Abstract
Background
Despite being frequently landed in fish markets along the Saudi Arabian Red Sea coast, information regarding fundamental biology of the Scalloped hammerhead shark (Sphyrna lewini) in this region is scarce. Satellite telemetry studies can generate important data on life history, describe critical habitats, and ultimately redefine management strategies for sharks. To better understand the horizontal and vertical habitat use of S. lewini in the Red Sea and to aid with potential future development of zoning and management plans for key habitats, we deployed a pop-up satellite archival transmitting tag to track a single female specimen (240 cm total length) for a tracking period of 182 days.
Results
The tag was physically recovered after a deployment period of 6 months, thus providing the complete archived dataset of more than one million depth and temperature records. Based on a reconstructed, most probable track, the shark travelled a circular distance of approximately 1000 km from the central Saudi Arabian Red Sea southeastward into Sudanese waters, returning to the tagging location toward the end of the tracking period. Mesopelagic excursions to depths between 650 and 971 m occurred on 174 of the 182 days of the tracking period. Intervals between such excursions were characterized by constant oscillatory diving in the upper 100 m of the water column.
Conclusions
This study provides evidence that mesopelagic habitats might be more commonly used by S. lewini than previously suggested. We identified deep diving behavior throughout the 24-h cycle over the entire 6-month tracking period. In addition to expected nightly vertical habitat use, the shark exhibited frequent mesopelagic excursions during daytime. Deep diving throughout the diel cycle has not been reported before and, while dive functionality remains unconfirmed, our study suggests that mesopelagic excursions may represent foraging events within and below deep scattering layers. Additional research aimed at resolving potential ecological, physiological and behavioral mechanisms underpinning vertical movement patterns of S. lewini will help to determine if the single individual reported here is representative of S. lewini populations in the Red Sea.
Similar content being viewed by others
Background
A better understanding of habitat use, the temporal and spatial scales of movements, and the utilization of key sites by animals are of vital importance to biologists and conservationists in virtually all ecological systems [1]. Yet, in marine ecosystems, such information can be difficult to acquire. Horizontal and vertical movements of animals across a variety of environments can be defined via satellite telemetry (see [2] for a review). Studies employing this tool have generated important data on life history, described critical habitats, and ultimately redefined management strategies for a large range of species, including sharks, in many ocean systems around the world (see [3] for a review). In the Red Sea, satellite telemetry studies have so far focused on Whale sharks (Rhincodon typus) [4] and Manta rays (Manta alfredi) [5, 6]. Other accounts of movements in elasmobranch species in this ocean basin are limited to an acoustic tracking study on Silky sharks (Carcharhinus falciformis) [7].
The scalloped hammerhead shark (Sphyrna lewini) is a slow-growing requiem shark with a circumglobal distribution, occurring in warm temperate and tropical seas. Juveniles of this species inhabit coastal waters, while adults are found in groups or as solitary individuals further offshore [8, 9]. For the Indian Ocean, the species’ maximum observed total length (TL) was reported as 316.8 cm and males and females in this region have been observed to reach maturity between 180–189.9 and 220–240 cm TL, respectively [10]. Adult S. lewini are pelagic apex predators (trophic level = 4.1 [11]) and feed opportunistically on a diet consisting of a wide variety of teleosts, cephalopods, crustaceans, and rays [12, 13]. Although S. lewini exhibit high fecundity compared to other shark species (14–41 pups [10]), resilience to exploitation is low due to the specific evolutionary and ecological traits and behaviors developed by sphyrnids [14], including late age at maturity (10–30 years [15]). Based on International Union for Conservation of Nature (IUCN) Red List criteria, S. lewini is globally listed as Endangered [15].
Despite legal protection, S. lewini is among the most landed shark species along the Saudi Arabian Red Sea coast [16]. Furthermore, S. lewini represents over 3% of all species traded in the Arabian Seas region [17]. At the same time, S. lewini populations in the western Indian Ocean appear fragmented with limited dispersal between the Arabian Seas region and other Indian Ocean regions [18]; yet, stock assessments and species-specific studies are missing in the area [19, 20]. Gathering ecological information of movement data is a critical first step toward appropriate management strategies of shark species, and several studies have examined movements of S. lewini in different ocean systems (e.g., [21,22,23,24,25,26,27]). This species is known to conduct offshore migrations and to inhabit a highly expanded vertical niche in the open ocean, tolerating large fluctuations in depth, temperature, and extremely low levels of dissolved oxygen [22, 23, 28]. Although several studies have investigated vertical distributions of S. lewini, the tracking duration in these studies was comparatively short and data resolution coarse [e.g., 21, 28]. The complex nature of vertical movements is hence still not well understood and might represent a range of behaviors such as foraging, thermoregulation, energetics, and/or reproduction [29].
To better understand the horizontal and vertical habitat use of this endangered predator in the Red Sea, and to aid with potential future development of zoning and management plans for key habitats, we used a MiniPAT tag (pop-up satellite archival transmitting tag) to track a single S. lewini for a period of 182 days and were able to describe migration patterns and fine-scale vertical movements over seasonal scales (April–November).
Results
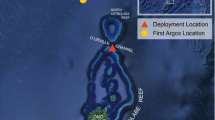
On April 25, 2012, one MiniPAT tag was deployed on a female S. lewini measuring 240 cm (TL) at the western end of Sh’ib Nazar reef (N22°19.089, E038°51.380) (Fig. 1). The shark was caught approximately 50 m off the outer reef wall in 60 m of depth. Based on previous studies in the Indian Ocean region, a body length of 240 cm indicates that the tagged individual was likely mature or nearing maturity [10].
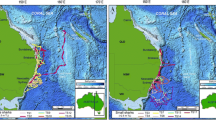
Reconstructed track based on daily records of light levels from April 25 to October 24, 2012. Green and red triangles denote the tagging and pop-up locations, respectively. Solid black line indicates missing positions between September 15 and October 7 due to poor geolocation around the equinox. Gray shading indicates confidence regions for position estimates
The tag initiated pop-off and started transmitting on October 24, 2012, <1 km (N22º19.228, E038°51.432) from the deployment site (Fig. 1). It was physically recovered on October 28, thus providing the complete archived dataset. The archived data contained >1.04-million time series data points for recorded depth, temperature, and light levels at 15-s intervals over the deployment period.
Horizontal and vertical movements
Based on the most probable track, the shark underwent a cyclical migration, approximately 1000 km in length (circle distance between the tagging location and the furthest recorded position). After its release on April 25, 2012, the tagged animal travelled southwestward for approximately 25 days, reaching the central rift of the Red Sea on May 19, approximately 300 km from the tagging location. It spent the following 97 days in coastal and offshore waters of the central and southern Sudanese Red Sea, before once more crossing the central Red Sea rift around September 10 moving northeast toward the Saudi Arabian Red Sea coast.
The next reliable position was recorded on October 7, 100 km south of the tagging location, from where the shark moved northward in coastal waters, before reaching the original tagging location approximately 15 days later (Fig. 1).
The tagged S. lewini occupied depths between 0 and 971 m (Fig. 2a; Additional file 1) with a mean (±SD) of 155 ± 230 m. It spent >70% of its time in the upper 100 m (Fig. 2a), yet relatively little time in surface waters (<2.5% of time in the upper 10 m; data not shown). Mean daytime and nighttime depths were 161 and 147 m, respectively, indicating relatively little difference in diel depth occupation. Similar results were found for diel temperature differences (means 25.5 and 25.9 °C for day and night, respectively). Temperatures ranged from 21.5 to 32.7 °C, with a mean of 25.7 ± 2.6 °C. The majority of temperature records fell between 25 and 29 °C, but the shark also spent nearly 23 and 17% of its time in 21–22 °C during day and night, respectively, generating a bimodal temperature use signature (Fig. 2b).
Excursions to mesopelagic depths interspersed with constant vertical movement (i.e., oscillatory diving) in the upper 100 m of the water column occurred throughout the entire deployment. Deep dives (beyond 850 m) mainly took place between 7 p.m. and 3 a.m., while shallower dives (<500 m) occurred more scattered throughout the diel cycle (Fig. 3; Additional file 1). As the deployment progressed, the frequency of mesopelagic excursions to depth >850 m increased (Fig. 4), first during nighttime (as reflected by a high coefficient of variation) and, starting in July, also during daytime, leading to a marked increase in time spent at greater depths over the deployment period (from 5% in May to 10% in October).
Depth profiles
Swimming depths generally followed one of three distinct patterns, differing by the amount of time spent at the deepest depth (Fig. 5). Vertical activities in the upper 100 m of the water column were dominated by rapid repetitions of symmetrical ‘V’ dives, in which the shark ascended immediately after reaching the deepest depth (Fig. 5a; Additional file 1). Although, ‘V’ patterns were also observed to reach depths >100 m, this behavior was less frequent and tended to intermix with other patterns. The second identified pattern, ‘U’ dives, was characterized by descending and then remaining at a certain depth for as long as 2.5 h (Fig. 5b–d; Additional file 1). We also identified a third pattern, ‘Uv’ dives. This pattern is characterized by extended bottom time, as seen in ‘U’ dives, but also shows one or several small vertical excursions during the ascents (Fig. 5b). The maximum depth reached during the majority of ‘U’ and ‘Uv’ dives was within two particular depth strata, 670–675.5 and 850–885 m (Fig. 5; Additional file 1). The maximum vertical descent speed was 6.43 ms−1. Descent speed on dives was 1.6× faster on average than ascent speed. Duration of dives within a shallower depth range (median depth down to 200 m) was proportionally shorter than dive duration for greater depths (median depths 400–800 m) to cover the longer vertical distance.
Discussion
Although the movement ecology of S. lewini has received much attention in the Pacific [e.g., 9, 21,22,23,24, 27, 30, 31], data for this species from the Indian Ocean are rare [28]. This study represents the first record of PSAT technology used to collect movement data for an S. lewini in the Indian Ocean and provides the longest high-resolution dataset of vertical movements of this species globally. While the low sample size in this study limits our capacity to draw broader conclusions on a species-level, it is clear that PSAT technology has the potential to offer great insights into this species movement ecology in the Red Sea.
The elongated nature of the Red Sea in concert with the lack of significant oceanographic features (e.g., strong surface thermal gradients) poses considerable challenges in reconstructing horizontal movements in this ocean basin. Despite these difficulties, we were able to extract a most probable track of the tagged animal based on available light-based geolocation data only. Although ETOPO2 bathymetry data (www.ngdc.noaa.gov/mgg/global/etopo2.html) are available for our study region, the quality is questionable. The data neither match regional admiralty charts, nor data obtained through recent underwater glider operations. The latter indicate depths exceeding 1000 m much closer to shore than suggested by ETOPO2 data (Burton Jones pers. comm.). The lack of accurate bathymetric data limits our ability to assess the accuracy of our reconstructed track given what we learnt from the shark’s routine use of depths >670 m. Nonetheless, when ETOPO2 depth estimates were overlaid with our reconstructed track and the associated confidence regions, areas with depths >500 m were found in reasonable proximity to the estimated shark’s daily positions (Fig. 1; Additional file 2).
The tagged animal conducted a cyclical migration to offshore habitats, similar to what has previously been reported for a conspecific in the Gulf of California [22]. Possible functions underlying such cyclical migratory behavior could be related to a number of factors, including social (e.g., sexual segregation and schooling), reproductive, or feeding activities [32, 33]. S. lewini is known to seasonally aggregate at offshore islands or seamounts in schools of up to 225 individuals, which are generally dominated by females [8]. Such schools have been observed at offshore reefs in Sudan, the majority of which lie within our tracking area [34, 35]. Furthermore, schools of 8–12 individuals have been observed in deeper waters (45–60 m) at offshore reefs in the northern and southern Saudi Arabian Red Sea (T.S. Habis and Dream Divers Jeddah, pers. comm.). The observed circular migration of the tagged individual might thus form the basis of temporal schooling behavior along one or both coasts of the Red Sea and suggest connectivity among aggregation areas.
While the tagged animal spent most of its time in the epipelagic zone, deep vertical movements, including mesopelagic excursions, occurred during day and night throughout the entire migratory circuit and increased over the deployment period (Fig. 4). Dives to >850 m occurred more frequently during the night, while dives between 650 and 700 m were common during the day, typically occurring 1–3 h after sunrise (Fig. 3; Additional file 1). Overall, there was no significant difference between the mean depths of the shark throughout the diel cycle. This result contrasts with those of previous studies where deep diving in S. lewini was reported to occur almost exclusively during nighttime and/or evening twilight [21, 22, 25, 30]. Changes in diving behavior of other shark species have previously been attributed to stress reactions in response to the capture and handling process [e.g., 36]. Sphyrnids in particular were shown to be inherently vulnerable to capture stress [e.g., 37, 38,39,40,]. However, our tagging operation (from the shark being hooked to its’ release) took less than 6 min; we thus consider it unlikely that the observed increase in mesopelagic excursions 2 months into the tracking period was related to a possible capture trauma. Furthermore, it has been hypothesized that alterations in diving behavior might be related to predator avoidance [39]. In the central Red Sea, however, the abundance of marine predators that could pose a threat to a shark the size of our study animal is extremely low [16, 20, 34]. In addition, one would expect vertical movements to be fast and unpredictable, if they were a response to predator encounters [39]. Yet, the observed diving behavior was characterized by constant and uniform oscillatory patterns, which are unlikely to be the result of predator avoidance.
Instead, the functional role of the observed dive patterns is likely attributable to a combination of various ecological, environmental, and physiological drivers, e.g., behavioral thermoregulation, navigation, energy conservation, or foraging [e.g., 40,41,42,43]. As previously suggested for S. lewini, constant oscillatory ‘V’ diving could serve navigational purposes based on the increase in intensity of local magnetic gradients with depth [30]. Based on the collected data alone and considering the lack of data on bottom topography, we are unable to determine possible point-to-point movements or directionality of the swimming behavior, which would be suggestive of a navigational function of the shark’s oscillatory diving [30]. Clearly, more experimental research on orientation behavior is required to uncover the underlying mechanisms governing shark navigation behavior and to find ways to identify such behaviors based on tracking data alone.
‘V’ dives observed here were characterized by a ‘fast descent, slow ascent’ dive profile, which in other shark species has been postulated to be motivated by prey searching behavior [44,45,46,47]. In air-breathing marine vertebrates, ‘U’ dives, including a bottom phase without wiggles, and ‘Uv’ dives, characterized by one or several wiggles during the bottom phase or during the ascent, have been associated with feeding events [48, 49]. Dive profiles and depth occupancy of the individual tagged in our study could hence indicate that dives in the 650–700 m layer and below 850 m may represent short and longer feeding events, respectively (Additional file 1). The observed preference of the shark for the 650–700 and >850 m depth layers may reflect foraging on mesopelagic fish, which are believed to be the main component of the deepest of four scattering layers at 600–800 m in the Red Sea [50]. Echosounder data collected in the broader tracking area suggest that squids, which can make up over 49% of S. lewini’s diet [51], form patches during the day in and below the deepest scattering layer and leave the deep waters at night (Stein Kaartvedt, unpublished data). The same data also suggest that prey density in mesopelagic depths is generally extremely low during nighttime, which might explain the observed increase in daytime vertical habitat use over the tracking period. Nightly mesopelagic excursions could be related to foraging on deep benthos based on previous observations on S. lewini hunting at night for benthic prey on or in sediments, probably using their bioelectric sensory system and other senses [52]. Dietary studies of S. lewini reported high proportions of mesopelagic prey items, such as teleosts, cephalopods, and crustaceans in gut content analyses, further pointing toward mesopelagic feeding behavior in this species [32, 52,53,54]. Potential daytime feeding of the focal shark at mesopelagic depths contrasts the findings of a number of previous studies suggesting that feeding in S. lewini occurs exclusively at night, while daytime serves as a resting phase, which is spent refuging in shallow waters close to seamounts or islands [9, 22, 32, 51,52,53,54,55]. While data from a single individual cannot be extrapolated to the species level, the repetitive nature of continuous vertical movements performed by this individual over the entire 182-day tracking period suggests that foraging may be continuous throughout day and night.
Intervals between deep dives generally lasted around 30 min and were characterized by oscillatory diving in the upper 100 m of the water column. One of the most widely proposed functions for oscillatory diving has been thermoregulation, with shallow depth intervals between deep diving events serving as a ‘warming period’ to recover from heat loss prior to the next dive into colder depths [25, 32, 44]. The water column in the Red Sea, however, is only poorly stratified [56, 57]. The minimum temperature experienced by the tagged individual during deep dives was relatively high compared to other regions (21.5 vs. 5.8 [25], 4.8 [23], 5.9 °C [28]), and the experienced maximum change in temperature was relatively low (depending on the season between 5 and 10 vs. 24.5 [25], 23 °C [23]), making it less likely that surface warming-intervals would be necessary to maximize time at depth. In addition, not all deep dives were interrupted by shallow water intervals, as would be expected if they were related to thermoregulatory behavior. Moreover, S. lewini are ectothermic fishes, lacking extensive retes for heat storage [40]. Other shark species have been shown to actively use shallow, warm waters to increase their core body temperature in order to optimize rates of digestion, growth, and gestation [58,59,60]. Juvenile S. lewini were found to have a Q 10 (the increase in the rate of biological functions caused by a 10 °C increase in temperature [61]) of 1.34 at 21–29 °C [62]. While extrapolating metabolic rate data from juvenile to adult sharks is problematic [63], it is expected that even in adults, movements between temperatures may result in changes in physiological rate functions. Every shallow water interval could hence indicate a preceding successful feeding event. Missing ‘warming intervals’ between dives on the contrary could indicate an unsuccessful hunting event during the preceding dive.
Conclusions
We present the first movement data for S. lewini in the Red Sea. Our results provide information on the vertical migrations of this species at hitherto unknown detail. We identified previously unreported continuous deep diving behavior throughout the 24-h cycle over a tracking period of 182 days. The increased daytime vertical habitat use observed over the tracking period may suggest adaptations to foraging behavior. However, additional studies addressing diet composition of S. lewini in concert with the fine-scale distribution of prey species throughout the water column in the Red Sea are needed.
The observed site fidelity to the tagging location and the migration pattern across the Red Sea provide evidence of complex spatial structure and dynamics that encompass both pelagic and heavily fished coastal environments. Local-scale no-take MPAs may hence not be effective at protecting S. lewini populations on a regional scale, and species-specific protection strategies are warranted.
Methods
Study site
The shark was tagged at the western end of Sh’ib Nazar, a submerged reef platform located about 24 km off the coast of the fishing village Thuwal and ~80 km north of Jeddah in the Saudi Arabian Red Sea (Fig. 1). It is the largest (~0.5 km2) and southernmost reef in a chain of barrier reefs arranged on a north–south axis. Sh’ib Nazar has a shallow (2–3 m deep) reef crest with a steep slope on its western (seaward-facing) side and southern tip. At a depth of about 20 m, the reef wall meets the sandy seabed, which slopes more gradually and reaches a depth of over 1000 m about 300 m away from the reef wall. On its eastern side, the reef slope is less steep and reaches the seabed at a shallower depth of about 10–15 m. The slope eventually reaches a depth of over 1000 m on the eastern side at a distance ranging from about 0.5 (near the south tip of the reef) to 3.2 km (further north) away from the reef.
Field techniques
Tagging was conducted on April 25, 2012. The shark was captured using a handline consisting of 85 m of nylon line (6 mm diameter), with an 18/0 non-offset circle hook attached to one end and baited with 200 g of Striped bonito (Sarda orientalis). As soon as the shark was hooked, a large float (45 cm diameter) was attached to the handline to provide buoyancy to the gear. Once brought alongside the vessel, the shark was measured for total length (TL) to the nearest cm and its sex visually determined by the absence of claspers (female). A small tissue sample was clipped from the left pectoral fin for genetic analysis [18]. One uniquely numbered external tag (FT-1 Dart Tag) was inserted in the basolateral dorsal musculature with a hollow canula (Floy Tag, Seattle, WA, USA). A MiniPAT tag (Wildlife Computers, Inc., WA, USA) was inserted into the musculature at the base of the dorsal fin with a handheld tagging lance. The tag was anchored with a plastic wilton anchor (Wildlife Computers, Inc., WA, USA) and trailed from 15 cm of 1.2-mm stainless steel wire-rope coated in heat shrink tubing. The hook was completely removed by cutting the barb and rotating the hook free immediately prior to release.
Tag details and programming
Pop-up satellite archival transmitting tags record time series of light levels, depth, and temperature during deployment and archive the data until the tag self-releases from the shark after a user-programmed amount of time. Once at the surface, it transmits summarized data to orbiting Argos satellites. MiniPAT tags are 12 cm in length, have a volume of 60 cm3, and weigh approximately 50 g. The MiniPAT tag was programmed to release from the animal after 180 days, to record water depth (±0.5 m) and temperature (±0.05 °C) every 15 s for a period of 182 days, and to summarize the data into 24-h temporal bins for satellite data transmission. Time-at-depth and time-at-temperature data were arranged in 12 strata.
Track reconstruction
Light-based geolocation was used to reconstruct a most probable track from the full-resolution light level and depth time series in the recovered tag. Light levels were first corrected as surface measurements following a simple two-layer depth model [64], implemented in TrackIt [65]. Scatter plots and histograms of daily light levels were then visually inspected to identify a single threshold (110 relative light units) for selecting times of sunrise and sunset. From these selected times, a draft track was calculated using GeoLight [66] and then refined by a state-space Kalman filter model, Kftrack [67]. The Kftrack model utilizes an underlying random walk movement model that describes the overall diffusion and advection for the entire track and provides error estimates in the form of confidence regions. The refined track, referred to as the most probable track, remained in the ocean for the entire deployment and thus required no further correction using bathymetry or sea surface temperature. Parameter estimates (see [67] for details) were as follows: u = 0.0025 nautical mile (nm) day−1, v = −0.0457 nm day−1, D = 83.435 nm2 day−1, sy = 2.1488 degree, a0 = 0 degree, and b0 = −9.8247 day. Averaged diffusive speed for the entire track (0.11 ms−1, calculated from D) is within the range of horizontal speed (maximum = 0.83 ms−1) obtained from short-term acoustic tracking [30]. Light-based geolocation is least reliable during times around the equinoxes, when day length is the same for anywhere on Earth [65, 68, 69]. In our tagged shark, positions could not be estimated for a period in September. We did not attempt to interpolate positions for this period, given no subsequent analyses required georeferenced information.
Statistical analysis
Basic statistics were derived using base functions in R (version 3.3.2) [70]. To characterize changes in swimming depth, depths were averaged hourly over the 24-h period for each month of the deployment. A coefficient of variation was also calculated that expressed the standard deviation in hourly depth as a percentage of the mean hourly depth. To aid analysis, a definition for a dive was arbitrarily defined, after visual examination of all swimming depths. A dive is defined to begin with the first instance that the shark descended below 100 m and to end with the first instance of ascending above 100 m. Swimming depths above 100 m were considered shallow activities.
References
Schneider DC. Quantitative ecology: spatial and temporal scaling. Amsterdam: Elsevier; 1994.
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF. Aquatic animal telemetry: a panoramic window into the underwater world. Science. 2015;348:1221–31.
Hammerschlag N, Gallagher AJ, Lazarre DM. A review of shark satellite tagging studies. J Exp Mar Biol Ecol. 2011;398:1–8.
Berumen ML, Braun CD, Cochran JEM, Skomal GB, Thorrold SR. Movement patterns of juvenile whale sharks tagged at an aggregation site in the Red Sea. PLoS ONE. 2014;9:e103536.
Braun CD, Skomal GB, Thorrold SR, Berumen ML. Movements of the reef manta ray (Manta alfredi) in the Red Sea using satellite and acoustic telemetry. Mar Biol. 2015;162:2351–62.
Braun CD, Skomal GB, Thorrold SR, Berumen ML. Diving behavior of the reef manta ray links coral reefs with adjacent deep pelagic habitats. PLoS ONE. 2014;9:e88170.
Clarke C, Lea JSE, Ormond RFG. Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea. Mar Freshw Res. 2011;62:668–75.
Klimley AP, Nelson DR. Schooling of the scalloped hammerhead shark, Sphyrna lewini, in the Gulf of California. Fish Bull. 1981;79:356–60.
Hearn A, Ketchum J, Klimley AP, Espinoza E, Penaherrera C. Hotspots within hotspots? Hammerhead shark movements around Wolf Island, Galapagos Marine Reserve. Mar Biol. 2010;157:1899–915.
White WT, Bartron C, Potter IC. Catch composition and reproductive biology of Sphyrna lewini (Griffith & Smith) (Carcharhiniformes, Sphyrnidae) in Indonesian waters. J Fish Biol. 2008;72:1675–7.
Cortés E. Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci. 1999;56:707–17.
Ebert DA, Fowler SL, Compagno LJ, Dando M. Sharks of the world: a fully illustrated guide. Plymouth: Wild Nature Press; 2013.
Bush A. Diet and diel feeding periodicity of juvenile scalloped hammerhead sharks, Sphyrna lewini, in Kāne’ohe Bay, Ō’ahu, Hawai’i. Environ Biol Fishes. 2003;67:1–11.
Gallagher AJ, Hammerschlag N, Shiffman DS, Giery ST. Evolved for extinction: the cost and conservation implications of specialization in hammerhead sharks. Bioscience. 2014;64:619–24.
Baum J, Clarke S, Domingo A, Ducrocq M, Lamónaca AF, Gaibar N, et al. Sphyrna lewini. The IUCN red list of threatened species. Version 2014.3. 2007.
Spaet JLY, Berumen ML. Fish market surveys indicate unsustainable elasmobranch fisheries in the Saudi Arabian Red Sea. Fish Res. 2015;161:356–64.
Jabado RW, Al Ghais SM, Hamza W, Henderson AC, Spaet JLY, Shivji MS, Hanner RH. The trade in sharks and their products in the United Arab Emirates. Biol Conserv. 2015;181:190–8.
Spaet JLY, Jabado RW, Henderson AC, Moore ABM, Berumen ML. Population genetics of four heavily exploited shark species around the Arabian Peninsula. Ecol Evolut. 2015;5:2317–32.
Moore ABM. Elasmobranchs of the Persian (Arabian) Gulf: ecology, human aspects and research priorities for their improved management. Rev Fish Biol Fish. 2011;22:35–61.
Spaet JLY, Thorrold SR, Berumen ML. A review of elasmobranch research in the Red Sea. J Fish Biol. 2012;80:952–65.
Bessudo S, Soler GA, Klimley AP, Ketchum JT, Hearn A, Arauz R. Residency of the scalloped hammerhead shark (Sphyrna lewini) at Malpelo Island and evidence of migration to other islands in the Eastern Tropical Pacific. Environ Biol Fishes. 2011;91:165–76.
Hoyos-Padilla EM, Ketchum JT, Klimley AP, Galván-Magaña F. Ontogenetic migration of a female scalloped hammerhead shark Sphyrna lewini in the Gulf of California. Anim Biotelemetry. 2014;2:17.
Jorgensen SJ, Klimley AP, Muhlia-Melo AF. Scalloped hammerhead shark Sphyrna lewini, utilizes deep-water, hypoxic zone in the Gulf of California. J Fish Biol. 2009;74:1682–7.
Ketchum JT, Hearn A, Klimley AP, Espinoza E, Peñaherrera C, Largier JL. Seasonal changes in movements and habitat preferences of the scalloped hammerhead shark (Sphyrna lewini) while refuging near an oceanic island. Mar Biol. 2014;161:755–67.
Hoffmayer ER, Franks JS, Driggers WB, Howey PW. Diel vertical movements of a scalloped hammerhead, Sphyrna lewini, in the northern Gulf of Mexico. Bull Mar Sci. 2013;89:551–7.
Klimley AP, Brown ST. Stereophotography for the field biologist: measurement of lengths and three-dimensional positions of free-swimming sharks. Mar Biol. 1983;74:175–85.
Klimley AP, Butler SB, Nelson DR, Stull AT. Diel movements of scalloped hammerhead sharks, Sphyrna lewini Griffith and Smith, to and from a seamount in the Gulf of California. J Fish Biol. 1988;33:751–61.
Moore ABM, Gates AR. Deep-water observation of scalloped hammerhead Sphyrna lewini in the western Indian Ocean off Tanzania. Mar Biodivers Rec. 2015;8:e91.
Shepard ELC, Ahmed MZ, Southall EJ, Witt MJ, Metcalfe JD, Sims DW. Diel and tidal rhythms in diving behaviour of pelagic sharks identified by signal processing of archival tagging data. Mar Ecol Ser. 2006;328:205–13.
Klimley AP. Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar Biol. 1993;117:1–22.
Ketchum JT, Hearn A, Klimley AP, Peñaherrera C, Espinoza E, Bessudo S, Soler G, Arauz R. Inter-island movements of scalloped hammerhead sharks (Sphyrna lewini) and seasonal connectivity in a marine protected area of the eastern tropical Pacific. Mar Biol. 2014;161:939–51.
Klimley AP. The determinants of sexual segregation in the scalloped hammerhead shark, Sphyrna lewini. Environ Biol Fishes. 1987;18:27–40.
Sims D. Differences in habitat selection and reproductive strategies of male and female sharks. In: Ruckstuhl KE, Neuhaus P, editors. Sexual segregation in vertebrates: ecology of the two sexes. Cambridge: Cambridge University Press; 2005. p. 127–47.
Spaet JLY, Nanninga GB, Berumen ML. Ongoing decline of shark populations in the Eastern Red Sea. Biol Conserv. 2016;201:20–8.
Kattan A, Cocker DJ, Berumen ML. Reef fish communities in the central Red Sea show evidence of asymmetrical fishing pressure. Mar Biodivers. 2017. doi:10.1007/s12526-017-0665-8.
Afonso AS, Hazin FHV. Post-release survival and behavior and exposure to fisheries in juvenile tiger sharks, Galeocerdo cuvier, from the South Atlantic. J Exp Mar Biol Ecol. 2014;454:55–62.
Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N. Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar Ecol Prog Ser. 2014;496:207–18.
Gulak SJB, de Ron Santiago AJ, Carlson JK. Hooking mortality of scalloped hammerhead Sphyrna lewini and great hammerhead Sphyrna mokarran sharks caught on bottom longlines. Afr J Mar Sci. 2015;37:267–73.
Andrews KS, Williams GD, Farrer D, Tolimieri N, Harvey CJ, Bargmann G, Levin PS. Diel activity patterns of sixgill sharks, Hexanchus griseus: the ups and downs of an apex predator. Anim Behav. 2009;78:525–36.
Klimley AP, Beavers SC, Curtis TH, Jorgensen SJ. Movements and swimming behavior of three species of sharks in La Jolla Canyon, California. Environ Biol Fishes. 2002;63:117–35.
Gleiss AC, Norman B, Wilson RP. Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct Ecol. 2011;25:595–607.
Weng KC, Boustany AM, Pyle P, Anderson SD, Brown A, Block BA. Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Mar Biol. 2007;152:877–94.
Nasby-Lucas N, Dewar H, Lam CH, Goldman KJ, Domeier ML. White shark offshore habitat: a behavioral and environmental characterization of the eastern Pacific shared offshore foraging area. PLoS ONE. 2009;4:e8163.
Carey FG, Scharold JV, Kalmijn AJ. Movements of blue sharks (Prionace glauca) in depth and course. Mar Biol. 1990;106:329–42.
Cartamil DP, Sepulveda CA, Wegner NC, Aalbers SA, Baquero A, Graham JB. Archival tagging of subadult and adult common thresher sharks (Alopias vulpinus) off the coast of southern California. Mar Biol. 2011;158:935–44.
Howey-Jordan LA, Brooks EJ, Abercrombie DL, Jordan LKB, Brooks A, Williams S, Gospodarczyk E, Chapman DD. Complex movements, philopatry and expanded depth range of a severely threatened pelagic shark, the oceanic whitetip (Carcharhinus longimanus) in the western North Atlantic. PLoS ONE. 2013;8:e56588.
Nakano H, Matsunaga H, Okamoto H, Okazaki M. Acoustic tracking of bigeye thresher shark Alopias superciliosus in the eastern Pacific Ocean. Mar Ecol Prog Ser. 2003;265:255–61.
Boeuf BJL, Naito Y, Asaga T, Crocker D, Costa DP. Swim speed in a female northern elephant seal: metabolic and foraging implications. Can J Zool. 1992;70:786–95.
Lesage V, Hammill MO, Kovacs KM. Functional classification of harbor seal (Phoca vitulina) dives using depth profiles, swimming velocity, and an index of foraging success. Can J Zool. 1999;77:74–87.
Klevjer TA, Torres DJ, Kaartvedt S. Distribution and diel vertical movements of mesopelagic scattering layers in the Red Sea. Mar Biol. 2012;159:1833–41.
Torres-Rojas Y, Hernandez-Herrera A, Galvan-Magana F. Feeding habits of the scalloped hammerhead shark, Sphyrna lewini, in Mazatlán waters, southern Gulf of California, Mexico. Cybium. 2006;30:85–90.
Smale MJ, Cliff G. Cephalopods in the diets of four shark species (Galeocerdo cuvier, Sphyrna lewini, S. zygaena and S. mokarran) from Kwazulu-Natal, South Africa. S Afr J Mar Sci. 1998;20:241–53.
Stevens JD, Lyle JM. Biology of three hammerhead sharks (Eusphyra blochii, Sphyrna mokarran and S. lewini) from northern Australia. Mar Freshw Res. 1989;40:129–46.
Vaske Júnior T, Vooren CM, Lessa RP. Feeding strategy of the night shark (Carcharhinus signatus) and scalloped hammerhead shark (Sphyrna lewini) near seamounts off northeastern Brazil. Braz J Oceanogr. 2009;57:97–104.
Klimley AP, Nelson DR. Diel movement patterns of the scalloped hammerhead shark (Sphyrna lewini) in relation to El Bajo Espiritu Santo: a refuging central-position social system. Behav Ecol Sociobiol. 1983;15:45–54.
Neumann AC, McGill DA. Circulation of the Red Sea in early summer. Deep Sea Res. 1961;8:223–35.
Sofianos SS, Johns WE. Observations of the summer Red Sea circulation. J Geophys Res Ocean. 2007;112:C6.
Hight BV, Lowe CG. Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: evidence for behavioral thermoregulation? J Exp Mar Bio Ecol. 2007;352:114–28.
Economakis AE, Lobel PS. Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, Central Pacific Ocean. Environ Biol Fishes. 1998;51:129–39.
Speed CW, Meekan MG, Field IC, McMahon CR, Bradshaw CJA. Heat-seeking sharks: support for behavioural thermo-regulation in reef sharks. Mar Ecol Prog Ser. 2012;463:231.
Schmidt-Nielsen K. Animal physiology: adaptation and environment. Cambridge: Cambridge University Press; 1997.
Lowe C. Metabolic rates of juvenile scalloped hammerhead sharks (Sphyrna lewini). Mar Biol. 2001;139:447–53.
Carlson JK, Goldman KJ, Lowe CG. Metabolism, energetic demand, and endothermy. Biol Sharks Relat. 2004;10:203–24.
Ekstrom PA. Blue twilight in a simple atmosphere. In: Proceedings of SPIE, conference 4815; paper 14; 2002.
Nielsen A, Sibert JR. State-space model for light-based tracking of marine animals. Can J Fish Aquat Sci. 2007;64:1055–68.
Lisovski S, Hahn S. GeoLight-processing and analysing light-based geolocator data in R. Methods Ecol Evol. 2012;3:1055–9.
Sibert JR, Musyl MK, Brill RW. Horizontal movements of bigeye tuna (Thunnus obesus) near Hawaii determined by Kalman filter analysis of archival tagging data. Fish Oceanogr. 2003;12:141–51.
Royer F, Lutcavage M: Positioning pelagic fish from sunrise and sunset times: complex observation errors call for constrained, robust modeling. In Tagging and tracking of marine animals with electronic devices. Springer; 2009. pp. 323–341.
Teo SLH, Boustany A, Blackwell S, Walli A, Weng KC, Block BA. Validation of geolocation estimates based on light level and sea surface temperature from electronic tags. Mar Ecol Prog Ser. 2004;283:81–98.
R Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2016. https://www.R-project.org/. Accessed 10 Mar 2017.
Authors’ contributions
JLYS and MLB designed the study. JLYS conducted the fieldwork of the study and wrote the manuscript. CHL and CDB analyzed the data and created the figures. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Abdullh A. Aljahdali, Esam S. Aljahdali, Gazzi J. Aljahdai, Abdulmohsen H. Aljahdali and the King Abdullah University of Science and Technology Coastal and Marine Resources Core Lab for facilitating the fieldwork of this study; Pedro R. de la Torre and Lloyd Smith for assistance with tag retrieval and Gregory B. Skomal for facilitating the tag data transfer. We are grateful to Stein Kaartvedt, Burton Jones, and Anders Røstad for helpful discussions of the data. We would also like to thank Austin Gallagher and one anonymous reviewer for helpful feedback on the manuscript.
Competing interests
The authors declare that they have no competing interests.
Declarations
The research was undertaken in accordance with the policies and procedures of the King Abdullah University of Science and Technology (KAUST). Permissions relevant for KAUST to undertake the research have been obtained from the applicable governmental agencies in the Kingdom of Saudi Arabia. The animal use protocol was performed in accordance with Woods Hole Oceanographic Institution’s Animal Care and Use Committee protocol #16518.
Availability of data and materials
The complete time series dataset used in this study is available from the Pangea Data Repository (https://doi.pangaea.de/10.1594/PANGAEA.880113).
Consent for publication
Not applicable.
Funding
Funding for the research was provided by the King Abdullah University of Science and Technology (baseline research funds to MLB).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
40317_2017_135_MOESM1_ESM.pdf
Additional file 1. Daily depth temperature profiles of the tagged S. lewini over 182 days at liberty. Depth and temperature profiles recorded at 15 s intervals from April 26th, 2012–October 22nd, 2012. Dashed lines indicate times of local sunrise and sunset. Dotted lines indicate depths of 650 and 850 m, respectively.
40317_2017_135_MOESM2_ESM.pdf
Additional file 2. Reconstructed track based on daily records of light levels from April 25th to October 24th, 2012. Green and red triangles denote the tagging and pop-up locations, respectively. Solid black line indicates missing positions between September 15th and October 7th due to poor geolocation around the equinox. Grey shading indicates confidence regions for position estimates. Bathymetry data based on ETOPO2.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Spaet, J.L.Y., Lam, C.H., Braun, C.D. et al. Extensive use of mesopelagic waters by a Scalloped hammerhead shark (Sphyrna lewini) in the Red Sea. Anim Biotelemetry 5, 20 (2017). https://doi.org/10.1186/s40317-017-0135-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-017-0135-x