Abstract
Background
It is generally considered that on relatively homogenous marine soft sediment habitats, such as sand, fish are unlikely to show site attachment. This poses challenges for management and the evaluation of the efficacy of marine protected areas, in which soft sediments often make up more than 70 % of habitats. The blue-spotted flathead is a commercially and recreationally targeted species found on soft sediments in coastal marine waters of south-eastern Australia. There are no published data on its movement patterns. Here, using active acoustic telemetry, we aim to (a) quantify movement and habitat use of blue-spotted flathead, (b) compare area usage to no-take sanctuary zone size and (c) obtain data to aid in the design of a large passive receiver array to be used in long-term comprehensive tracking of soft sediment fish.
Results
Three of five blue-spotted flathead that were tagged exhibited strong site attachment and were detected close to their release points for the entire 60-day study period. The two other fish were not detected after 4 and 25 days and were likely to have moved out of the study area (search radius ≈ 3 km). For the three fish tracked over 60 days, the area used was compact (mean ± SE = 0.021 km2 ± 0.037) and two patterns of movement were apparent: (1) a small activity space used in its entirety each day (two fish) and (2) a larger activity space in which a separate area is utilised each day (one fish).
Conclusions
Our study is the first to document the movement of blue-spotted flathead, and these preliminary results demonstrate two broad movement patterns shown by this species on soft sediments in Jervis Bay. Over the course of 60 days, a majority of fish in this study showed strong site attachment; however, a number of fish also made larger-scale movements. Finally, our study suggests that a tightly spaced, passive acoustic array would provide meaningful results for this species, although strategically placed receivers outside this array would be required to detect any longer range movements.
Similar content being viewed by others
Background
Soundly and effectively implementing and managing marine protected areas (MPAs) requires knowledge of species presence, abundance, size structure and also site attachment and broader range movements [1]. An understanding of movement is particularly important as reserve effectiveness is dependent on the scale of movement of species in relation to reserve size [2, 3]. Frequent and large-scale movement of animals has been used to argue that MPAs are unlikely to have tangible benefits for wide ranging taxa [3]. For example, a spatial closure to fishing such as a no-take sanctuary zone is thought to be less effective if the movement of the fish intended to be protected covers an area much larger than the area closed to fishing [4]. If species display site attachment to areas well within reserve boundaries, then MPAs may have potential value; however, if significant numbers of individuals have no site attachment and move between different habitats or areas outside of the reserve boundaries, then alternate management strategies may be more effective [5].
In many cases, particularly on marine soft sediments, little information on the habitat use and movement of fishes is available to inform MPA design and location. Consequently, MPAs may not be of a suitable size or in the correct location to provide effective protection. Understanding the habitats used, degree of site attachment and patterns of movement will substantially aid in the design and management of MPAs, particularly where preferred fish habitat (such as spawning or aggregation grounds) can be identified [6]. Without such data, this is impossible to assess or to infer the effectiveness of a marine reserve on soft sediments.
The homogeneous nature of marine soft sediments, with little obvious structure or habitat differentiation, appears to lead to a general assumption that fish will not show appreciable site attachment [7]. This is in comparison with reef-associated fishes which are often found to show high levels of site attachment [8–11]. This assumption, however, is based on very little data, as relatively few studies look at the movement of demersal fish species on open coastal marine soft sediments. This knowledge gap appears incongruous with the fact that marine soft sediments are the most common habitat on Earth [12], and comprises most of the habitat within near- and off-shore areas. Furthermore, although we have little data for the effect of MPAs on soft sediment systems [13], marine soft sediments are often the major habitat type protected within MPAs [7].
The blue-spotted flathead (Platycephalus caeruleopunctatus) is a common species found on marine sands in south-eastern Australia and is both commercially and recreationally exploited [14]. Despite this, there are currently no published data on blue-spotted flathead movement patterns. This study sought to provide a preliminary assessment of movement patterns within a temperate MPA (Jervis Bay Marine Park—JBMP, NSW, Australia) to test the hypothesis that blue-spotted flathead would not show any sign of site attachment (the consistent positioning of a fish within an area over the study period). This study was carried out to inform the management of the MPA, and more broadly, these preliminary data are essential to aid in the design of marine reserves on soft sediments and will go some way to filling a substantial knowledge gap for this habitat. In terms of future studies, these data will provide a basis for the design of a large passive receiver array for long-term tracking of large numbers of soft sediment fish in a marine park (JBMP) over appropriate spatial scales. The specific aims of this paper are to: (1) use active telemetry to examine blue-spotted flathead movement patterns, behaviour and area use, (2) compare movement to current no-take sanctuary zone size and (3) visualise patterns in activity space of blue-spotted flathead to better inform decision-making on future tracking array design.
Methods
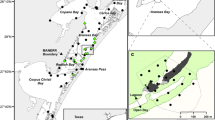
The study was undertaken in JBMP on the south coast of NSW, Australia. Jervis Bay (Fig. 1) is approximately 50 km2 and dominated by sub-tidal soft sediments (predominately coarse sand). A mosaic of rocky intertidal, subtidal reefs and seagrass beds are scattered around the edge of the bay. In addition, there are five designated no-take sanctuary zones within Jervis Bay where fishing is not permitted; the remainder of the bay has zoning that allows for recreational fishing and limited forms of commercial fishing (e.g. not trawling). The current zoning within the bay was implemented in 2002.

Sources: NSW Department of Primary Industries, NSW Office of Environment and Heritage, Geoscience Australia. Mangrove, seagrass and saltmarsh boundaries as defined in [31]
Study location in Jervis Bay, NSW, Australia. Area where tagged fish were captured and released in Hare Bay no-take sanctuary zone is shown within the black square. All areas in shades of blue are marine sand; other major habitat types are indicated in the legend. Inset map: location of Jervis Bay in Australia. Subtidal reef features digitised preferentially from swath bathymetry, LADS, and ADS40 aerial imagery.
On the 22 August 2011, blue-spotted flathead (n = 5) were line caught on sand at a depth of 10 m in the Hare Bay no-take sanctuary zone (Fig. 1). The fish were then anaesthetised in seawater containing 60 mg L−1 of Aqui-S® before a transmitter (Vemco V9 model; 21 mm length, 9 mm diameter, 1.6 g in the water, battery life 80 days, nominal ping interval 120 s) was inserted through a 1-cm mid-ventral incision in the abdomen. Surgery lasted <2 min and the incision was closed with one or two dissolving stitches tied with a double surgeon’s knot. Fish were then transferred to a holding tank and monitored for around 20 min, before releasing them at the site of capture.
We actively tracked blue-spotted flathead for 12 days over a 60-day period between 22 August and 20 October 2011, using a boat-based mobile receiver and directional hydrophone (Vemco VR100 and VH110). For the first 4 days post-release, fish were tracked in daylight hours, and we attempted to position each fish repeatedly throughout each day. Fish were then tracked on 8 random follow-up days in daylight hours, and we attempted to position the fish at least once on each of these days. Previous trapping data in Jervis Bay suggested that blue-spotted flathead were not active at night. Therefore, we decided not to track at night in this study and redirect the associated costs and effort to increase the study length. Fish were sequentially located, and after we located the fish, which generally took between 10 and 20 min to position to within 10 m, the position of the fish was recorded on a hand-held Garmin GPS 60 when the signal strength was at its maximum (i.e. between 70 and 90 dB). Previous range testing indicated that we could reliably get to within 10 m of a tag to take a position. Subsequent searches commenced at the last known position, and if the fish was not detected within 30 min, we then searched for the next fish. Once several locations were recorded for each fish, a broader search pattern was implemented to try and locate any undetected fish. This involved returning to the last known position and searching for the fish in circles of ever increasing size out to maximum of 3 km.
Data analysis
Positional data were visualised to evaluate movement patterns and site attachment. To estimate the activity space for each fish, we used a fixed kernel method to produce 95 % kernel utilisation distributions (KUDs; default grid size/search radius of 50 × 50 m and extent of 1) which were visualised as 95 % probability contours. We calculated KUDs for the first 4 days of tracking and the entire tracking period to assess both post-release and short-term space use. KUDs were produced using the ‘adehabitatHR’ package in the statistical software R [15] and plotted as 95 % probability contours in the ZOATRACK interface [16]. To avoid fragmentation of estimated activity spaces, Kie’s rule-based ad hoc method [17] was used to estimate a suitable smoothing parameter (h). The smoothing parameter was sequentially increased or decreased if required from the reference smoothing (h ref) value by 0.10 increments, until the smallest continuous (rather than a number of discrete) 95 % KUD probability contour that did not cut off any obvious paths between two subsequent detections was attained. We assumed uniform use of space within the 95 % probability contour as the tracking strategy employed did not allow a true estimate of core area use within the activity space.
To indicate activity level, we used a minimum activity index (MAI m h−1) [18] which was calculated by the distance between two points divided by the time elapsed between observations, averaged across all points for each fish. The nature of the data collection meant that this was only possible for the first 4 days of intense tracking. A residency index (RI), as a proportion of total tracking days detected, was calculated to give an estimate of site attachment. We make the assumption that where fish are not detected for two tracking days in succession they have left the study area. We also assume that fish remain in the study area between two tracking days where they are detected (e.g. if a fish is detected on day 18 of tracking and then again on the next tracking day, day 24, we assume the fish stayed in the study area between those days).We used displacement (D) given as distance in metres from the release point to the final position after 60 days and furthest distance (FD) from first release position (calculated for 4 and 60 days) to indicate straight line distance that fish moved from the release point over the study period. An additional file shows a detailed quantitative summary of movement pattern metrics including final h values and proportion of h reference (see Additional file 1: Table S1).
Results
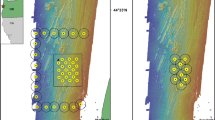
All five of the tagged blue-spotted flathead (F1–F5) were active after tagging and detected on each of the first 4 days of post-tagging, and moved over a scale of 10–100s of metres within a day (Figs. 2, 3). The activity space (95 % KUD) over this time was generally compact with a mean of 0.046 km2 ± 0.025 (±SE). Most fish (F2–F5) were continually reusing the same areas within their activity space, with each animal’s positions being intermingled through time over the 4 days (Fig. 3; Table 1). The exception was F1 which used a much larger area than the other fish and used a separate area on each of the 4 days (Fig. 3; Table 1). F1 also moved a much further distance from tagging location, 534 m compared with between 108 and 149 m for all other fish (209 ± 82 m; mean ± SE). Activity over the 4 days was similar for all fish with a MAI over the first 4 days ranging from 22.11 to 44.96 m h−1 (29.34 m h−1 ± 4.15; mean ± SE, Table 1).
Four-day activity space (95 % KUD) of five blue-spotted flathead (F1–F5). Calculated with positions obtained using active acoustic tracking over initial 4 days of continuous tracking between 22 and 25 August 2011. D1–D4 indicate tracking day for F1 (daily positions of F2–F5 were intermingled within their respective activity spaces)
Over 60 days, residency for the five fish averaged 74 % (SE ± 14 %) suggesting strong site attachment (Table 1). Two fish (F2 and F3) appeared to move outside the no-take sanctuary zone after the first 4 days of intensive tracking, as searches well beyond the no-take sanctuary zone failed to detect these fish. Fish F2 did move back into the sanctuary zone, and was subsequently detected on 2 days (days 24 and 25) to the south of the study area (Fig. 4). Despite extensive searches of the no-take sanctuary zone and surrounding areas covering a minimum of 3-km radius around release point, we did not detect either fish again during the study. The three remaining fish (including F5 which had the largest activity space over the first 4 days) showed strong site attachment and were still being detected in Hare Bay sanctuary zone after 60 days when the study concluded. The activity space (95 % KUD) for the three fish remaining after 60 days (0.121 km2 ± 0.037; mean ± SE) was compact and much smaller than the ≈5.50 km2 of soft sediments within Hare Bay sanctuary zone. F1 and F4 were detected on all of the 12-day tracking which was undertaken, and F5 was detected on all but one tracking day (Fig. 2). Again, F1 covered the greatest amount of area, which was 2–4 times greater than F4 and F5. Fish F1 also moved the furthest distance from the tagging location over the 60 days (541 m), although its displacement at the end of the study was only 108 m from the release point, compared with 305 and 240 m for F4 and F5, respectively (Table 1).
Sixty-day activity space (95 % KUD) of three blue-spotted flathead (F1, F4, F5). Calculated with positions obtained using active acoustic tracking over 60 days between 22 August and 20 October 2011. D1–D60 indicate tracking day for F1 and F4. Daily positions for F5 were intermingled within its activity space. The final 2 days of detections for F2 are also shown towards the southern edge of the figure
The three fish that were detected for the full 60-day study length within the main study area each used a relatively small area but showed different movement patterns within their activity space (Fig. 4). F5 repeatedly used the same area within its activity space. F4 used two areas relatively evenly within its activity space. F1 used the largest activity space and was detected in a separate area on each day it was tracked, but over the long-term revisited parts of its range visited earlier. Hence, for these fish, there was consistency in terms of the usage of relatively small areas, though the pattern of use varied greatly among individual fish. The remaining two fish appeared to make much larger-scale movements. Tagged fish were only detected on soft sediments for the whole study period, and we did not detect blue-spotted flathead moving onto adjacent seagrass or reef habitats despite these areas being searched.
Discussion
This study demonstrated that a number of movement patterns are exhibited by tagged blue-spotted flathead (Platycephalus caeruleopunctatus) found on soft sediments in Jervis Bay. Over a daily timescale, all fish in our study used small relatively compact areas each day when actively tracked across daylight hours. Over periods of up to 60 days, blue-spotted flathead in our study showed two broad movement patterns; three out of five tagged fish showed strong site attachment and were detected on each day of tracking within the Hare Bay no-take sanctuary zone. The remaining two fish appear to have moved much larger distances of more than 3 km away from tagging location. Given the perception that soft sediment fishes are unlikely to show site attachment [7], and observations that blue-spotted flathead can be strong active swimmers (Fetterplace personal observation from baited underwater video; see data and materials section), it is particularly interesting that the majority of tagged fish in our study showed such strong site attachment. The ability of blue-spotted flathead to target many types of prey [19] coupled with the expected ambush predation by flathead species in general [20] could explain why blue-spotted flathead generally utilise relatively small areas over a day. Why some individuals continue to show this compact space use over periods of 60 days and others move away is not clear.
Intriguingly, the movement patterns of the oceanic blue-spotted flathead assessed in this study are consistent with those for estuarine dusky flathead (Platycephalus fuscus) found in southern Australia [21]. Dusky flathead were found to be largely sedentary, often remaining in one section of Gippsland Lakes for months. A small number of dusky flathead, however, were recorded moving up to 30 km over a few days. The use of active tracking in our study provided high-resolution movement and space-use patterns over a much smaller scale (10–100s of metres). Unexpectedly, and contrary to suggestions that fish on soft sediments would likely move over larger distances than those on hard substrata [7], blue-spotted flathead in our study also exhibited short-term site attachment comparable to many temperate reef fishes (e.g. [11, 22]). In addition, blue-spotted flathead MAI of 22.11–44.96 m h−1 (mean ± SE = 29.34 ± 4.15) is much lower than the reef-associated luderick (Girella tricuspidata, 165.4 ± 74.87 m h−1; mean ± SE) assessed within the same embayment and with the same tracking technique [23].
Two fish were lost from the study after 4 and 25 days. This was despite extensive searches of at least 3 km from their last recorded positions. The underlying reason for this is unclear but could conceivably include capture, tag failure, predation, or movement out of the study site. Our observations suggest that blue-spotted flathead are robust and survive surgery well; they recover readily from anaesthetic and, lacking a swim-bladder, are unaffected by barotrauma. Previous tagging effects studies have indicated that ‘tagging-induced’ mortality tends to occur within the first 24 h after release [24]. Four out of five of our tagged fish were detected moving up to 25 days after surgery. This suggests that mortality from surgery in our study was unlikely. We would argue instead that the two fish that were not detected for the entire study simply moved out of the study area. Capture is unlikely, at least in the study area, due to the study area being in a no-take sanctuary zone. As these two fish may in fact have travelled outside of tracking range, it follows that some part of the population moves much greater distances than the averages estimated here. Why they moved remains unclear and as our study is preliminary with a small sample size it not possible to estimate exactly what portion of the blue-spotted flathead population makes these larger-scale movements or how large these movements may be.
The larger-scale movements shown by two fish do not appear to be driven by size, as both small and larger fish left the study area and conversely both small and larger fish also showed site attachment. As it is not possible to distinguish the sexes of blue-spotted flathead based on markings or size (they are not known to show sexual size dimorphism), it is more difficult to assess whether these movements may be related to the sex of the fish. Many fish make seasonal migrations at specific times of year (e.g. [25]), and the closely related dusky flathead have been reported to seasonally migrate in order to spawn, based on indirect evidence such as aggregation sightings and the capture of spawning females around the mouths of estuaries [28]. While blue-spotted flathead are thought to spawn year round [26], there are no published evidence to support this and no evidence of migration movements to date. Further investigation is required to determine whether or not the larger movements shown by some of our tagged fish are just roaming movements over scales greater than our study size or are linked to spawning movements.
We did not catch any blue-spotted flathead on, or detect tagged fish blue-spotted flathead moving onto seagrass or surrounding reef, suggesting that they are exclusively soft sediment fish. Our movement data supports findings of recent baited remote underwater video (BRUV) studies where no blue-spotted flathead were recorded on reef within Jervis Bay (Rees, Davis and Knott, unpublished data and Coleman et al. [27]). However, other BRUV studies have found very small numbers of blue-spotted flathead on reef habitat; for example in Batemans Marine Park, Kelaher et al. [28] recorded blue-spotted flathead on five out of 384 drops over 5 years; this raises the possibility that blue-spotted flathead occasionally venture into edge areas of reef and seagrass habitats or reside there in very low numbers.
Many studies on the effectiveness or impacts of MPAs have focused on changes in abundances and diversity, without taking into account critical information on movement patterns of the species within them [2, 29]. This is often because this information is not available or because while potentially very useful, quantifying the movement patterns and observing the natural behaviour of marine fish in the field is difficult to achieve. Without knowledge of the basic movement patterns of a species, it is difficult to predict effectiveness of spatial protection measures such as MPAs [6]. Our study indicates that no-take sanctuary zones protecting soft sediment habitats in JBMP appear large enough to adequately encompass the expected short-term movement of blue-spotted flathead exhibiting site attachment. However, our data suggest that two movement patterns are likely to exist within the population, one that is highly site attached, and thus would potentially benefit from MPAs, and one that tends to roam, and thus may not benefit as much. If these preliminary data are found to be representative of longer-term patterns of movement and activity space use by a large part of the blue-spotted flathead population, then it is likely that the Hare Bay no-take sanctuary zone is sufficiently large to provide protection for a large number of blue-spotted flathead. If this is the case, we would suggest that comparably sized zones on soft sediments in other areas of temperate Australia may also be appropriate. Though it is beyond the scope of this study, investigating what portion of the blue-spotted flathead population would need to show site attachment for spatial closures like MPAs to be effective will require tagging of much larger numbers of fish and deserves further attention.
As this investigation was a preliminary assessment for movement of blue-spotted flathead with a view to expanding the duration and area of coverage, the current study has a number of implications for design of a large-scale tracking array. As a large tracking array can be expensive and time-consuming to install, our data provide guidance to best place passive receivers to cover this movement most efficiently. Our results indicate that using a tightly spaced passive acoustic array for investigation of the movement of this species is feasible and would yield meaningful results. However, given the potential wider ranging movements of this species, using multiple approaches would be useful to provide a more comprehensive understanding of their movement patterns. At the current study site, the entrance to Jervis Bay has now been gated and an array of receivers placed around the edge of the bay. These extra receivers (also part of other ongoing studies) should provide a good idea of visitation to other sections of Jervis Bay and also detect if fish leave Jervis Bay.
Conclusions
Our study, the first to document the movement of blue-spotted flathead, provides clear evidence of short-term site attachment and compact space use by part of the blue-spotted flathead population in Jervis Bay. We also highlight the benefit of using active tracking as a first step in understanding the movement of unstudied species. The area used by tagged fish showing site attachment over a 60-day study period was much smaller than no-take sanctuary zones on soft sediments in Jervis Bay Marine Park. However, our results also suggest that part of the population is also non-resident. While these results suggest that blue-spotted flathead may respond positively to protection provided by the no-take sanctuary zones in place, further tracking on a larger number of fish is needed to determine exactly what proportion of the population shows site attachment and if it continues over the long term. Lastly, our results demonstrate that if we are to effectively manage fish found on soft sediments we need to revisit the current view that fish on this habitat are unlikely to show site attachment.
Abbreviations
- KUD:
-
kernel utilisation distribution
- JBMP:
-
Jervis Bay Marine Park
- MPA:
-
marine protected area
- RI:
-
residency index
- D:
-
displacement
- FD:
-
furthest distance
- h :
-
smoothing parameter
- BRUV:
-
baited remote underwater video
References
Topping D, Lowe C, Caselle J. Home range and habitat utilization of adult California sheephead-Semicossyphus pulcher (Labridae), in a temperate no-take marine reserve. Mar Biol. 2005;147(2):301–11.
Gerber LR, Botsford LW, Hastings A, Possingham HP, Gaines SD, Palumbi SR, Andelman S. Population models for marine reserve design: a retrospective and prospective synthesis. Ecol Appl. 2003;13(sp1):47–64.
Kramer D, Chapman M. Implications of fish home range size and relocation for marine reserve function. Environ Biol Fishes. 1999;55(1–2):65–79.
Gaines SD, White C, Carr MH, Palumbi SR. Designing marine reserve networks for both conservation and fisheries management. Proc Natl Acad Sci. 2010;107(43):18286–93.
Palumbi SR. Marine reserves and ocean neighborhoods: the spatial scale of marine populations and their management. Annu Rev Environ Resour. 2004;29(1):31–68.
Grüss A, Kaplan DM, Guénette S, Roberts CM, Botsford LW. Consequences of adult and juvenile movement for marine protected areas. Biol Conserv. 2011;144(2):692–702.
Caveen AJ, Sweeting CJ, Willis TJ, Polunin NVC. Are the scientific foundations of temperate marine reserves too warm and hard? Environ Conserv. 2012;39(3):199–203.
Ferguson AM, Harvey ES, Knott NA. Herbivore abundance, site fidelity and grazing rates on temperate reefs inside and outside marine reserves. J Exp Mar Biol Ecol. 2016;478:96–105.
Lee KA, Huveneers C, Macdonald T, Harcourt RG. Size isn’t everything: movements, home range, and habitat preferences of eastern blue gropers (Achoerodus viridis) demonstrate the efficacy of a small marine reserve. Aquat Conserv Mar Freshw Ecosyst. 2015;25(2):174–86.
Harasti D, Lee KA, Gallen C, Hughes JM, Stewart J. Movements, home range and site fidelity of snapper (Chrysophrys auratus) within a temperate marine protected area. PLoS ONE. 2015;10(11):e0142454.
Willis TJ, Parsons DM, Babcock RC. Evidence for long-term site fidelity of snapper (Pagrus auratus) within a marine reserve. NZ J Mar Freshw Res. 2001;35(3):581–90.
Wilson WH. Competition and predation in marine soft-sediment communities. Annu Rev Ecol Syst. 1991;21:221–41.
Sciberras M, Jenkins SR, Kaiser MJ, Hawkins SJ, Pullin AS. Evaluating the biological effectiveness of fully and partially protected marine areas. Environ Evid. 2013;2(1):1–31.
McGrouther M. Bluespotted Flathead, Platycephalus caeruleopunctatus McCulloch, 1922. Australian Museum Website. Published 15 September 2012. http://australianmuseum.net.au/eastern-blue-spotted-flathead-platycephalus-caeruleopunctatus-mcculloch-1922. Accessed 20 June 2016.
R Development Core Team R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN: 3-900051-07-0. 2010. http://wwwR-project.org.
Dwyer R, Brooking C, Brimblecombe W, Campbell H, Hunter J, Watts M, Franklin C. An open Web-based system for the analysis and sharing of animal tracking data. Anim Biotelem. 2015;3(1):1.
Kie JG. A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses. Anim Biotelem. 2013;1(1):1–12.
Taylor MD, Laffan SD, Fielder DS, Suthers IM. Key habitat and home range of mulloway Argyrosomus japonicus in a south-east Australian estuary: finding the estuarine niche to optimise stocking. Mar Ecol Prog Ser. 2006;328:237–47.
Barnes L, Leclerc M, Gray C, Williamson J. Dietary niche differentiation of five sympatric species of Platycephalidae. Environ Biol Fishes. 2011;90(4):429–41.
Platell ME, Potter IC. Distributions, size compositions and diets of two abundant benthic ambush-feeding teleosts in coastal waters of south-western Australia. J Mar Biol Assoc UK. 1998;78(02):587–608.
Hindell JS. Determining patterns of use by black bream Acanthopagrus butcheri (Munro, 1949) of re-established habitat in a south-eastern Australian estuary. J Fish Biol. 2007;71(5):1331–46.
Edgar GJ, Barrett NS, Morton AJ. Patterns of fish movement on eastern Tasmanian rocky reefs. Environ Biol Fishes. 2004;70(3):273–84.
Ferguson AM, Harvey ES, Taylor MD, Knott NA. A herbivore knows its patch: luderick, Girella tricuspidata, exhibit strong site fidelity on shallow subtidal reefs in a temperate marine park. PLoS ONE. 2013;8(5):e65838.
Thorstad EB, Næsje TF, Fiske P, Finstad B. Effects of hook and release on Atlantic salmon in the River Alta, northern Norway. Fish Res. 2003;60(2–3):293–307.
Hirose T, Minami T. Spawning grounds and maturation status in adult flathead flounder (Hippoglossoides dubius); off Niigata Prefecture, Sea of Japan. Fish Sci. 2007;73(1):81–6.
Rowling K, Hegarty A, Ives M. Bluespotted flathead (Playtcephalus caeruleopunctatus). In status of fisheries resources in NSW 2008/09, NSW Industry & Investment, Cronulla; 2010, p. 47–9.
Coleman MA, Bates AE, Stuart-Smith RD, Malcolm HA, Harasti D, Jordan A, Knott NA, Edgar GJ, Kelaher BP. Functional traits reveal early responses in marine reserves following protection from fishing. Divers Distrib. 2015;21(8):876–87.
Kelaher BP, Coleman MA, Broad A, Rees MJ, Jordan A, Davis AR. Changes in fish assemblages following the establishment of a network of no-take marine reserves and partially-protected areas. PLoS ONE. 2014;9(1):e85825.
Claudet J, Osenberg CW, Benedetti-Cecchi L, Domenici P, García-Charton J-A, Pérez-Ruzafa Á, Badalamenti F, Bayle-Sempere J, Brito A, Bulleri F, Culioli J-M, Dimech M, Falcón JM, Guala I, Milazzo M, Sánchez-Meca J, Somerfield PJ, Stobart B, Vandeperre F, Valle C. Marine reserves: size and age do matter. London: Wiley-Blackwell; 2008. p. 481–9.
Fetterplace LC, Taylor MD, Knott NA. Data from: ‘Jervis Bay Marine Park: active tracking of blue-spotted flathead’. http://ZoaTrack.org; 2016.
Williams RJ, West G, Morrison D, Creese RG. Estuarine resources of NSW. In: The NSW comprehensive coastal assessment toolkit. CD ROM. ISBN: 0 7347 5805 7. NSW Department of Planning; 2007.
Authors’ contributions
LF designed the study, conducted fieldwork, analysed data, drafted the manuscript and created the figures and graphics; AD designed the study, provided materials and field resources and assisted in drafting of the manuscript; NK designed the study, conducted fieldwork, provided materials and field resources and assisted in analyses and drafting of the manuscript; JN created the figures and graphics; MT provided materials and field resources, assisted in analyses and assisted in drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We wish to thank the staff at Jervis Bay Marine Park that assisted in tracking of flathead, in particular Ian Osterloh, Adrian Ferguson, Mark Fackerell, Matt Carr, Matt Rees and Marie Claire Demers. We also thank Duane Byrnes for providing valuable GIS assistance and Margie Andreason for proof reading a number of drafts. We acknowledge the efforts of two anonymous reviewers who helped to improve the initial manuscript substantially.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data set supporting the conclusions of this article is available in the Zoatrack repository (publisher: the Atlas of Living Australia), DOI: 10.4226/68/5701CE37BD10D, DOI URL: http://dx.doi.org/10.4226/68/5701CE37BD10D [30]. Blue-spotted flathead baited underwater footage can be found at: https://vimeo.com/162039402.
Ethics approval
The relevant ethics approval for this study was granted by a Department of Primary Industries NSW Animal Ethics Committee of qualified scientific and lay members (AEC Number 100802/04). Field work was carried out under NSW Fisheries Scientific Collection Permit P01/0059(A)-2.0.
Funding
Funding and in-kind support for this work was provided by the NSW Department of Primary Industries. Additional student funding was provided by the University of Wollongong.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fetterplace, L.C., Davis, A.R., Neilson, J.M. et al. Active acoustic tracking suggests that soft sediment fishes can show site attachment: a preliminary assessment of the movement patterns of the blue-spotted flathead (Platycephalus caeruleopunctatus). Anim Biotelemetry 4, 15 (2016). https://doi.org/10.1186/s40317-016-0107-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-016-0107-6