Abstract
Compared with common dust storms, saline dust storms transport high concentrations of fine-grain saline and alkaline material. The saline dust storm differs from common dust storm, especially considering the sources of the suspended particulate matter (PM), chemical composition, grain size, and circulation processes. Atmospheric particulate matters (TSP, PM10, PM2.5, and PM1) and their water-soluble ions were concurrently measured at two sites located at north and southeast part of Urmia lake from January 2013 to September 2013. Particulate matters (PMs) were measured using high volume sampler and HAZ-DUST EPAM-5000 particulate air monitors. In both of the sampling sites, the highest concentration of PM was observed during the summer season (521.6, 329.1, 42.6, and 36.5 for TSP, PM10, PM2.5, and PM1, respectively). A total of 11 inorganic water-soluble ions in the TSP and PM10 were identified by ion chromatography (IC). No statistically significant difference was found between PM’s ions concentrations of two sampling sites. The average of the total measured water-soluble ions in the sampling sites was 28.75 ± 12.9 μg/m3 (11.9 ± 4.8% of total TSP mass) for TSP and 14.65 ± 7.1μg/m3 (8.7 ± 4.4 of total PM10 mass) for PM10. Among all detected ions, sulfate was the dominant constituent followed by nitrate and sodium. This study showed that the water soluble salts compose 3–20% of the total mass of TSP and PM10. The PCA analysis showed that saline particulates formed from Urmia lake bed were the dominant source (57.6 %) of TSP. In addition, saline particulates together with crustal materials resulted from resuspension were the main source (59.9%) of PM10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Dust Storm phenomenon could change global climate and affect the economy and quality of human life [1]. In addition to contribution of the formation of gypcrete and calcrete, dust may lead to accumulation of more water soluble salts in soil profiles and thus contribute to salinization [2]. The saline dust storm differs from common dust storm, especially considering the sources of the suspended particulate matter (PM), chemical composition, grain size, and circulation processes. Saline dust storms are defined as “a kind of environmental disaster phenomenon in arid and semiarid regions that has been caused by dust deflated from the salt-rich sediments of dried lake beds and strongly salinized soils on the margins of lake floors” [3, 4]. Such a phenomena has been registered in many parts of the world, including the Aral Sea region in Kazakhstan and Uzbekistan, Lake Balkhash region in Kazakhstan, the Inner Mongolian region of China, southeastern Australia, and many other regions with arid and semiarid climate [5]. It was cleared that due to salt accumulation, sparse or absent of vegetation, and easily occurrences of wind erosion, these surface sediments will form a sleazy texture [3].
Compared with common dust storms, saline dust storms transport high concentrations of fine-grain saline and alkaline material, such as sodium sulfate, sodium chloride, and other potentially toxic components which could be threaten to the ecological security and humans health in the arid regions [3, 6, 7].
As mentioned above, chemical composition of saline dust storms is concerned to the characterization of region soil properties, and this redistribution leads to dual effects depending on the nature of transported material. Based on studies, saline dust storms are rich in CaCl2, NaCl, MgCl2 and other chloride particles, which can be suspended in the atmosphere for long periods of time [8].
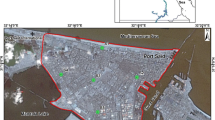
Urmia lake, located in northwest of Iran, is the second great saline lake in the world and the largest lake in Iran. In recent decade, the water level at Urmia lake has fallen more than 5 meters and the concentration of salt in this lake has increased from 185 to 220 g/L [9]. In addition to decrease in water level and increase in salt concentration, the unique ecosystem of the lake is being destroyed. As a result, a salt desert would be created with an area of more than 5000 km2 (Fig. 1), overlaid with a 50–60 cm thick salt deposits [10]. In the warm season, wind could carry out these salts and transport those to adjacent areas as far as 300 km. The transported PM could damage agricultural lands, pollute the ecosystem and cause variety of diseases in nearby urban and rural areas [9].
Hitherto, no study has been conducted about atmospheric PM in Urmia Lake bed. The present study was therefore carried out to determine the mass levels of total suspended particulate (TSP), PM10, PM2.5 and PM1 (aerodynamic diameter smaller than10, 2.5 and 1μm, respectively) along with the variations of water-soluble ionic species associated with TSP and PM10 in the floor of Urmia Lake.
Methods
Study area, sampling sites and schedule
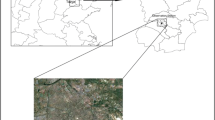
Based on the region wind direction, two sites were selected (Fig. 2); 1) Tasuj site, located in the north of Urmia lake (38° 13' 17.8" N - 45° 24' 38.5" E), and 2) Ajabshir site, located in the southeast of Urmia lake (37° 31' 12.9" N - 45° 47' 53.0" E). The level of TSP, PM10, PM2.5 and PM1.0 were measured 8 times every season in the period of January 2013 to September 2013. In total, 48 samples of TSP and PM10 were collected and analyzed during the study period.
PMs measurement
TSP and PM10 samples were collected by two high volume samplers manufactured by Graseby–Andersen at flow rates of 1.13–1.41 m3/min for 24 h. Both TSP and PM10 were collected on a 20.3cm X 25.4cm Whatman glass micro fiber filter. Before and after sampling, filters were set under 40% relative humidity (RH) at 25°C for over 48h, afterward at room condition for 2 h; then were weighed three times using an A&D electronic balance with the reading precision of 0.1mg. PM2.5 and PM1.0 were measured using two portable HAZ-DUST EPAM-5000 particulate air monitors.
Chemical analysis
For water soluble ions analysis, one quarter of each filter was placed in a glass vial and then 40 mL ultra pure water (specific resistance ≥18 Ω cm) was added. The vials were shaken for 2h, and subsequently were ultra-sonicated for 30min. The extracted solutions were filtered through a micro porous membrane (with the pore size 0.45 μm) [11]. An ion chromatograph (Metrohm 850 Professional IC, Switzerland) with operating flow rate of 0.7 mL/min was used to analyze water-soluble ions. Field and laboratory blanks and spiked samples were analyzed along with the PM samples. For all ions, method detection limits (MDLs) were calculated by adding three standard deviations of the blank readings to the average of five replicates of the blank. The obtained MDLs and the recovery efficiencies for water-soluble ions are presented in Table 1.
Size and morphology
To determine the morphology and the elemental composition of the collected particles, TSP and PM10 samples were analyzed separately using the SEM-EDX system at the Razi Metallurgical Research Center.
Data analysis
Data were analyzed (with SPSS20 statistical software, SPSS Inc.) by means of the linear regression (for correlation coefficients among water-soluble ions), the bivariate correlations (to quantify the relation between the elemental concentrations), dimension reduction factor (for the quality principal components (PCs) of TSP and PM10), and multivariate test (to quantify significance different between ions concentrations in Tasuj site vs. Ajabshir site). Differences and correlations were considered significant at the 0.05 level.
Meteorological data were obtained from the national climatic data center [12] and East Azerbaijan Meteorological Organization. The obtained data were examined for the missing values and outliers, and then were entered into WRPLOT View Freeware 7.0.0 to plot the wind-rose. The concentrations of PM were analyzed using Microsoft Excel 2010.
Results and discussion
Meteorological dates
Based on the collected meteorological dates, in both sampling sites, February was the coldest month with the monthly mean temperature of −1.0°C, while August was the warmest month with the mean temperature of 28.0°C. Also the RH varied from 25 to 72%. Dates of wind speed and the direction in both sampling sites revealed that in Ajabshir township, seasonal mean wind speed were 2.8, 2.72, 2.48 and 2.12 m/s, while in the Tasuj township were 2.36, 3.28, 3.25 and 1.97 m/s in winter, spring, summer and autumn, respectively. Annual wind rose plots for both sampling sites are shown in Fig. 3.
In Tasuj Township, the annual prevailing wind blew from South and South West with the speed of 0.5–13.6 m/s. The annual mean wind speed was 2.57 m/s. Calm wind (0 m/s) frequencies were 0.13, 0.6, 1.0, and 2.24% in spring, summer, autumn and winter, respectively. In Ajabshir Township, wind speed varied from 0.5 to 11.12 m/s and annual mean wind speed was 2.57 m/s. In this region the annual prevailing wind blew from South and South East. Also, calm wind (0 m/s) frequencies were 0.5, 0.5, 2.9, and 1.0% in spring, summer, autumn and winter, respectively.
It was cleared that a drought associated with strong wind is regarded as the climatic background for the formation of dust storms [13, 14]. With respect to meteorological dates, June to September have the greatest potential to create dust storm, therefore, in order to predict the direction of probable saline dust storm, wind rose was plotted for mentioned months
(Fig. 3). As it can be seen in Fig. 3, in Tasuj sampling site prevailing wind blew from North East and South West during dry season; and in Ajabshir Township prevailing wind was from South to North and from
The PM mass concentrations and relations
Descriptive statistics of PM mass concentrations and the ratio of PMs’ species in two sampling sites are presented in Table 2. According to Table 2, the average concentrations of TSP, PM10, PM2.5, and PM1 in Tasuj sampling site were 230.1 ± 71.7, 140.3 ± 37.2, 28.3 ± 6.3, and 23.0 ± 5.6 μg/m3, respectively. In Ajabshir sampling site the average concentrations were 291.4 ± 140.3, 220.1 ± 108.6, 31.8 ± 8.7, and 27.4 ± 7.6 μg/m3 for TSP, PM10, PM2.5, and PM1, respectively. In two sampling sites, the highest concentration of PM was observed during the summer season followed by spring.
Also, Table 2 represents the ratio of PMs’ species in two sampling sites. The average of PM10/TSP ratio in Ajabshir site (0.75) was higher than those for Tasuj site (0.64). Also the PM2.5/PM10 ratio for Ajabshir and Tasuj sampling sites ranged between 0.10–0.24 and 0.16–0.16, respectively. There was no significance difference between PM’s ratios obtained from Tasuj and Ajabshir sampling sites (Wilks’ Lambda value = 0.73, F = 0.65 and Pvalue = 0.65). Compared to our previous study on Tabriz urban and industrial suburban PM ratios, PM2.5/PM10 in Urmia lake region was smaller than urban (0.48) and industrial zone (0.38) of Tabriz [15]. It seems due to significant differences in particle sources and also some geographical and meteorological conditions the obtained results from one study could not be directly compared with the findings of other studies.
Ionic composition of PM
The statistical analysis of water soluble ions concentration in the TSP and PM10 showed that there was no significant difference between PM’s ions concentrations at Tasuj and Ajabshir sites (Wilks’ Lambda value = 0.022, F = 4.35 and Pvalue = 0.36 for TSP and Wilks’ Lambda value = 0.019 F = 5.1 and Pvalue = 0.33 for PM10). Therefore, the concentrations of water soluble ions were reported together as Urmia lake PM’s ionic characterization.
The descriptive statistics of water-soluble ions concentration in TSP and PM10 collected from Urmia lake region are given in Table 3; expressed by cubic meter of sampled air (species in mass per volume units: μg/m3) and by mg per g of sampled particles (species in mass/mass units: mg/g of TSP and PM10). Also, the average of water-soluble ions concentration in TSP and PM10 are presented in Fig. 4(a and b); expressed by cubic meter of sampled air (μg/m3) and percentage of species in the measured water soluble ions mass concentration.
The average of the total measured water-soluble ions in TSP was 28.75 ± 12.9 μg/m3 (11.9 ± 4.8% of total TSP mass) and in PM10 was 14.65 ± 7.1μg/m3 (8.7 ± 4.4 of total PM10 mass). As it can be seen in Fig. 4, about 90% of total detected water-soluble ions mass in PM10 included by S0 2 −4 (29%), NO −3 (21%), Na + (14%), Cl −(13%), and Ca 2+(100%) in TSP, and SO 2 −4 (29%), NO −3 (20%), Na +(15%), Cl −(12%), and Ca 2+(12%). Among all detected ions, sulfate was the dominant constituent followed by nitrate and sodium. These results are in agreement with the study by Hassanzadeh et al. [16] which Na+, K+, Ca2+, Li+, and Mg2+ were the main cations, while Cl−, SO 2 −4 , and HCO −3 were the main anions in Urmia lake’s water. The Urmia lake is a hyper-saline lake and the concentrations of Na+ and Cl− were roughly 4 times the concentration of natural seawater [16]. High concentrations of Na+ and Cl− (especially for TSP) could be caused by the higher and persistent on-shore winds which create abundant sea water droplets and marine aerosols.
Our study show that the water soluble salts compose 3–20% of the total mass of TSP and PM10, while Abuduwaili et al. [3] stated that in the Ebinur region, the soluble salts compose 10–25% of the total mass of the saline dust, and salts are predominantly represented by sodium and calcium chlorides and sulfates. Gholampour et al. [15] reported that in the Tabriz urban and suburban region, that are the near to the Urmia lake, water soluble ions accounted for approximately 20 ± 10% of total TSP mass and 25 ± 12 of total PM10 mass. Low percentage of total ions in the PM mass could be due to the small amount of secondary ion, especially non sea salt sulfate and ammonium, in the PM of Urmia lake bed.
Despite of higher PM and ions concentration during warm season, the comparison of mass percentage of water soluble ions in the ambient air PM of Urmia lake during cold and warm seasons showed that there was no significant difference between the mass percentage of PM’s ions in the various seasons (Wilks’ Lambda value = 0.4, F = 1.64 and Pvalue = 0.2). Therefore, it could be concluded that in the Urmia lake region, the sources of PMs are same during various seasons.
Size and morphology of aerosol particles
SEM-EDX photographs for TSP and PM10 samples are shown as an example in Figs. 5(a) and 6(a); and the SEM-EDX spectra of those are shown in Figs. 5(b) and 6 (b), respectively. Quantitative estimates of the particulate’s (TSP sample) elemental composition are given in Table 4. As shown in the table; Oxygen (30.7%) and Silicon (20.4%) are the major component of the analyzed particulate. However, other elements, such as Iron (8.7%), Magnesium (6.9%), Calcium (6.6%), Aluminum (4%), Sodium (1.2%), and Potassium (1%) are also of great importance. Also, elemental composition quantitative estimates of the PM10 sample are given in Table 5. It can be seen that Oxygen (29.1%) and Silicon (14.4%) are the major component of the analyzed particulate. However, other elements, such as Aluminum (8%), Zinc (7.1%), Iron (6.7%), Carbon (3.6%), Magnesium (1.7%), Chlorine (1.6%), and Sodium (1.3) are with great importance.
Comparison of the TSP and PM10 elemental composition with world average values revealed that amount of Silicon in the Urmia lake dusts is less rich (20.4% for TSP and 14.4% for PM10vs. the world average of 59.9%). However, the TSP resulted from Urmia lake dusts are richer in Fe (8.7% vs. the world average of 6.85%), Ca (6.6% vs. the world average of 3.94%), Mg (6.9% vs. the world average of 2.60%),and sodium (1.2% vs. the world average of 0.5–1%) than the Saharan, Harmattan, Chinese, and North American dusts [2].
It is important to note that the concentration of Chloride in the world average values is negligible; whereas our results indicated that chloride is one of the main ions in the Urmia lake dusts. This finding indicates that sea salt particles contribute large to the total mass of the particles collected. On the other hand, crustal dusts have also major role in the composition of TSP and PM10 in Urmia lake region.
Ionic balance of PM
Often the ionic balance is employed to determine the potentially ions missing, which are not measured during the extraction and the detection. The plots of total cations versus the total anions equivalents (neq/m 3) are presented in Fig. 7. The slope of the regression line for PM indicated a value greater than unity (slope = 2.95, R2 = 0.79 for TSP and slope = 2.22, R2 = 0.7 for PM10), which might be due to high level of carbonate and bicarbonate, which were not analyzed. The main ions that could not be detected by our used method are HCO −3 and H+.
On the other hand, as it was shown in Fig. 8, investigation of the relation between Cl − with Na + and K + revealed strong correlation between Cl − and Na + (R2 = 0.91 and slope = 0.9 for TSP and R2 = 0.76 and slope = 0.8 for PM10) and Cl − with K +. But, the slope of relation between Cl − with K + as smaller than Na +. These results allow us to conclude that the main chlorinated compound in PM was NaCl followed by KCl.
Ionic correlation of PM
Ions correlation matrixes were calculated using bivariate correlations to quantify the relation between the elemental concentrations. As it is shown in Table 6, the number of significant correlations of the elements in PM10 is higher and also stronger than the TSP elements. Yatkin et al. [17] has stated that values of elemental correlations in PM2.5 were much higher than PM10. Therefore, it could be concluded that with decreasing the size of PM, the elemental correlation increases and it might show that the sources of smaller PM were limited compared to larger ones.
High level of correlations was obtained between the terrestrial elements in TSP and PM10. For example, the correlation between Ca 2+ and Mg 2+ were 0.81 and 0.88 in TSP and PM10, respectively (P <0.01). These results suggest that the sources of the terrestrial elements are most probably soil and soil-related activities. Also, the high and strong correlation between Cl −, Na +, Ca +, Mg 2+ and K + indicates that a main part of PM was originated from marine salt. Correlations between NH +4 with NO −3 and SO 2 −4 were weak; 0.35 and 0.46 in TSP and 0.31 and 0.46 for PM10, respectively (p > 0.05). It could be concluded that secondary ions have a few roles in the formation of TSP and PM10 in the Urmia lake region.
The Cl −/Na + equivalent ratio for the whole study period ranged from 0.85, in warm seasons, to 1.76, in cold seasons, for TSP. This ratio for PM10 was 0.67, in warm seasons, to 2.15, in cold seasons. These results reveal that the loss of particulate Cl − during warm seasons caused by the formation of gaseous HCl from NaCl and acidic gases [18]. The chloride loss may be illustrated by the reaction between HNO3/or H2SO4 and NaCl in sea-salt particles generally formulated by:
The Cl− /Na + ratio in Urmia Lake region is broader and higher than the measured ratio in PM of urban and industrial zones of Tabriz (0.41–1.07 for TSP and 0.42–0.78 for PM10) [15].
Principal components analysis of PM
Three air quality principal components (PCs) for TSP and two PCs for PM10 with eigenvalues exceeding 1.0 were found. Based on Table 7, it was indicated that these PCs have a significant influence on the PM quality in Urmia Lake. For the TSP, the first PC showed high loading of Na+ , NH +4 , K+, Cl−, F−, NO −3 , and SO 2 −4 with a total variance of 57.6 %. These ions are saline particulates and formed from Urmia lake bed. The second PC showed the high loading of Mg2+ and Ca2+ with a total variance of 18.41%. Theses ions are typically associated with crustal materials in windblown dust and the resuspended dust from around lands. Finally, the third PC showed high loading of NO −2 and PO 2 −4 with a total variance of 13.08 %. Application of chemical fertilizer in agricultural lands around sampling sites could be the origin of third PC.
As mentioned above, two PCs were found for PM10. The first PC showed high loading of Na+, K+, Mg2+, Ca2+, Cl−, F−, NO −3 , and SO 2 −4 with a total variance of 59.9 %. These ions typically associated with saline particulates and crustal materials in windblown dust and resuspended dust from Urmia lake bed and sampling sites around lands. The second PC showed high loading of NH +4 , NO −2 and PO 2 −4 with a total variance of 17.1%. High loading of these ions allows us to conclude that the main source of second PC was application of chemical fertilizer in agricultural lands around sampling sites.
Conclusion
The implemented prevention and control measures are mainly the diversion or conservation of water to recover dried lake beds to reduce wind erosion. However, water-saving potential is limited due to continuing social and economical development in arid regions. On the other hand, evaporative losses from lake surfaces are high because of intense sunshine, large water areas, shallow water depths, and vigorous evaporation from the water surface.
As mentioned above, the water level of Urmia Lake has been decreased up to 6 m during last decade. Along with decreasing of water level, a salt desert is created with area more than 5000 km2. According to the dynamic characteristics of dust, grains with diameters smaller than 10 μm can be transported by wind for several thousands of kilometers under common wind power conditions, and those with diameters of 10–20 μm can be transported for hundreds or up to several thousands of kilometers with wind speeds > 15 m/s. Therefore, at Urmia Lake region and especially during the warm season, wind could carry on saline dust to adjacent areas as far as hundreds of kilometers. The transported PM could damage agricultural lands, pollute the ecosystem and cause variety of disease in some state of Iran, Azerbaijan and other neighboring countries.
References
Okin GS, Mahowald N, Chadwick OA, Artaxo P. Impact of desert dust on the biogeochemistry of phosphorus in terrestrial ecosystems. Global Biogeochemical Cycles. 2004;18(2).
Goudie AS, Middleton NJ. Desert dust in the global system. Springer; 2006
Abuduwaili J, Liu D, Wu G. Saline dust storms and their ecological impacts in arid regions. J Arid Land. 2010;2(2):144–50.
Micklin P. The Aral sea disaster. Annu Rev Earth Planet Sci. 2007;35:47–72.
Gill TE. Eolian sediments generated by anthropogenic disturbance of playas: human impacts on the geomorphic system and geomorphic impacts on the human system. Geomorphology. 1996;17(1):207–28.
Small I, Van der Meer J, Upshur R. Acting on an environmental health disaster: the case of the Aral Sea. Environ Health Perspect. 2001;109(6):547.
Mu G, Yan S, Jilil A, He Q, Xai X. Wind erosion at the dry-up bottom of Aiby Lake. Sci China Ser D Earth Sci. 2002;45(1):157–64.
Liu D, Abuduwaili J, Lei J, Wu G. Deposition rate and chemical composition of the Aeolian dust from a bare saline playa, Ebinur Lake, Xinjiang, China. Water Air Soil Pollut. 2011;218(1–4):175–84.
Hoseinpour M, Fakheri Fard A, Naghili R, editors. Death Of Urmia Lake, a Silent Disaster Investigating Causes, Results and Solutions of Urmia Lake drying. 1st International Applied Geological Congress, Department of Geology, Islamic Azad University, Islamic Azad University-Mashad Branch, Iran; 2010.
Eimanifar A, Mohebbi F. Urmia Lake (northwest Iran): a brief review. Saline Systems. 2007;3(5):1–8.
Cheng M-T, Chou W-C, Chio C-P, Hsu S-C, Su Y-R, Kuo P-H, et al. Compositions and source apportionments of atmospheric aerosol during Asian dust storm and local pollution in central Taiwan. J Atmos Chem. 2008;61(2):155–73.
NCDC. 2013. http://www7.ncdc.noaa.gov/CDO/cdoselect.cmd.
Fu P, Huang J, Li C, Zhong S. The properties of dust aerosol and reducing tendency of the dust storms in northwest China. Atmos Environ. 2008;42(23):5896–904.
Ta W, Dong Z, Sanzhi C. Effect of the 1950s large-scale migration for land reclamation on spring dust storms in Northwest China. Atmos Environ. 2006;40(30):5815–23.
Gholampour A, Nabizadeh R, Naseri S, Yunesian M, Taghipour H, Rastkari N, et al. Exposure and health impacts of outdoor particulate matter in two urban and industrialized area of Tabriz, Iran. J Environ Health Sci Eng. 2014;12(1):27.
Hassanzadeh E, Zarghami M, Hassanzadeh Y. Determining the main factors in declining the Urmia Lake level by using system dynamics modeling. Water Resour Manag. 2012;26(1):129–45.
Yatkin S, Bayram A. Elemental composition and sources of particulate matter in the ambient air of a Metropolitan City. Atmos Res. 2007;85(1):126–39.
Galindo N, Yubero E, Nicolas JF, Crespo J. Water-soluble ions measured in fine particulate matter next to cement works. Atmos Environ. 2011;45(12):2043–9.
Acknowledgments
The authors would like to thank financial support for this research by Institute for Environmental Research (IER), Tehran University of Medical Sciences (grant number 92-01-46-21258). Also, we would like to acknowledge General Administration of Education at East Azarbayejan University of Medical Sciences for providing us with sampling locations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gholampour, A., Nabizadeh, R., Hassanvand, M.S. et al. Characterization of saline dust emission resulted from Urmia Lake drying. J Environ Health Sci Engineer 13, 82 (2015). https://doi.org/10.1186/s40201-015-0238-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40201-015-0238-3