:167–77.
Fontana R, Gianaroli M, Amorosi F, Lanzi A. Effects of boar hunting on space utilization of three Red deer (Cervus elaphus) in the Northern Apennines. 1st International Conference on Genus Cervus, Italy. 2007
Fontana R, Lanzi A, Musarò C, Reggioni W, Riga F, Viliani M. Relazione consuntiva 2011–2012 e programma annuale operativo di gestione del cervo 2012–2013 del comprensorio Acater occidentale. Regione Emilia Romagna. 2012.
Forrester TD, Casady DS, Wittmer HU. Home sweet home: fitness consequences of site familiarity in female black- tailed deer. Behav Ecol Sociobiol. 2015;69:603–12.
Fryxell JM, Sinclair AR. Causes and consequences of migration by large herbivores. Trends Ecol Evol. 1988;3(9):237–41.
Gugiatti A, Pedrotti L, Nicoloso S, Bonardi A. Analisi della densità, dinamica e costituzione delle popolazioni di cervo (Cervus elaphus, L.). Quadro dello status delle popolazioni di cervo del settore lombardo del Parco Nazionale dello Stelvio. Technical report for Comitato di gestione per la regione Lombardia del Consorzio del Parco Nazionale dello Stelvio. 2006.
Hagen R, Haydn A, Suchant R. Estimating red deer (Cervus elaphus) population size in the Southern Black Forest: the role of hunting in population control. Eur J Wildl Res. 2018;64(4):1–8.
Hebblewhite M, Merrill E, Mcdermid GA. Multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecol Monogr. 2008;78:141–66.
Hjeljord O. Dispersal and migration in northern forest deer - Are there unifying concepts? Alces. 2001;37:353–70.
Hofman MPG, Hayward MW, Hei M, Marchand P, Rolandsen CM, Mattisson J, et al. Right on track? Performance of satellite telemetry in terrestrial wildlife research. PLoSONE. 2019;14(5): e0216223.
Hudson RJ, White RG. Bioenergetics of wild herbivores. Boca Raton: CRC Press; 1985.
Iuell, B. Wildlife and Traffic-a European handbook for identifying conflicts and designing solutions. The XXIInd PIARC World Road CongressWorld Road Association (PIARC). 2003.
Janovsky M, Tataruch F, Ambuehl M, Giacometti M. A Zoletil-Rompun mixture as an alternative to the use of opioids for immobilization of feral red deer. J Wildl Diseases. 2000;36:663–9.
Jarnemo A. Seasonal migration of male red deer (Cervus elaphus) in southern Sweden and consequences for management. Eur J Wildl Res. 2008;54(2):327–33.
Jesmer BR, Merkle JA, Goheen JR, Aikens EO, Beck JL, Courtemanch AB, et al. Is ungulate migration culturally transmitted? Evidence of social learning from translocated animals. Science. 2018;361:1023–5.
Kropil R, Smolko P, Garaj P. Home range and migration patterns of male red deer Cervus elaphus in Western Carpathians. Eur J Wildl Res. 2015;61(1):63–72.
Langvatn R, Loison A. Consequences of harvesting on age structure, sex ratio and population dynamics of red deer Cervus elaphus in central Norway. Wildl Biol. 1999;5(4):213–23.
Li X, Zhang X, Ren X, Fritsche M, Wickert J, Schuh H. Precise positioning with current multi-constellation global navigation satellite systems: GPS, GLONASS. Galileo and BeiDou Sci Rep. 2015;5:8328.
Loe LE, Rivrud IM, Meisingset EL, Bøe S, Hamnes M, Veiberg V, et al. Timing of the hunting season as a tool to redistribute harvest of migratory deer across the landscape. Eur J Wildl Res. 2016;62(3):315–23.
Lovari S, Bartolommei P, Meschi F, Pezzo F. Going out to mate: excursion behaviour of female roe deer. Ethology. 2008;114(9):886–96.
Luccarini S, Mauri L, Ciuti S, Lamberti P, Apollonio M. Red deer (Cervus elaphus) spatial use in the Italian Alps: home range patterns, seasonal migrations, and effects of snow and winter feeding. Ethol Ecol Evol. 2006;18(2):127–45.
Mattioli S, Meneguz PG, Brugnoli A, Nicoloso S. Red deer in Italy: recent changes in range and numbers. Hystrix, It J Mamm. 2001;12(1).
McPeek MA, Holt RD. The evolution of dispersal in spatially and temporally varying environments. Am Nat. 1992;140:1010–27.
Meisingset EL, Loe LE, Brekkum Ø, Bischof R, Rivrud IM, Lande US, et al. Spatial mismatch between management units and movement ecology of a partially migratory ungulate. J Appl Ecol. 2018;55(2):745–53.
Miller B, Muller L, Doherty T, Osborn D, Miller K, Warren R. Effectiveness of antagonists for tiletamine–zolazepam/xylazine immobilization in female white-tailed deer. J Wildl Dis. 2004;40:533–7.
Mysterud A, Loe LE, Zimmermann B, Bischof R, Veiberg V, Meisingset EL. Partial migration in expanding red deer populations at northern latitudes - a role for density dependence? Oikos. 2011;120:1817–25.
Mysterud A, Vike BK, Meisingset EL, Rivrud IM. The role of landscape characteristics for forage maturation and nutritional benefits of migration in red deer. Ecol Evol. 2017;7(12):4448–55.
Mols B, Lambers E, Cromsigt JP, Kuijper DP, Smit C. Recreation and hunting differentially affect deer behaviour and sapling performance. Oikos. 2022. https://doi.org/10.1111/oik.08448.
Morato RG, Stabach JA, Fleming CH, Calabrese JM, De Paula RC, Ferraz KM, et al. Space use and movement of a neotropical top predator: the endangered jaguar. PLoSONE. 2016;11(12): e0168176.
Nelson ME. Winter range arrival and departure of white- tailed deer in northeastern Minnesota. Can J Zool. 1995;73:1069–76.
Nelson ME. Development of migratory behavior in northern white-tailed deer. Can J Zool. 1998;76:426–32.
Nicolini R, Fontana R, Rigotto F, Sola G, Malagoli F, Bracco G. Piano Faunistico-Venatorio Provinciale 2008–2012 della Provincia di Modena. Provincia di Modena, Area Ambiente e Sviluppo Sostenibile. 2008.
Patin R, Etienne MP, Lebarbier E, Chamaillé-Jammes S, Benhamou S. Identifying stationary phases in multivariate time series for highlighting behavioural modes and home range settlements. J Anim Ecol. 2020;89(1):44–56.
Peters W, Hebblewhite M, Mysterud A, Eacker D, Hewison AM, Linnell JD, et al. Large herbivore migration plasticity along environmental gradients in Europe: life-history traits modulate forage effects. Oikos. 2019;128(3):416–29.
Putman R, Apollonio M, Andersen R. Ungulate management in Europe: problems and practices. Cambridge University Press; 2011.
Putman R, Watson P, Langbein J. Assessing deer densities and impacts at the appropriate level for management: a review of methodologies for use beyond the site scale. Mamm Rev. 2011;41(3):197–219.
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. http://qgis.osgeo.org. Accessed Apr 2022.
Raganella Pelliccioni E, Riga F, Toso S. Linee Guida per la gestione degli Ungulati – Cervidi e Bovidi. ISPRA Manuali e Linee guida n.91/2013. 2013. https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/linee-guida-per-la-gestione-degli-ungulati.-cervidi-e-bovidi.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. https://www.R-project.org/. Accessed 25 July 2022.
Reinecke H, Leinen L, Thihen I, Meihner M, Herzog S, Schütz S, Kiffner C. Home range size estimates of red deer in Germany: Environmental, individual, and methodological correlates. Eur J Wildl Res. 2014;60:237–47.
Regione Emila Romagna. Geoportale and metadata. Last accessed. (https://geoportale.regione.emilia-romagna.it/download/download-data?type=dbtopo) https://servizigis.regione.emilia-romagna.it/ctwmetadatiRER/metadatoISO.ejb?stato_FileIdentifier=iOrg01iEnP1fileIDr_emiro:2017-11-24T133826). 2021.
Richard E, Morellet N, Cargnelutti B, Angibault JM, Vanpé C, Hewison AM. Ranging behaviour and excursions of female roe deer during the rut. Behav Processes. 2008;79(1):28–35.
Sawyer H, Merkle JA, Middleton AD, Dwinnell SP, Monteith KL. Migratory plasticity is not ubiquitous among large herbivores. J Anim Ecol. 2018;88(3):450–60.
Stopher KV, Nussey DH, Clutton-Brock TH, Guinness F, Morris A, Pemberton JM. The red deer rut revisited: female excursions but no evidence females move to mate with preferred males. Behav Ecol. 2011;22(4):808–18.
Sweanor PY, Sandegren F. Migratory behavior of related moose. Holarctic Ecol. 1988;11:190–3.
Winner K, Noonan MJ, Fleming CH, Olson KA, Mueller T, Sheldon D, Calabrese JM. Statistical inference for home range overlap. Methods Ecol Evol. 2018;9(7):1679–91.
Zanni ML, Fontana R, Armaroli E, Cianfanelli L, Filetto P, Giardarello M, et al. Piano Faunistico-Venatorio Regionale 2018–2023. Boll. Uff. Regione Emilia-Romagna. 361 (II). https://agricoltura.regione.emilia-romagna.it/caccia/temi/pianificazione. 2018.
Acknowledgements
We wish to thank the Provinces of Modena and Reggio Emilia for allowing the project and supporting it for as long as necessary. We are particularly grateful to the Polizia Provinciale of Reggio Emilia, the volunteers who collaborated on the capture nights, and Luca Pedrotti and Sandro Gugiatti (Stelvio National Park) for their suggestions. Finally, a special consideration goes to the memory of Oscar Rocchi.
Funding
This work was supported by the private sponsors listed below: ATC RE4 Montagna (RE), ATC RE3 Collina (RE), AFV “La Mandria” (MO), Graniti Fiandre S.P.A (MO), AFV “Canossa” (RE), Schiatti Class Concessionaria (RE), Luca Gomme (RE).
Author information
Authors and Affiliations
Contributions
RF and AL conceived and designed the study; EA provided veterinary support in handling and sedation procedures; ERP supervised the scientific efforts; RF, AL and EA collected the data; LC, ERP and RF analysed the data; ERP, LC and RF wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All data reported in this study were collected in accordance with a protocol approved first by Istituto Nazionale per la Fauna Selvatica (2010/12) and then by the Istituto Superiore per la Protezione e la Ricerca Ambientale (2013/09).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fontana, R., Calabrese, L., Lanzi, A. et al. Spatial behavior of red deer (Cervus elaphus) in Northern Apennines: are we managing them correctly?. Anim Biotelemetry 10, 30 (2022). https://doi.org/10.1186/s40317-022-00300-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-022-00300-3