Abstract
Background
Tuberculosis is a bacterial infectious disease, which affects different parts of a human body, mainly lungs and can lead to the patient’s death. The aim of this study is to investigate the global prevalence of drug-resistant tuberculosis using a systematic review and meta-analysis.
Methods
In this study, the PubMed, Scopus, Web of Science, Embase, ScienceDirect and Google Scholar repositories were systematically searched to find studies reporting the global prevalence of drug-resistant tuberculosis. The search did not entail a lower time limit, and articles published up until August 2022 were considered. Random effects model was used to perform the analysis. The heterogeneity of the studies was examined with the I2 test. Data analysis was conducted within the Comprehensive Meta-Analysis software.
Results
In the review of 148 studies with a sample size of 318,430 people, the I2 index showed high heterogeneity (I2 = 99.6), and accordingly random effects method was used to analyze the results. Publication bias was also examined using the Begg and Mazumdar correlation test which indicated the existence of publication bias in the studies (P = 0.008). According to our meta-analysis, the global pooled prevalence of multi-drug resistant TB is 11.6% (95% CI: 9.1–14.5%).
Conclusions
The global prevalence of drug-resistant tuberculosis was found to be very high, thus health authorities should consider ways to control and manage the disease to prevent a wider spread of tuberculosis and potentially subsequent deaths.
Similar content being viewed by others
Background
Tuberculosis (TB) is one of the most common infectious diseases, which is the main cause of widespread mortality, especially among people living with HIV (PLHIV) [1, 2]. The disease is caused by a type of bacteria called Mycobacterium TB [3]. Different types of TB are multi drug-resistant (MDR), pre-extensively drug-resistant (Pre-XDR), and extensively drug-resistant (XDR) [4]. TB usually affects the lungs, however it can also affect other parts of the body, such as the kidneys and the brain [4].
There were an estimated 450,000 incident cases of MDR in 2021, up 3.1% from 437,000 in 2020, three countries accounted for 42% of global cases in 202: India (26%), the Russian Federation (8.5%), and Pakistan (7.9%) [5]. A study by Baya et al., reported that the average age of patients was 39.31 ± 14.64 years, whilst 62.6% of patients were less than 40 years old. Patients were predominantly male 76.2%, and 77.1% were married [6]. Additionally, the prevalence of latent MDR TB has been reported in some countries of Eastern Europe and Central Asia, such as China (6 million people), India (4 million people), and Russia (1.8 million people) [4,5,6].
According to the existing literature, risk factors of tuberculosis include demographic characteristics such as gender, age, place of residence, education, marital status, bad habits such as alcohol abuse and smoking, and concomitant infections including diabetes mellitus, HIV, Acid-Fast Bacilli (AFB) smear, pulmonary space, history of tuberculosis, and history of anti-tuberculosis treatment are significant risk factors for MDR TB [7].
TB often impacts patients with other diseases such as diabetes, HIV, and chronic obstructive pulmonary disease (COPD) [7]. Cough or fever for > 2 weeks, weight loss, or hemoptysis are among the symptoms of TB which are also associated with lack of health insurance, tuberculin skin test, diagnosis through a process not entailing screening, and ethnicities other than Asian [8]. Complications of this disease include bronchial stenosis, severe airway obstruction, pneumonia, and hemoptysis, the most common of which is liver damage [9, 10]. Vocal cord paralysis, associated with laryngeal TB, can also be found among the patients [10]. To treat TB, a combination of isoniazid, rifampin, ethambutol, and pyrazinamide, followed by a combination of isoniazid and rifampin are used [9].
Several studies have been conducted on the prevalence of drug-resistant tuberculosis worldwide. These studies have reported different rates, yet their reported results are heterogeneous and are not aligned. The aim of this systematic review and meta-analysis is to pool the reported results of the existing studies and offer a scientifically consistent prevalence for drug resistant TB. The findings of our study can provide useful insights to health policymakers to devised appropriate interventions, with a view to reducing the subsequent complications from the disease.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The keywords of prevalence, drug-resistant tuberculosis, burden, outbreak and their combination using the (AND) and (OR) operators, were used to search the PubMed, Google Scholar, Science Direct, Embase, Scopus and Web of Science databases. The search was conducted with no lower time limit and until August 2022. The reference lists within the identified studies were also manually searched to ensure the comprehensive of the collected articles. The information of the identified studies was transferred into the EndNote reference management software, and studies that had reported the prevalence of drug-resistant tuberculosis by continent and were satisfying the inclusion criteria, were selected for final analysis.
Inclusion and exclusion criteria
The following criteria were used to keep an identified study in the systematic review and for meta-analysis: Studies that reported the prevalence of drug-resistant tuberculosis (including cross-sectional, case–control, and cohort studies), Studies with their full-text available, Studies that provided sufficient data (sample size, prevalence), Studies written and published in English. In contrary, the following criteria resulted in excluding identified articles: Case report studies, case series studies, duplicate studies and meta-analysis studies.
Study selection
Similarly, selection of studies was conducted in accordance with the PRISMA guidelines. Initially, articles that were duplicates in different databases were excluded, and only one copy was retained. Subsequently, the initial screening of articles was conducted through reviewing the titles and abstracts, and irrelevant articles were omitted based on the inclusion and exclusion criteria. Then their full text of articles was reviewed in line with the inclusion and exclusion criteria, and at this stage further irrelevant studies were removed. To avoid any potential bias, all the steps of reviews and data extraction were conducted by two reviewers independently. In cases where there was a difference of opinion between two reviewers, the review of the article was finalized by a third reviewer.
Quality evaluation
To evaluate the quality of articles, a checklist appropriate to observational studies was selected. The Strengthening the Reporting of Observational Studies in Epidemiology checklist (STROBE) consists of six scales including: title, abstract, introduction, methods, results, and discussion. In total, this instruction consists of 32 subscales. These 32 subscales denote different methodological aspects of the study, i.e., title, statement of the problem, study objectives, type of study, statistical population of the study, sampling method, determining the appropriate sample size, definition of variables and procedures, study data collection tools, statistical analysis methods and findings. Consider that the fulfilment of each of the subscales award a point, and based on this, articles with scores of 16 and above were considered to be of medium and high methodological quality articles respectively. Articles with a score below 16 were considered to be of poor quality and were therefore excluded from our study.
Data extraction
Data extraction was completed by two researchers using a different pre-prepared checklist. This checklist included: first author's name, year of publication, study location, sample size, age group of men and women, global prevalence of drug-resistant tuberculosis, and research instruments.
Statistical analysis
The extracted information were structured and were inputted into Comprehensive Meta-Analysis software (Version 2, Biostat, Inc., 14 North Dean Street, Englewood, NJ 07631 USA). The heterogeneity of the studies was then assessed using the I2 test. In order to check the publication bias, the Begg’s test was used at a significance level of 0.1, and associated Funnel plots were drawn.
Results
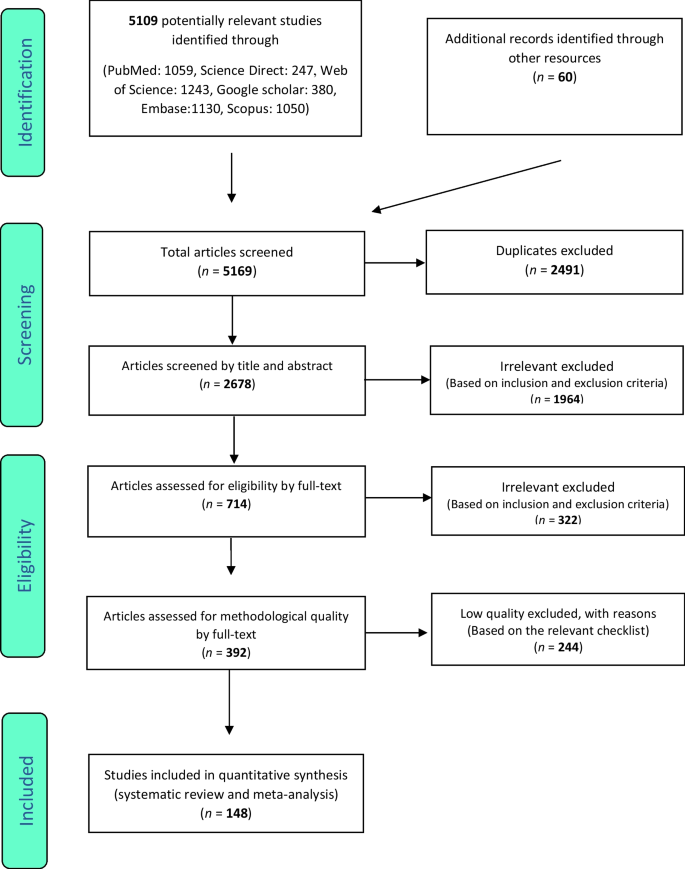
Following the initial search, 5109 articles were identified from the databases. An additional 60 related articles were also included following manual searches. Information of all identified articles were then transferred into the EndNote reference management software. Throughout the PRISMA’s identification stage, 2491 articles were excluded due to being repeated in various databases, and only one copy was retained. In the screening stage, the title and abstract of the studies were reviewed and 1964 further articles were excluded based on the inclusion and exclusion criteria. In the eligibility evaluation phase, 323 articles were omitted, after examination of the full text of the articles. As part of quality evaluation, and through the evaluation of the full text of the articles and based on the scores obtained from the STROBE checklist, studies with poor methodological quality were removed, and finally 148 studies were kept for analysis. All included studies were cross-sectional and most of the reviewed studies were conducted in Africa (continent). The information related to the 148 included studies is presented in Fig. 1 and Additional file 1: Tables S1 to S6 [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179].
Multi drug-resistant TB
In the review of 148 studies that had studied multi drug resistant TB (sample size of 318,430 people), the I2 test showed a high heterogeneity (I2 = 99.6), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the global pooled prevalence of multi-drug resistant TB was found to be 11.6% (95% CI: 9.1–14.5%). Test of publication bias in the studies through the Begg and Mazumdar correlation test showed the existence of publication bias among the studies (P = 0.008) (Table 1) (Figs. S1, S2 in Additional file 2).
Isoniazid resistant TB
In 98 studies with a focus on Isoniazid resistant TB (sample size of 102,260 people), the I2 heterogeneity test showed a high heterogeneity (I2 = 99.03), and accordingly, random effects method was used to analyze the results. Considering the meta-analysis, the pooled global prevalence of isoniazid resistant TB was found to be 15.7% (95% CI: 13.7–17.9%). The study of publication bias in the studies through the Begg and Mazumdar correlation test showed the existence of publication bias in the studies (P = 0.02) (Table 1) (Figs. S3, S4 in Additional file 2).
Rifampin resistant TB
In the review of 109 studies that had researched rifampin resistant TB (sample size of 215,660 people), the I2 heterogeneity test showed a high heterogeneity (I2 = 98.9), and similarly, random effects method was used to analyze the results. Based on the meta-analysis, the pooled global prevalence of rifampin- resistant TB was found as 9.4% (95% CI: 7.8–11.2%). The study of publication bias in the studies through the Begg and Mazumdar correlation test indicated the existence of publication bias in the studies (P = 0.00045) (Table 1) (Figs. S5, S6 in Additional file 2).
Single drug resistant TB
In the review of 35 studies with a focus on single drug resistant TB (sample size of 45,147 people), the I2 heterogeneity test showed a high heterogeneity (I2 = 98.5). Hence, random effects method was used to analyze the results. Considering the meta-analysis results, the pooled global prevalence of single drug resistant TB was found as 11.8% (95% CI: 9.2–15.2%). The study of publication bias in the studies through the Begg and Mazumdar correlation test showed the absence of publication bias in the studies (P = 0.139) (Table 1) (Figs. S7, S8 in Additional file 2).
Extensive drug resistant TB
In the review of 56 studies on extensive drug resistant TB (sample size of 350,420 people), the I2 heterogeneity test showed high heterogeneity (I2 = 98.8), and therefore, random effects method was used to analyze the results. Considering the meta-analysis results, the pooled global prevalence of extensive drug resistant TB was found to be 2.5% (95% CI: 2–3%). The study of publication bias using the Begg and Mazumdar correlation test indicated the absence of publication bias in the studies (P = 0.938) (Table 1) (Figs. S9, S10 in Additional file 2).
Information in Table 2 outlines the subgroup analysis of the types of tuberculosis resistance among patients by gender, and by TB type. Accordingly, male patients have a higher prevalence in multi-drug resistant TB, Isoniazid resistant TB and Rifampin-resistant TB, compared to female patients, with prevalence of 20% (95% CI: 11.9–31.8%), 17.5% (95% CI: 9.6–29.8%), and 12.7% (95% CI: 5.7–25.9%) respectively. Given that the articles did not report gender-segregated data for single drug-resistant TB and extensively drug-resistant TB, the authors could not include these results in the subgroup analysis.
Discussion
Tuberculosis is a very common infection with a bacterial agent called Mycobacterium [22, 45, 180,181,182]. MDR-TB is a strain of Tuberculosis (TB) that is resistant to at least two of the most important anti-tuberculosis drugs (INH and RIF) [180,181,182,183,184,185].
This systematic review and meta-analysis was conducted to identify and review existing research works that had examined prevalence of different types of TB. It was also aimed to obtain pooled prevalence of TB types globally. Accordingly, we did not find a specific study on the prevalence of drug-resistant tuberculosis at the global level, despite the fact that there are many articles that have reported the prevalence of this disease at country level, or at most in a continent.
Considering the reported results of an all included studies, the global pooled prevalence of different types of drug-resistant tuberculosis, namely MDR, Isoniazid (INH), Rifampcin (RIF), and XDR were calculated as 11.6%, 15.7%, 9.4%, and 2.5%, respectively.
Eastern European countries, the Russia and Central Asian countries, and parts of China have a high rate of MDR-TB infection [184, 186]. In the study by Kindu Alem Mola et al., the authors reported that the level of MDR-TB in East Africa is higher than other regions globally [187]. In this work, based on the relevant reports from the World Health Organization (WHO) in 2015, the prevalence of global MDR TB in new and previous TB cases were 3.5% and 20.5%, respectively, while countries in southern regions of Africa have greater rates [187, 188].
The main reasons for the emergence of MDR TB globally numerous [187], and they are mostly related to living conditions [189], lifestyle [190], previous medical history [111, 191], history of diabetes [192, 193] and Human Immunodeficiency Viruses (HIV) infection [194]. A study conducted in Ethiopia shows that HIV infection is one of the most important factors associated with MDR TB [187, 195]. In addition, HIV patients, due to the length of hospitalization in hospitals with poorer hygiene and infection control, are more exposed to MDR TB and hence the rate of infection is higher among these patients [187]. In another study by Al-Derraji et al. [187, 196], the incidence of MDR TB among HIV-positive patients was reported to be 20% higher compared to that of HIV-negatives [187].
In densely populated and poor families, the spread of TB disease is also more prevalent [187]. According to the literature, unhealthy or poor lifestyles which entail alcohol abuse, smoking, drug use, etc. are the main risk factors related to the spread of MDR TB [187]. It was also stated that smokers, especially men, are more likely to be infected with MDR TB compared to female smokers [187, 197,198,199].
According to an article by Jilani Talha et al., tuberculosis complications are usually seen more among elderly patients, young children, people with severe respiratory disorders or patients who do not receive proper treatment. Accordingly, patients who do not receive proper treatment are more exposed to tuberculosis complications. Some of these complications are acute respiratory distress syndrome, extensive lung destruction, empyema, pneumothorax, disseminated tuberculosis infection (including tuberculosis meningitis), bronchiectasis, fibrothorax, aspergilloma, and hemoptysis [200].
According to a study conducted by Jilani et al., with a focus on treating active tuberculosis, a combination of drugs is required during the two intensive and the continuous phases; the first-line drugs that are the most common regimen for tuberculosis treatment include: (1) isoniazid, (2) rifampin, (3) ethambutol, and (4) pyrazinamide [200].
The intensive phase in the treatment of Tuberculosis includes the combination of the above 4 drugs that are prescribed for 2 months, yet the continuation phase includes the combination of isoniazid and rifampin for an additional 4 months. The second line drugs include: (1) Injectable aminoglycoside: streptomycin, amikacin, kanamycin; (2) Injectable polypeptides: viomycin and capreomycin; (3) Fluoroquinolones: levofloxacin, gatifloxacin, ofloxacin and moxifloxacin, and (4) Para-amino salicylic acid, ethionamide, cycloserine, prothionamide, trazodone, linezolid [200].
In a study conducted, the side effects of each anti-tuberculosis drug were described as follows: (1) Isoniazid: liver damage (fatigue, nausea, lethargy, abdominal pain, and vomiting), skin rash, numbness, headache and tingling of limbs; (2) Rifampin: jaundice, arthralgia (joint stiffness), and fever; (3) Ethambutol: visual impairment including blurred or reduced vision and blindness, liver damage, headache, and nausea, and (4) Pyrazinamide: nausea, painful or swollen joints, and liver damage [200]. According to the reported results of the same study, the highest prevalence of multi-drug resistant tuberculosis was reported in males.
Considering the ratio of infections among males vs females, one study reported that the split between males and females with multi-drug resistant tuberculosis was 70.4% and 29.6% respectively [201], In a study conducted in patients with resistant tuberculosis in Ghana, the ratio of males and females was 69.6% and 30.4% respectively [202], whilst in another study conducted in Egypt, the ratio of males and females was reported as 67.5% and 32.5%, respectively [203]. Moreover, in a similar research work conducted in Ethiopia 65.3% male and 34.7% female has multi drug resistant TB [204].
Our study shows that different strains of Tuberculosis, including drug-resistant TBs, have a high prevalence. On the other hand, these strains can be treated, and there are similar strategies and interventions to control existing and new infections. Considering the complications that this disease may cause, its control and management are vital, since it would be possible to reduce the Tuberculosis induced mortality rate through controlling its different strains.
The main limitation of the present meta-analysis is related to the significant publication bias among the identified studies, and therefore, the results should be considered with caution. Moreover, it is recommended that future meta-analysis studies in this field are conducted using more keywords and databases to potentially eliminate this bias.
Conclusions
According to the results of the present study, the global prevalence of multidrug-resistant, mono drug-resistant, isoniazid, and rifampicin tuberculosis are 11.6%, 11.8%, 15.7%, and 9.4%, respectively. The results of this study can offer some consistency to the heterogeneous results from studies conducted around the world and provide reliable insights to health policymakers. Such insights would be instrumental to devise appropriate preventive, therapeutic and diagnostic measures.
Availability of data and materials
Datasets are available through the corresponding author upon reasonable request.
Abbreviations
- DST:
-
Drug susceptibility testing
- WGS:
-
Whole genome sequencing
- RFLP:
-
Restriction fragment length polymorphism
- ZN:
-
Decontamination and Ziehl–Neelsen
- CXR:
-
Chest X-ray
- AST:
-
Antimicrobial susceptibility testing
- CI :
-
Confidence interval
- TB:
-
Tuberculosis
References
Iacobino A, Fattorini L, Giannoni F. Drug-resistant tuberculosis 2020: where we stand. Appl Sci. 2020;10(6):2153.
Asgedom SW, Teweldemedhin M, Gebreyesus H. Prevalence of multidrug-resistant tuberculosis and associated factors in Ethiopia: a systematic review. J Pathogens. 2018;2018:7104921.
Lange C, Chesov D, Heyckendorf J, Leung CC, Udwadia Z, Dheda K. Drug-resistant tuberculosis: an update on disease burden, diagnosis and treatment. Respirology. 2018;23(7):656–73.
Mazurek GH. Division of tuberculosis elimination, national center for HIV, STD, and TB prevention, centers for disease control and prevention (CDC). Guidelines for using the QuantiFERON-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55.
Global Tuberculosis Report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
Baya B, Achenbach CJ, Kone B, Toloba Y, Dabitao DK, Diarra B, et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. IJID. 2019;81:149–55.
Xi Y, Zhang W, Qiao R-J, Tang J. Risk factors for multidrug-resistant tuberculosis: a worldwide systematic review and meta-analysis. PLoS ONE. 2022;17(6): e0270003.
Miller LG, Asch SM, Yu EI, Knowles L, Gelberg L, Davidson P. A population-based survey of tuberculosis symptoms: how atypical are atypical presentations? Clin Infect Dis. 2000;30(2):293–9.
Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. DÄ International. 2019;116(43)
Hsu D, Irfan M, Jabeen K, Iqbal N, Hasan R, Migliori GB, et al. Post tuberculosis treatment infectious complications. IJID. 2020;92:S41–5.
Wang W, Wang J, Zhao Q, Darling ND, Yu M, Zhou B, et al. Contribution of rural-to-urban migration in the prevalence of drug resistant tuberculosis in China. Eur J Clin Microbiol Infect Dis ESCMID. 2011;30(4):581–6.
Timire C, Metcalfe JZ, Chirenda J, Scholten JN, Manyame-Murwira B, Ngwenya M, et al. Prevalence of drug-resistant tuberculosis in Zimbabwe: a health facility-based cross-sectional survey. IJID. 2019;87:119–25.
Ahmad N, Javaid A, Sulaiman SA, Ming LC, Ahmad I, Khan AH. Resistance patterns, prevalence, and predictors of fluoroquinolones resistance in multidrug resistant tuberculosis patients. Braz J Infect Dis SBI. 2016;20(1):41–7.
Mohammed KAS, Khudhair GS, Bekheet A-R. Prevalence and drug resistance pattern of Mycobacterium tuberculosis isolated from tuberculosis patients in Basra, Iraq. Pol J Microbiol. 2022;71(2):205–15.
Hu Y, Mathema B, Zhao Q, Chen L, Lu W, Wang W, et al. Acquisition of second-line drug resistance and extensive drug resistance during recent transmission of Mycobacterium tuberculosis in rural China. Clin Microbiol Infect ESCMID. 2015;21(12):1093.
Mazahir R, Beig FK, Ahmed Z, Alam S. Burden of tuberculosis among household children of adult multi drug resistant patients and their response to first line anti tubercular drugs. Egypt Pediatr Assoc Gazette. 2017;65(4):122–6.
Ayaz A, Hasan Z, Jafri S, Inayat R, Mangi R, Channa AA, et al. Characterizing Mycobacterium tuberculosis isolates from Karachi, Pakistan: drug resistance and genotypes. IJID. 2012;16(4):e303–9.
Al-Dabbagh M, Lapphra K, McGloin R, Inrig K, Schaaf HS, Marais BJ, et al. Drug-resistant tuberculosis: pediatric guidelines. PIDJ. 2011;30(6):501–5.
Yang Y, Zhou C, Shi L, Meng H, Yan H. Prevalence and characterization of drug-resistant tuberculosis in a local hospital of Northeast China. IJID. 2014;22:83–6.
Magula NP, Madala ND, Kriel Y, Bayi V, Duze NP, Manzini TC, et al. Prevalence of drug resistant tuberculosis in patients presenting with a large pericardial effusion at King Edward VIII Hospital. IJID. 2014;21:87.
Mahabeer P, Khan M, Mlisana K. Drug-resistant tuberculosis in children less than 5 years old with culture positive mycobacterium tuberculosis. IJID. 2016;45:212–3.
Berberian G, Gonzalez S, Reijtman V, Miño N, Casimir L, Sarkis C, et al. Seventeen years of drug-resistant tuberculosis in Argentinian children. IJID. 2016;45:387.
Singhal R, Arora J, Sah GC, Bhalla M, Sarin R, Prasad MV. Frequency of multi-drug resistance and mutations in Mycobacterium tuberculosis isolates from Punjab state of India. J Epidemiol Glob Health. 2017;7(3):175–80.
Prakash R, Kumar D, Gupta VK, Jain S, Chauhan DS, Tiwari PK, et al. Status of multidrug resistant tuberculosis (MDR-TB) among the Sahariya tribe of North Central India. J Infect Public Health. 2016;9(3):289–97.
Dujaili JA, Blebil AQ, Dujaili MA, Awaisu A, Hassali MA, Syed Sulaiman SA. Prevelance of pulmonary tuberculosis and multi drug resistant tuberculosis patients in baghdad, Iraq. Value Health. 2013;16(3):A82.
Santos LC, Bousquet Hde M, Pereira AM, Junqueira-Kipnis AP, Kipnis A. A high prevalence of resistance in new tuberculosis cases of midwestern Brazil. Infect Genet Evol. 2010;10(7):1052–7.
Kontsevaya I, Nikolayevskyy V, Kovalyov A, Ignatyeva O, Sadykhova A, Simak T, et al. Tuberculosis cases caused by heterogeneous infection in Eastern Europe and their influence on outcomes. Infect Genet Evol. 2017;48:76–82.
Bhembe NL, Green E. Molecular epidemiological study of multidrug-resistant tuberculosis isolated from sputum samples in Eastern Cape, South Africa. Infect Genet Evol. 2020;80: 104182.
Ghebremichael S, Petersson R, Koivula T, Pennhag A, Romanus V, Berggren I, et al. Molecular epidemiology of drug-resistant tuberculosis in Sweden. Microbes Infect. 2008;10(6):699–705.
Montoro E, Lemus D, Echemendía M, Armas L, González-Ochoa E, Llanes MJ, et al. Drug-resistant tuberculosis in Cuba results of the three global projects. Tuberculosis. 2006;86(3–4):319–23.
Pardini M, Niemann S, Varaine F, Iona E, Meacci F, Orrù G, et al. Characteristics of drug-resistant tuberculosis in Abkhazia (Georgia), a high-prevalence area in Eastern Europe. Tuberculosis. 2009;89(4):317–24.
Jiao W-W, Liu Z-G, Han R, Zhao X-Q, Dong F, Dong H-Y, et al. Prevalence of drug resistant Mycobacterium tuberculosis among children in China. Tuberculosis. 2015;95(3):315–20.
Brandao AP, Pinhata JMW, Simonsen V, Oliveira RS, Ghisi KT, Rabello MCS, et al. Transmission of Mycobacterium tuberculosis presenting unusually high discordance between genotypic and phenotypic resistance to rifampicin in an endemic tuberculosis setting. Tuberculosis. 2020;125: 102004.
Van Rie A, Warren R, Richardson M, Gie RP, Enarson DA, Beyers N, et al. Classification of drug-resistant tuberculosis in an epidemic area. Lancet. 2000;356(9223):22–5.
Chand K, Tewari S, Varghese S. Prevalence of drug resistant tuberculosis in armed forces-study from a tertiary referral chest diseases hospital at Pune. MJAFI. 2000;56(2):130–4.
Ismail NA, Mvusi L, Nanoo A, Dreyer A, Omar SV, Babatunde S, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18(7):779–87.
Daniel O, Osman E. Prevalence and risk factors associated with drug resistant TB in South West, Nigeria. Asian Pac J Trop Med. 2011;4(2):148–51.
Wang G, Peng YL, Zhang G, Zhang L, Xing J, Li D, et al. Sample survey of drug-resistant tuberculosis in Henan, China, 1996. Respirology. 2002;7(1):67–72.
Hannan MM, Peres H, Maltez F, Hayward AC, Machado J, Morgado A, et al. Investigation and control of a large outbreak of multi-drug resistant tuberculosis at a central Lisbon hospital. J Hosp Infect. 2001;47(2):91–7.
Wu B, Zhang L, Liu Z, He H, Pan A, Wang F, et al. Drug-resistant tuberculosis in Zhejiang Province, China: an updated analysis of time trends, 1999–2013. Glob Health Action. 2017;10(1):1293925.
Phyu S, Lwin T, Ti T, Maung W, Mar WW, Shein SS, et al. Drug-resistant tuberculosis in Yangon, Myanmar. Scand J Infect Dis. 2005;37(11–12):846–51.
Hu Y, Mathema B, Wang W, Hoffner S, Kreiswirth B, Xu B. Prevalence of multidrug-resistant pulmonary tuberculosis in counties with different duration of DOTS implementation in rural China. Microb Drug Resist. 2008;14(3):227–32.
Shah MA, Shah I. Increasing prevalence of pediatric drug-resistant tuberculosis in Mumbai, India, and its outcome. PIDJ. 2018;37(12):1261–3.
Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. Predominance of multi-drug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. J Med Microbiol. 2006;55(10):1413–8.
Kapata N, Chanda-Kapata P, Bates M, Mwaba P, Cobelens F, Grobusch MP, et al. Multidrug-resistant TB in Zambia: review of national data from 2000 to 2011. TM & IH. 2013;18(11):1386–91.
Kamolwat P, Nateniyom S, Chaiprasert A, Disratthakit A, Mahasirimongkol S, Yamada N, et al. Prevalence and associated risk factors of drug-resistant tuberculosis in Thailand: results from the fifth national anti-tuberculosis drug resistance survey. TM & IH. 2021;26(1):45–53.
Hu Y, Hoffner S, Wu L, Zhao Q, Jiang W, Xu B. Prevalence and genetic characterization of second-line drug-resistant and extensively drug-resistant Mycobacterium tuberculosis in Rural China. Antimicrob Agents Chemother. 2013;57(8):3857–63.
Huo F, Lu J, Zong Z, Jing W, Shi J, Ma Y, et al. Change in prevalence and molecular characteristics of isoniazid-resistant tuberculosis over a 10-year period in China. BMC Infect Dis. 2019;19(1):689.
Zhao LL, Chen Y, Chen ZN, Liu HC, Hu PL, Sun Q, et al. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Hunan, China. Antimicrob Agents Chemother. 2014;58(6):3475–80.
Daum LT, Konstantynovska OS, Solodiankin OS, Liashenko OO, Poteiko PI, Bolotin VI, et al. Next-generation sequencing for characterizing drug resistance-conferring Mycobacterium tuberculosis genes from clinical isolates in the Ukraine. J Clin Microbiol. 2018;56: e00009.
Dinic L, Akande P, Idigbe EO, Ani A, Onwujekwe D, Agbaji O, et al. Genetic determinants of drug-resistant tuberculosis among HIV-infected patients in Nigeria. J Clin Microbiol. 2012;50(9):2905–9.
Agarwal M, Gunal S, Durmaz R, Yang Z. Integration of Mycobacterium tuberculosis drug susceptibility testing and genotyping with epidemiological data analysis to gain insight into the epidemiology of drug-resistant tuberculosis in Malatya, Turkey. J Clin Microbiol. 2010;48(9):3301–5.
Djuretic T, Herbert J, Drobniewski F, Yates M, Smith EG, Magee JG, et al. Antibiotic resistant tuberculosis in the United Kingdom: 1993–1999. Thorax. 2002;57(6):477–82.
Lv XT, Lu XW, Shi XY, Zhou L. Prevalence and risk factors of multi-drug resistant tuberculosis in Dalian, China. J Int Med Res. 2017;45(6):1779–86.
Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC Infect Dis. 2011;11:28.
Sharaf Eldin GS, Fadl-Elmula I, Ali MS, Ali AB, Salih AL, Mallard K, et al. Tuberculosis in Sudan: a study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect Dis. 2011;11:219.
Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15:461.
Ba Diallo A, Ossoga GW, Daneau G, Lo S, Ngandolo R, Djaibé CD, et al. Emergence and clonal transmission of multi-drug-resistant tuberculosis among patients in Chad. BMC Infect Dis. 2017;17(1):579.
Juma SP, Maro A, Pholwat S, Mpagama SG, Gratz J, Liyoyo A, et al. Underestimated pyrazinamide resistance may compromise outcomes of pyrazinamide containing regimens for treatment of drug susceptible and multi-drug-resistant tuberculosis in Tanzania. BMC Infect Dis. 2019;19(1):129.
Ogari CO, Nyamache AK, Nonoh J, Amukoye E. Prevalence and detection of drug resistant mutations in Mycobacterium tuberculosis among drug naïve patients in Nairobi, Kenya. BMC Infect Dis. 2019;19(1):279.
Saldanha N, Runwal K, Ghanekar C, Gaikwad S, Sane S, Pujari S. High prevalence of multi drug resistant tuberculosis in people living with HIV in Western India. BMC Infect Dis. 2019;19(1):391.
Gehre F, Otu J, Kendall L, Forson A, Kwara A, Kudzawu S, et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Med. 2016;14:160.
Diriba G, Kebede A, Tola HH, Alemu A, Tadesse M, Tesfaye E, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):54.
Cox HS, McDermid C, Azevedo V, Muller O, Coetzee D, Simpson J, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS ONE. 2010;5(11): e13901.
Hom JK, Wang B, Chetty S, Giddy J, Mazibuko M, Allen J, et al. Drug-resistant tuberculosis among HIV-infected patients starting antiretroviral therapy in Durban, South Africa. PLoS ONE. 2012;7(8): e43281.
Porwal C, Kaushik A, Makkar N, Banavaliker JN, Hanif M, Singla R, et al. Incidence and risk factors for extensively drug-resistant tuberculosis in Delhi region. PLoS ONE. 2013;8(2): e55299.
Isaakidis P, Das M, Kumar AMV, Peskett C, Khetarpal M, Bamne A, et al. Alarming levels of drug-resistant tuberculosis in HIV-infected patients in metropolitan Mumbai, India. PLoS ONE. 2014;9(10): e110461.
Ullah I, Shah AA, Basit A, Ali M, Khan A, Ullah U, et al. Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert MTB/RIF in Khyber Pakhtunkhwa, Pakistan: a retrospective study. BMC Infect Dis. 2016;16:143.
Mesfin EA, Beyene D, Tesfaye A, Admasu A, Addise D, Amare M, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS ONE. 2018;13(6): e0197737.
Kigozi E, Kasule GW, Musisi K, Lukoye D, Kyobe S, Katabazi FA, et al. Prevalence and patterns of rifampicin and isoniazid resistance conferring mutations in Mycobacterium tuberculosis isolates from Uganda. PLoS ONE. 2018;13(5): e0198091.
Shibabaw A, Gelaw B, Gebreyes W, Robinson R, Wang SH, Tessema B. The burden of pre-extensively and extensively drug-resistant tuberculosis among MDR-TB patients in the Amhara region, Ethiopia. PLoS ONE. 2020;15(2): e0229040.
Gilad J, Borer A, Riesenberg K, Peled N, Schlaeffer F. Epidemiology and ethnic distribution of multidrug-resistant tuberculosis in Southern Israel, 1992–1997. Chest. 2000;117(3):738–43.
Ramzan MM, Sabayev V, Anwar N, Patel A, Asnis D, Avaiya A, et al. Prevalence of drug resistant tuberculosis among asians: a flushing hospital experience. Chest. 2004;126(4, Supplement):753S.
Um S-J, Son C, Roh MS, Lee S-K, Kim KH, Huh J, et al. Prevalence Of Multi Drug Resistant Pulmonary Tuberculosis In Intermediate Endemism Country. A55 Multi-drug resistant and extensively drug-resistant tuberculosis: American Thoracic Society; 2011. p. A1824-A.
Diandé S, Badoum G, Combary A, Zombra I, Saouadogo T, Sawadogo LT, et al. Multidrug-resistant tuberculosis in Burkina Faso from 2006 to 2017: results of national surveys. Eur J Microbiol Immunol. 2019;9(1):23–8.
Becerril-Montes P, Said-Fernández S, Luna-Herrera J, Caballero-Olín G, Enciso-Moreno JA, Martínez-Rodríguez HG, et al. A population-based study of first and second-line drug-resistant tuberculosis in a high-burden area of the Mexico/United States border. Mem Inst Oswaldo Cruz. 2013;108(2):160–6.
Bastos GM, Cezar MC, Mello FC, Conde MB. Prevalence of primary drug resistance in pulmonary tuberculosis patients with no known risk factors for such. J Bras Pneumol. 2012;38(6):733.
Micheletti VCD, Moreira JS, Ribeiro MO, Kritski AL, Braga JU. Drug-resistant tuberculosis in subjects included in the second national survey on antituberculosis drug resistance in Porto Alegre, Brazil. J Bras Pneumol. 2014;40(2):155–63.
Zhao LL, Huang MX, Xiao TY, Liu HC, Li MC, Zhao XQ, et al. Prevalence, risk and genetic characteristics of drug-resistant tuberculosis in a tertiary care tuberculosis hospital in China. Infect Drug Resist. 2019;12:2457–65.
Migliori GB, Ortmann J, Girardi E, Besozzi G, Lange C, Cirillo DM, et al. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg Infect Dis. 2007;13(5):780.
Deng Y, Wang Y, Wang J, Jing H, Yu C, Wang H, et al. Laboratory-based surveillance of extensively drug-resistant tuberculosis, China. Emerg Infect Dis. 2011;17(3):495.
Wallengren K, Scano F, Nunn P, Margot B, Buthelezi SS, Williams B, et al. Drug-resistant tuberculosis, KwaZulu-Natal, South Africa, 2001–2007. Emerg Infect Dis. 2011;17(10):1913.
El Achkar S, Demanche C, Osman M, Rafei R, Ismail MB, Yaacoub H, et al. Drug-resistant tuberculosis, Lebanon, 2016–2017. Emerg Infect Dis. 2019;25(3):564.
Lee SW, Jeon K, Kim KH, Min KH. Multidrug-resistant pulmonary tuberculosis among young Korean soldiers in a communal setting. J Korean Med Sci. 2009;24(4):592–5.
Buyankhishig B, Naranbat N, Mitarai S, Rieder HL. Nationwide survey of anti-tuberculosis drug resistance in Mongolia. Int J Tuberculosis Lung Dis. 2011;15(9):1201–5.
Seddon JA, Hesseling AC, Marais BJ, Jordaan A, Victor T, Schaaf HS. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. IJTLD. 2012;16(7):928–33.
Bojorquez-Chapela I, Bäcker CE, Orejel I, López A, Díaz-Quiñonez A, Hernández-Serrato MI, et al. Drug resistance in Mexico: results from the national survey on drug-resistant tuberculosis. IJTLD. 2013;17(4):514–9.
Ei PW, Aung WW, Nyunt WW, Swe TL, Htwe MM, Win SM, et al. Extensively drug-resistant tuberculosis in Myanmar: burden and mutations causing second-line drug resistance. IJTLD. 2018;22(1):47–53.
Smith CM, Lessells R, Grant AD, Herbst K, Tanser F. Spatial clustering of drug-resistant tuberculosis in Hlabisa subdistrict, KwaZulu-Natal, 2011–2015. IJTLD. 2018;22(3):287–93.
Alikhanova N, Akhundova I, Seyfaddinova M, Mammadbayov E, Mirtskulava V, Rüsch-Gerdes S, et al. First national survey of anti-tuberculosis drug resistance in Azerbaijan and risk factors analysis. Public Health Action. 2014;4(Suppl 2):S17-23.
Tasbiti AH, Yari S, Ghanei M, Shokrgozar MA, Fateh A, Bahrmand A. Low levels of extensively drug-resistant tuberculosis among multidrug resistant tuberculosis isolates and their relationship to risk factors: surveillance in Tehran, Iran; 2006 to 2014. PHRP. 2017;8(2):116–23.
Cox HS, Orozco JD, Male R, Ruesch-Gerdes S, Falzon D, Small I, et al. Multidrug-resistant tuberculosis in central Asia. Emerg Infect Dis. 2004;10(5):865.
Otokunefor K, Otokunefor TV, Omakwele G. Multi-drug resistant Mycobacterium tuberculosis in Port Harcourt, Nigeria. Afr J Lab Med. 2018;7(2):805.
Mehdi RM, Reza MS, Mohammad R. Study prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Tuberculosis in East Azerbaijan province of Iran. HealthMED. 2012;6(9):3091–4.
Israel K, Jean-Baptiste G, Julceus E, Docteur W, Sohler N, editors. Prevalence of Multi-Drug Resistant Tuberculosis in Zanmi Lasante Network among Patients who had a Gene Xpert Testing from October 2014 to September 20152018: 9th Annual CUGH Conference.
Wang SF, Yang Z, Yu P, Hui Wen Z, Yan LZ. Prevalence and risk factors of primary drug-resistant tuberculosis in China. Biomed Environ Sci. 2016;29(2):91–8.
Wang D, Yang C, Kuang T, Lei H, Meng X, Tong A, et al. Prevalence of multidrug and extensively drug-resistant tuberculosis in Beijing, China: a hospital-based retrospective study. Jpn J Infect Dis. 2010;63(5):368–71.
Laghari GS, Hussain Z, Khemani L, Hussain SZM, Yaqoob U. Burden of drug-resistant pulmonary tuberculosis in Pakistani children: a cross-sectional study. F1000Research. 2019;8:344.
Afroz H, Ali MA, Fakruddin M, Kamrunnahar DS. Prevalence and treatment follow-up of drug-resistant extra-pulmonary tuberculosis in rural communities in Narshingdi, Bangladesh. Int J Adv Med. 2014;1:71–7.
Faridi M, Shukla I, Fatima N, Varshney S, Shameem M. Prevalence of primary pulmonary multi-drug resistant tuberculosis in and around Aligarh Region. J Med Microb Diagn. 2018;7(285):2161.
Aguiar F, Vieira M, Staviack A, Buarque C, Marsico A, Fonseca L, et al. Prevalence of anti-tuberculosis drug resistance in an HIV/AIDS reference hospital in Rio de Janeiro, Brazil. IJTLD. 2009;13(1):54–61.
Rashedi J, Mahdavi Poor B, Rafi A, Asgharzadeh M, Abdolalizadeh J, Moaddab SR. Multidrug-resistant tuberculosis in north-west of Iran and Republic of Azerbaijan: a major public health concern for Iranian people. J Res Health Sci. 2015;15(2):101–3.
Akhtar AM, Arif MA, Kanwal S, Majeed S. Prevalence and drug resistance pattern of MDR TB in retreatment cases of Punjab, Pakistan. JPMA. 2016;66(8):989–93.
Anunnatsiri S, Chetchotisakd P, Wanke C. Factors associated with treatment outcomes in pulmonary tuberculosis in northeastern Thailand. Southeast Asian J Trop Med Public Health. 2005;36(2):324–30.
Surucuoglu S, Ozkutuk N, Celik P, Gazi H, Dinc G, Kurutepe S, et al. Drug-resistant pulmonary tuberculosis in western Turkey: prevalence, clinical characteristics and treatment outcome. Ann Saudi Med. 2005;25(4):313–8.
Bhat J, Rao VG, Yadav R, Muniyandi M, Sharma R, Karfarma C, et al. Situation of drug resistant tuberculosis in Saharia tribe of central India. Indian J Med Res. 2015;141(5):636–9.
Kinjal R, Firoz G, Iva C, Gaurang K, Pradeep P. Study On Prevalence Of Drug Resistance And Genetic Mutation Pattern Among Suspected Drug Resistant Pulmonary Tuberculosis Cases In Jamnagar District. National Journal of Integrated Research in Medicine. 2019;10(4)
Jacobs MG, Pinto VL. Characterization of drug-resistant tuberculosis in Brazil, 2014. Epidemiologia e Serviços de Saúd. 2020;28: e2018294.
Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16(10):1193–201.
Abouyannis M, Dacombe R, Dambe I, Mpunga J, Faragher B, Gausi F, et al. Drug resistance of Mycobacterium tuberculosis in Malawi: a cross-sectional survey. Bull World Health Organ. 2014;92(11):798–806.
Abdella K, Abdissa K, Kebede W, Abebe G. Drug resistance patterns of Mycobacterium tuberculosis complex and associated factors among retreatment cases around Jimma, Southwest Ethiopia. BMC Public Health. 2015;15:599.
Weyer K, Brand J, Lancaster J, Levin J, Van der Walt M. Determinants of multidrug-resistant tuberculosis in South Africa: results from a national survey. SAMJ. 2007;97(11):1120–8.
Soliman NS. Prevalence of multidrug-resistant tuberculosis using phenotypic drug susceptibility testing and GeneXpert MTB/RIF with characterization of non-tuberculous mycobacteria using MALDI-TOF. EJMM. 2021;30(3):143–51.
Villa-Rosas C, Laniado-Laborín R, Oceguera-Palao L. Primary drug resistance in a region with high burden of tuberculosis. A critical problem. Salud Publica Mex. 2015;57(2):177–9.
Aia P, Kal M, Lavu E, John LN, Johnson K, Coulter C, et al. The burden of drug-resistant tuberculosis in Papua New Guinea: results of a large population-based survey. PLoS ONE. 2016;11(3): e0149806.
Hang NTL, Maeda S, Lien LT, Thuong PH, Hung NV, Thuy TB, et al. Primary drug-resistant tuberculosis in Hanoi, Viet Nam: present status and risk factors. PLoS ONE. 2013;8(8): e71867.
Minion J, Pai M. Assays for drug resistant tuberculosis in high burden countries reply. Lancet Infect Dis. 2011;11(3):162.
Gallo JF, Pinhata JMW, Simonsen V, Galesi VMN, Ferrazoli L, Oliveira RS. Prevalence, associated factors, outcomes and transmission of extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in São Paulo, Brazil: a cross-sectional study. ESCMID. 2018;24(8):889–95.
Bedaso MH, Kalil FS. Trends of Drug Resistance Tuberculosis from 2014 to 2018, Bale Zone, Oromia Region, Ethiopia. Infect Drug Resist. 2021;14:2073–8.
Anwaierjiang A, Wang Q, Liu H, Yin C, Xu M, Li M, et al. Prevalence and molecular characteristics based on whole genome sequencing of Mycobacterium tuberculosis resistant to four anti-tuberculosis drugs from Southern Xinjiang, China. Infect Drug Resist. 2021;14:3379–91.
Patil S, Nakate P, Patil S, Shelke Y. Prevalence of multi-drug resistant (MDR) pulmonary tuberculosis in a tertiary care rural hospital in Western Maharashtra, India. 2019.
Kulkarni GS, Palwe SD, Patil NP, Telkhade AJ, Kadukar J. Prevalence of multidrug-resistant tuberculosis at a regional drug-resistant tuberculosis center of Maharashtra. Indian J Respir Care. 2020;9(1):30.
Adwani S, Desai UD, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai. JKIMSU. 2016;5:13–9.
Dagne B, Desta K, Fekade R, Amare M, Tadesse M, Diriba G, et al. The Epidemiology of first and second-line drug-resistance Mycobacterium tuberculosis complex common species: evidence from selected TB treatment initiating centers in Ethiopia. PLoS ONE. 2021;16(1): e0245687.
Micheni LN, Kassaza K, Kinyi H, Ntulume I, Bazira J. Rifampicin and isoniazid drug resistance among patients diagnosed with pulmonary tuberculosis in southwestern Uganda. PLoS ONE. 2021;16(10): e0259221.
Muhmmad A, Muhammad N, Khan ZU, Jamal T, Ishaq M. Burden of multi-drug resistant and extensive drug resistant of Mycobacterium tuberculosis. KJMS. 2019;12(2):1.
Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, et al. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect. 2008;47(7):e57-63.
Lukoye D, Adatu F, Musisi K, Kasule GW, Were W, Odeke R, et al. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS ONE. 2013;8(8): e70763.
Oyedeji GJ, Adeyemo C, Dissou A, Abiodun T, Alli OAT, Onaolapo OJ, et al. Prevalence of multi-drug resistant tuberculosis among tuberculosis patients attending chest clinics in Osun-State, Nigeria. Curr Pharm Biotechnol. 2020;21(10):939–47.
Masood R, Muhammad IN, Siddiqui T, Mushtaque M, Irshad A. High prevalence of DR-TB (drug-resistant tuberculosis): an Indicator of public health negligence. Pak J Pharm Sci. 2019;32(4):1529–36.
Safwat TM, Elmasry AA, Mohamed AKM. Prevalence of multi drug-resistant tuberculosis at Abbassia Chest Hospital from July 2006 to 2009. Egypt J Bronchol. 2011;5(2):124–30.
Abebe G, Abdissa K, Abdissa A, Apers L, Agonafir M, de Jong BC, et al. Relatively low primary drug resistant tuberculosis in southwestern Ethiopia. BMC Res Notes. 2012;5:225.
Yoshiyama T, Supawitkul S, Kunyanone N, Riengthong D, Yanai H, Abe C, et al. Prevalence of drug-resistant tuberculosis in an HIV endemic area in northern Thailand. Int J Tuberc Lung Dis. 2001;5(1):32–9.
Dewan P, Sosnovskaja A, Thomsen V, Cicenaite J, Laserson K, Johansen I, et al. High prevalence of drug-resistant tuberculosis, Republic of Lithuania, 2002. IJTLD. 2005;9(2):170–4.
Pleumpanupat W, Jittimanee S, Akarasewi P, Rienthong S, Jittimanee S, Chiewlian Y, et al. Resistance to anti-tuberculosis drugs among smear-positive cases in Thai prisons 2 years after the implementation of the DOTS strategy. IJTLD. 2003;7(5):472–7.
Sidze LK, Mouafo Tekwu E, Kuaban C, Assam Assam JP, Tedom JC, Eyangoh S, et al. Strong decrease in streptomycin-resistance and absence of XDR 12 years after the Reorganization of the National Tuberculosis Control Program in the Central Region of Cameroon. PLoS ONE. 2014;9(6): e98374.
Nigus DM, Lingerew W, Beyene B, Tamiru A, Lemma M, Melaku MY. Prevalence of multi drug resistant tuberculosis among presumptive multi drug resistant tuberculosis cases in Amhara National Regional State, Ethiopia. J Mycobac Dis. 2014;4(152):2161.
Alberte-Castiñeiras A, Brezmes-Valdivieso MF, Campos-Bueno A, Montes-Martinez I, López-Medrano R, Avellaneda C, et al. Drug-resistant tuberculosis in Castilla-León, Spain, 1996–2000. IJTLD. 2006;10(5):554–8.
Elmi OS, Hasan H, Abdullah S, Jeab MZM, Zilfalil B, Naing NN. Prevalence and associated factors with transmission of latent tuberculosis among household contacts of multi-drug resistant tuberculosis patients in Malaysia. World J Med Sci. 2014;10(3):285–94.
Amala SE, Silas G. The prevalence of tuberculosis (TB) and multiple drug resistant tuberculosis (MDR-TB) in Bayelsa state. NIGERIA AJHR. 2019;5:1–5.
Brito RC, Mello FCQ, Andrade MK, Oliveira H, Costa W, Matos HJ, et al. Drug-resistant tuberculosis in six hospitals in Rio de Janeiro, Brazil. IJTLD. 2010;14(1):24–33.
Wang G, Jiang G, Jing W, Zong Z, Yu X, Chen S, et al. Prevalence and molecular characterizations of seven additional drug resistance among multidrug-resistant tuberculosis in China: a subsequent study of a national survey. J Infect. 2021;82(3):371–7.
Xiang Y, Ying L, Liu J, Su Q, Shen J, Zhan J, et al. An epidemiological study of resistant tuberculosis in Chongqing, China. J Med Colleges of PLA. 2011;26(3):158–73.
Yang X, Yuan Y, Pang Y, Wang B, Bai Y, Wang Y, et al. The burden of MDR/XDR tuberculosis in coastal plains population of China. PLoS ONE. 2015;10(2): e0117361.
Zazueta-Beltran J, Leon-Sicairos N, Muro-Amador S, Flores-Gaxiola A, Velazquez-Roman J, Flores-Villasenor H, et al. Increasing drug resistance of Mycobacterium tuberculosis in Sinaloa, Mexico, 1997–2005. IJID. 2011;15(4):E272–6.
Pang Y, Zhu D, Zheng H, Shen J, Hu Y, Liu J, et al. Prevalence and molecular characterization of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis isolates from Southern China. BMC Infect Dis. 2017;17(1):711.
Lapphra K, Sutthipong C, Vanprapar N, Phongsamart W, Wittawatmongkol O, Udompornwattana S, et al. Drug-resistant tuberculosis in Thai children. IJID. 2012;16: e26.
Liu ZY, Shilkret KI, Finelli L. Epidemiology of drug-resistant tuberculosis in New Jersey from 1991 to 1995. Int J Epidemiol. 1998;27(1):121–6.
Huo F, Luo J, Shi J, Zong Z, Jing W, Dong W, et al. A 10-year comparative analysis shows that increasing prevalence of rifampin-resistant mycobacterium tuberculosis in china is associated with the transmission of strains harboring compensatory mutations. Antimicrob Agents Chemother. 2018;62(4): e02303.
Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Ghebremichael S, Hoffner S, et al. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates from Ethiopian pulmonary tuberculosis patients with and without human immunodeficiency virus infection. J Clin Microbiol. 2002;40(5):1636–43.
Amanullah F, Ashfaq M, Khowaja S, Parekh A, Salahuddin N, Lotia-Farrukh I, et al. High tuberculosis prevalence in children exposed at home to drug-resistant tuberculosis. IJTLD. 2014;18(5):520–7.
Ombura IP, Onyango N, Odera S, Mutua F, Nyagol J. Prevalence of drug resistance Mycobacterium tuberculosis among patients seen in coast Provincial General Hospital, Mombasa, Kenya. PLoS ONE. 2016;11(10): e0163994.
Gebeyehu M, Lemma E, Eyob G. Prevalence of drug resistant tuberculosis in Arsi Zone, Ethiopia. The Ethiopian Journal of Health Development. 2001;15(1).
Madukaji L, Okohu I, Usman S, Oyedum U, Enagi A, Usman A, et al. Early detection of Pre-XDR TB with line probe assay in a high TB burden country. Afr Health Sci. 2021;21(3):968–74.
Khunjeli R, Mohsin U, Shrestha S, Adhikari S, Srivastava B, Shrestha B. Prevalence of primary drug resistant tuberculosis in a tertiary care hospital. Nepal JCMC. 2014;4(4):36–8.
Pande JN, Singh UB, Sinha S, Agarwal RC, Singh SPN. Evaluation of risk factors and prevalence of drug resistant tuberculosis in north India. Chest. 2005;128(4):404S.
Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338(23):1641–9.
Committee TR. Drug-resistant Mycobacterium tuberculosis in Japan: a nationwide survey, 2002. IJTLD. 2007;11(10):1129–35.
Dorjee K, Sadutshang TD, Rana RS, Topgyal S, Phunkyi D, Choetso T, et al. High prevalence of rifampin-resistant tuberculosis in mountainous districts of India. IJTB. 2020;67(1):59–64.
Mohajeri P, Sadri H, Farahani A, Norozi B, Atashi S. Frequency of mutations associated with rifampicin resistance in Mycobacterium tuberculosis strains isolated from patients in west of Iran. Microb Drug Resist. 2015;21(3):315–9.
Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185–92.
Araya S, Negesso AE, Tamir Z. Rifampicin-resistant Mycobacterium tuberculosis among patients with presumptive tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist. 2020;13:3451–9.
Wang X, Fu Q, Li Z, Chen S, Liu Z, Nelson H, et al. Drug-resistant tuberculosis in Zhejiang province, China, 1999–2008. Emerg Infect Dis. 2012;18(3):496.
El Achkar S. Prevalence of drug-resistant tuberculosis assessed by next-generation sequencing: an 18-month nationwide study in Lebanon: Université de Lille; 2019.
Adejumo OA, Olusola-Faleye B, Adepoju V, Bowale A, Adesola S, Falana A, et al. Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. Afr Health Sci. 2018;18(3):472–8.
Gebrehiwet GB, Kahsay AG, Welekidan LN, Hagos AK, Abay GK, Hagos DG. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J Infect Dev Ctries. 2019;13(1):21–7.
Bitet DE, Kumurya SA, Joseph L, Bathelomow P. Rifampicin resistant tuberculosis among patients attending General Hospital, Kagarko, Kaduna State, Nigeria. Afr J Clin Exp Microbiol. 2020;21(3):250–4.
Awais M, Ahmad R, Jan F, Anwar B, Rehman R, Mujtaba G, et al. Prevalence and detection of drug-resistant tuberculosis in Hazara Division, Pakistan. Acad J Biotechnol. 2018;6(9):116–23.
Ikuabe PO, Ebuenyi ID. Prevalence of rifampicin resistance by automated Genexpert rifampicin assay in patients with pulmonary tuberculosis in Yenagoa, Nigeria. Pan Afr Med J. 2018;29:204.
Meaza A, Tesfaye E, Mohamed Z, Zerihun B, Seid G, Eshetu K, et al. Diagnostic accuracy of Truenat Tuberculosis and Rifampicin-Resistance assays in Addis Ababa, Ethiopia. PLoS ONE. 2021;16(12): e0261084.
Fadeyi A, Desalu OO, Ugwuoke C, Opanwa OA, Nwabuisi C, Salami AK. Prevalence of rifampicin-resistant tuberculosis among patients previously treated for pulmonary tuberculosis in North-Western, Nigeria. NMJ. 2017;58(6):161–6.
Elion Assiana DO, Abdul J, Linguissi LSG, Epola M, Vouvoungui JC, Mabiala A, et al. Epidemiological profile of multidrug-resistant and extensively drug-resistant Mycobacterium tubrculosis among Congolese patients. Ann Clin Microbiol Antimicrob. 2021;20(1):84.
Huo F, Zhang F, Xue Y, Shang Y, Liang Q, Ma Y, et al. Increased prevalence of levofloxacin-resistant Mycobacterium tuberculosis in China is associated with specific mutations within the gyrA gene. IJID. 2020;92:241–6.
Rivière E, Verboven L, Dippenaar A, Goossens S, De Vos E, Streicher E, et al. Variants in bedaquiline-candidate-resistance genes: prevalence in bedaquiline-naive patients, effect on MIC, and association with Mycobacterium tuberculosis Lineage. Antimicrob Agents Chemother. 2022;66(7): e0032222.
Latrilha FO, Simonsen V, Pinhata JM, Brandao AP, Galesi VMN, Waldman EA, et al. Transmission and prevalence of drug-resistant tuberculosis in a Brazilian setting under a directly observed therapy short-course strategy. Rev Soc Bras Med Trop. 2020;53: e20190404.
Ahmed I, Jabeen K, Inayat R, Hasan R. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob Agents Chemother. 2013;57(6):2522–5.
Kumar RS. Prevalence of pre-extensively drug-resistant tuberculosis and extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in South Tamil Nadu. Int J Sci Stud. 2020;8(9):96–9.
Chuchottaworn C. Extensively drug resistant tuberculosis (XDR-TB) in Chest Disease Institute, 197–205. J Med Assoc Thailand. 2010;93(1):34–7.
Pang Y, Lu J, Huo F, Ma Y, Zhao L, Li Y, et al. Prevalence and treatment outcome of extensively drug-resistant tuberculosis plus additional drug resistance from the National Clinical Center for Tuberculosis in China: a five-year review. J Infect. 2017;75(5):433–40.
Diriba G, Alemu A, Tola HH, Yenew B, Amare M, Eshetu K, et al. Pre-extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in Ethiopia: a laboratory-based surveillance study. IJID Regions. 2022;5:39–43.
Mellor Y, Herron D. Disease management: an introduction to tuberculosis. Aust Pharm. 2020;39(2):52–9.
Agyeman AA, Ofori-Asenso R. Tuberculosis—an overview. J Public Health Emerg. 2017;1(7):1–11.
Onyedum CC, Alobu I, Ukwaja KN. Prevalence of drug-resistant tuberculosis in Nigeria: a systematic review and meta-analysis. PLoS ONE. 2017;12(7): e0180996.
Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8(1):1–5.
Duan Q, Chen Z, Chen C, Zhang Z, Lu Z, Yang Y, et al. The prevalence of drug-resistant tuberculosis in mainland China: an updated systematic review and meta-analysis. PLoS ONE. 2016;11(2): e0148041.
Organization WH. Anti-tuberculosis drug resistance in the world. Report No. 4: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. Anti-tuberculosis drug resistance in the world Report No 4: The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. 2008.
Molla KA, Reta MA, Ayene YY. Prevalence of multidrug-resistant tuberculosis in East Africa: a systematic review and meta-analysis. PLoS ONE. 2022;17(6): e0270272.
Migliori GB, Dheda K, Centis R, Mwaba P, Bates M, O’Grady J, et al. Review of multidrug-resistant and extensively drug-resistant TB: global perspectives with a focus on sub-Saharan Africa. Trop Med Int Health. 2010;15(9):1052–66.
Chen S, Huai P, Wang X, Zhong J, Wang X, Wang K, et al. Risk factors for multidrug resistance among previously treated patients with tuberculosis in eastern China: a case–control study. Int J Infect Dis. 2013;17(12):e1116–20.
Lema NA, Mbelele PM, Majigo M, Abade A, Matee MI. Risk factors associated with multidrug resistant tuberculosis among patients referred to Kibong’oto Infectious Disease Hospital in northern Tanzania. TJHR. 2016;18(4).
Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS ONE. 2015;10(3): e0119332.
Alkabab YM, Al-Abdely HM, Heysell SK. Diabetes-related tuberculosis in the Middle East: an urgent need for regional research. Int J Infect Dis. 2015;40:64–70.
Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):1–15.
Mesfin YM, Hailemariam D, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2014;9(1): e82235.
Demile B, Zenebu A, Shewaye H, Xia S, Guadie A. Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in a tertiary armed force referral and teaching hospital, Ethiopia. BMC Infect Dis. 2018;18(1):1–10.
Al-Darraji HAA, Tan C, Kamarulzaman A, Altice FL. Prevalence and correlates of latent tuberculosis infection among employees of a high security prison in Malaysia. Occup Environ Med. 2015;72(6):442–7.
Gobena D, Ameya G, Haile K, Abreha G, Worku Y, Debela T. Predictor of multidrug resistant tuberculosis in southwestern part of Ethiopia: a case control study. Ann Clin Microbiol Antimicrob. 2018;17(1):1–7.
Dujaili JA, Syed Sulaiman SA, Awaisu A, Muttalif AR, Blebil AQ. Outcomes of tuberculosis treatment: a retrospective cohort analysis of smoking versus non-smoking patients in Penang, Malaysia. J Public Health. 2011;19(2):183–9.
Rajendran M, Zaki RA, Aghamohammadi N. Contributing risk factors towards the prevalence of multidrug-resistant tuberculosis in Malaysia: a systematic review. Tuberculosis. 2020;122: 101925.
Jilani TN, Avula A, Gondal Z, Siddiqui AH. Active tuberculosis. 2018.
Dirie AMH, Çolakoğlu S, Abdulle OM, Abdi BM, Osman MA, Shire AM, Hussein AM. Prevalence of multidrug-resistant TB among smear-positive pulmonary TB patients in Banadir, Somalia: a multicenter study. Infect Drug Resist. 2022;15:7241–8.
Sylverken AA, Kwarteng A, Twumasi-Ankrah S, et al. The burden of drug resistance tuberculosis in Ghana; results of the First National Survey. PLoS ONE. 2021;16(6):1–14.
Emam SA, Kasem EM, Sedhom AE. Characteristics of multidrug resistant tuberculosis in Minia, Egypt. Medico Legal Updat. 2020;20(1):446–52.
Welekidan LN, Skjerve E, Dejene TA, et al. Characteristics of pulmonary multidrug-resistant tuberculosis patients in Tigray Region, Ethiopia: a cross-sectional study. PLoS ONE. 2020;15(8):1–20.
Acknowledgements
We would like to thank the support provided by the Student Research Committee of Kermanshah University of Medical Sciences.
Funding
By Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (50002460). This deputy has no role in the study process.
Author information
Authors and Affiliations
Contributions
NS and RH and AK contributed to the design, MM statistical analysis, participated in most of the study steps. MM and RH and AHF prepared the manuscript. AK and RH and KM assisted in designing the study, and helped in the interpretation of results. All authors have read and approved the content of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was received from the ethics committee of deputy of research and technology, Kermanshah University of Medical Sciences (50002460).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Supplementary Information
Additional file 1: Table S1.
Summary of Characteristics of Included Studies of Prevalence of MDR-TB. TableS2. Summary of Characteristics of Included Studies of Prevalence of Isoniazid Resistant-TB.Table S3. Summary of Characteristics of Included Studies of Prevalence of Rifampcin Resistant-TB.Table S4.Summary of Characteristics of Included Studies of Prevalence of Single Drug Resistant-TB.Table S5.Summary of characteristics of included studies of prevalence of XDR-TB.TableS6. Summary of characteristics of included studies of prevalence of pre-XDR TB.
Additional file 2: Figure S1.
Forest plot of the global prevalence ofmulti-drug resistant TB based on the random effects method. Figure S2. Funnel plotof publication bias in reviewed studies. Figure S3.Forest plot of global prevalence of isoniazid resistant TB based on randomeffects method. Figure S4. Funnel plot of publication biasin reviewed studies. Figure S5. Forest plot ofglobal prevalence of rifampin-resistant TB based on random effects method. FigureS6.Funnel plot of publication bias in reviewed studies. FigureS7. Forest plot of globalprevalence of single drug resistant TB based on random effects method. FigureS8.Funnel plot of publication bias in reviewed studies.Figure S9. Forest plot of global prevalence of extensively drugresistant TB based on random effects method. Figure S10. Funnel Plot of Publication Bias in ReviewedStudies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salari, N., Kanjoori, A.H., Hosseinian-Far, A. et al. Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis. Infect Dis Poverty 12, 57 (2023). https://doi.org/10.1186/s40249-023-01107-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-023-01107-x