Abstract
Background
Primary health care settings and hospitals of low- and middle-income countries have few accessible diagnostic tools and limited laboratory and human resources capacity to identify multiple pathogens with high accuracy. In addition, there is a paucity of information on fever and its underlying aetiology in the adolescent and adult population in East Africa. The purpose of this study was to estimate the pooled prevalence of fever of unidentified aetiology among adolescent and adult febrile patients seeking health care in East Africa.
Methods
We pursued a systematic review using readily available electronic databases (i.e. PubMed, Cumulative Index to Nursing & Allied Health Literature, Scopus, Cochrane Library and Web of Science) without language restriction from inception date of the respective databases to October 31, 2022. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Identified studies were screened for relevance. Further analyses based on pre-set eligibility criteria were carried out for final inclusion. Two reviewers independently screened and extracted data. Risk of study bias was assessed. Meta-analysis of the prevalence of fever of unidentified aetiology was performed.
Results
We identified 14,029 articles of which 25 were eligible for inclusion, reporting data from 8538 participants. The pooled prevalence of febrile cases with unidentified aetiology was 64% [95% confidence interval (CI): 51–77%, I2 = 99.6%] among febrile adolescents and adults in East Africa. For the proportion of patients with identified aetiology, the studies documented bacterial pathogens (human bloodstream infections), bacterial zoonotic pathogens and arboviruses as the main non-malarial causative agents in East Africa.
Conclusions
Our study provides evidence that almost two-thirds of adolescent and adult febrile patients attending health care facilities in East Africa might receive inappropriate treatments due to unidentified potential life-threatening fever aetiology. Hence, we call for a comprehensive fever syndromic surveillance to broaden a consequential differential diagnosis of syndromic fever and to considerably improve the course of patients’ disease and treatment outcomes.
Similar content being viewed by others
Background
Fever is the temporary elevation in body temperature in response to a disease or illness [1, 2]. It is the cardinal sign for an acute infection [3]. Fever is also one of the most common complaints of patients seeking care at hospitals and primary health care settings in low- and middle-income countries (LMICs) [4, 5]. The burden of febrile illnesses is usually estimated based on the identified aetiology. In recent years, attempts were made to address the combined burden of fever-characterised conditions [6]. Crump and Kirk [6] proposed a syndromic approach to all febrile illnesses to enable the assignment of disability-adjusted life years (DALYs) and deaths to specific aetiologic agents.
In primary health care settings of LMICs, fever poses a potential diagnostic challenge. Approximately one-third to half of patients present with fever [7]. Children and seriously ill patients make up the highest percentage of people affected by fever. Importantly, case fatality ratio among patients with fever requiring hospital admission may exceed 20% [8,9,10] and accurate determination of the underlying cause of fever is challenging due to the wide spectrum of fever aetiologies, the lack of differential diagnostic tools and limitations in access to care and human resources.
Notwithstanding the paucity of fever studies in adults in LMICs, the presence of infections other than malaria underscore the need for evidence-based algorithms to help clinicians manage febrile illnesses [11]. Despite the progress made with analysis by multiplex polymerase chain reaction (PCR) that increases the recognition of multiple possible aetiologies of pathogen-initiated fever [12], hospitals in East Africa currently still have few accessible diagnostic tools and limited laboratory capacity to identify multiple pathogens with high accuracy [13]. It follows that diagnosis of fever predominantly relies on single-disease based investigation in East Africa. As a result, clinicians rely on non-specific clinical data to judge empirical therapy.
The purpose of this paper was to determine the extent of reported fever cases with unidentified aetiologies in adolescents and adults (i.e. individuals aged ≥ 13 years) in East Africa. We pursued a systematic review and meta-analysis to establish an evidence-base for an appropriate fever case management in East Africa. Additionally, our findings should help policy-makers to prioritise healthcare resources and funding towards programmes that strengthen surveillance-response systems to better address fever of unidentified aetiology.
Methods
We pursued a systematic review and meta-analysis to estimate the pooled prevalence of fever of unidentified aetiology in adolescents and adults in East Africa, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. We also determined whether patient characteristics, study setting and study design contribute to the observed prevalence of fever of unidentified aetiology in the included studies.
In the context of this paper, we use the term “fever of unidentified aetiology” to describe any documented febrile illness with no identified aetiologic agent and no limitation of fever duration. Of note, this term is distinct from “fever of unknown origin” (FUO) defined as a febrile illness that did not resolve, and with no obvious source despite full investigation, persisting for more than 3 weeks [15].
According to the African Union (AU), Africa comprises of five geographic sub-regions, namely: (i) Central Africa; (ii) Eastern Africa; (iii) Northern Africa; (iv) Southern Africa; and (v) Western Africa [16]. We focus on Eastern Africa (also called East Africa) that consists of the following 14 countries: Comoros, Djibouti, Eritrea, Ethiopia, Kenya, Madagascar, Mauritius, Rwanda, Seychelles, Somalia, South Sudan, Sudan, Tanzania and Uganda.
Eligibility criteria
We included studies that reported undifferentiated fever (UDF) among febrile patients that met the following criteria. First, studies that reported the proportion of UDF among adolescent and adult febrile patients (aged ≥ 13 years) from health facilities and community-based programmes. Second, UDF studies that were conducted in East Africa. Third, primary UDF studies of both observational and interventional designs (longitudinal and cross-sectional, randomised and non-randomised community trials, controlled and uncontrolled before/after studies).
Any study that met at least one of the following exclusion criteria was removed:
-
(i)
Studies that evaluated patients with a focus on a single aetiologic agent. The focus of this meta-analysis was on fever with unidentified aetiology, and studies that only investigated a specific aetiologic agent would not contribute to the overall understanding of the topic.
-
(ii)
Editorials, reviews, policy statements, case reports, case series studies, perspectives and author replies. These types of studies were excluded because they are not original research studies and do not provide new data that would contribute to the overall analysis. Including them would introduce bias and could potentially skew the results.
-
(iii)
Studies on fever associated with malignancies, autoimmune disorders and immunodeficiency. These studies were excluded because fever in these conditions has a known aetiology, and hence, including them would not contribute to the understanding of fever with unknown aetiology.
Data sources
We systematically searched the following readily available electronic databases: PubMed, Cumulative Index to Nursing & Allied Health Literature (CINAHL), Scopus, Cochrane Library and Web of Science. We searched from inception to October 31, 2022, without language restriction. The reference lists of relevant studies were hand-searched for additional studies.
Search strategy
The following strings of word combinations were employed to identify relevant studies:
-
1.
“Fever” OR “fever of unknown origin” OR “FUO” OR “febrile” OR “fever without apparent source” OR “FWAS” OR “undifferentiated fever” OR “febrile state” OR “hyperthermia” OR “pyrexia” OR “febrile syndrome*” OR “fever without source” OR “FWS” OR “fever without a source” OR “acute undifferentiated fever” OR “AUF” OR “acute febrile illness*” OR “undifferentiated fever”.
-
2.
“Diagnosis” OR “diagnostic*” OR “screening” OR “test*” OR “management” OR “clinical”.
-
3.
“Comoros” OR “Djibouti” OR “East* Africa” OR “Eritrea” OR “Ethiopia” OR “Horn of Africa” OR “Kenya” OR “Madagascar” OR “Mauritius” OR “Rwanda” OR “Seychelles” OR “Somalia” OR “South Sudan” OR “sub-Sahara*” OR “Sudan” OR “Tanzania” OR “Uganda”.
Study records
We screened the identified items and assessed for inclusion. Retrieved studies were transferred to the bibliographic software EndNote™ X9 (Clarivate Analytics; Philadelphia, USA), screened for relevance and checked for duplication. The criterion for relevance was based on the scope and objective of our review. Further analyses based on the eligibility criteria identified the relevant documents for final inclusion. Key data from the included studies were extracted using Microsoft® Excel 2016 (Microsoft; Washington, USA). The retrieved data included: study characteristics, participant characteristics and major findings.
Risk of bias in individual studies
Two authors (FN and AC) independently assessed and rated the risk of bias on individual studies, following the Joana Briggs Institute (JBI) critical appraisal tool for prevalence studies [17]. Disagreements between the two reviewers were discussed and, if need be, a third author was consulted until consensus was reached.
Data synthesis
For the included studies, descriptive findings were summarised in tables accompanied by text. We explored the types of diagnostic tests commonly reported by the studies and the aetiologic agents that were identified. We documented the extent of the diagnostic tests performed in each study before any clinical decision was made.
We performed descriptive tasks including comparisons of the studies and patient characteristics. We used metaprop package in STATA version 16 (StataCorp; Texas, USA) to calculate the pooled prevalence of the reported fever of unidentified aetiology [18]. We calculated weighted country-specific and overall pooled prevalence from a random-effects model using inverse-variance weights. The study-specific 95% confidence interval (CI) was computed using the exact method. We applied the Freeman-Tukey double arcsine transformation to correct extreme values.
Results
Description of search results
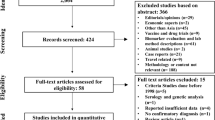
The first database search identified a total of 15,579 items. Scopus (35.5%) and Web of Science (28.5%) contributed the majority of items. PubMed added another 26.4%. We reduced the search results to 14,018 articles after exclusion of reviews. We then imported the refined results into EndNote. Among these articles, Scopus contributed 30.7%, while PubMed and Web of Science contributed 26.4% and 25.0%, respectively (Fig. 1).
Additional searches (e.g. hand searching reference lists of relevant documents) identified another 11 articles (including two items from Google scholar). A total of 6729 duplicates were removed and another 7259 Items were excluded while screening titles and abstracts. As a result, full texts of 35 documents were assessed. Risk of bias was evaluated in these 35 articles. Ten of these articles (29.4%) were excluded due to high risk of bias (Additional file 1: Table S1). Hence, 25 records were retained for qualitative analysis. Overall, 20 studies were included for meta-analysis (Fig. 1).
Description of studies
The 25 records included into the qualitative synthesis [8, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] reported data from studies conducted in six East African countries: Tanzania (n = 15), Ethiopia (n = 5), Kenya (n = 2), Madagascar, South Sudan and Uganda (n = 1 each). The studies were all published in English between 1988 and October 2022. Four articles reported findings from one study of 403 patients in a single hospital [21, 23, 25, 29]. We combined the findings of these articles into a single study. Similarly, another four articles [26, 27, 34, 41] reported results from two studies. Hence, the 25 articles reviewed here reported findings from 20 different studies. Of these studies, six were community- or population-based prevalence studies [22, 32, 35, 37, 39, 40], one study was conducted in a primary health care centre [31] and the remaining 13 studies were hospital-based [8, 19, 20, 24, 27,28,29,30, 33, 36, 38, 41, 42]. From the non-hospital based studies, we extracted data reported from febrile individuals only. The duration of data collection varied from one month to more than a year. None of the studies followed patients longitudinally.
Overall, the included studies reported data from 8538 participants, of these 5045 were from hospital and 3493 were from non-hospital-based settings. Few studies enrolled children but described results from children and adults separately. The number of patients in each study ranged from 90 [38] to 1425 [8]. Data extracted from the included studies were obtained from participants aged 13 years and above. Table 1 summarises the characteristics of the 20 included studies.
Pooled prevalence of fever of unidentified aetiology
The pooled prevalence of fever with unidentified aetiology was 64% (95% CI: 51–77%) among adolescent and adult febrile patients seeking health care in East Africa (Fig. 2). Five of the studies had estimates below 50% of which two studies had relatively large CIs. High variability was observed among the studies in our meta-analysis, indicated by I2 = 99.6%.
The analysis by country showed a prevalence of 69% (95% CI: 53–84%) in Ethiopia, 67% (95% CI: 65–68%) in Kenya and 61% (95% CI: 37–86%) in Tanzania. The highest prevalence of fever of unidentified aetiology was observed in Ethiopia, while Tanzania showed the lowest prevalence. The studies from Tanzania showed the highest variability with the largest CI compared to the other subgroups of studies. The lowest variability was observed in the studies from Kenya. The studies from Kenya were also focusing on the identification of arboviruses. Studies from Ethiopia focused on isolation of bacterial infections, whereas those from Tanzania demonstrated concomitant identification of bacterial and viral pathogens.
Separate analysis of publications from 14 studies conducted at health care facilities showed an overall prevalence of 62% (95% CI: 46–97%) of fever of unidentified aetiology. Ethiopian studies (n = 5) showed the highest prevalence (72%; 95% CI: 46–97%) and Tanzanian studies (n = 10) exhibited highest variability (59%; 95% CI: 32–85%). The majority of facility-based studies were either conducted in Tanzania (n = 7) or in Ethiopia (n = 3). Both groups of studies exhibited high variability. Both groups also showed considerable overlap in their group-specific 95% CIs. The estimates from the two other studies in Uganda and South Sudan lied within the shared CIs (Fig. 3).
Identified aetiologic agents of febrile illnesses
Overall, the included studies showed a multitude of causative agents of fever in East Africa. The most prevalent pathogen was chikungunya virus (with a prevalence of 17.5% in 6 studies) followed by Plasmodium spp. (with a prevalence of 16.4% in 10 studies), Haemophilus influenzae (with a prevalence of 14.0% in 2 studies) and dengue fever virus (with a prevalence of 10.1% in 7 studies). Rickettsia spp. (with a prevalence of 8.7% in 8 studies) and coagulase negative staphylococci (CoNS) (with a prevalence of 6.5% in 2 studies) were the most prevalent among bacterial zoonotic and bloodstream infections, respectively. Taken together, the included studies identified two main groups of infectious agents; namely, bloodstream infectious and zoonotic pathogens. Out of the six population-based studies, four identified only viral pathogens, whereas the remaining two identified bacterial pathogens. In contrast, all types of pathogens were identified in the health facility-based studies.
Bloodstream infectious pathogens
The most frequently reported bloodstream infectious pathogens in the included 20 studies were Staphylococcus aureus and Streptococcus pneumoniae, each recorded in 9 (45%) studies. Following these were Salmonella Typhi and Escherichia coli, both recorded in 7 (37%) studies each (Fig. 4). A total of 4176 patients were tested in the studies for S. aureus of whom 170 were tested positive owing to a prevalence of 4.1%. The highest prevalence was observed in this group for H. influenza, reported in two studies and tested positive for 14.0% of 813 individuals.
Frequency and percentage of patients (adults and adolescents in East Africa) with reported blood stream pathogens (the pathogens at the right side of the dotted vertical line rarely appear in the blood stream) and number of studies that identified specific pathogens in 20 studies, published from 1988 to 2022
Apart from H. influenzae and CoNS (6.5%), which were the most prevalent pathogens reported in the 20 studies, Salmonella Typhi (5.3%; 132/2475), S. pneumoniae (4.2%; 170/4003), Salmonella spp. (4.2%; 60/1424) and Mycobacterium tuberculosis (4.0%; 144/3577) were prevalent bloodstream infections.
Zoonotic pathogens
Ten viral zoonotic pathogens were reported in the 20 articles analysed (Fig. 5). Chikungunya virus and dengue fever virus were the most prevalent viral pathogens with prevalence of 17.5% and 10.1% in 3529 and 4001 tested patients, respectively. Yellow fever (9.5%) and Rift Valley fever viruses (6.0%) were the next most prevalent pathogens detected. Moreover, West Nile fever virus, human rhinovirus and Epstein-Barr virus were also reported. Furthermore, five bacterial zoonotic pathogens were reported, among which Rickettsia spp. [8 studies (40%)] and Leptospira spp. [5 studies (25%)] were most frequently reported. Among the other pathogens of this group, the prevalence was highest for Borrelia spp. and Rickettsia spp.
Diagnostic techniques used
Various diagnostic methods were applied in the included studies (Table 2). Enzyme-linked immunosorbent assay (ELISA) [in 12 studies (60%)] was the most frequently used technique, most often applying the direct IgM detection. Microscopy, blood culture, rapid diagnostic test (RDT) and PCR were each used in 9 (45%) studies. RDTs were applied for the detection of Plasmodium, human immunodeficiency virus (HIV) and dengue virus. In 5 (20%) studies, multiplex PCR analyses were used. Three of these studies (15%) applied TaqMan array cards (TAC) reporting the lowest prevalence of undifferentiated fever. Immunofluorescence assay (IFA) and Western blot were applied in 3 (15%) and 2 (10%) studies, respectively.
Discussion
In this systematic review and meta-analysis, we found that causes of fever in a large proportion (pooled prevalence = 62%; 95% CI: 48–77%) of adolescent and adult febrile patients in East Africa remain unidentified. However, knowledge of pathogens that cause fever is indispensable to inform case management, and hence, there is an urgent need for improved access to diagnostics for patients presenting with a febrile illness. The majority of reviewed studies reported misdiagnoses of febrile cases as “malaria”, thereby underappreciating other causes of fever. Previous studies highlighted important mismatches between clinical diagnosis and case management with confirmed diagnoses [11, 43,44,45,46,47]. This was attributed to the heavy reliance of clinicians on empirical diagnoses, due to limited access to readily available clinical decision support systems and diagnostic tests. Consequently, the burden of disease for various aetiologic agents might be considerably under- or over-estimated. It is important to note that inappropriate and unnecessary use of anti-malarial medications and broad-spectrum antibiotics is a major concern in areas with limited diagnostic capacity [48,49,50,51]. For example, a study in Tanzania estimated that approximately 56% of patients with suspected malaria were treated with antimalarials without laboratory confirmation [52]. Similarly, a study in India reported that up to 82% of adult patients with febrile illnesses received antibiotics without microbiological confirmation [53]. A systematic review estimated that up to 69% of antibiotic use in sub-Saharan Africa is inappropriate [54]. Another study showed that 30% of antibiotics used in hospitals were inappropriate in the United States of America [48]. Inappropriate and unnecessary use of antimicrobials can lead to development and spread of antimicrobial resistance (AMR), treatment failure and adverse patient outcomes, unsolicited drug reactions and increased healthcare costs [55]. All of these represent major challenges for global health [56, 57]. Indeed, adequate identification and characterization of potential pathogens as well as increased awareness of clinicians, patients and communities of potential fever aetiologies are crucial to enhance prevention, prevent spread of these pathogens and further enhance early detection and adequate case management.
The included studies revealed common causes of acute febrile illnesses in both hospitalized and ambulatory adolescent and adult patients. Major non-malarial causes of fever were bacterial pathogens (human bloodstream infection), bacterial or viral zoonotic pathogens. Similar findings have been reported recently in another review on non-malarial febrile illnesses with slight differences in the common types of pathogens reported from East Africa. In their review, Elven and colleagues [58] showed a surge of non-typhoidal Salmonella spp., while typhoidal Salmonella spp. are predominantly reported in our review. The difference might be explained by specific inclusion and exclusion criteria, particularly regarding the age range of individuals included. In our review, we included adolescents and adults (aged ≥ 13 years), while a large proportion of studies reviewed by Elven and colleagues [58] included children. Despite this difference in age profiles, there was a similar viral distribution in the two studies. A narrative review pertaining to the epidemiology of febrile illnesses in sub-Saharan Africa reported observations similar to our findings [11]. Indeed, Maze and colleagues [11] reported that pathogens isolated from hospitalized patients were more likely to be bloodstream infections, while common causes of fever among ambulatory patients were due to arboviruses and other respiratory pathogens [44].
Evidence of exposure to, and infection by, these common agents suggests their potential endemicity in East Africa, and hence, highlighting the clinical importance of the necessity of preventive measures targeting these pathogens and of the implementation of improved diagnostic techniques for their timely and reliable detection and effective treatment. Integrated approaches of pathogen detection, including setting-specific surveillance-response systems and application of multiple-pathogen detection technologies are, therefore, of paramount importance [59].
Collation of high-quality data is central to setting up effective surveillance-response systems. Yet, in East Africa, resources are often limited [60]. Health system strengthening to enhance surveillance-response activities will increase the potential capacity to detect causative agents in East Africa [38]. In this regard, establishing comprehensive fever syndromic surveillance-response approaches [61], with improved local availability of inexpensive, rapid, reliable and integrated diagnostic techniques that are suitable for point-of-care (POC), multi-pathogen detection using single-sample would lead to considerable impact by supporting health care professionals to offer more accurate and certain diagnosis and management, which would improve patient outcomes. Pilot studies employing a metagenomics approach are warranted and should determine costs, feasibility and scalability [62, 63].
Implications of our findings
The findings of this review suggest that a large share of febrile adolescents and adults in East Africa experience inappropriate care by either receiving unnecessary or ineffective medications or being withheld from essential medications. Hence, while diagnostic capacity of East African countries is limited due to lack of human and financial resources, health systems strengthening and integrated approaches for detecting pathogens will support improved detection of aetiologic agents of febrile illnesses in East Africa. In turn, this will improve febrile case management, inform clinical epidemiology, refine understanding of the endemic disease profiles and thus positively impact on the health and well-being of the affected communities.
Recommendations for clinicians, hospital and public health communities
Based on the findings of this study, there are a couple of specific recommendations that can be drawn for policy and practice in East Africa. First, the study suggests that a considerable proportion of fevers in East Africa is of unknown or unidentified aetiology. The high prevalence of fever of unidentified aetiology indicates that there are significant gaps in diagnostic capabilities and surveillance systems, particularly in remote rural areas. Strengthening diagnostic capacity and surveillance-response systems for fever of unidentified aetiology could lead to more accurate diagnoses and more effective treatment, as well as more timely detection and control of febrile illnesses. This could include the use of multiplex PCR technologies, which have shown promise in identifying a wider range of pathogens compared to traditional diagnostic methods. Second, the study emphasises the importance of continued investment in public health initiatives aimed at preventing the transmission of arboviral diseases and reducing the burden of bacterial infections, which were identified as the most common causes of febrile illnesses in our analysis. Third, our study suggests the need for increased collaboration and coordination among public health authorities and healthcare providers in the region. This could involve the development of regional networks for surveillance, diagnosis and treatment of fever of unidentified aetiology, as well as the sharing of best practices and resources among healthcare providers. By working together to improve diagnostic capacity and strengthen surveillance-response systems, policy-makers and practitioners in East Africa could make significant progress towards reducing the burden of fever of unidentified aetiology in the region.
Strengths and limitations
In this review, we did not restrict our search terms to capture the keywords that show up in the title or abstract of published articles only. Consequently, a very large number of hits resulted from our initial search (> 14,000). We also hand-searched references of included studies to identify potential additional documents not identified by our electronic search. Furthermore, to increase the robustness of the review, we extracted information from included studies by strictly following a systematic procedure. However, a limitation of this review is that we did not perform a search of the grey literature (literature produced outside of the indexed databases, such as government reports, policy statements, pre-prints, etc.). In addition, the search strategy may have missed studies published in local journals that are not indexed in the selected databases. Likewise, the final set of studies that met our inclusion criteria were all published in English, though it is unlikely that this biased our analysis, since we did not apply any language restriction. Moreover, although some of the studies provided supplementary materials detailing the tests performed and the number and types of pathogens tested per patient, most of the included studies did not publish this fine-grained level of detail. Hence, our findings should be considered with some caution, as we could only capture the full range of pathogens reported in all included studies. Also, due to the limited data available, the findings were not further stratified by quality of diagnostic evidence. Finally, it is important to acknowledge that meta-analyses are inevitably constrained by the quality and diversity of the available data. As a result, despite our efforts to address heterogeneity, some degree of residual heterogeneity may remain.
Conclusions
Febrile patients, both ambulatory and hospitalised, require appropriate diagnosis to receive adequate management and therapy. This systematic review and meta-analysis provides new evidence that causes of fever in a large proportion (over 60%) of febrile adolescent and adult patients in East Africa remain unidentified. The outcome of this meta-analysis and the results of the individual studies reviewed support the notion that the majority of febrile patients attending health care facilities experience inappropriate care by either receiving unnecessary medications or being withheld from essential effective ones. Hence, we call for increased awareness of health professionals and policy-makers, improved access and availability of affordable and accurate diagnostic tests and the use of integrated approaches of multi-pathogen detection. Together, such a package holds high potential to improve patient outcomes in East Africa and elsewhere in LMICs.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional information files.
Abbreviations
- AMR:
-
Antimicrobial resistance
- AU:
-
African Union
- AUF:
-
Acute undifferentiated fever
- CI:
-
Confidence interval
- CINAHL:
-
Cumulative Index to Nursing & Allied Health Literature
- CoNS:
-
Coagulase negative staphylococci
- DALYs:
-
Disability-adjusted life years
- ELISA:
-
Enzyme-linked immunosorbent assay
- FUO:
-
Fever of unknown origin
- FWAS:
-
Fever without apparent source
- FWS:
-
Fever without source
- HIV:
-
Human immunodeficiency virus
- IFA:
-
Immunofluorescence assay
- JBI:
-
Joana Briggs Institute
- LMICs:
-
Low- and middle-income countries
- PCR:
-
Polymerase chain reaction
- POC:
-
Point-of-care
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RDT:
-
Rapid diagnostic test
- TAC:
-
TaqMan array cards
- UDF:
-
Undifferentiated fever
References
Leggett J. Approach to fever or suspected infection in the normal host. In: Goldman L, Schafer A, editors. Goldman’s Cecil Medicine, vol. 2. Philadelphia: Elsevier; 2020.
Steele GM, Franco-Paredes C, Chastain DB. Noninfectious causes of fever in adults. Nurse Pract. 2018;43:38–44.
Ogoina D. Fever, fever patterns and diseases called ’fever’-a review. J Infect Public Health. 2011;4:108–24.
Nawar EW, Niska RW, Xu J. National hospital ambulatory medical care survey: 2005 emergency department summary. Adv Data. 2007;386:1–32.
Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, Aura B, et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS ONE. 2011;6: e16085.
Crump JA, Kirk MD. Estimating the burden of febrile illnesses. PLoS Negl Trop Dis. 2015;9: e0004040.
D’Acremont V, Bosman A. WHO informal consultation on fever management in peripheral health care settings: a global review of evidence and practice. Geneva: World Health Organization; 2013.
Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis. 1998;26:290–6.
Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, Molyneux ME, et al. Bacteraemia and mortality among adult medical admissions in Malawi—predominance of non-typhi salmonellae and Streptococcus pneumoniae. J Infect. 2001;42:44–9.
Chheng K, Carter MJ, Emary K, Chanpheaktra N, Moore CE, Stoesser N, et al. A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS ONE. 2013;8: e60634.
Maze MJ, Bassat Q, Feasey NA, Mandomando I, Musicha P, Crump JA. The epidemiology of febrile illness in sub-Saharan Africa: implications for diagnosis and management. Clin Microbiol Infect. 2018;24:808–14.
Robinson ML, Manabe YC. Reducing uncertainty for acute febrile illness in resource-limited settings: the current diagnostic landscape. Am J Trop Med Hyg. 2017;96:1285–95.
Schroeder LF, Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141:791–5.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097.
Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore). 1961;40:1–30.
African Union. Member states. https://au.int/en/member_states/countryprofiles2. Accessed 22 March 2023.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors., et al., JBI manual for evidence synthesis, vol. 5. Adelaide: JBI; 2020.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39.
Woodruff PW, Morrill JC, Burans JP, Hyams KC, Woody JN. A study of viral and rickettsial exposure and causes of fever in Juba, southern Sudan. Trans R Soc Trop Med Hyg. 1988;82:761–6.
Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, Williams D, et al. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:484–9.
Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011;52:341–8.
Mease LE, Coldren RL, Musila LA, Prosser T, Ogolla F, Ofula VO, et al. Seroprevalence and distribution of arboviral infections among rural Kenyan adults: a cross-sectional study. Virol J. 2011;8:371.
Prabhu M, Nicholson WL, Roche AJ, Kersh GJ, Fitzpatrick KA, Oliver LD, et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis. 2011;53:e8-15.
Zenebe T, Kannan S, Yilma D, Beyene G. Invasive bacterial pathogens and their antibiotic susceptibility patterns in Jimma University Specialized Hospital, Jimma, southwest Ethiopia. Ethiop J Health Sci. 2011;21:1–8.
Hertz JT, Munishi OM, Ooi EE, Howe S, Lim WY, Chow A, et al. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–7.
Meremo AJ, Kidenya BR, Mshana SE, Kabangila R, Kataraihya JB. High prevalence of tuberculosis among adults with fever admitted at a tertiary hospital in north-western Tanzania. Tanzan J Health Res. 2012;14:183–8.
Meremo A, Mshana SE, Kidenya BR, Kabangila R, Peck R, Kataraihya JB. High prevalence of non-typhoid salmonella bacteraemia among febrile HIV adult patients admitted at a tertiary hospital, north-western Tanzania. Int Arch Med. 2012;5:28.
Nadjm B, Mtove G, Amos B, Walker NF, Diefendal H, Reyburn H, et al. Severe febrile illness in adult hospital admissions in Tanzania: a prospective study in an area of high malaria transmission. Trans R Soc Trop Med Hyg. 2012;106:688–95.
Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7: e2324.
Moon AM, Biggs HM, Rubach MP, Crump JA, Maro VP, Saganda W, et al. Evaluation of in-hospital management for febrile illness in northern Tanzania before and after 2010 World Health Organization guidelines for the treatment of malaria. PLoS ONE. 2014;9: e89814.
Feleke SM, Animut A, Belay M. Prevalence of malaria among acute febrile patients clinically suspected of having malaria in the Zeway Health Center, Ethiopia. Jpn J Infect Dis. 2015;68:55–9.
Ochieng C, Ahenda P, Vittor AY, Nyoka R, Gikunju S, Wachira C, et al. Seroprevalence of infections with dengue, Rift Valley fever and chikungunya viruses in Kenya, 2007. PLoS ONE. 2015;10: e0132645.
Hercik C, Cosmas L, Mogeni OD, Wamola N, Kohi W, Omballa V, et al. A diagnostic and epidemiologic investigation of acute febrile illness (AFI) in Kilombero, Tanzania. PLoS ONE. 2017;12: e0189712.
Boillat-Blanco N, Mbarack Z, Samaka J, Mlaganile T, Mamin A, Genton B, et al. Prognostic value of quickSOFA as a predictor of 28-day mortality among febrile adult patients presenting to emergency departments in Dar es Salaam, Tanzania. PLoS ONE. 2018;13: e0197982.
Guillebaud J, Bernardson B, Randriambolamanantsoa TH, Randrianasolo L, Randriamampionona JL, Marino CA, et al. Study on causes of fever in primary healthcare center uncovers pathogens of public health concern in Madagascar. PLoS Negl Trop Dis. 2018;12: e0006642.
Hercik C, Cosmas L, Mogeni OD, Wamola N, Kohi W, Houpt E, et al. A combined syndromic approach to examine viral, bacterial, and parasitic agents among febrile patients: a pilot study in Kilombero, Tanzania. Am J Trop Med Hyg. 2018;98:625–32.
Zerfu B, Medhin G, Mamo G, Getahun G, Tschopp R, Legesse M. Community-based prevalence of typhoid fever, typhus, brucellosis and malaria among symptomatic individuals in Afar Region, Ethiopia. PLoS Negl Trop Dis. 2018;12: e0006749.
Ali MA, James OC, Mohamed AA, Joachim A, Mubi M, Omodior O. Etiologic agents of fever of unknown origin among patients attending Mnazi Mmoja Hospital, Zanzibar. J Community Health. 2020;45:1073–80.
Budodo RM, Horumpende PG, Mkumbaye SI, Mmbaga BT, Mwakapuja RS, Chilongola JO. Serological evidence of exposure to Rift Valley, dengue and chikungunya viruses among agropastoral communities in Manyara and Morogoro regions in Tanzania: a community survey. PLoS Negl Trop Dis. 2020;14: e0008061.
Endale A, Michlmayr D, Abegaz WE, Asebe G, Larrick JW, Medhin G, et al. Community-based sero-prevalence of chikungunya and yellow fever in the South Omo Valley of Southern Ethiopia. PLoS Negl Trop Dis. 2020;14: e0008549.
Boillat-Blanco N, Mbarack Z, Samaka J, Mlaganile T, Kazimoto T, Mamin A, et al. Causes of fever in Tanzanian adults attending outpatient clinics: a prospective cohort study. Clin Microbiol Infect. 2021;27:913.e1-e7.
Akelew Y, Pareyn M, Lemma M, Negash M, Bewket G, Derbew A, et al. Aetiologies of acute undifferentiated febrile illness at the emergency ward of the University of Gondar Hospital. Ethiopia Trop Med Int Health. 2022;27:271–9.
Manock SR, Jacobsen KH, de Bravo NB, Russell KL, Negrete M, Olson JG, et al. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg. 2009;81:146–51.
Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, et al. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health. 2013;1:e46-54.
D’Acremont V, Kilowoko M, Kyungu E, Philipina S, Sangu W, Kahama-Maro J, et al. Beyond malaria–causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370:809–17.
Mueller TC, Siv S, Khim N, Kim S, Fleischmann E, Ariey F, et al. Acute undifferentiated febrile illness in rural Cambodia: a 3-year prospective observational study. PLoS ONE. 2014;9: e95868.
Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS ONE. 2015;10: e0127962.
Fridkin S, Baggs J, Fagan R, Magill S, Pollack LA, Malpiedi P, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194–200.
Vaughn VM, Flanders SA, Snyder A, Conlon A, Rogers MAM, Malani AN, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med. 2019;171:153–63.
Landstedt K, Sharma A, Johansson F, Lundborg CS, Sharma M. Antibiotic prescriptions for inpatients having non-bacterial diagnosis at medicine departments of two private sector hospitals in Madhya Pradesh, India: a cross-sectional study. BMJ Open. 2017;7: e012974.
Acam J, Kuodi P, Medhin G, Makonnen E. Antimicrobial prescription patterns in East Africa: a systematic review. Syst Rev. 2023;12:18.
Njozi M, Amuri M, Selemani M, Masanja I, Kigahe B, Khatib R, et al. Predictors of antibiotics co-prescription with antimalarials for patients presenting with fever in rural Tanzania. BMC Public Health. 2013;13:1097.
Robinson ML, Kadam D, Kagal A, Khadse S, Kinikar A, Valvi C, et al. Antibiotic utilization and the role of suspected and diagnosed mosquito-borne illness among adults and children with acute febrile illness in Pune. India Clin Infect Dis. 2018;66:1602–9.
Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10:13.
Cantón R, Horcajada JP, Oliver A, Garbajosa PR, Vila J. Inappropriate use of antibiotics in hospitals: the complex relationship between antibiotic use and antimicrobial resistance. Enferm Infecc Microbiol Clin. 2013;31(Suppl 4):3–11.
Daily J. Fever diagnostic technology landscape. Geneva: World Health Organization; 2018.
Semret M, Ndao M, Jacobs J, Yansouni CP. Point-of-care and point-of-’can’: leveraging reference-laboratory capacity for integrated diagnosis of fever syndromes in the tropics. Clin Microbiol Infect. 2018;24:836–44.
Elven J, Dahal P, Ashley EA, Thomas NV, Shrestha P, Stepniewska K, et al. Non-malarial febrile illness: a systematic review of published aetiological studies and case reports from Africa, 1980–2015. BMC Med. 2020;18:279.
Yansouni CP, Bottieau E, Chappuis F, Phoba MF, Lunguya O, Ifeka BB, et al. Rapid diagnostic tests for a coordinated approach to fever syndromes in low-resource settings. Clin Infect Dis. 2012;55:610–1.
Lamorde M, Mpimbaza A, Walwema R, Kamya M, Kapisi J, Kajumbula H, et al. A cross-cutting approach to surveillance and laboratory capacity as a platform to improve health security in Uganda. Health Secur. 2018;16:S76–86.
Uscher-Pines L, Babin SM, Farrell CL, Hsieh YH, Moskal MD, Gaydos CA, et al. Research priorities for syndromic surveillance systems response: consensus development using nominal group technique. J Public Health Manag Pract. 2010;16:529–34.
Ko KKK, Chng KR, Nagarajan N. Metagenomics-enabled microbial surveillance. Nat Microbiol. 2022;7:486–96.
Marais G, Hardie D, Brink A. A case for investment in clinical metagenomics in low-income and middle-income countries. Lancet Microbe. 2023;4:e192–9.
Acknowledgements
Our deep appreciation goes to Ms. Christine Mensch for her kind support. We also thank Prof. Valérie D’Acremont for her valuable comments on a previous version of the manuscript. FN is grateful for the generous support of his PhD by the Swiss Tropical and Public Health Institute. The contents in this article are the sole responsibility of the authors and do not necessarily reflect the views of any of the institutions named here.
Funding
Open access funding provided by University of Basel This work was supported by the Swiss Tropical and Public Health Institute.
Author information
Authors and Affiliations
Contributions
FN and AC searched, screened and selected the studies. JU and NP-H supervised the conduct of the review. FN, DHP and AD conceptualised the review. FN analysed the data, interpreted the findings and drafted the manuscript. KR, JO, NWB, JU, NP-H, DHP and AD contributed to the data analysis, interpretation of the findings and critically reviewed the manuscript. JU, DHP and AD provided overall guidance and were involved in the conceptualisation of the review, data analysis and data interpretation, assessed the methodological quality and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Table S1.
Summary of quality assessment of included studies by the Joanna Briggs Institute critical appraisal tool assessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nooh, F., Chernet, A., Reither, K. et al. Prevalence of fever of unidentified aetiology in East African adolescents and adults: a systematic review and meta-analysis. Infect Dis Poverty 12, 55 (2023). https://doi.org/10.1186/s40249-023-01105-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-023-01105-z