Abstract
Background
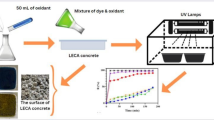
In this study, concrete application as a substrate for TiO2 nano powder immobilization in heterogeneous photocatalytic process was evaluated. TiO2 immobilization on the pervious concrete surface was done by different procedures containing slurry method (SM), cement mixed method (CMM) and different concrete sealer formulations. Irradiation of TiO2 was prepared by UV-A and UV-C lamps. Phenolic wastewater was selected as a pollutant and efficiency of the process was determined in various operation conditions including influent phenol concentration, pH, TiO2 concentration, immobilization method and UV lamp intensity.
Findings
The removal efficiency of photocatalytic process in 4 h irradiation time and phenol concentration ranges of 25–500 mg/L was more than 80 %. Intermediates were identified by GC/Mass and spectrophotometric analysis.
Conclusions
According to the results, photocatalytic reactions followed the pseudo-first-order kinetics and can effectively treate phenol under optimal conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The ability of Advanced Oxidation Processes (AOPs) in treating a wide range of hazardous wastes has brought this technology to the forefront of research over the last decade. Among AOPs, application of heterogeneous photo-catalysis by using semiconductors has been proved to be real interest as an efficient tool for degrading both aquatic and atmospheric organic contaminants [1]. Semiconductors are photo-reactive metal oxides for contaminants eradication that refer to photo-catalysts [2]. Titanium dioxide (TiO2) is an established photocatalyst utilized in the photo-oxidation process. When TiO2 is exposed to the appropriate wavelength of ultra-violet light (UV-A), electrons in the low-energy valence band will absorb the photon’s energy and move into the high-energy conduction band. The result of this electron excitation is a hole, or positive charge, in the valence band (h+) and an electron in the conduction band (e−) [3]. Reaction yield and photocatalytic activity will be increased when the diameter of TiO2 particles becomes smaller especially below 100A° [1].
Photocatalytic reactors for water and wastewater treatment can be classified to slurry and photocatalytic ones. In the slurry reactors, the catalyst particles are freely dispersed in the fluid phase (water) and consequently the photo-catalyst is fully integrated in the liquid mobile phase [4]. Whereas the immobilized catalyst reactor design features a catalyst anchored to a fixed support and dispersed on the stationary phase. Slurry systems require the separation of the fine sub micron particles TiO2 from the treated milk-like water suspension. Separation steps cause to complicate the treatment process and decrease the economical viability of the slurry reactor approach [5]. Therefore, these difficulties have led many researchers to study reactors with thin immobilized films of catalyst bonded to a solid substrate such as activated carbon [6], fiber optic cables [7] fiberglass [8], glass beads [9], quartz sand [10], silica gel [11] and stainless steel [12]. Dip coating from suspension, spray coating, sputtering, sol–gel related methods, and electrophoretic deposition [4, 13] are techniques developed for immobilizing TiO2 catalysts on these substrates. Although these techniques and substrates had a suitable performance for treating different types of wastewater in laboratory scale experiments, but their application in wastewater treatment plants and pilot scale studies are questionable.
Most applications of concrete modified by TiO2 were done for air pollutant removal and self-cleaning surfaces [14]. Although many studies in the field of air pollution have been done by concrete-TiO2 photocatalyst process [15, 16], but the number of wastewater treatment researches is very low. Application of concrete as TiO2 substrate has unique characteristics such as porosity, natural abundance, absence of toxicity, and low price. This construction material is used in all of the water and wastewater treatment plants (WWTP) all over the world. Therefore, the photocatalytic process by TiO2 photocatalyst that immobilized on concrete surfaces can be used in large scale WWTP.
Phenols and its compounds are widely found in paint, leather and textile, disinfectants, medicine, oil refinery and lubricant production wastewater industries in Iran [17–19]. Phenol is rapidly absorbed through the skin and can cause skin and eye burns upon contact. Comas, convulsions, cyanosis and death can be resulted from its overexposure [20, 21]. Therefore Phenol-containing wastewater may not be conducted in open water without treatment because of its toxicity.
Several investigations using different physical, chemical and biological systems and their combination for phenol elimination from wastewater have been reported [22–27]. Biological processes are preferred to other conventional methods due to their ability to effectively destroy the pollutants in an environmentally benign and cost effective way [19]. Sensitive of process to organic shocking load and need to high control for microbial acclimation for hard degradable compound such as phenol are limiting factor in biological treatment. Photocatalytic process didn’t have these limitations and can be used separately or in joint with biological process. Different researches were done related study to photocatytic process in wastewater treatment. Chiou et al. (2008) have studied degradation of phenol and m-nitrophenol using a photocatalytic process in aqueous solution by commercial TiO2 powders (Degussa P-25) under UV irradiation [28]. The optimal solution pH of single phenol and m-NP was at around 7.4 and 8.9, respectively. Immobilization of TiO2 on perlit granules for photocatalytic degradation of phenol was done by Hosseini et al. (2009) [13]. The Results showed uniform coating on perlit and good photocatalytic activity for the catalysts. Pumic stone was applied as TiO2 substrate for degradation of dyes and dye industry pollutants [29]. Real wastewaters collected before biological treatment treated in photocatalytic reactor and color disappeared after 4-h.
The main objective of this research was to study the feasibility of using concrete as substrate for TiO2 nano powder immobilization to treat phenolic wastewater. For this reason, four methods were applied for immobilization of nano TiO2 on concrete. Kinetic of the reactions, long term use of the process and intermediates formed during the photo degradation were other objectives of this research.
Material and methods
Chemicals
Cement in the concrete was an ordinary Portland type V that is usually applied in WWTP construction in Iran. Selection of concrete mix proportion was done according to standard practice for selecting proportions of normal concrete (ACI 211.1 :1996) [30]. Light expanded clay aggregate (LECA) was used as light coarse aggregate to lead specific gravity of concrete to 1200 kg/m3. LECA application increased surface porosity of concrete and promoted the specific surface area as an important immobilization parameter. Concrete surface was fabricated in wooden moulds with an internal dimension of 500 × 250 × 50 mm. TiO2 Degussa P25, anatase/rutile ca. 70/30, with an average particle size of 20 nm and a BET surface area 55 ± 15 m2.g−1 was used as photocatalyst. UV radiation was provided by different powers of UV-A Philips medium pressure lamps. The spectral irradiance of UV-A lamp ranges from 300 to 400 nm. The primary wavelength distribution of the UV-A lamp was 365 nm and the light intensity was among 4.42-8.9 mW.cm−2. The incident UV-A light intensity was measured by a UV power meter (UVA-365- Lutron Taiwan). Also UV illumination was provided by UV-C lamps (20 Watts, maximal light intensity at 256 nm).
Phenol (purity above 99 %) as a contaminant, NaOH, HCl for pH adjustment, NH4OH, K2HPO4, KH2PO4, K3Fe(CN)6 and 4-aminoantiprine as phenol concentration reagents and other chemicals all offered by Merck Co., as an analytical reagent grade. Wastewater produced by adding deionized water and phenol in various concentrations (25–500 mg/L).
Immobilization methods
Immobilization of TiO2 nano powder on concrete surfaces was carried out by four different procedures. Adhesion properties of cement and concrete sealers were applied to fix photocatalyst on pervious concrete surfaces.
Slurry method (SM)
The details of this technique are given as follows:
-
20 g/L slurry TiO2 was prepared using 25 % (v/v) methanol in deionized water. Methanol would help with the attachment of TiO2 to the surface.
-
The solution was stirred vigorously for 10 min at 20 °C.
-
The slurry was sonicated for 5 min to separate the flocculated TiO2 and to obtain more uniform slurry.
-
One half of slurry sprayed on fresh concrete to use adhesive properties of cement.
-
The coated surface was placed in oven at 100 °C for 2 h to remove the moisture content.
-
The rest of the slurry sprayed on hardened concrete and annealed at 450 °C for 2 h to remove any organics from the surface.
-
The support was let to dry and was washed with pure water to eliminate the excess of the catalyst.
Cement mixed method (CMM)
In this method, 20 g/L TiO2 was mixed by cement grout (40 g cement + 20 mL water) and distributed on hardened concrete base by an ordinary brush. After 8 h the concrete surface was placed in oven at 100 °C for 2 h to be dried. The support was washed with pure water to eliminate the excess of the catalyst.
Epoxy sealer method (ESM) & Waterproof sealer method (WSM)
In these procedures, the effect of epoxy concrete sealer (Nitofix- Fars Iran Company) and waterproof concrete sealer (Nitotile- Fars Iran Company) for adhesion of TiO2 was examined. The details of this technique are as follows:
-
100 mL of the selected concrete sealer mixed with 1000 mL of pure water and stirred vigorously for 10 min at 20 °C.
-
Prepared emulsion was distributed on the hardened concrete surface with a trowel and let to dry for 20 min.
-
After that time, 20 gr/L of TiO2 poured on concrete surface for adhesion on sealer.
-
The support was let to dry and was washed with pure water to eliminate the excess of the catalyst.
Photocatalytic reactor setup
The immobilized concrete surfaces were placed in the pilot scale photocatalytic reactor (Fig. 1). A dosing pump was used to feed the phenol-laden wastewater into photocatalytic zone and distributed by a pipe with 1 cm mesh in its length. Reactor hydraulics parameters were controlled by water depth limited to 4 mm. Three concrete surfaces and circulation mode was used to prepare efficient contact time between photocatalyst and wastewater. Dissolved oxygen (DO) and pH was monitored in all the experimental period time by Crison-Oxi45 and Metrohm690, respectively. UV radiation was provided by UV-A and UV-C Philips medium pressure lamps. Samples were taken periodically form the sample ports for phenol concentration analysis. Prior to measurement, the liquid samples were centrifuged by Sigma101 at about 4000 rpm for 10 min to remove detached TiO2 particles. The phenol concentrations were measured by colorimetric 4-aminoantipyrine procedure using a Perkin Elmer-Lambda EZ 150 UV/vis spectrophotometer as described in standard methods (2005). The structure and morphology of prepared catalysts and concrete surface were determined using Philips XL30 scanning electron microscope (SEM) followed by AU-coated by sputtering method using a coater sputter (Bal-Tec, Switzerland). All other parameters were determined according to the standard methods (2005). The intermediates determined by GC/Mass (column: chrompack CP-Sil 8 CB 50 m × 250 μm × 0.12 μm). The gas vector used was helium and the detector was a FID. The program of temperature was: detector temperature = 240 °C, injector temperature = 230 °C, oven initial and final temperatures = 105 °C & 190 °C, oven rise 10 °C min−1, initial and final time = 2 & 15 min). All other parameters were determined according to the Standard Methods [31].
Results and discussion
Characteristics of immobilized concrete surfaces
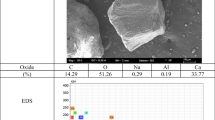
In all immobilization procedures, SEM and energy dispersive X-ray microanalysis (EDX) were done to confirm the presence of TiO2 on concrete surfaces (Fig. 2). Images of non-immobilized surface proved high surface porosity of concrete as a good support for TiO2. SEM analysis showed a uniform appearance of TiO2 catalyst in ESM, WSM and SM but dispersed coating in CMM.
Mixing TiO2 by cement as an adhesive agent caused the minimum level of active surface catalyst in CMM (Fig. 2c). In other methods, catalyst was poured on concrete surface that covered by adhesive agent and consequently uniform TiO2 cover was prepared. EDX analysis of TiO2 coatings showed no significant levels of noticeable impurities in all immobilizations cases.
Effect of influent phenol concentration
Effect of different initial concentrations (C0 = 25–500 ppm) in removal efficiency is shown in Fig. 3.
At first, in all experiments the removal efficiency was measured in pH = 7 without UV lamps for 60 min to determine the pollutants adsorption to concrete and phenol volatility. Removal efficiency in these conditions was less than 5 % and stripping of phenol by aeration was negligible because of its very low Henry’s constant [32]. Photocatalytic process was then provided by turning on UV lamps and removal efficiency was measured in different retention times. In SM method after 4 h, 95 and 69 % of phenol was degraded in 25 and 500 ppm initial concentration, respectively. Equal degradation was achieved in ESM when C0 was 25 ppm but the removal efficiency in WSM was 75 % at the same conditions. The removal efficiency of the CMM was lower than that of other methods. So that after 4 h, 28 % phenol with initial concentration of 500 ppm was degraded. At the same conditions removal efficiency for ESM and WSM were 56 and 43 %, respectively. The results of this study are comparable with similar researches in recent years. For example Chiou et al. (2008) in slurry photo-reactor treated 60 % of 0.52 mM (~49 mg/L) phenol after 3 h and 400 W UV lamp. In other research, Hosseini et al. (2007) reached to 88.3 % phenol degradation after 4 h in initial concentration = 1 mM (~94 mg/L) and UV lamp intensity = 120 W [13].
Effect of pH solution
Electrostatic interaction between semiconductor surface, solvent molecules, substrate and charged radicals formed during photocatalytic oxidation is strongly dependent on the pH of the solution [28]. Solution pH dominates photo-degradation process due to the strong pH dependence of many related properties such as the semiconductor’s surface charge state, flat band potential, and the solution dissociation. In alkaline pH, phenol was converted to phenoxide group (remove of H+ and creation of negative charge on hydroxyl group) that was more reactive than phenol in a solution [33]. In the other hand, the ionization state of the surface of the photocatalyst can also be protonated and deprotonated under acidic and alkaline conditions. The pHZPC of Degussa P-25 TiO2 used here is 6.5, and phenol is 9.89, respectively [28]. While under acidic conditions, the positive charge of the TiO2 surface increases as the pH decreases (TiOH2 +); above pH 6.5 the negative charge at the surface of the TiO2 increases with increasing pH (TiO−). The optimum pH can be obtained between pHZPC of photocatalyst and contaminant. Effect of pH in the range of 4–12 on phenol removal efficiency in ESM was evaluated (Fig. 4).
The highest efficiency was observed at pH of 9–12. The difference between phenol removal efficiency at pH of 12 and 4 was determined 39 % in ESM (52 and 86 % removal efficiency) when initial concentration was 100 mg/L and UV-A lamp intensity was equal to 5.33 mW.cm−2. The rate of removal efficiency in different pH was similar in different immobilization methods and determined between pHZPC of phenol and TiO2. Several researchers have observed different results about the effect of the pH on the TiO2 photocatalytic decomposition of phenol. This discrepancy on the optimum pH may be a function of the various operating conditions considered.
Effect of immobilization methods
The photocatalytic activities for all combinations of coating methods are summarized in Fig. 5. Influent phenol concentration was 50 ppm, pH = 7, UV intensity = 5.33 mW.cm−2 and UV lamp distance to concrete surface = 10 cm in these experiments. Results showed ESM had the highest photocatalytic activities so that the removal efficiency of this method was more than 90 % after 4 h. The photocatalytic efficiency of the coating methods was in the following order: ESM > SM > WSM > CMM. The removal efficiency of CMM method after 4 h was 42 % that was the minimum efficiency between other immobilization techniques. The most important reason that proved by SEM-EDX analysis was reduction levels of active surface of nano particles that mixed with cement. While in other methods, TiO2 nano particles attached to a concrete top surface, in the CMM nano TiO2 particles mixed with cement as cohesive agent and a lot of percents of photoctalyst disappeared. Comparison between ESM and WSM showed that ESM had better performance due to hydrophobic properties of waterproof sealers that decreased connection between contaminants and immobilized pohotocatalyst.
Effect of UV lamp Intensity
The effect of UV lamp intensity on the phenol photo degradation at constant initial concentration (100 ppm) and pH = 7 is presented in Fig. 6. The results showed that phenol removal efficiency increased when the UV lamps intensity promoted. In other words, photocatalytic efficiency in all immobilization methods improved about 50 % when UV-A lamp intensity increased from 4.42 to 8.9 mW.cm−2. This is reasonable because the stronger the irradiating UV, the more the UV penetrating. A few differences between phenol degradation since 8.9 and 8.1 UV intensity was confirmed that further increase in UV intensity couldn’t increase the amount of phenol destroyed.
Application of UV-C lamp promoted phenol removal efficiency more than 18 % compared to UV-A lamps at a constant irradiation time. Capability of UV-C lamp in degradation of 50 % of phenol without TiO2 photocatalyst showed proper spectral irradiance of UV-A lamp that degraded only 7 % of phenol when photocatalyst was deleted. On the other hand, UV-A lamps had appreciated feature in the photocatalytic process compare to UV-C lamps.
Effect of long-term use
One of the most important parameters in photocatalytic reactors with immobilized photocatalyst is a reduction in removal efficiency because of TiO2 particles detachment and catalyst surface fouling by formation of by-products during the degradation process. This limitation inhibited the application of immobilized procedure as a long term process for wastewater treatment. The influence of long term use in the degradation efficiency has been examined at a constant initial concentration (100 ppm), pH = 7 and UV lamp intensity of 5.33 mW.cm−2. Proper connection between TiO2 nano particles and concrete surface led to 2 and 3.5 % reduction in phenol removal efficiency for ESM and WSM, respectively. In SM that TiO2 poured on concrete surfaces and cement was used as a cohesive agent instead of concrete sealers, the reduction in removal efficiency after 45 times iterations was more than 21 %. Mixing cement by TiO2 caused 4 % removal efficiency reduction after 45 times in CMM. In other research, the elimination of some TiO2 from the pumice stone surface showed significant decrease of photocatalytic efficiency in long term use of process when SM was used [29].
Kinetics of the reactions
Langmuir-Hinshelwood kinetic has been used to charac-terize the destruction of many contaminants in different structure of catalyst [34]. The final form of this model can be expressed as Eq. 1:
Where (r) is the rate of the photocatalytic degradation, (C0) is the organic concentration, (Kr) the reaction rate constant, (Ks) the apparent adsorption constant and (t) is the time of reaction. The term KsC0 is often negligible when the concentration is low, and the reaction rate can be expressed as pseudo-first-order model as shown in Eq. 2:
Where Kapp is called apparent first order reaction rate. Integration of the equation yields to Eq. 3:
A plot of –ln(C/C0) against (t) and slope of linear regression analysis is equal to the value of Kapp. The calculated result indicated that photocatalytic degradation of phenol at the reactions conditions follows a pseudo-first-order kinetics that compared with other researches [13, 35].
Regression coefficients (R2) and Kapp for the photo degradation of phenol at different immobilization method, pH values and initial concentration are shown in Table 1.
The results showed that Kapp increased in all immobilization methods and different initial concentration when pH value changes from 4 to 12. Comparison among different immobilization methods approved that ESM had the highest removal efficiency compared to the other methods that had the most Kapp.
Phenol photo degradation intermediates
The intermediates of photo degradation of 100 mg/L phenol at pH = 9 and intensity = 8.1 mW.cm−2 were obtained by GC/Mass analysis. The results showed phenol hydroxylated via hydroxyl radical attack to different extent forming a variety of intermediates from the very beginning of the reaction. Firstly, aromatic ring (hydroquinone, catechol and benzoquinone) produced and after 60 min, intermediates convert to linear compounds such as oxalic acids and formic acids. Lastly, phenol is completely mineralized to CO2 and H2O. This can be explained given the fact that adsorbed phenol molecules on TiO2 are subject to variable interaction with hydroxyl radicals.
Changes of the UV spectrum with respect to destruction of aromatic ring and phenol and variation of the absorbance at 268 nm in different irradiation time are shown in Fig. 7. It can be seen that when the irradiation time increased, maximum peak of the absorption spectrum decreased and finally a spectrum without noticeable peak was observed. At the end, a residual absorbance around 200 nm corresponding to the organic acid formation as final degradation products could be seen.
Conclusions
In this study, the performances of various coating methods alternatives on concrete surfaces for treating phenolic wastewater were compared. Based on the findings, the following conclusions can be drawn:
-
1.
SEM determination has showed well uniformity of coating processes and EDX spectrum approved nano TiO2 on concrete surface.
-
2.
The photocatalytic tests of phenol degradation showed a good photocatalytic activity for TiO2.
-
3.
The ESM coating method was found to be the best technique, with the highest photocatalytic activity and adhesion. The removal efficiency of this method was more than 90 % after 4 h.
-
4.
Effect of pH showed that the removal efficiency of phenol increased as pH value increased from 4 to 9 while phenol was converted to phenoxide group.
-
5.
Photocatalytic efficiency in all immobilization methods improved about 50 % when UV lamp intensity increased 2 times.
-
6.
Process kinetics by Langmuir-Hinshelwood model was approved pseudo-first-order reaction for phenol photo degradation.
-
7.
Reduction of phenol removal efficiency was done as a reverse method for evaluation of nano TiO2 detachment from concrete surfaces.
-
8.
Results showed that application of concrete sealers had better performance in comparison with SM and CMM that cement was used as a cohesive agent. In WSM and ESM, reduction of removal efficiency was less than 2 and 3.5 % after 45 times iteration of process, respectively.
-
9.
Consequently, concrete due to its special characteristics and large consumption in WWTP as a construction material seems to be an ideal support for the immobilization of TiO2 photo-catalysts. Also concrete sealers showed good capability for attachment of TiO2 nano particles to concrete surfaces.
References
Gaya UI, Abdullah AH. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals. Progress and problems, J Photoch Photobio C. 2008;9:1–12.
Li Y, Li S, Li Y, Guo Y, Wang J. Visible-light driven photocatalyst (Er3+:YAlO3/Pt–NaTaO3) for hydrogen production from water splitting. Int J Hydrogen Energ. 2014;39:17608–16.
Rizzo L, Koch J, Belgiorno V, Anderson MA. Removal of methylene blue in a photocatalytic reactor using polymethylmethacrylate supported TiO2 nanofilm. Desalination. 2007;211:1–9.
De Lasa H, Errano B, Salaices M. Photocatalytic reaction engineering. USA: Springer Science Pub; 2005.
Bickley RI, Slater MJ, Wang WJ. Engineering development of a photocatalytic reactor for wastewater treatment. Process Saf Environ. 2005;83:205–16.
Shi J, Zheng J, Wu P, Ji X. Immobilization of TiO2 films on activated carbon fiber and their photocatalytic degradation properties for dye compounds with different molecular size. Catal Commun. 2008;9:1846–50.
Lim LLP, Lynch RJ, In SI. Comparison of simple and economical photocatalyst immobilisation procedures. Appl Catal A-Gen. 2009;365:214–21.
Horikoshi S, Watanabe N, Onishi H, Hidaka H, Serpone N. Photodecomposition of a nonylphenol polyethoxylate surfactant in a cylindrical photoreactor with TiO2 immobilized fiberglass cloth. Appl Catal B-Environ. 2002;37:117–29.
Sakthivel S, Shankar MV, Palanichamy M, Arabindoo B, Murugesan V. Photocatalytic decomposition of leather dye: comparative study of TiO2 supported on alumina and glass beads. J Photoch Photobio A. 2002;148:153–9.
Choy CC, Wazne M, Meng X. Application of an empirical transport model to simulate retention of nanocrystalline titanium dioxide in sand columns. Chemosphere. 2008;7:1794–01.
Zainudin NF, Abdullah AZ, Mohamed AR. Characteristics of supported nano-TiO2/ZSM-5/silica gel (SNTZS): photocatalytic degradation of phenol. J Hazard Mater. 2010;174:299–06.
Chen Y, Dionysiou DD. TiO2 photocatalytic films on stainless steel: the role of Degussa P-25 in modified sol–gel methods. Appl Catal B-Environ. 2006;62:255–64.
Hosseini SN, Borghei SM, Vossoughi M, Taghavinia N. Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Appl Catal B-Environ. 2007;74:53–62.
Chen J, Poon CS. Photocatalytic activity of titanium dioxide modified concrete materials – influence of utilizing recycled glass cullets as aggregates. J Environ Manage. 2009;90:3436–42.
Poon CS, Cheung E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr Build Mater. 2007;21:1746–53.
Husken G, Hunger M, Brouwers HJH. Experimental study of photocatalytic concrete products for air purification. Build Environ. 2009;44:2463–74.
Mortazavi SB, Sabzali A, Rezaee A. Sequence-Fenton Reaction for decreasing phenol formation during benzene chemical conversion in aqueous solutions. Iranian J Environ Health Sci Eng. 2005;2:62–71.
Kidak R, Ince NH. Ultrasonic destruction of phenol and substituted phenols: a review of current research. Ultrason Sonochem. 2006;13:195–9.
Moussavi G, Mahmoudi M, Barikbin B. Biological removal of phenol from strong wastewaters using a novel MSBR. Water Res. 2009;43:1095–302.
Busca G, Berardinelli S, Resini C, Arrighi L. Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater. 2008;160:265–88.
Gholizadeh A, Kermani M, Gholami M, Farzadkia M. Kinetic and isotherm studies of adsorption and biosorption processes in the removal of phenolic compounds from aqueous solutions: comparative study. J Environ Health Sci Eng. 2013;11:29.
Asadgol Z, Forootanfar H, Rezaei S, Mahvi AH, Faramarzi MA. Removal of phenol and bisphenol-a catalyzed by laccase in aqueous solution. J Environ Health Sci Eng. 2014;12:93.
Hemmati Borji S, Nasseri S, Mahvi A, Nabizadeh R, Javadi A. Investigation of photocatalytic degradation of phenol by Fe(III)-doped TiO2 and TiO2 nanoparticles. J Environ Health Sci Eng. 2014;12:101.
Mahvi AH, Maleki A, Alimohamadi M, Ghasri A. Photo-oxidation of phenol in aqueous solution: toxicity of intermediates. Korean J Chem Eng. 2007;24:79–82.
Mahvi AH, Maleki A. Photosonochemical degradation of phenol in water. Deaslin Water Treat. 2010;20:197–02.
Maleki A, Mahvi AH, Mesdaghinia A, Naddafi K. Degradation and toxicity reduction of phenol by ultrasound wavesSoc. Bull Chem Ethiop. 2007;21:33–8.
Shahamat YD, Farzadkia M, Nasseri S, Mahvi A, Gholami M, Esrafili A. Magnetic heterogeneous catalytic ozonation: a new removal method for phenol in industrial wastewater. J Environ Health Sci Eng. 2014;12:50.
Chiou CH, Wu CY, Juang RS. Photocatalytic degradation of phenol and m-nitrophenol using irradiated TiO2 in aqueous solutions. Sep Purif Technol. 2008;62:559–64.
Venkata Subba Rao K. Immobilization of TiO2 on pumice stone for the photo-catalytic degradation of dyes and dye industry pollutants. Appl Catal B-Environ. 2003;46:77–85.
ACI 211. Manual of concrete practice. Farmington Hill: ACI; 1996.
APHA, AWWA, WEF. Standard methods for the examination of water and wastewater. 21st ed. Washington: American Public Health Association; 2005.
Feigenbrugel V, Le Calve S, Mirabel P, Louis F. Henry’s law constant measurements for phenol, o-, m-, and p-cresol as a function of temperature. Atmos Environ. 2004;38:5577–88.
Morrison RT, Boyd RN. Organic chemistry. 6th ed. USA: McGraw-Hill; 2000.
Zheng S, Cai Y, O’Shea KE. TiO2 photocatalytic degradation of phenylarsonic acid. J Photoch Photobio A. 2010;210:61–8.
Royaee SJ, Sohrabi M. Application of photo-impinging streams reactor in degradation of phenol in aqueous phase. Desalination. 2010;253:57–61.
Acknowledgements
The authors would like to acknowledge Iranian Nano Technology Initiative Council and Vice-Chancellor for Research Affairs of Tarbiat Modares University for their partial financial support in this research. The valuable supports of Environmental Engineering Laboratory of Civil and Environmental Engineering Faculty of Tarbiat Modares University are also appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors participated in conception and design, generation of data, analysis of data, interpretation of data and revision of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Delnavaz, M., Ayati, B., Ganjidoust, H. et al. Application of concrete surfaces as novel substrate for immobilization of TiO2 nano powder in photocatalytic treatment of phenolic water. J Environ Health Sci Engineer 13, 58 (2015). https://doi.org/10.1186/s40201-015-0214-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40201-015-0214-y