Abstract
Background
Toxoplasma gondii is an obligate intracellular apicomplexan parasite and is responsible for zoonotic toxoplasmosis. It is essential to develop an effective anti-T. gondii vaccine for the control of toxoplasmosis, and this study is to explore the immunoprotective effects of a live attenuated vaccine in mice and cats.
Methods
First, the ompdc and uprt genes of T. gondii were deleted through the CRISPR-Cas9 system. Then, the intracellular proliferation and virulence of this mutant strain were evaluated. Subsequently, the immune responses induced by this mutant in mice and cats were detected, including antibody titers, cytokine levels, and subsets of T lymphocytes. Finally, the immunoprotective effects were evaluated by challenge with tachyzoites of different strains in mice or cysts of the ME49 strain in cats. Furthermore, to discover the effective immune element against toxoplasmosis, passive immunizations were carried out. GraphPad Prism software was used to conduct the log-rank (Mantel–Cox) test, Student’s t test and one-way ANOVA.
Results
The RHΔompdcΔuprt were constructed by the CRISPR-Cas9 system. Compared with the wild-type strain, the mutant notably reduced proliferation (P < 0.05). In addition, the mutant exhibited virulence attenuation in both murine (BALB/c and BALB/c-nu) and cat models. Notably, limited pathological changes were found in tissues from RHΔompdcΔuprt-injected mice. Furthermore, compared with nonimmunized group, high levels of IgG (IgG1 and IgG2a) antibodies and cytokines (IFN-γ, IL-4, IL-10, IL-2 and IL-12) in mice were detected by the mutant (P < 0.05). Remarkably, all RHΔompdcΔuprt-vaccinated mice survived a lethal challenge with RHΔku80 and ME49 and WH6 strains. The immunized sera and splenocytes, especially CD8+ T cells, could significantly extend (P < 0.05) the survival time of mice challenged with the RHΔku80 strain compared with naïve mice. In addition, compared with nonimmunized cats, cats immunized with the mutant produced high levels of antibodies and cytokines (P < 0.05), and notably decreased the shedding numbers of oocysts in feces (95.3%).
Conclusions
The avirulent RHΔompdcΔuprt strain can provide strong anti-T. gondii immune responses, and is a promising candidate for developing a safe and effective live attenuated vaccine.
Graphical abstract

Similar content being viewed by others
Background
Toxoplasma gondii is an intracellular protozoan parasite and can infect nearly all warm-blooded animals including humans [1]. It is estimated that approximately one-third of the world's population is infected with T. gondii [2]. Humans acquire T. gondii infection usually through ingestion of tissue cysts in raw or undercooked meat, oocysts in food or water, or congenitally via the placenta [3, 4]. Although T. gondii usually appears as a latent infection in people with normal immunity, it can cause serious complications in individuals with suppressed immune systems such as AIDS patients or people with malignant tumors [5, 6]. Pregnant women infected with T. gondii may experience miscarriage and stillbirth, and the fetus will have deformities or severe intellectual disability after birth [7]. The infection of intermediate animals including cattle, sheep and pigs may result in huge economic losses to the farm and potential health risks to humans [8].
Prevention or treatment of toxoplasmosis is difficult due to the complex life cycle and elaborate immune escape system of the parasite to establish chronic infection in most hosts [3, 9]. So far, toxoplasmosis is mainly treated with drugs such as pyrimethamine and sulfadiazine. However, these drug treatments are only effective in the acute infection stage and may cause serious side effects and promote the development of drug-resistant strains [10,11,12]. Therefore, novel drugs and effective treatments for T. gondii infection should be discovered and developed.
Vaccination is a promising and effective long-term approach for disease control and prevention [13]. The current anti-toxoplasmosis vaccines mainly include protein vaccine, DNA vaccine, live vector vaccine and live attenuated vaccine [13]. Among the existing vaccines against T. gondii, live attenuated vaccines provided the best protection with robust cellular and humoral responses to toxoplasmosis in murine models [14]. Moreover, the only commercial T. gondii vaccine (Toxovax®) is also a live attenuated vaccine developed through continuous subculture from S48 T. gondii tachyzoites [15, 16]. However, due to the potential risk of virulence recovery, the vaccine can’t be used in humans and is mainly used in sheep. In contrast, genetic deletions may completely prevent virulence regression compared to naturally attenuated strains [7]. Recently, several studies have demonstrated the stability and protective effect of gene knockout live attenuated vaccines against T. gondii. For example, Li et al. developed a double gene deletion mutant of gra17 and npt1 in the RH strain of T. gondii, which can protect mice from acute, chronic and congenital toxoplasmosis [17].
Pyrimidine plays an important role in parasite proliferation. Thus, as the precursor of all pyrimidines, uridine monophosphate (UMP) is one of the most important nucleotides in RNA for T. gondii [18, 19]. Through de novo biosynthesis or salvage pathways, T. gondii can acquire adequate pyrimidine and UMP for propagation [20]. The de novo pyrimidine biosynthesis pathway mainly functions under normal circumstances, while the salvage pathway only functions when the de novo biosynthesis pathway is blocked [21]. Studies have shown that when the de novo pyrimidine biosynthesis pathway of T. gondii is disrupted, it has little effect on the ability of T. gondii to invade host cells, but its proliferation and virulence are significantly weakened [20]. Orotidine-5'-monophosphate decarboxylase (OMPDC) is the terminal enzyme belonging to the de novo biosynthesis pathway, and studies have found that after knocking out the ompdc gene of T. gondii, the mutant strain lost its replication ability and virulence [22, 23]. According to these studies, the pyrimidine auxotrophic strain of T. gondii can be used as an alternative live attenuated vaccine. However, we cannot ignore the fact that T. gondii with ompdc gene knockout can still proliferate weakly in vitro [24]. As mentioned above, uracil phosphoribosyltransferase (UPRT) is a key enzyme in the UMP salvage pathway [25], which could contribute to parasite proliferation when the de novo biosynthesis pathway is blocked. Thus, to completely block the uracil synthesis pathway, here we generated a mutant with double gene deletion of ompdc and uprt in the RHΔku80Δhxgprt strain by a clustered regularly interspaced short palindromic repeats (CRISPR)/cas9 system and evaluated the immune protection of this strain against toxoplasmosis.
Methods
Animals and ethics statement
Six- to eight-week-old BALB/c and BALB/c-nu mice were purchased from the Center of Laboratory Animal of Hangzhou Medical College and 3-month-old cats were purchased from a local breeder. All cats were tested serologically and found to be free of T. gondii and viruses, including feline calicivirus, coronavirus, feline immunodeficiency virus, feline leukemia virus, and feline parvovirus. All animals were raised under standard conditions according to the Animal Management Regulations of the People's Republic of China. Animal experiments were approved by the Animal Care and Use Committee of Hangzhou Medical College (2018–027).
Parasite and cell culture
Tachyzoites of the ME49, WH6, RHΔku80Δhxgprt, RHΔku80Δuprt::HXGPRT and RHΔku80ΔuprtΔhxgprt strains were maintained in human foreskin fibroblasts (HFFs) in our laboratory in high-glucose Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Thermo Fisher Scientific, MA, USA) supplemented with 5% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, MA, USA), penicillin (100 units/ml; Thermo Fisher Scientific, MA, USA), and streptomycin (100 µg/ml; Thermo Fisher Scientific, MA, USA). The RHΔku80ΔuprtΔompdc::HXGPRT strain was additionally treated with 250 μmol/L uracil and 200 μmol/L UMP (Sigma-Aldrich, MO, USA) [20]. HFFs were cultured in high-glucose DMEM supplemented with 10% FBS at 37 °C, and 5% CO2. In all experiments, freshly egressed tachyzoites were filtered with 5-µm polycarbonate membranes to remove host cell debris.
Preparation of soluble T. gondii antigens (STAg)
Soluble T. gondii antigens were prepared as previously described [26]. In brief, suspensions of T. gondii RHΔku80 tachyzoites were collected in phosphate buffered saline (PBS), subjected to repeated freeze and thaw cycles, and then sonicated on ice at 80 W/s. The prepared product was centrifuged at 14,000 × g for 30 min at 4 °C. The supernatant was filtered through 0.22 μm sterile nitrocellulose filters. The STAg concentration was determined by the Bradford kit (Beyotime, Shanghai, China), and aliquots were stored at − 80 °C until use.
Construction of RHΔuprt and RHΔompdcΔuprt mutant strains
The primers involved in this experiment are listed in Additional file 1: Table S1. The mutant strains were constructed using the CRISPR/Cas9 approach based on the RHΔku80Δhxgprt strain according to Shen B’s protocol [27]. Briefly, the hxgprt and ompdc targeting CRISPR plasmids were generated by replacing the uprt targeting guide RNA in pSAG1::CAS9-U6::sgUPRT with an hxgprt and ompdc single-guide RNA by site-directed mutagenesis (New England Biolabs, MA, USA). All plasmids were verified by DNA sequencing prior to use. First, the uprt CRISPR plasmid and HXGPRT homologous template were electro transfected into RHΔku80Δhxgprt tachyzoites. The uprt-deleted parasites were screened with 25 μg/ml xanthine (Sigma-Aldrich, MO, USA) and 25 μg/ml mycophenolic acid (Sigma-Aldrich, MO, USA), and single-cloned by limiting the dilution. RHΔku80Δuprt::HXGPRT single positive clones were identified by PCR and qRT-PCR. Second, the hxgprt CRISPR plasmid and uprt5’UTR-3’UTR homologous template were electro transfected into RHΔku80Δuprt::HXGPRT tachyzoites. The RHΔku80ΔuprtΔhxgprt strain was screened with 10 μmol/L 5-fluorodeoxyuracil (Sigma-Aldrich, MO, USA) and identified by PCR. Finally, the ompdc CRISPR plasmid and HXGPRT homologous template were electro transfected into RHΔku80ΔuprtΔhxgprt tachyzoites. The RHΔku80ΔuprtΔompdc::HXGPRT strain was screened and identified in the same way as described above. The PCRs were carried out in a volume of 25 μl containing 12.5 μl of 2 × Taq PCR Master Mix (TIANGEN, Beijing, China), 1 μl of each primer (10 µmol/L), 1 μl genomic DNA template, and 9.5 μl of sterile distilled water by the conditions with an initial melting step at 98 ℃ for 3 min, followed by 30 cycles with each cycle at 98 ℃ for 30 s, 60 ℃ for 30 s, and 72 ℃ for 1 min, followed by a final extension at 72 ℃ for 10 min.
Total RNA of T. gondii tachyzoites was extracted using the TRIzol reagent (Invitrogen, CA, USA). The cDNA was synthesized using a First Strand cDNA Synthesis Kit (ReverTra Ace -α-, Toyobo, Osaka, Japan). qRT-PCR was carried out in a volume of 20 μl containing 10 μl of 2 × Real time PCR Master Mix (Toyobo, Osaka, Japan), 0.4 μl of each primer (10 µmol/L), 1 μl cDNA template, and 8.2 μl of sterile distilled water and amplification was performed on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, CA, USA). The relative mRNA levels were calculated using the comparative ΔCt method using the formula 2−ΔΔCt.
Parasite intracellular replication assay
An indirect immunofluorescence assay (IFA) was used to detect the intracellular proliferation of parasites. Equal amounts of RHΔku80ΔompdcΔuprt and RHΔku80 strains were inoculated in plates filled with HFF. The wells inoculated with RHΔku80ΔompdcΔuprt strains were cultured with or without 250 μmol/L uracil and 200 μmol/L UMP for 24 h and 48 h, and the cells were fixed with 4% paraformaldehyde (Beyotime, Shanghai, China) solution for 30 min. The cells were incubated with rabbit anti-GRA7 polyclonal antibody and Alexa Fluor488-conjugated goat anti-rabbit IgG (Abcam, Oxford, UK). The intracellular parasites at different stages of proliferation (i.e., 1, 2, 4, 8, 16 or more than 16 tachyzoites) were counted from 100 parasitophorous vacuoles under fluorescence microscopy (Nikon eclipse 80i, Tokyo, Japan). The experiment was performed in three independent biological repeats.
Parasite plaque assay
HFF monolayers grown in six-well plates were infected with 500 tachyzoites of RHΔku80 or RHΔku80ΔompdcΔuprt strains in each well. RHΔku80ΔompdcΔuprt strains were cultured with or without 250 μmol/L uracil and 200 μmol/L UMP. After 7 days, the cells were fixed with 4% paraformaldehyde (Beyotime, Shanghai, China) and stained with crystal violet. Finally, the number and size of plaques were analyzed.
Evaluation of the infectivity of the mutant parasites in mice
Tachyzoites of the RHΔku80 and RHΔku80ΔompdcΔuprt strains were washed and resuspended in PBS. Four groups of mice were injected intraperitoneally (i.p.) with 0.1 ml of PBS, 1 × 102 tachyzoites of the RHΔku80 strain or 1 × 105 or 1 × 106 tachyzoites of the RHΔku80ΔompdcΔuprt strain (5 BALB/c mice per group). Moreover, serial doses (1 × 102, 1 × 103, 1 × 104, 1 × 105, 1 × 106 tachyzoites) of the RHΔompdcΔuprt strain or 1 × 102 tachyzoites of RHΔku80 strain were i.p. injected into immunodeficient mice (5 BALAB/c-nu mice per group). Survival of mice was monitored daily for 30 days.
Evaluation of the infectivity of the mutant parasites in cats
Two groups of cats were injected intramuscularly (i.m.) with 0.1 ml of PBS or 1 × 107 tachyzoites of the RHΔku80ΔompdcΔuprt strain, faecal samples were collected daily from 1 day post infection (dpi) to 10 dpi and monitored for T. gondii oocysts. The oocysts were purified as described previously [28]. Briefly, first, 1 g of feces was weighed and mixed with an appropriate amount of water and centrifuged to collect the sediment. Next, the sediment was mixed with 10 times the volume of sucrose solution with a specific gravity of 1.15 and centrifuged (1500 × g). Finally, approximately 5 ml of the supernatant was mixed with 45 ml of water and centrifuged (1500 × g); the sediment was resuspended in 1 ml of water and counted under a light microscope (Nikon Ti-S, Tokyo, Japan).
Detection of parasite load
Six- to eight-week-old BALB/c mice (36 per group) were infected i.p. with 1 × 106 tachyzoites of the RHΔku80 and RHΔku80ΔompdcΔuprt strains. The ascites of mice (30 per group) were collected from day 1 to day 5, and mice (6 per group) were executed on the fourth day to collect liver and lung tissues. Genomic DNA was sacrificed by using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Amplifications of genomic DNA were carried out with primers targeting the repeated 529 bp gene of T. gondii. The qPCR system was as described above and the amplification was performed on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, CA, USA). The parasite burden was subsequently determined through a standard curve of the 529 bp gene.
Immunization and challenge in mice
Mice (30 per group) were intraperitoneally immunized with 1 × 106/100 µl RHΔku80ΔompdcΔuprt strain tachyzoites or an equivalent amount of PBS, once every two weeks, for a total of three immunizations. Mouse sera were collected from the tail vein at 0, 2, 4, and 6 weeks. After centrifugation at 4000 × g for 5 min, the sera were collected and stored at − 20 °C until further use. Two weeks after the last immunization, mice were intraperitoneally injected with 1 × 103 tachyzoites of the RHΔku80 strain, 1 × 103 bradyzoites of the ME49 strain, or 1 × 103 tachyzoites of the WH6, and the survival rate was recorded daily.
Immunization and challenge in cats
Cats (6 per group) were intramuscularly immunized with 1 × 107/100 µl RHΔku80ΔompdcΔuprt strain tachyzoites or an equivalent amount of PBS once every three weeks for a total of two immunizations. Sera samples were collected at 0, 2 and 4 weeks and stored at − 20 °C until further use. One week after the last immunization, oral administration of 200 ME49 cysts was carried out in immunized and control cats. According to the above method, feces were collected and purified for oocyst counting for 15 consecutive days after the challenge.
Measurement of antibody responses in mice and cats
Antibody levels of IgG, IgG1, and IgG2a of mice or IgG of cats were detected by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtiter plates were coated with 100 μl (10 µg/ml) STAg (diluted in PBS) and incubated at 4 °C overnight. Then the plates were washed five times with PBS containing 0.05% Tween 20 (PBST) and blocked with PBST containing 5% non-fat milk powder for 1 h at 37 °C. The plates were washed five times with PBST. Then, 100 µl of sera samples diluted in PBST (1:100) containing 5% non-fat milk powder were added to the wells and incubated at 37 °C for 1 h. The plates were washed five times with PBST, then 100 µl of diluted horseradish-peroxidase-conjugated goat anti-mouse IgG (Abcam, Oxford, UK, 1:10,000), anti-mouse IgG1 (Abcam, Oxford, UK, 1:5000), IgG2a (Abcam, Oxford, UK, 1:5000) or horseradish-peroxidase-conjugated goat anti-cat IgG (Abcam, Oxford, UK, 1:5000) was added for incubation for 1 h at 37 °C. After washing five times, 100 µl of 3,3′,5,5′-tetramethylbenzidine (TMB) chromogen solution (Beyotime, Shanghai, China) was added to each well and the plates were incubated at 37 °C for 15 min. After adding 100 µl of stop solution for TMB substrate (Beyotime, Shanghai, China), the absorbance was measured by an ELISA plate reader at 450 nm.
Cytokine assay
Mice (5 per group) were sacrificed two weeks after the last immunization, and the spleens were aseptically removed to prepare a single-cell suspension. Briefly, the spleen was placed on a 70 μm cell filter mesh and then added into a 50 ml centrifuge tube by using a 5 ml syringe plunger to grind the spleen. Hank's solution was added dropwise while grinding, and the mesh was rinsed with Hank's solution after grinding to obtain a single cell suspension. The supernatant was discarded after centrifugation, and then 5 times the cell volume erythrocyte lysing solution was added to the cell. Afterwards, the mixture was gently mixed by pipetting and lysed for 2 min. After centrifugation again, the supernatant was discarded, and the cells were resuspended in high-glucose DMEM containing 20% FBS and counted on a bovine abalone counter. Splenocytes (1 × 106) from different groups of mice were seeded in sterile 96-well cell culture plates with a final volume of 100 µl. The culture supernatant was added to STAg at a final concentration of 10 μg/ml and the supernatant was collected for 24 h, 72 h, 96 h. The levels of secreted interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-10 (IL-10), interleukin-12 (IL-12) and interferon-γ (IFN-γ) were measured by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) using a BD™ Cytometric Bead Array (CBA) kit (BD Biosciences, Franklin Lakes, NJ, USA).
Lymphocyte proliferation assay
Two weeks after the last immunization, the spleens of three mice from each group were prepared and resuspended as described above. Splenocytes (1 × 105) from different groups of mice were added to 96-wellplates and stimulated with STAg (10 µg/ml) or DMEM high-glucose medium of equal volume (negative control). Moreover, no cells were added, and only the wells of medium were added as a blank control. Then, the splenic lymphocytes were incubated at 37 °C for 96 h with 5% CO2. Cell Counting Kit 8 (CCK-8, Solarbio, Beijing, China) (10 µl) was added according to the instructions and incubated for 4 h. Subsequently, the absorbance was measured by an ELISA plate reader at 450 nm to illustrate lymphocyte proliferation. The cell proliferation activity was calculated using the following formula: cell proliferation activity = (OD450 STAg−OD450 Blank)/(OD450 Control−OD450 Blank).
Flow cytometry analysis of T cell subsets
To analyze the percentage of CD4+ and CD8+ T lymphocytes, 1 × 106 splenocytes were prepared as described above and suspended in 100 µl PBS. After incubation with fluorochrome-labelled mAbs including FITC-CD3, APC-Cy7-CD4 and PE-CD8 (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature for 15 min in the dark, the cultures were washed with 2 ml PBS. After centrifugation, the samples were suspended in 500 µl PBS and fluorescence profiles were analyzed on a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) by FlowJo software (BD Biosciences, Franklin Lakes, NJ, USA, version 10.8.1).
Analysis of hematoxylin–eosin (HE) staining of the liver, spleen, and lung
The liver, spleen, and lung tissues of mice from each group (3 mice per group) were removed and soaked in 4% paraformaldehyde (Beyotime, Shanghai, China) at room temperature for 24 h, dehydrated with ethanol, cleared with xylene, embedded in wax and sliced with a slicer. Sections were then stained with hematoxylin and eosin (H&E) as described previously [29].
Passive immunization of sera and splenocytes from RHΔompdcΔuprt -vaccinated mice
Splenocyte suspensions without erythrocytes were prepared as described above, CD19+ B cells, CD8+ T cells or CD4+ T cells were purified (> 90% purity) using Miltenyi Mouse positive selection kits (Miltenyi Biotec, Cologne, Germany). Naïve mice received splenocytes (1 × 107), CD19+ B cells (2 × 106), CD4+ T cells (2 × 106) or CD8+T cells (2 × 106) from RHΔku80ΔompdcΔuprt-vaccinated mice, or splenocytes (1 × 107) from naïve mice via tail vein injection. After cell transfer for 24 h, mice were intraperitoneally injected with 1 × 103 tachyzoites of the RHΔku80 strain (5 mice per group) [30]. Two weeks after the last immunization, sera from immunized mice were collected as positive sera. In addition, sera from naïve mice were collected as negative sera. Subsequently, BALB/c mice (5 per group) were i.p. challenged with 1 × 103 RHΔku80 tachyzoites, and injected with positive or negative sera (200 μl/mouse) via the tail vein from day 0 to day 4. Survival of mice was monitored daily and the parasitic load in mouse (5 per group) peritoneal fluid was detected one day after the end of sera treatment.
Statistical analyses
Statistical analyses in this study were performed with GraphPad Prism version 8 (GraphPad Software Inc, CA, USA). The log-rank (Mantel–Cox) test was used to compare the survival curves. Independent Student’s t-test was conducted to compare two groups and one-way ANOVA was conducted to compare ≥ 3 groups. Quantitative variables were presented as the means ± standard deviations (SD). In all analyses, P < 0.05 was considered statistically significant.
Results
Generation of the RHΔompdcΔuprt Strains Using the CRISPR/Cas9 System
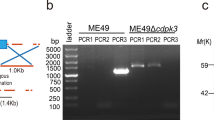
The HXGPRT marker was inserted into the uprt-specific guide RNA-targeted sequence region (Additional file 1: Fig. S1a). The single and stable RHΔku80Δuprt::HXGPRT clone was verified by PCR detection (Fig. 1a). Under the effects of sgRNA of hxgprt and enzyme Cas9, the HXGPRT marker was deleted (Additional file 1: Fig. S1b), and PCR results indicated that a single RHΔku80ΔuprtΔhxgprt strain was successfully selected (Fig. 1b). Subsequently, the ompdc gene was replaced by the HXGPRT marker in the RHΔku80ΔuprtΔhxgprt strain to yield the RHΔompdcΔuprt mutant (Additional file 1: Fig. S1c), and the stable mutant clone was determined by PCR and qPCR assays (Fig. 1c, d). In summary, the double gene knockout RHΔku80ΔuprtΔhxgprt strain was successfully generated.
CRISPR/Cas9-mediated generation of the ompdc-uprt deletion mutant in the Toxoplasma gondii type I RH strain. a Diagnostic PCR of the RHΔuprt::HXGPRT clone. PCR1 is used to detect drug gene and PCR2 is used to detect uprt gene b Diagnostic PCR confirming the removal of hxgprt. PCR1 is used to detect hxgprt gene, PCR2 is used to detect uprt gene and PCR3 is used to detect internal reference 529 bp gene c Diagnostic PCR of the RHΔuprtΔompdc::HXGPRT clone. PCR1 is used to detect drug gene, PCR2 and PCR3 is used to detect ompdc gene d qRT-PCR of the RHΔuprtΔompdc::HXGPRT clone. ***P < 0.001 by Student’s t test. CRISPR clustered regularly interspaced short palindromic repeats, PCR polymerase chain reaction, HXGPRT hypoxanthine–guanine phosphoribosyltransferase, qRT-PCR quantitative reverse transcription polymerase chain reaction
The RHΔompdcΔuprt mutant exhibited reduced cellular replication ability
To explore the biological characteristics of the RHΔompdcΔuprt mutant, invasion, parasite replication, and plaque assays were carried out. Plaque assay results showed that in the presence of uracil and ump, the mutant could grow as normally as the wild type RHΔku80 strain to form plagues, while plagues were rarely observed when the uracil and ump were removed from the culture medium of the RHΔompdcΔuprt mutant (Fig. 2a–c). In addition, the parasite replication assay showed that at 24 h and 48 h post infection, the number of RHΔompdcΔuprt mutant tachyzoites per parasitophorous vacuole (PV) was notably lower (P < 0.05) than that of wild type RHΔku80 tachyzoites in the absence of uracil and ump, and most PVs of this mutant consisted of 1 or 2 tachyzoites indicating a nearly quiescent condition of cellular proliferation (Fig. 2d, e, Additional file 1: Fig. S2). However, the number of RHΔompdcΔuprt mutant tachyzoites per PV presented a similar level to that of the wild type once supplied with uracil and ump, indicating the replication restoration. Furthermore, the results of the invasion assay showed limited differences between the wild type strain and the RHΔompdcΔuprt mutant regardless of whether they were supplied with or without uracil and ump (Additional file 1: Fig. S3), suggesting that the deletion of ompdc and uprt had no effect on invasion.
Deficiency of ompdc and uprt in the RH strain severely reduced parasitic proliferation in vitro. a Plaque assay comparing the growth of the RHΔku80 strain and RHΔku80ΔompdcΔuprt strain with or without the addition of 250 µmol/L uracil and 200 µmol/L UMP. b–c Number and size of the plaques. d–e Intracellular proliferation of the RHΔku80 strain and RHΔku80ΔompdcΔuprt strain with or without the addition of 250 µmol/L uracil and 200 µmol/L UMP. The number of parasites in each parasitophorous vacuole (PV) was determined at 24 h and 48 h. These results are from three independent trials. ***P < 0.001 by Student’s t test and one-way ANOVA, ns not significant. UMP uridine monophosphate, PV parasitophorous vacuole
The virulence of the RHΔompdcΔuprt mutant was severely attenuated in mice
The survival of mice was monitored daily, and the results showed that mice injected with the wild-type strain all died within 9 days, while the survival rates of RHΔompdcΔuprt mutant-injected mice were 100%, even when challenged with an infectious high dose of 106 tachyzoites (Fig. 3a, b), indicating that the virulence of this mutant was significantly attenuated. Subsequently, ascites samples from different groups of mice were collected from 1 to 5 dpi for parasite examination. The parasite number in wild type-infected mice increased remarkably day by day, while that of RHΔompdcΔuprt-injected mice notably decreased (P < 0.05) every day (Fig. 3c). Similarly, a large number of T. gondii tachyzoites were detected in the liver and lung tissues of RHΔku80-injected mice, while limited tachyzoites were detected by qPCR in these tissues from RHΔompdcΔuprt-injected mice on the 4th day post infection (Fig. 3d, e). These results demonstrated a significant reduction (P < 0.05) in parasite burden caused by the RHΔompdcΔuprt mutant. Next, the histological sections of liver, spleen, and lung tissues were subjected to HE staining for pathological examination. As shown in Fig. 3f, few changes were observed in tissue sections from RHΔompdcΔuprt-injected mice compared with naïve mice, while in wild type infected mice, large amounts of necrotic cells and absent lymphoid follicles were appeared in spleen tissue, obvious cellular separation was displayed in liver, and notably thicker alveolar walls were observed in lung tissues.
The virulence of the RHΔku80ΔompdcΔuprt mutant was severely attenuated in mice. a Tachyzoites of RHΔku80ΔompdcΔuprt or RHΔku80 tachyzoites were injected intraperitoneally (i.p.) into BALB/c mice (n = 5) and monitored for more than 30 d. b Tachyzoites of RHΔku80ΔompdcΔuprt or RHΔku80 tachyzoites were injected i.p. into BALB/c-nu mice (n = 5) and monitored for more than 30 d. c The parasite numbers in ascitic fluid of BALB/C mice infected with the 1 × 106 RHΔku80ΔompdcΔuprt strain and RHΔku80 strain from 1 to 5 days. d–e The parasite burden of liver and lung tissues infected with the RHΔku80ΔompdcΔuprt strain and RHΔku80 strain for 4 days. f Tissue damage in the spleen, liver and lung of naïve mice inoculated with the RHΔku80 strain and defective strain respectively. The 529 bp gene was detected by qPCR to demonstrate the number of T. gondii tachyzoites in each sample. ***P < 0.001 by Student’s t test and log-rank (Mantel–Cox) test. i.p. intraperitoneally, T. gondii Toxoplasma gondii, qPCR quantitative polymerase chain reaction
Robust humoral and cellular immune responses were elicited by vaccination with the RHΔompdcΔuprt mutant in mice
Significantly high levels of anti-T. gondii IgG were detected in vaccinated mice, and the IgG titer was increased remarkably (P < 0.05) after every vaccination (Fig. 4b), indicating that a robust humoral response was induced. The levels of IgG subclasses (IgG1 and IgG2a) were tested to characterize the immune response type. Both the levels of IgG1 and IgG2a were significantly higher (P < 0.05) in RHΔompdcΔuprt-vaccinated mice than in control mice (Fig. 4c). In addition, the level of IgG2a was notably higher than that of IgG1, indicating that vaccination with RHΔompdcΔuprt in mice elicited a Th1/Th2 mixed and Th1-biased immune response.
Immunization with the RHΔku80ΔompdcΔuprt mutant vaccine induced specific humoral and cellular responses. a Determination of IgG antibodies in the sera of BALB/c mice at 0, 2, 4, and 6 weeks. b Detection of antibody subtypes (IgG1 and IgG2a) in the sera of immunized mice 2 weeks after the last immunization. c–f Percentages of CD4+ T cells and CD8+ T cells subsets in immunized BALB/c mice. The results are shown as the means ± SD from three independent experiments. *P < 0.05, **P < 0.01 ***P < 0.001 by Student’s t test, ns not significant. SD standard deviation
After immunization with the RHΔompdcΔuprt mutant, a significant increase (P < 0.05) in the percentage of CD3+CD4+ T cells by 2 weeks post vaccination was observed compared with that of the controls (Fig. 4d, e). Additionally, the percentage of CD3+CD8+ T cells in vaccinated mice was increased to a much higher degree (P < 0.05) (Fig. 4f, g). In parallel, the rest of splenocytes from vaccinated and unvaccinated mice were cultured in vitro for further stimulation with STAg. The cytokine levels in the splenocyte supernatant were then detected by flow cytometry. Consistent with the levels of IgG1 and IgG2a, the proinflammatory cytokine levels, including Th1-type cytokines (IL-2, IL-12, IFN-γ) and Th2-type cytokines (IL-10, IL-4) of the immunized mice were notably higher (IL-2: 24.27 ± 3.35 pg/ml; IL-12: 83.83 ± 14.15 pg/ml; IFN-γ: 26.61 ± 2.68 ng/ml; IL-10: 1380 ± 357.7 pg/ml; IL-4: 92.22 ± 21.46 pg/ml) than those of the control mice (P < 0.05) (Fig. 5a–e). The results also showed a quick and robust proliferation (P < 0.05) of splenocytes once stimulated by the STAg (Fig. 5f), indicating the efficient cellular immune response induced by the immunization of the mutant. Of note, IL-12 and IFN-γ which are the key factors in cellular immune clearance of tachyzoites were also found significantly increased (P < 0.05) in sera samples of immunized mice and lasted for nearly a week post vaccination (Additional file 1: Fig. S4).
Pro-Inflammatory cytokines were elicited by immunization with RHΔku80ΔompdcΔuprt. Splenocytes collected from the immunized and non-immunized (6 weeks after immunization) mice were co-incubated with STAg (10 µg/ml). The levels of Th1 [IFN-γ (a), IL-2 (b), and IL-12 (c)] and Th2 [IL-4 (d) and IL-10 (e)] in the culture supernatants were measured by flow cytometry. (f) The proliferative responses of splenocytes were measured by a CCK8 kit. The results are presented as the means ± SD. (n = 5, ***P < 0.001 by Student’s t test). STAg soluble Toxoplasma gondii antigens, CCK Cell counting kit, SD standard deviation
RHΔompdcΔuprt immunization confers protection against infection with various types of T. gondii tachyzoites in mice
Now that the above results demonstrated that the strong immune responses were successfully stimulated, the protective efficacy provoked by the RHΔompdcΔuprt mutant was evaluated. Two weeks after the third vaccination, naïve and vaccinated mice were challenged with lethal doses of type I RHΔku80 strains. All naïve mice died within 10 days, while 100% of the vaccinated mice completely survived (Fig. 6a). In addition, when challenged with type II ME49 tachyzoites or Chinese locally isolated strain WH6, RHΔompdcΔuprt mutant-vaccinated mice presented 100% survival, while naïve mice all died within 13 or 14 days post infection (Fig. 6b, c). Subsequently, tissues including liver, spleen, and lung were collected for pathological examination after 6 days (RHΔku80) or 12 days (ME49) of challenged with tachyzoites. Obvious changes were observed in unvaccinated mice, such as liver cell necrosis, spleen body destruction, and lung congestion as described above, while the tissue sections of vaccinated mice showed limited changes (Fig. 6d).
Protective immunity induced by the RHΔku80ΔompdcΔuprt vaccine against T. gondii challenge infection in mice. a–c Survival curves of naïve or RHΔku80ΔompdcΔuprt immunized mice infected with RHΔku80, ME49 and WH6. Two weeks after the last immunization, mice were intraperitoneally (i.p.) challenged with 1 × 103 T. gondii tachyzoites of RHΔku80, ME49 and WH6 strains (5 mice/strain), and monitored for 30 days. d Organ damage in vaccinated mice and naïve mice after challenge with the RHΔku80 and ME49 strains. e Two weeks after the last immunization, whole splenocytes, CD4+, CD8+ and CD19+ splenocytes were harvested, and 1 × 107 total immune splenocytes, 2 × 106 CD8+ T cells, 2 × 106 CD4+ T cells, 2 × 106 CD19+ B cells, or 1 × 107 total naïve splenocytes were transferred to naïve recipient mice. Twenty-four hours after transfer mice mice were challenged with 1 × 103 T. gondii RHΔku80 i.p. and monitored for survival. f From day 0 to day 4 after infection, mice were treated with sera from RHΔku80ΔompdcΔuprt-vaccinated and naïve mice. g Parasite load detection after one day of sera treatment. *P < 0.05, **P < 0.01, *** P < 0.001 by log-rank (Mantel–Cox) test, ns not significant
The immune protection induced by RHΔompdcΔuprt vaccination could be adoptively transferred against acute infection in mice
Mice that received naïve splenocytes or RHΔompdcΔuprt-vaccinated CD4+ T cells succumbed to death by 9 dpi (Fig. 6e), in contrast, the passive immunization of purified CD19+ B cells, CD8+ T cells, or total splenocytes from RHΔompdcΔuprt-vaccinated mice survived a significantly longer (P < 0.05) time than naïve mice (Fig. 6e). The data showed a relative but significantly longer (P < 0.05) survival rate in passively immunized mice than in control mice (Fig. 6f). Consistent with this result, a significantly lower parasite load was observed in passively immunized mice (Fig. 6g).
Vaccination with the RHΔompdcΔuprt mutant was determined to be safe in cats and to induce a robust immune response
As showed in Table 2S, no oocysts were found in any of the RHΔompdcΔuprt-injected cats. Then, the immune response in cats was further evaluated. After vaccination for 2 doses, sera obtained from cats were used to determine the specific antibody response. Significantly high levels of anti-T. gondii IgG were detected in vaccinated cats, and the IgG titer was increased remarkably (P < 0.05) after every vaccination (Fig. 7a), indicating that a robust humoral response was induced by the mutant.
Protective immunity induced by the RHΔku80ΔompdcΔuprt vaccine against T. gondii challenge infection in cats. a Determination of IgG antibodies in the sera of cats at 0, 2 and 4 weeks. b Daily oocyst emissions of naïve or RHΔku80ΔompdcΔuprt immunized cats orally administered 200 cysts of ME49. c Total oocyst emissions of naïve or RHΔku80ΔompdcΔuprt immunized cats orally administered 200 cysts of ME49. (n = 6, *P < 0.05, ***P < 0.001 by Student’s t test, ns not significant.)
RHΔompdcΔuprt-vaccinated cats notably reduced the oocyst shed number and period
To evaluate the possibility of applying the vaccine in cats, we performed a challenge and tested the expulsion of oocysts. Oocysts were detectable at 3 dpi for all-infected cats, while RHΔompdcΔuprt-vaccinated cats notably reduced (P < 0.05) the shedding period (6 days) compared with that of naïve cats (11 days) as shown in Fig. 7b. Furthermore, the results showed a remarkable decrease (P < 0.05) in the total number of oocysts (95.3%) in vaccinated cats compared with naïve cats (Fig. 7c).
Discussion
Control of T. gondii is a major challenge since the parasite can cross the blood–brain barrier to develop a persistent infection where most chemicals are inaccessible [31]. Currently, the combination of pyrimethamine and sulfadiazine is commonly used clinically for the treatment of acute toxoplasmosis [32]. However, these drugs have limited effects on T. gondii cysts. It has become a popular theory that preventative strategies such as vaccination are a more effective way to provide protection against T. gondii. Attempts to develop vaccines over the last 60 years have acquired a few successful experimental candidates, among which live attenuated vaccines are the most promising ones. Live attenuated vaccines are non-disease cause mutants created by weakening or altering the pathogen. In this study, we applied the double gene knockout strain RHΔompdcΔuprt as a live attenuated vaccine and explored the induction of protective immunity against T. gondii challenge.
Undoubtedly, safety and effectiveness are the primary factors for a vaccine. Currently, there is only one vaccine called Toxovax on the market specifically derived from the S48 strain to reduce fetal abortion in sheep. However, this vaccine is not safe enough to use in humans due to the possibility that the strain may revert the ability to form cysts as the data show that it could cause acute infection and lead to death in murine models [33]. Recently, several studies with emphasis on gene modified mutants proved that robust protective immune responses had been elicited by these vaccines, such as RHΔgra17Δntp1 [17] and ME49Δldh [34] and the uracil auxotroph mutants (ΔcpsII and Δompdc) [20, 23]. With defective de novo UMP biosynthesis activity, uracil auxotroph mutants lose the ability to propagate in vivo and induce long-term protection against acute and chronic T. gondii infection. However, we cannot ignore the fact that their protection is not always 100%. As reported by Peng [24], the Δompdc strain supplied without uracil could still replicate relatively slow but never stop way in vitro, indicating that the salvage pathway may play a compensatory role. Although they concluded that in ompdc disrupted strains, the potential salvage of host cell uracil and nucleosides is not sufficient to support a significant rate of parasite replication in vitro [23], safety cannot be guaranteed when this live attenuated vaccine is used in meat producing animals or cats. Thus, according to the UMP synthesis and salvage pathways, we specifically deleted the ompdc and uprt through a CRISPR/Cas9 system to completely block the production or intake of UMP. Consistent with the predicted model, the RHΔompdcΔuprt strain lost viability in vitro, as confirmed by parasite replication and plaque assays. Furthermore, no deaths were observed when the RHΔompdcΔuprt strains were subjected to BALB/c or BALB/c-nu mice, demonstrating that this strain is safe in immunodeficient individuals. As documented and evaluated here, the tachyzoites could be found in ascitic fluids as early as 1 dpi in acute infection mice and parasite loads in tissues such as liver and lung increased notably [35]. However, we found a rather small number of parasites in ascitic fluids and tissues, which means that the RHΔompdcΔuprt strain is avirulent to mice. Additional visual evidence is that limited pathological changes or tachyzoites were found in histological sections from RHΔompdcΔuprt strain injected mice. To our knowledge, once infected by T. gondii, mouse tissues display obvious changes, such as clear cellular separation in the liver indicting hepatocellular dysfunction, thicker alveolar walls in the lung representing interstitial pneumonia, and plenty of necrotic cells in the spleen with absent lymphoid follicles [36]. Thus, this avirulent RHΔompdcΔuprt strain is nearly harmless to mice. In addition, the daily analysis of cat feces after vaccination with the RHΔompdcΔuprt strain revealed no oocysts, indicating that this attenuated mutant also lost the ability of sexual reproduction compared with previous results [37]. Consequently, we conclude that the attenuated strain is unable to reproduce in mouse and cat models with limited harm or side effects.
Effectiveness is a more crucial factor for an ideal vaccine. We noticed that the type of attenuated T. gondii strain for vaccine development is also of great importance. As reported by Xia et al. [34], the ME49Δldh mutant induced long-term protection against type 2 and 3 strains, while short-term protection against the type 1 RH strain. Therefore, we chose the RH strain to construct the live attenuated vaccine. Consistently, this defective vaccine contributed 100% protection against the RHΔku80, ME49 and WH6 strains through a humoral and cellular mixed immune response in a murine model. In consideration of the data, we gathered above that RHΔompdcΔuprt would be cleared within 1 week, we proceeded with a three-dose inoculation procedure rather than a single inoculation procedure, as reported by others [14, 22, 24, 34] to achieve a higher and longer immune response. As expected, high levels of specific anti-T. gondii IgG antibodies were gradually induced by the RHΔompdcΔuprt strain, particularly both IgG2a and IgG1 were significantly increased indicating a mixed Th1/Th2 immune response which is consistent with other live attenuated T. gondii vaccines [17, 38, 39]. These specific antibodies play a protective role by neutralizing the attachment of T. gondii. [40,41,42,43]. In addition, the level of IgG2a was significantly higher than that of IgG1, suggesting that the immunized mice successfully induced a significant Th1-type humoral immune response. This is consistent with previous reports that IgG2a is more efficient than IgG1 in clearing T. gondii tachyzoites [44]. In addition, we demonstrated that the adoptive transfer of sera from RHΔompdcΔuprt immunized mice to naïve recipients provided short-term protection against virulent challenge. The protection of sera is most likely provided by the IFN-γ and IL-12p70 cytokines since we observed that they were significantly induced by the injection of the RHΔompdcΔuprt strain and sustained for a quite long period, but on the other hand, the eventual death of mice was probably due to the depletion of cytokines succumbing to virulent challenge.
Understanding the mechanism of immunity is essential for the development of vaccines. Since immunity has been correlated with a Th1 biased inflammatory response to T. gondii, most vaccines attempt to achieve a higher Th1 response. Evidence from other studies particularly supports that CD8+ T cells and IFN-γ are absolutely essential to combat T. gondii infection. Our analysis indicated that both CD4+ and CD8+ T cell subsets were recruited by RHΔompdcΔuprt. Despite the fact that both CD4+ and CD8+ T cells correspond to the production of IFN-γ, infected mice developed partial protection against RHΔku80 challenge provided by passive immunization of purified CD8+ T cells instead of CD4+ T cells. Interestingly, we also established that adoptive transfer of purified B cells to naïve recipients provided protection against virulent challenge. However, the protective effective of cells, as well as sera, from RHΔku80-infected mice remained unclear since these passive immunizations were difficult to carried out due to the persistent presence of parasites in these cells that could result in T. gondii infection. Collectively with the results of the humoral protective immune response above, we assume that protective immunity would be enhanced by memory B cells through a more efficient antigen processing and presentation producer via multi-point inoculation. Therefore, a CD8+ cells dominant and B cells required immunity was elicited in response to vaccination with RHΔompdcΔuprt for the protection of T. gondii.
IFN-γ can induce various intracellular mechanisms to kill parasites or inhibit their replication and proliferation [45,46,47]. We observed that the expression levels of IFN-γ in the culture supernatant of splenocytes and sera from the vaccinated mice were remarkably higher than those of the unvaccinated mice, which in turn provided effective protection against a high dose virulent challenge. Correspondingly, we found that mice vaccinated with RHΔompdcΔuprt exhibited 100% protection against type I, type II, or Chinese isolated strains, in accordance with the protective effects of the Δgra7Δnpt1 and ΔcpsII strains, while those immunized with ME49Δldh or ME49Δcdpk3 [48] only provided short-term protection against the type I RH strain. In addition, we also revealed that RHΔompdcΔuprt can induce and sustain significant IL-12p70 production, indicating that the innate immune response is also activated. IL-12p70 is mainly produced by B cells and neutrophils, as reported by Gigley [39]. Infection with live attenuated tachyzoites recruits neutrophils early and acts as an innate effector cell, resulting in the early secretion of IL-12 to destroy intracellular parasites by autophagy. Our data indicate a rapid increase in IL-12p70 and a daily decrease in response to vaccination with RHΔompdcΔuprt, while IFN-γ presents a gradually increasing pattern with a peak production level at 4 dpi. However, other studies have shown that excessive levels of Th1 inflammatory factors may also cause pathological damage to mice or even lead to the death of mice [49, 50]. At this time, IL-10 and IL-4 are needed to regulate the inflammatory response during T. gondii infection [51,52,53]. In accordance with this theory, we discovered that Th2-type cytokines (IL-4 and IL-10) were notably increased in response to RHΔompdcΔuprt infection to balance the high production of Th1 cytokines. As the results, immunized mice developed immune profiles capable of clearing high doses of type I and type II T. gondii tachyzoites challenge with limited pathological change.
It is well known that as the definitive host, cats infected with T. gondii can excrete a large number of infective oocysts with feces lasting for about two weeks, while there are few studies on anti-T. gondii vaccines in cats. Given the crucial role of cats in the transmission of T. gondii, there is an urgent need to develop vaccines for cats. The mutant of the T. gondii bradyzoite (T-263) was the first report of a feline T. gondii vaccine [54]. By oral administration of T-263 bradyzoites obtained from brain cysts, 84% of immunized cats excreted no oocysts [54], while immunization with T-263 tachyzoites did not completely induce protective immunity against oocyst shedding [55]. We should not ignore that vaccination with brayzoites from brain cysts is impractical in clinical use in consideration of production and cost. Later, some studies have shown that rhoptry protein vaccines could induce a 67% preventable fraction of oocysts in cats [56]. In the present study, we found that cats immunized with this live attenuated strain developed high antibody titers and showed a 95.3% reduction in oocyst shedding after challenge. Of note, all cats were successfully immunized with a significantly shorter period of oocyst shedding indicating effective protection against toxoplasmosis in cats.
Although RHΔompdcΔuprt strains as attenuated live vaccine showed a protective effect against feline toxoplasmosis, they failed to reach the 100% blocking level of oocyst, which may be related to the immune dose level, immune frequency and immune interval. In the future, we expect to further enhance immune protection by optimizing the immune program.
Conclusions
Our study shows that the attenuated ompdc-uprt double knockout strains from the RH strain of T. gondii are safe and avirulent, can protect mice from challenge with high doses of RHΔku80, ME49 and WH6 strain tachyzoites and can allow the cat to reduce the excretion of oocysts. These data suggest that the RHΔompdcΔuprt mutant has the potential to be used as a candidate for a live attenuated vaccine. Although this mutant strain has great potential as a vaccine in mice, we also need to further study whether this vaccine has similar efficacy in other animals.
Availability of data and materials
The original data that supports the conclusions of this article are presented in the article or in the supplementary information.
Abbreviations
- OMPDC:
-
Orotidine—5'—monophosphate decarboxylase
- UPRT:
-
Uracil phosphoribosyltransferase
- UMP:
-
Uridine monophosphate
- HFFs:
-
Human foreskin fibroblasts
- DMEM:
-
Dulbecco’s Modified Eagle medium
- i.p.:
-
Intraperitoneally
- FBS:
-
Fetal bovine serum
- PBS:
-
Phosphate buffered saline
- IFA:
-
Immunofluorescence assay
- ELISA:
-
Enzyme-linked immunosorbent assay
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
- CBA:
-
Cytometric bead array
- CCK-8:
-
Cell counting kit 8
- PV:
-
Parasitophorous vacuole
References
Ybañez RHD, Ybañez AP, Nishikawa Y. Review on the current trends of toxoplasmosis serodiagnosis in humans. Front Cell Infect Microbiol. 2020;10:204.
Innes EA. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health. 2010;57(1):1–7.
Zhuo X, Du K, Ding H, Lou D, Zheng B, Lu S. A carbamoyl phosphate synthetase II (CPSII) deletion mutant of Toxoplasma gondii induces partial protective immunity in mice. Front Microbiol. 2020;11: 616688.
Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–58.
Ferguson DJ. Toxoplasma gondii: 1908–2008, homage to Nicolle, Manceaux and Splendore. Mem Inst Oswaldo Cruz. 2009;104(2):133–48.
Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. 2017;4(4):e177–88.
Wang JL, Huang SY, Behnke MS, Chen K, Shen B, Zhu XQ. The past, present, and future of genetic manipulation in Toxoplasma gondii. Trends Parasitol. 2016;32(7):542–53.
De Berardinis A, Paludi D, Pennisi L, Vergara A. Toxoplasma gondii, a foodborne pathogen in the swine production chain from a European perspective. Foodborne Pathog Dis. 2017;14(11):637–48.
Lang D, Schott BH, van Ham M, Morton L, Kulikovskaja L, Herrera-Molina R, et al. Chronic Toxoplasma infection is associated with distinct alterations in the synaptic protein composition. J Neuroinflamm. 2018;15(1):216.
Alday PH, Doggett JS. Drugs in development for toxoplasmosis: advances, challenges, and current status. Drug Des Devel Ther. 2017;11:273–93.
Katlama C, De Wit S, O’Doherty E, Van Glabeke M, Clumeck N. Pyrimethamine-clindamycin vs pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1996;22(2):268–75.
Montazeri M, Mehrzadi S, Sharif M, Sarvi S, Tanzifi A, Aghayan SA, et al. Drug resistance in Toxoplasma gondii. Front Microbiol. 2018;9:2587.
Wang JL, Zhang NZ, Li TT, He JJ, Elsheikha HM, Zhu XQ. Advances in the development of anti-Toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol. 2019;35(3):239–53.
Yang WB, Wang JL, Gui Q, Zou Y, Chen K, Liu Q, et al. Immunization with a live-attenuated RH:DeltaNPT1 strain of Toxoplasma gondii induces strong protective immunity against toxoplasmosis in mice. Front Microbiol. 2019;10:1875.
Jaurigue JA, Seeberger PH. Parasite carbohydrate vaccines. Front Cell Infect Microbiol. 2017;7:248.
Buxton D, Innes EA. A commercial vaccine for ovine toxoplasmosis. Parasitology. 1995;110(Suppl):S11–6.
Liang QL, Sun LX, Elsheikha HM, Cao XZ, Nie LB, Li TT, et al. RHDeltagra17Deltanpt1 strain of Toxoplasma gondii elicits protective immunity against acute, chronic and congenital toxoplasmosis in mice. Microorganisms. 2020;8(3):352.
Hortua Triana MA, Cajiao Herrera D, Zimmermann BH, Fox BA, Bzik DJ. Pyrimidine pathway-dependent and -independent functions of the Toxoplasma gondii mitochondrial dihydroorotate dehydrogenase. Infect Immun. 2016;84(10):2974–81.
Luft BJ. Potent in vivo activity of arprinocid, a purine analogue, against murine toxoplasmosis. J Infect Dis. 1986;154(4):692–4.
Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415(6874):926–9.
Pfefferkorn ER, Pfefferkorn LC. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977;24(3):449–53.
Fox BA, Bzik DJ. Nonreplicating, cyst-defective type II Toxoplasma gondii vaccine strains stimulate protective immunity against acute and chronic infection. Infect Immun. 2015;83(5):2148–55.
Fox BA, Bzik DJ. Avirulent uracil auxotrophs based on disruption of orotidine-5’-monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun. 2010;78(9):3744–52.
Xu LQ, Yao LJ, Jiang D, Zhou LJ, Chen M, Liao WZ, et al. A uracil auxotroph Toxoplasma gondii exerting immunomodulation to inhibit breast cancer growth and metastasis. Parasit Vectors. 2021;14(1):601.
Donald RG, Roos DS. Insertional mutagenesis and marker rescue in a protozoan parasite: cloning of the uracil phosphoribosyltransferase locus from Toxoplasma gondii. Proc Natl Acad Sci U S A. 1995;92(12):5749–53.
Wang JL, Elsheikha HM, Zhu WN, Chen K, Li TT, Yue DM, et al. Immunization with Toxoplasma gondii GRA17 deletion mutant induces partial protection and survival in challenged mice. Front Immunol. 2017;8:730.
Shen B, Brown K, Long S, Sibley LD. Development of CRISPR/Cas9 for efficient genome editing in Toxoplasma gondii. Methods Mol Biol. 2017;1498:79–103.
Villena I, Aubert D, Gomis P, Ferte H, Inglard JC, Denis-Bisiaux H, et al. Evaluation of a strategy for Toxoplasma gondii oocyst detection in water. Appl Environ Microbiol. 2004;70(7):4035–9.
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008(5):pdb-prot4986.
Ely KH, Kasper LH, Khan IA. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J Immunol. 1999;162(9):5449–54.
Schluter D, Barragan A. Advances and challenges in understanding cerebral toxoplasmosis. Front Immunol. 2019;10:242.
Weglinska L, Bekier A, Trotsko N, Kapron B, Plech T, Dzitko K, et al. Inhibition of Toxoplasma gondii by 1,2,4-triazole-based compounds: marked improvement in selectivity relative to the standard therapy pyrimethamine and sulfadiazine. J Enzyme Inhib Med Chem. 2022;37(1):2621–34.
Burrells A, Benavides J, Canton G, Garcia JL, Bartley PM, Nath M, et al. Vaccination of pigs with the S48 strain of Toxoplasma gondii—safer meat for human consumption. Vet Res. 2015;46:47.
Xia N, Zhou T, Liang X, Ye S, Zhao P, Yang J, et al. A lactate fermentation mutant of Toxoplasma stimulates protective immunity against acute and chronic toxoplasmosis. Front Immunol. 2018;9:1814.
Zenner L, Foulet A, Caudrelier Y, Darcy F, Gosselin B, Capron A, et al. Infection with Toxoplasma gondii RH and Prugniaud strains in mice, rats and nude rats: kinetics of infection in blood and tissues related to pathology in acute and chronic infection. Pathol Res Pract. 1999;195(7):475–85.
Zhuo XH, Sun HC, Huang B, Yu HJ, Shan Y, Du AF. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J Zhejiang Univ Sci B. 2017;18(6):453–61.
Le Roux D, Djokic V, Morisse S, Chauvin C, Doré V, Lagrée A-C, et al. Evaluation of immunogenicity and protection of the Mic1-3 knockout Toxoplasma gondii live attenuated strain in the feline host. Vaccine. 2020;38(6):1457–66.
Li TT, Wang JL, Liang QL, Sun LX, Zhang HS, Zhang ZW, et al. Effect of deletion of gra17 and gra23 genes on the growth, virulence, and immunogenicity of type II Toxoplasma gondii. Parasitol Res. 2020;119(9):2907–16.
Gigley JP, Fox BA, Bzik DJ. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol. 2009;182(2):1069–78.
Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000;68(3):1026–33.
Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10(11):766–78.
Pifer R, Yarovinsky F. Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 2011;27(9):388–93.
Spellberg B, Edwards JE Jr. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32(1):76–102.
Zhang NZ, Gao Q, Wang M, Elsheikha HM, Wang B, Wang JL, et al. Immunization with a DNA vaccine cocktail encoding TgPF, TgROP16, TgROP18, TgMIC6, and TgCDPK3 genes protects mice against chronic toxoplasmosis. Front Immunol. 2018;9:1505.
Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol. 2012;34(6):793–813.
Sasai M, Pradipta A, Yamamoto M. Host immune responses to Toxoplasma gondii. Int Immunol. 2018;30(3):113–9.
Ivanova DL, Fatima R, Gigley JP. Comparative analysis of conventional natural killer cell responses to acute infection with Toxoplasma gondii strains of different virulence. Front Immunol. 2016;7:347.
Wu M, Liu S, Chen Y, Liu D, An R, Cai H, et al. Live-attenuated ME49Δcdpk3 strain of Toxoplasma gondii protects against acute and chronic toxoplasmosis. NPJ Vaccines. 2022;7(1):98.
Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167(8):4574–84.
Nguyen TD, Bigaignon G, Markine-Goriaynoff D, Heremans H, Nguyen TN, Warnier G, et al. Virulent Toxoplasma gondii strain RH promotes T-cell-independent overproduction of proinflammatory cytokines IL12 and gamma-interferon. J Med Microbiol. 2003;52(Pt 10):869–76.
Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157(2):798–805.
Roberts CW, Ferguson DJ, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64(3):897–904.
Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164(10):5375–82.
Frenkel JK, Pfefferkorn ER, Smith DD, Fishback JL. Prospective vaccine prepared from a new mutant of Toxoplasma gondii for use in cats. Am J Vet Res. 1991;52(5):759–63.
Freyre A, Choromanski L, Fishback JL, Popiel I. Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J Parasitol. 1993;79(5):716–9.
Garcia JL, Navarro IT, Biazzono L, Freire RL, da Silva Guimaraes Junior J, Cryssafidis AL, et al. Protective activity against oocyst shedding in cats vaccinated with crude rhoptry proteins of the Toxoplasma gondii by the intranasal route. Vet Parasitol. 2007;145(3–4):197–206.
Acknowledgements
The authors would like to thank the participants in this study.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81871684, No. 81802037), the Provincial Key R & D program of Zhejiang Department of Science and Technology (No. 2019C03057), the Zhejiang Provincial Natural Science Foundation of China (No. LY22H190003), the Zhejiang Medical and Health Science and Technology Plan (WKJ-ZJ-2203), the Central Leading Local Science and Technology Development Fund Project (2023ZY1019) and the Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A), Hangzhou Medical College.
Author information
Authors and Affiliations
Contributions
SL, XZ, YS and BZ designed the experiments. YS, BZ, HS, SW, JF, JD, MG, QK, DL and HD performed the experiments. YS, BZ and HS collected and analyzed the data. YS made major contributions to the writing of the manuscript. SL and XZ revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experiments were approved by the Animal Care and Use Committee of Hangzhou Medical College (2018-027).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Supplementary Information
Additional file 1: Table S1.
The primers used in this study. Table S2. This table shows the oocysts in the feces of cats from 1 to 10 days after being infected with RHΔompdcΔuprt strain. Figure S1. The diagram of knocking out. (a) Schematic illustration of knocking out uprt by homologous gene replacement in RHΔku80Δhxgprt strain. (b) Schematic showing deletion of hxgprt gene by insertion of a uprt5’utr-3’utr into hxgprt gene. (c) Diagram illustrating the deletion of ompdc in RHΔuprtΔhxgprt to make the double mutant RHΔuprtΔompdc::HXGPRT by CRISPR/Cas9-mediated homologous gene replacement. Figure S2. Intracellular proliferation of OMPDC-UPRT deletion mutant under the fluorescence microscope. (a) 24 hours of intracellular proliferation. (b) 48 hours of intracellular proliferation. Figure S3. Invasion and attachment assay for RHΔku80 and RHΔompdcΔuprt strains. (a) The invasion and attachment of T. gondii RHΔku80 and RHΔompdcΔuprt were evaluated by indirect immunofluorescence assay. (b) The analysis of the invasion and attachment. Figure S4. The levels of cytokines in the serum of mice injected with RHΔompdcΔuprt. The levels of IFN-γ (a) and IL-12 (b) were measured by flow cytometer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shen, Y., Zheng, B., Sun, H. et al. A live attenuated RHΔompdcΔuprt mutant of Toxoplasma gondii induces strong protective immunity against toxoplasmosis in mice and cats. Infect Dis Poverty 12, 60 (2023). https://doi.org/10.1186/s40249-023-01109-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-023-01109-9