Abstract
Background
Malaria continues to cause burden in various parts of the world. Haiti, a Caribbean country, is among those aiming to eliminate malaria within a few years. Two surveys were conducted in Haiti during which we aimed to evaluate the performance of the simple and rapid procedure for ultra-rapid extraction–loop-mediated isothermal amplification (PURE-LAMP) method with dried blood spots as an alternative diagnostic method for malaria in the context of low to very low rates of transmission.
Methods
Febrile and afebrile people were recruited from three administrative divisions within Haiti: Nippes, Sud and Grand’Anse, during the summers of 2017 (early August to early September) and 2018 (late July to late August). Their blood samples were tested by microscopy, rapid diagnostic tests (RDT), PURE-LAMP and nested PCR to detect Plasmodium infection. Sensitivity, specificity, positive and negative predictive values and kappa statistics were estimated with the nested PCR results as the gold standard.
Results
Among 1074 samples analyzed, a positive rate of 8.3% was calculated based on the nested PCR results. Among febrile participants, the rates in 2017 and 2018 were 14.6% and 1.4%, respectively. Three positives were detected among 172 afebrile participants in 2018 by PURE-LAMP and nested PCR, and all three were from the same locality. There was no afebrile participants recruited in 2017. The PURE-LAMP, RDT and microscopy had respective sensitivities of 100%, 85.4% and 49.4%. All of the testing methods had specificities over 99%.
Conclusions
This study confirmed the high performance of the PURE-LAMP method to detect Plasmodium infection with dried blood spots and recommends its use in targeted mass screening and treatment activities in low endemic areas of malaria.
Similar content being viewed by others
Background
The World Health Organization estimated 247 million cases and 691 thousand deaths of malaria worldwide in 2021. Some regional progress has been made from the E-2020 countries (identified as having the potential to be malaria free by 2020). China and El Salvador were certified as malaria free in 2021. Iran, and Malaysia have accomplished their goal of three consecutive years without indigenous cases. In the Americas, Paraguay and Argentina achieved malaria elimination [1, 2]. There remain 18 malaria-endemic countries and territories in the Americas, which includes only 2 in the Caribbean: the Dominican Republic and Haiti, on the sole island of Hispaniola on which malaria elimination is also considered feasible [3, 4]. Plasmodium falciparum is the endemic species, transmission is mainly focal, and the annual incidence was < 1% in Haiti and reached < 0.1% in 2018 whereas < 1000 cases were reported for the Dominican Republic [1, 5]. Both countries have a population of approximately 11 million inhabitants.
When transmission reaches a very low level, it is important to have sensitive tools that help detect the remaining reservoirs that need to be cleared to avoid the reestablishment of high positive rates within the population [6, 7]. Nucleic acid amplification techniques are the most accurate diagnostic choice [7]. Although polymerase chain reaction (PCR) assays to diagnose malaria have been developed since the 1990s, they remain centralized and too demanding for regular use in the endemic field [8]. The loop-mediated isothermal amplification method (LAMP) is becoming a formidable alternative [9,10,11]. LAMP is fast, robust and less expensive than PCR and, subsequently, is applicable in less well-equipped laboratories although the current cost per sample is still higher than ideal.
Among the different malaria LAMP assays developed, commercial kits have become available from two companies: the Loopamp™ MALARIA Pan/Pf Detection Kit (Eiken Chemical, Tokyo, Japan) [12,13,14,15,16,17,18,19,20,21,22] and the Illumigene Malaria LAMP (Meridian Bioscience Inc., Cincinnati, OH, USA) [23, 24]. The Loopamp™ MALARIA Pan/Pf Detection Kit has been evaluated with a significant number of samples in various studies conducted in both endemic [13, 15, 19,20,21,22] and non-endemic settings [12, 16], using whole blood [12, 21] or dried blood spots [15, 16], and coupled with diverse DNA extraction methods. One of these methods is the procedure for ultra-rapid extraction (PURE), a quick and simple DNA extraction method, also developed by Eiken Chemical to prepare DNA solution suitable for the LAMP reaction. We previously evaluated the accuracy of the combination of PURE and Loopamp™ MALARIA Pan/Pf (PURE-LAMP) methods on dried blood spots of suspected and confirmed cases of malaria imported to Japan [16]. The performance of this system was proved at a Japanese laboratory. However, the greater impact of a LAMP-based system is expected in malaria-endemic areas through its application in screening and confirmation of a malaria diagnostic or parasitic clearance. Thus, the present study aimed to evaluate the PURE-LAMP malaria detection method in the low transmission context of Haiti along with concurrent observation of the epidemiological situation.
Methods
Study sites
Based on data from the national malaria control program, three departments (main administrative divisions in Haiti) with the highest positivity rates in 2016 and early 2017 were selected as study sites. They were Nippes, Sud, and Grand’Anse in the southwestern part of the country (Fig. 1), with respective annual parasite indexes of 2.5, 4.4 and 22.3 in 2016 and 2.9, 7.6, and 18.4 in 2017 per 1000 population. Sud and Grand’Anse shared 63.6% of the malaria cases registered in 2016 in the country. Although a descending or at least stable incidence trend has been registered in the other 7 departments since 2015, the three chosen departments have shown an increasing or stable trend. Recruitment was carried out in small coastal cities with less than 50,000 inhabitants and which were located remotely from the capital cities of each department. At the start of the study, the sequels of hurricane Matthew that had hit 10 months earlier (October 2016) could still be spotted in a recovering environment.
Location of the study area. Haiti is located in the Americas. The three departments (administrative divisions) of the study are highlighted in grey, and the others are shown in white. The number of participants by department, year and nested PCR diagnosis, is shown based on recruitment setting, hospital or community
Light microscopy is dictated as the gold standard for malaria diagnosis by the Haitian Ministry of Health; however, after allowing the alternative use of rapid diagnostic tests (RDT) in recent years, they are more widely used [4, 25, 26]. Besides the availability of these tests and malaria treatment free of charge for patients, vector control activities had been carried out under the national malaria control program.
Recruitment
People aged 1 year and over were recruited following two approaches: (i) hospital-based recruitment targeting participants with fever on the day of their hospital visit or anytime up to 2 weeks earlier, and (ii) community-based recruitment targeting the general population during social gatherings. There was no criterion for fever. Most social group participants were afebrile, but some people recruited there reported a history of fever.
Recruitment was carried out simultaneously at the same three health facilities, Centre de santé de Baradères (Nippes), Hôpital de Port-à-Piment (Sud) and Hôpital de la communauté de Dame-Marie (Grand’Anse), during the summers of 2017 (early August to early September) and 2018 (late July to late August). Community-based recruitment was carried out in Port-à-Piment, Port-Salut (Sud) and Petite Rivière de Dame-Marie. At the point of recruitment, participants had to submit a consent form, answers to a short questionnaire about demography, and their fever history and preventive practices, and venous blood samples were collected by a qualified staff member either in ethylenediaminetetraacetic acid (EDTA)- or heparin-containing blood tubes. After blood collection, the participants were tested immediately with an SD Bioline Malaria Ag Pf/Pan RDT (Standard Diagnostics, Inc., Suwon, the Republic of Korea) in accordance with the manufacturer’s guidelines, and blood smears were prepared and 100 μl of blood was seeded on filter paper (Whatman™ FTA™ classic cards; GE Healthcare, Tokyo, Japan) and air dried. After drying, each card was stored in a plastic bag containing a small desiccant bag. The participants were treated immediately if the RDT result was positive. Blood smears were fixed, stained and transported to the same laboratory along with the filter papers. The other tests (PURE-LAMP, PCR, microscopy) were conducted afterwards.
This study used venous blood to avoid double invasion of the participants as the majority seeking care were also predicted to require other blood tests. A small volume (100 μl) was used to prepare dried blood spots to approximate capillary blood sampling.
Microscopy
Two microscopists based at a Haitian reference laboratory visualized thick and thin Giemsa-stained smears using a × 1000 light microscope for each participant. Three hundred microscopic fields of the thick smear were visualized before declaring a negative sample. Parasite density was determined as percentage of parasitized red blood cells after observing about 5000 of them using the thin smear. Any positive was seen by a third microscopist who also assessed 10% of the negatives. The microscopists were blinded to the other test results of the participants.
PURE-LAMP
DNA was extracted with the Loopamp™ PURE DNA Extraction Kit from three dried blood spots with a diameter of 3 mm following the manufacturer’s guidelines and as reported elsewhere [16]. Reactions were carried in two tubes for all samples with the Loopamp™ MALARIA Pan/Pf Detection Kit, a Pan (targeting Plasmodium spp.) and a Pf (P. falciparum-specific) tube for each. Negative and positive controls (provided in the kit) were also included in each run.
The presence of amplified DNA inside the reaction tubes may be recognized in two speedy ways: (i) by fluorescence under UV excitation due to calcein unquenched during reactions, and (ii) by turbidity due to the precipitation of magnesium pyrophosphate [27]. In this study, only end-point evaluation by fluorescence under UV excitation was conducted for samples collected in heparin tubes giving a dichotomous positive or negative result; while real-time turbidity was measured with a Loopamp EXIA real-time turbidimeter (Eiken Chemical, Tokyo, Japan) for hospital samples collected in EDTA blood tubes, giving a turbidity threshold time (tt) for amplified samples. This was done to exclude any possibility of false positives due to EDTA chelating properties.
DNA extraction for PCR
DNA samples were extracted from three dried blood spots with a diameter of 3 mm using a Maxwell® RSC DNA FFPE Kit (Promega, Madison, WI, USA) and the automated Maxwell® RSC Instrument (Promega, Madison, WI, USA). Minor modifications to the manufacturer’s guidelines can be found in a previous report [28]. DNA was eluted in 50 μl of elution buffer and stored at 4 °C.
Nested PCR
The reference test in this study was a nested PCR system targeting the Plasmodium 18S ribosomal RNA gene [29]. This method generally implies a secondary step with species-specific primer sets for all human Plasmodium. However, secondary PCRs in this study were conducted only with a primer set specific to P. falciparum, which is the species expected in Haiti. For discrepant samples between PURE-LAMP ( +) and nested PCR (−), both tests were repeated, and the number of secondary PCR cycles was increased from 20 to 30. The nested PCR diagnosis was updated if a previously negative sample became positive at this step.
Statistical analysis
Assuming 5% positivity rate among febrile participants, PURE-LAMP sensitivity of 97%, specificity of 99% [16], at least 895 participants samples had to be analyzed to estimate the performance of PURE-LAMP with a marginal error of 5% and confidence level of 95%.
Data analysis was performed with Stata (StataCorp, TX, USA). Sensitivity, specificity, positive predictive value and negative predictive value with their respective 95% confidence intervals (CIs) and kappa statistics were estimated with nested PCR as the reference.
Results
Characteristics of the study participants
In total, 1115 people gave consent to participate in this study: 265 in community settings and 850 at the hospitals. After exclusions due to missing samples, 554 blood samples were analyzed from those recruited during the summer of 2017, all from hospitals, and 520 from those of summer 2018, including 242 from community-based recruitment (Fig. 1). The participants ranged in age from 1 to 85 years old (mean: 31.0; SD: 20.5 years) and included 366 (34.7%) males and 690 (65.3%) females. Among them, 225 (21.1%) had a personal history of fever on the day of recruitment, 488 (45.7%) from a day before up to 7 days earlier and 183 (17.1%) from 8 days up to 2 weeks earlier, and 284 (26.9%) reported having a household member with fever and 92 (8.7%) a close neighbor. The history of having a relative with fever was significantly related to being tested positive for malaria by nested PCR or PURE-LAMP (P-value: 0.000). Preventive behaviors were quite common: 948 (88.3%) participants reported at least one, with the use of a bed net being the most reported (584 participants) (Table 1).
Rates of positivity
The rates of positivity for malaria as estimated by each method and by year are summarized in Table 1. As concluded by nested PCR, there were 89 (8.3%) positives among 1074 samples that had been tested by all methods. The positive rate varied from 14.6% among samples collected in 2017 to 1.5% for samples from 2018. Although all participants in 2017 were febrile, the positive rate among febrile participants in 2018 was 1.4%. The positive rate among the afebrile participants was 1.7%. RDT and microscopy did not detect any Plasmodium infection among the afebrile participants. Microscopic parasitemia varied, ranging from 0.02% to 20.0% (mean: 2.3, SD: 3.2) when countable.
Performance parameters
An almost perfect agreement was calculated between the nested-PCR and PURE-LAMP (Kappa: 0.95). The PURE-LAMP Pan and PURE-LAMP Pf had respective sensitivities of 100% (95% CI: 95.9–100) and 98.9% (95% CI: 93.9–100), and their specificities were 99.1% (95% CI: 98.3–99.6) and 99.2% (95% CI: 98.4–99.6). The RDT Pf band had a sensitivity of 85.4% (95% CI: 76.3–92.0) and specificity of 99.5% (95% CI: 98.8–99.8), whereas for the RDT Pan band, values of 60.7% (95% CI: 49.7–70.9) and 99.8% (95% CI: 99.3–100), respectively, were calculated. Microscopy sensitivity was 49.4% (95% CI: 38.7–60.2), and its specificity was 99.3% (95% CI: 98.5–99.7) (Fig. 2 and Table 2).
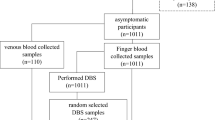
Flowchart of the study participants results by nested PCR, PURE-LAMP, RDT and microscopy. PURE-LAMP procedure for ultra-rapid extraction-loop-mediated isothermal amplification, PCR polymerase chain reaction, Pan tubes for diagnosis of Plasmodium genus, Pf P. falciparum-specific tubes, RDT rapid diagnostic test
Discussion
The results of the present study showed high sensitivity and specificity for a malaria diagnosis by the PURE-LAMP method on dried blood spots in a setting of low P. falciparum transmission. There was an important decrease of the positivity rate between the two study periods. The reason for it might be varied including weather patterns or effective interventions.
Our previous evaluation of the PURE-LAMP method with dried blood spots conducted with imported cases in a non-endemic setting showed a sensitivity over 96% and specificity of 100% [16]. A review of previously reported evaluations of the Loopamp™ MALARIA Pan/Pf Detection Kit with > 35 samples per group in diverse settings showed sensitivity in a range from about 90% up to 100% and specificity from about 85% to 100% [12, 13, 15, 16, 21, 22]. The results of the present study also lie within these ranges.
In the present study, all discrepancies between the nested PCR and the PURE-LAMP Pan results were negative by the nested PCR and positive by the PURE-LAMP Pan; they were also negative by microscopy and mostly negative by the RDT (except for one RDT Pf-positive result). We think it is possible that some of these samples had very low amount of parasites DNA, as the precision is lower for samples with parasitemia around PCR’s limit of detection. Three samples became positive by nested PCR after repeating the test. A limit of detection of 0.01 parasites/μl had been found for this nested PCR with DNA extracted from 200 μl of blood, eluted in the same volume and starting with 2 μl of template [29]. The discrepant samples had tt values longer than the 15 min expected after the start of the reaction (Table 3) [27]. This could indicate a very low amount of the parasites’ DNA on the template, which required longer amplification time to produce enough magnesium pyrophosphate to be detected by the turbidimeter. However, we did not find a strong correlation between tt values and parasitemia in this dataset. A stricter experimental approach regarding blood sample volume and cell distribution will be necessary to determine whether this parameter can be helpful to estimate parasitemia.
Some difference in the starting volume and extraction methods might have had some impact on the results. Although 3 DBS (~ 15 μl of blood) had been used for DNA extraction for LAMP by the PURE method and by Maxwell for nested PCR, there is inherent difference between the 2 methods. The PURE extraction renders about 120 μl of DNA solution of which 30 μl (corresponding to ~ 3 μl of blood) were used for each tube of LAMP reaction. From the 50 μl of DNA solution eluted at the end of the extraction by Maxwell, 2 μl were used for the primary PCR (corresponding to < 1 μl of blood). In this study we were interested in the PURE-LAMP system as diagnostic method due to its field-friendliness, not only to evaluate the LAMP kits. Thus, we had to use a different extraction method for PCR because the PURE is not validated for PCR.
RDT Pf band had a sensitivity over 85% and also agreed almost perfectly with the nested PCR results (Kappa: 0.88). Being among the three brands validated in Haiti [26]—the First Response Malaria Ag HRP2 (Premier Medical Corporation Ltd., Watchung, NJ, USA), CareStart Malaria HRP2 (Pf) (Access Bio, Inc., Monmouth Junction, NJ, USA) and SD Bioline- the RDT Pf band of the latter test was confirmed here to be an efficient option for the diagnosis of febrile cases. Rogier et al. evaluated the concordance between this RDT and a high-sensitivity (hs) RDT (Alere Malaria Ag P.f., Standard Diagnostics) in Haiti; they concluded that a hsRDT may have limited utility in a malaria setting like Haiti because of the minimal gain in sensitivity (0.2% more of the total population detected positive with the hsRDT compared to the conventional one) [30]. Here the gain in true positive by the PURE-LAMP Pan or the nested PCR represents 1.2% of the total population. However, as we consider the history of fever, only the PURE-LAMP and nested PCR allowed the detection of afebrile infections.
Among the RDT-negative samples, two were observed with microscopic parasitemia of > 0.6%, although microscopy had the lowest sensitivity in the present study. We considered the possibility of having some RDT Pf false negatives due to histidine rich protein 2 (hrp2) gene deletion, but hrp2 and 3 could be amplified for those two samples and eight more that were negative by the RDT Pf band but positive by the nested PCR and the PURE-LAMP. Three more samples- RDT Pf (-), nested PCR and PURE-LAMP ( +)- could not be amplified for hrp2, nor two among them for hrp3. Other genes such as pfcrt and k13 could not be amplified for those three samples either, so this does not prove deletion. Herman et al. reported no evidence of hrp2/3 deletion in Haiti as well [31]. This result could have been caused by variation in antigen expression, blood concentration or a technical error.
Five RDT false-positives were also found in this study, one coinciding with a PURE-LAMP false-positive. RDT false-positive may be caused by antigen persistence in the blood after an episode of malaria. Those cases reported personal history of fever within 2 weeks of the recruitment. It is possible that their recent fever was due to malaria. We think that the false positives by microscopy were due to artifacts during slide coloration.
In general, a decrease of malaria prevalence was observed between 2017 and 2018 in Haiti [3, 5]. The impact of hurricane Matthew might also have influenced malaria transmission in 2017 whereas malaria control interventions might have had a better impact in 2018.
While the prevalence of infection was significantly decreasing in hospitals and was reported to the national control program, some malaria foci may require special intervention including a different method of testing. In Dame-Marie (Grand’Anse), absolutely no febrile patients were diagnosed as malaria positive during the weeks of hospital recruitment in 2018 (Fig. 1). However, when a single-day community recruitment effort was organized in a locality about 3 km away from the hospital, two positives were first detected by the RDT Pf band. The number of positives from that day increased to five after performing PURE-LAMP and nested PCR. The first two positives were people with history of fever, whereas the other three had no recent history of fever. It is not known whether the febrile patients who participated in the community screening had planned to visit the hospital. We may also note that despite free malaria care, in Haiti visiting even a public hospital generally requires a small fee which is supported by patients out of pocket. The locality in question was known by the regional staff for having a higher risk of malaria infection and seemed to correlate well with a hotspot as defined by Bousema et al. [32]. Still, the present study showed that the RDT and microscopy methods were not exposing an accurate picture of malaria positivity.
Several studies have highlighted the importance of asymptomatic infections for transmission in low-transmission settings [6, 33,34,35]. It was estimated that submicroscopic carriers are the source of 20–50% of all human-to-mosquito transmissions when transmission reaches very low levels [6]. The present study provides data about an eligible method that can help detect those submicroscopic carriers that may be used for mass or reactive screening for downstream treatment.
The cost of PURE-LAMP (USD 40 per sample) is surely a barrier for its adoption in Haiti although performant. We don't think it would be cost-effective for this system to replace RDT at all levels of care. However, its introduction in regional laboratories could benefit the malaria program. Dried blood spots can be prepared by finger pricking at any health facility, gathering place or home, and be shipped. We think this test is better applied in focused community screening and reactive screening in low-endemic settings. Our opinion is pended to a cost-effectiveness analysis.
Despite the difference observed in positive rates between the periods studied, the challenge to achieve malaria elimination is enormous. This small number of afebrile infections is important for the community, considering that submicroscopic infections may still be transmitted and that the possible worst effect will occur in non-immune people. In future molecular studies in Haiti, the distribution and significance of these afebrile infections need to be explored in a larger sample size.
Conclusions
The present study confirmed the high performance of the PURE-LAMP method for malaria testing with dried blood spots. It allowed the detection of febrile cases and afebrile infections missed by the RDT and microscopy in a low-transmission setting. Malaria prevalence was found to be significantly lower in 2018 compared to 2017 across the study sites in Haiti; a specific focus of infection appeared to require intervention with sensitive malaria diagnostic methods and treatment. We recommend the use of this diagnostic system in targeted mass screening and treatment to detect the remaining human infectious reservoirs for their clearance as a means to accelerate the elimination of malaria.
Availability of data and materials
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- EDTA:
-
Ethylenediaminetetraacetic acid
- HRP:
-
Histidine rich protein
- LAMP:
-
Loop-mediated isothermal amplification
- NPV:
-
Negative predictive value
- PCR:
-
Polymerase chain reaction
- PPV:
-
Positive predictive value
- PURE:
-
Procedure for ultra-rapid extraction
- RDT:
-
Rapid diagnostic test
References
World Health Organization. World malaria report 2022. Geneva: World Health Organization; 2022.
World Health Organization. Update on the E-2020 initiative of 21 malaria-eliminating countries. Geneva: World Health Organization; 2020.
Jules JR, Alencar J, Suárez-Mutis MC, Baptiste EJ, de Albuquerque H, Rosa-Freitas MG, et al. Malaria in Haiti: a descriptive study on spatial and temporal profile from 2009 to 2018. Rev Soc Bras Med Trop. 2022;55:1–8. https://doi.org/10.1590/0037-8682-0355-2021.
Lemoine JF, Boncy J, Filler S, Kachur SP, Fitter D, Chang MA. Haiti’s commitment to malaria elimination: progress in the face of challenges, 2010–2016. Am J Trop Med Hyg. 2017;97:43–8. https://doi.org/10.4269/ajtmh.16-0902.
World Health Organization. World malaria report 2018. Geneva: World Health Organization; 2018.
Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. https://doi.org/10.1038/ncomms2241.
Imwong M, Hanchana S, Malleret B, Rénia L, Day NPJ, Dondorp A, et al. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol. 2014;52:3303–9. https://doi.org/10.1128/JCM.01057-14.
Georges S, Viriyakbosola S, Ping X, Jarra W, Snounou G, Viriyakosol S, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. https://doi.org/10.1016/0166-6851(93)90077-B.
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63.
Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122:233–40. https://doi.org/10.1016/j.actatropica.2012.02.004.
Britton S, Cheng Q, Mccarthy JS. Novel molecular diagnostic tools for malaria elimination : a review of options from the point of view of high-throughput and applicability in resource limited settings. Malar J. 2016. https://doi.org/10.1186/s12936-016-1158-0.
Polley SD, González IJ, Mohamed D, Daly R, Bowers K, Watson J, et al. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis. 2013;208:637–44. https://doi.org/10.1093/infdis/jit183.
Hopkins H, González IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis. 2013;208:645–52. https://doi.org/10.1093/infdis/jit184.
Mohon AN, Lee LD-Y, Bayih AG, Folefoc A, Guelig D, Burton RA, et al. NINA-LAMP compared to microscopy, RDT, and nested PCR for the detection of imported malaria. Diagn Microbiol Infect Dis. 2016;85:149–53. https://doi.org/10.1016/j.diagmicrobio.2015.11.009.
Aydin-Schmidt B, Xu W, González IJ, Polley SD, Bell D, Shakely D, et al. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS ONE. 2014;9: e103905. https://doi.org/10.1371/journal.pone.0103905.
Vincent JP, Komaki-Yasuda K, Iwagami M, Kawai S, Kano S. Combination of PURE-DNA extraction and LAMP-DNA amplification methods for accurate malaria diagnosis on dried blood spots. Malar J. 2018;17:373. https://doi.org/10.1186/s12936-018-2527-7.
Komaki-Yasuda K, Matsumoto-Takahashi ELA, Kawai S, Kano S. Application of PURE/LAMP method for malaria diagnosis. Clin Parasitol. 2015;26:83–5.
Sema M, Alemu A, Bayih AG, Getie S, Getnet G, Guelig D, et al. Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in Northwest Ethiopia. Malar J. 2015;14:44. https://doi.org/10.1186/s12936-015-0559-9.
Morris U, Khamis M, Aydin-Schmidt B, Abass AK, Msellem MI, Nassor MH, et al. Field deployment of loop-mediated isothermal amplification for centralized mass-screening of asymptomatic malaria in Zanzibar: a pre-elimination setting. Malar J. 2015;14:205. https://doi.org/10.1186/s12936-015-0731-2.
Cook J, Aydin-Schmidt B, González IJ, Bell D, Edlund E, Nassor MH, et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J. 2015;14:43. https://doi.org/10.1186/s12936-015-0573-y.
Vallejo AF, Martínez NL, González IJ, Arévalo-Herrera M, Herrera S. Evaluation of the loop mediated isothermal DNA amplification (LAMP) kit for malaria diagnosis in P. vivax endemic settings of Colombia. PLoS Negl Trop Dis. 2015;9: e3453. https://doi.org/10.1371/journal.pntd.0003453.
Serra-Casas E, Manrique P, Ding XC, Carrasco-Escobar G, Alava F, Gave A, et al. Loop-mediated isothermal DNA amplification for asymptomatic malaria detection in challenging field settings: technical performance and pilot implementation in the Peruvian Amazon. PLoS ONE. 2017;12: e0185742. https://doi.org/10.1371/journal.pone.0185742.
Lucchi NW, Gaye M, Diallo MA, Goldman IF, Ljolje D, Deme AB, et al. Evaluation of the Illumigene Malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep. 2016;6:36808. https://doi.org/10.1038/srep36808.
De Koninck A-S, Cnops L, Hofmans M, Jacobs J, Van den Bossche D, Philippé J. Diagnostic performance of the loop-mediated isothermal amplification (LAMP) based illumigene(®) malaria assay in a non-endemic region. Malar J. 2017;16:418. https://doi.org/10.1186/s12936-017-2065-8.
Ministère de la Santé et de la Population/Programme National de Controle de la Malaria. Manuel de Normes de Prise en Charge. 2018.
Existe A, Freeman N, Boncy J, Magloire R, Vely J-F, Chang M, et al. Rapid diagnostic tests for malaria-Haiti, 2010. Morb Mortal Wkly Rep. 2010;59:1372–3.
Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–4. https://doi.org/10.1006/bbrc.2001.5921.
Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Keomalaphet S, Lorpachan L, et al. Heterogeneous distribution of k13 mutations in Plasmodium falciparum in Laos. Malar J. 2018. https://doi.org/10.1186/s12936-018-2625-6.
Komaki-Yasuda K, Vincent JP, Nakatsu M, Kato Y, Ohmagari N, Kano S. A novel PCR-based system for the detection of four species of human malaria parasites and Plasmodium knowlesi. PLoS ONE. 2018;13: e0191886. https://doi.org/10.1371/journal.pone.0191886.
Rogier E, Hamre KES, Joseph V, Plucinski MM, Presume J, Romilus I, et al. Conventional and high-sensitivity malaria rapid diagnostic test performance in 2 transmission settings: Haiti 2017. J Infect Dis. 2020;221:786–95. https://doi.org/10.1093/infdis/jiz525.
Herman C, Huber CS, Jones S, Steinhardt L, Plucinski MM, Lemoine JF, et al. Multiplex malaria antigen detection by bead-based assay and molecular confirmation by PCR shows no evidence of Pfhrp2 and Pfhrp3 deletion in Haiti. Malar J. 2019;18:380. https://doi.org/10.1186/s12936-019-3010-9.
Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: Spatial targeting of malaria for control and elimination. PLoS Med. 2012;9: e1001165. https://doi.org/10.1371/journal.pmed.1001165.
Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in Western Thailand. J Med Entomol. 2004;41:201–8. https://doi.org/10.1603/0022-2585-41.2.201.
Imwong M, Stepniewska K, Tripura R, Peto TJ, Lwin KM, Vihokhern B, et al. Numerical distributions of parasite densities during asymptomatic malaria. J Infect Dis. 2016;213:1322–9. https://doi.org/10.1093/infdis/jiv596.
Chaumeau V, Kajeechiwa L, Fustec B, Landier J, Nyo SN, Hsel SN, et al. Contribution of asymptomatic Plasmodium infections to the transmission of malaria in Kayin State, Myanmar. J Infect Dis. 2019;219:1499–509. https://doi.org/10.1093/infdis/jiv596.
Acknowledgements
We acknowledge Fétise Gaie, Micheline Cicéron from the parasitology unit of Laboratoire National de Santé Publique and all the research staff for their help. We thank the study participants.
Funding
The kits used for this study and partial funding for field operations were provided by Eiken Chemical Co. Ltd. The funding source had no role in the study.
Author information
Authors and Affiliations
Contributions
JPV, study design, data collection, experiments, data analysis, writing—original draft, writing—review; AVE, study design, ethical review in Haiti, data collection, experiments; KKY, ethical review in Japan, project administration, writing—review; JB, fieldworks supervision; SK, study design, funding acquisition, project administration, supervision, writing—review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this study was reviewed and approved by the NCGM Ethics Committee (Ref: NCGM-G-002260-00) in Japan and the Haitian National Bioethics committee (Ref: 1617-48). Consent was obtained in person from participants over the age of majority and from a tutor in the case of minors.
Consent for publication
Not applicable.
Competing interests
KS received funding from Eiken Chemical Co. Ltd.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vincent, J.P., Existe, A.V., Komaki-Yasuda, K. et al. Performance of the procedure for ultra-rapid extraction and loop-mediated isothermal amplification (PURE-LAMP) method to detect malaria in Haiti. Infect Dis Poverty 12, 53 (2023). https://doi.org/10.1186/s40249-023-01097-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-023-01097-w