Abstract
Background
Carbon monoxide (CO), hypothetically linked to prebiotic biosynthesis and possibly the origin of the life, emerges as a substantive growth substrate for numerous microorganisms. In anoxic environments, the coupling of CO oxidation with hydrogen (H2) production is an essential source of electrons, which can subsequently be utilized by hydrogenotrophic bacteria (e.g., organohalide-respring bacteria). While Dehalococcoides strains assume pivotal roles in the natural turnover of halogenated organics and the bioremediation of chlorinated ethenes, relying on external H2 as their electron donor and acetate as their carbon source, the synergistic dynamics within the anaerobic microbiome have received comparatively less scrutiny. This study delves into the intriguing prospect of CO serving as both the exclusive carbon source and electron donor, thereby supporting the reductive dechlorination of trichloroethene (TCE).
Results
The metabolic pathway involved anaerobic CO oxidation, specifically the Wood-Ljungdahl pathway, which produced H2 and acetate as primary metabolic products. In an intricate microbial interplay, these H2 and acetate were subsequently utilized by Dehalococcoides, facilitating the dechlorination of TCE. Notably, Acetobacterium emerged as one of the pivotal collaborators for Dehalococcoides, furnishing not only a crucial carbon source essential for its growth and proliferation but also providing a defense against CO inhibition.
Conclusions
This research expands our understanding of CO’s versatility as a microbial energy and carbon source and unveils the intricate syntrophic dynamics underlying reductive dechlorination.
Graphical Abstract

Video Abstract
Similar content being viewed by others
Introduction
Carbon monoxide (CO), presumed to be abundant in the prebiotic Earth, holds compelling evidence for its involvement in prebiotic synthesis and probably the origin of life. Early life on Earth thrived amidst high levels of CO exposure [1,2,3], a phenomenon traced back to the primordial atmospheric conditions that prevailed around 4 billion years ago when life first emerged. The Wood-Ljungdahl pathway (WLP), an ancient carbon fixation pathway consisting of the methyl and the carbonyl branches, is hypothesized to have played a pivotal role in the origin of life on ancient Earth as well as microbial energy conservation and carbon assimilation under anoxic conditions [4,5,6]. CO, serving as both a carbon and electron source, finds relevance for microorganisms utilizing WLP [7, 8].
CO, once thought of as a toxic gas, has emerged as a fascinating and surprisingly versatile metabolic substrate for diverse microorganisms through both fermentative and respiratory pathways [8]. Certain aerobic bacteria, such as Oligotropha carboxidivorans, Ruegeria pomeroyi, and Mycobacterium smegmatis [9], are capable of oxidizing atmospheric CO, where CO serves as both electron donor and carbon source, with O2 as the electron acceptor. Under anoxic conditions, CO is typically metabolized through fermentative pathways, with nickel-dependent CODHs (Ni-CODH) playing a crucial role in oxidizing CO to various products, including CO2 plus H2, acetate, ethanol, or methane plus CO2 [7, 10, 11]. Certain groups of microorganisms, such as sulfate-reducing bacteria and iron-reducing bacteria, employ respiratory metabolism to utilize CO. In this respiratory process, CO is oxidized to CO2 with either SO42− or Fe3+ serving as the electron acceptor and subsequently being reduced to S2− or Fe2+ [7, 12]. CO’s diverse metabolic pathways highlight the remarkable adaptability of microorganisms to different environmental conditions.
Organohalide-respiring bacteria (OHRB) not only gained recognition as crucial microorganisms for the bioremediation of chlorinated solvents, but it has also played pivotal roles in the natural turnover of halogenated organics [13]. Dehalococcoides mccartyi populations are obligate OHRB, and its noteworthy ability to convert the ubiquitous groundwater contaminants chlorinated ethenes (strains 195, FL2, BAV1, GT), chlorinated ethanes (strains 195, BAV1, VS, 11a), pentachlorophenol (strain JNA), chlorinated biphenyls (strain CG1), and chlorinated benzenes (strain CBDB1) to the low or non-toxic end products has made it a key player in environmental detoxification [14,15,16,17,18,19,20,21,22,23,24]. While Dehalococcoides excel at detoxification, their own metabolic processes, specifically the incomplete WLP for methionine biosynthesis, can turn against them, leading to CO accumulation and potentially hindering their effectiveness [25,26,27]. The toxicity of CO, detrimental to Dehalococcoides strains, could be alleviated by other CO-utilizing bacteria within the microbial community (e.g., Desulfovibrio, Acetobacterium) [10, 27,28,29]. CO produced by Dehalococcoides may also serve as an energy source for anaerobically CO-oxidizing bacteria. In addition to protections offered by other microbes (e.g., fermenters, acetogens), Dehalococcoides thrives optimally in microbial communities by acquiring essential resources including the electron donor H2 and carbon source acetate. For instance, the H2 and acetate, generated through lactate fermentation by Desulfovibrio desulfuricans strain F1, were harnessed by Dehalococcoides strain CG1 for organohalide respiration, highlighting a synergistic interaction [30]. CO could have served as a more thermodynamically favorable “first” electron donor than H2 [31] and a potential carbon source; nonetheless, evidence regarding on CO’s role in supporting reductive dechlorination remains rare. In contrast to OHRB, Acetobacterium dehalogenans utilizes chloromethane and produces acetate as a fermentation end product [32,33,34]. This distinction highlights the diverse metabolic strategies employed by microorganisms for dehalogenation. While Acetobacterium species are primarily known as acetogens that indirectly support dechlorination by providing essential cofactors like vitamin B12 and acetate [35,36,37,38], some strains, such as Acetobacterium strain AG, have demonstrated the ability to directly debrominate polybrominated diphenyl ethers (PBDEs) under various growth conditions, including organic-carbon-free medium [37]. This finding expands the known capabilities of Acetobacterium in halogenated organic compound transformations.
CO is a naturally occurring compound in underground environments, potentially serving both as an electron donor and a carbon source for a variety of microorganisms. In this study, we hypothesize that CO could fuel reductive dechlorination within mixed microbial communities, specifically supporting the activities of OHRB (e.g., Dehalococcoides). To test this hypothesis, we established microcosms using river sediment as a microbial source, exploring the potential of CO as a supportive factor for sustained dechlorination. Our investigations revealed that CO, even at concentrations exceeding 2.2 µM—previously deemed detrimental to Dehalococcoides growth [27]—effectively promoted the survival, growth, and enrichment of the key dechlorinator. These revelations carry substantial implications, shedding light on the conceivable role of CO as both electron donor and carbon source for diverse microorganisms inhabiting subterranean environments, including the less-recognized OHRB. Additionally, they underscore the significance of CO in facilitating the reductive dechlorination activities of OHRB beyond the scope previously acknowledged.
Materials and methods
Chemicals
Trichloroethylene (TCE) and cis-1,2-dichloroethylene (cDCE) were purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Vinyl chloride (VC) and ethene (both ≥ 99%) were purchased from Dalian Special Gases Co., Ltd (Dalian, China). H2, nitrogen (N2), carbon dioxide (CO2), and CO (all ≥ 99.999%) were purchased from Shuntai Special Gases Co., Ltd (Shenyang, China). All other chemicals used in this study were analytical grade or of higher purity.
Microcosm setup and enrichment cultures
Medium preparation and anaerobic cultivation were performed following established protocols [39,40,41]. Briefly, a reduced, bicarbonate-buffered mineral salt medium was boiled under an atmosphere of N2 to remove dissolved oxygen, cooled down to room temperature, and then dispensed into serum bottles flushed with N2/CO2 (80/20, v/v). Sediment samples were collected from a location (41° 39′ 46″ N, 123° 6′ 20″ E) at Xi River, Shenyang, Liaoning Province, China, as described [42]. Microcosms were constructed inside an anoxic chamber (Coy Laboratory Inc., MI, USA) filled with N2/H2 (97/3, v/v). An aliquot of 2 mL sediment sludge was pipetted into 120-mL glass serum bottles prefilled with 80 mL of medium mentioned above as described [40, 43]. Bottles were sealed with butyl rubber stoppers (Fushiyuan rubber and plastic products factory, Shenzhen, Guangdong, China) and crimped with aluminum caps (Hongpu Instrument Technology, Ningbo, Zhejiang, China). Initially, acetate (5 mM) was provided as the carbon source, and CO (2 mL) was provided as the electron donor. In the incubation period, CO was added to several doses (2 mL each). Neat TCE (3 µL, ca. 0.3 mM or 43.4 mg/L aqueous phase concentration) was added as the electron acceptor. All bottles were amended with Wolin vitamin mix [44]. Following the complete dechlorination of TCE to ethene, 1 mL culture suspension was transferred into a fresh medium following the same procedures (Fig. S1). The bottles were incubated statically in the dark at 30 °C. Microcosms and enrichment cultures were established in triplicate bottles. Cultures amended without CO or with H2 as the electron donor served as controls.
Sequencing, assembly, and binning
Metagenome sequencing was performed by Novogene Co., Ltd. (Beijing, China). DNA samples were extracted from the fourth transfer enrichment cultures with CO as both carbon source and electron donor using the CTAB protocol [45]. DNA degradation and potential contamination were monitored on 1% agarose gels. DNA concentration was measured using Qubit® dsDNA Assay Kit in Qubit® 2.0 Fluorometer (Life Technologies, CA, USA). Sequencing libraries were generated using NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. The clustering of the index-coded samples was performed on a cBot Cluster Generation System. After cluster generation, the library preparations were sequenced on an Illumina Hiseq platform, and paired-end reads were generated. Raw sequence data were processed with Readfq v8 (https://github.com/cjfields/readfq) to acquire the filtered sequence data for subsequent analysis. After being trimmed and filtered, the resulting 35,338,878 paired-end reads were assembled using the JGI Metagenome Assembly Pipeline (https://github.com/kbaseapps/jgi_mg_assembly) [46]. Metagenomic short-read profiling and taxonomic classification were performed using Kaiju v1.7.3 [47]. Metagenomic contigs were classified with Maxbin2 v2.2.4 [48]. The metagenome-assembled genomes (MAGs) were assessed with CheckM [49] using default settings for completeness and contamination evaluation. High-quality MAGs that included the draft genome sequence of a Dehalococcoides strain, designated as strain CO, were annotated using BV-BRC v3.29.20 (https://www.bv-brc.org/) and RAST v2.0 (https://rast.nmpdr.org/) with default parameters. The procedures for DNA extraction, amplicon sequencing, Sanger sequencing, PCR, and qPCR are elaborated in the Supplementary Information (SI).
Analytical methods
Ethene, methane, and chlorinated compounds were analyzed using an Agilent 7890B gas chromatography (GC) equipped with an Agilent 7697A automatic headspace sampler (Agilent Technologies, Santa Clara, CA, USA), a flame ionization detector (FID) (method detection limit ~ 0.2 µM) and an Agilent DB-624 capillary column (60 m length × 0.32 mm inner diameter × 1.8 μm film thickness) as described [40]. Oven temperature was initially held at 60 °C for 2 min, increased to 200 °C at 25 °C/ min, and held at 200 °C for 1 min. Inlet and FID temperatures were set at 200 °C and 300 °C, respectively [42].
H2 and CO were analyzed using a Peak Performer 1 (PP1) 910-100 trace level gas chromatography equipped with a reducing compound photometer (RCP) (method detection limit ~ 1 ppb) (Peak Laboratories, CA, USA). Column and RCP bed temperatures were set at 105 °C and 265 °C, respectively.
Acetate was analyzed using an Agilent 1260 high-performance liquid chromatography (HPLC) system (Agilent Technologies, Santa Clara, CA, USA) equipped with an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) and a diode array detector (DAD) set at 210 nm; samples were separated at a flow rate of 0.6 mL/min using 4 mM H2SO4 as the mobile phase [50].
Data availability
The BioProject accession number is PRJNA1042952. The 16S rRNA gene amplicon sequencing data are available in the Sequence Read Archive of GenBank under the accession number SRR27024736 (CO, 5th transfer, HEPES buffer), SRR27024737 (CO, 5th transfer, bicarbonate buffer), SRR27024738 (CO, 3rd transfer, bicarbonate buffer), and SRR27024739 (CO and acetate, 3rd transfer, bicarbonate buffer). The binning genomic sequence of Dehalococcoides sp. strain CO is available in GenBank under the accession number JBDODF000000000. The binning genomic sequence of Acetobacterium sp. strain Z1 is available in GenBank under the accession number JBDODG000000000. Three rdhA genes (RdhA1, RdhA2, and RdhA3) annotated from the draft Dehalococcoides sp. strain CO are available in GenBank under the accession number PP060998, PP060999, and PP061000. The partial 16S rRNA gene sequence of Dehalococcoides sp. strain CO is available under the accession number OQ946896.
Results
CO as an electron source for reductive dechlorination of TCE
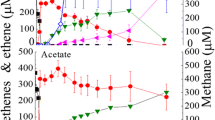
After a 3-month incubation period, the sediment microcosms supplemented with 5 mM acetate and 2 mL of CO completely dechlorinated the initial 33.9 ± 1.6 µmol of TCE to ethene. In contrast, microcosms supplemented only with 5 mM acetate and without CO were unable to achieve complete TCE dechlorination, with the process stalling at the cDCE stage with negligible amount of VC (data not shown). Over the incubation period, five doses of CO (i.e., 10 mL in total) were added into the bottles. This led to the reductive dechlorination of TCE to ethene in approximately 90 days, with cDCE and VC produced sequentially (Fig. S2A). Following four consecutive transfers, the culture maintained the ability of complete reductive dechlorination of TCE to ethene within ~ 50 days (Fig. 1A). Specifically, TCE was dechlorinated to ethene within 30 days when H2 was provided as the electron donor (Fig. 1B), while TCE dechlorination stalled in approximately 100 days without H2 or CO amendment (Fig. 1C).
Reductive dechlorination of TCE in enrichment cultures amended with CO plus acetate. Hydrogenolysis of TCE to ethene using CO (A) or H2 (B) as the electron donor. Stalled dechlorination of TCE in the absence of an electron donor (C). Error bars represent the standard deviations of triplicate samples, omitted when smaller than the symbol. The red arrows indicate CO supplementation (2 mL/dose)
Exclusive utilization of CO to support TCE reductive dechlorination
Separate microcosms were established solely with CO as the hypothesized electron and carbon source (Fig. S1). After an extended incubation period of exceeding 6 months, the sediment microcosm amended with only 2 mL of CO unexpectedly achieved the dechlorination of the initial 32.5 ± 1.6 µmol of TCE to stoichiometric amounts of VC (data not shown), and the transferred cultures dechlorinated the same amount of TCE to ethene with increased dechlorination rates (Fig. S3). Subsequently, the fourth transfer, cultivated in the bicarbonate-buffered medium amended with thirteen doses of CO (i.e., 26 mL in total), demonstrated stepwise reductive dechlorination of TCE to ethene in approximately 160 days (Fig. 2A). To further substantiate the role of CO as the sole electron and carbon source, the bicarbonate buffer system was replaced with HEPES buffer, and the headspaces were purged with pure N2 gas. By comparison, the transferred cultures, cultivated in the HEPES-buffered medium and amended with only CO (~ 24 mL in total), also stepwise dechlorinated the same amount of TCE to 31.7 ± 0.1 µmol ethene in about 190 days, affirming that CO served not only as the electron donor but also as the carbon source (Fig. 2B).
Reductive dechlorination of TCE to ethene by enrichment cultures only supplemented with CO in bicarbonate-buffered medium (A) or in HEPES-buffered medium (B). Error bars represent the standard deviations of triplicate samples, omitted when smaller than the symbol. The red arrows indicate the time points of CO supplementation (2 mL/dose)
Microbial community profiles of TCE-dechlorinating enrichment cultures sustained by CO
Amplicon sequencing of the 16S rRNA gene was applied to investigate the microbial population(s) responsible for CO-fueled TCE dechlorination. The enrichment cultures cultivated under different conditions exhibited varying compositions at the phylum level, with Firmicutes, Bacteroidota, Chloroflexi, Proteobacteria, Halobacterota, and Cloacimonadota being the major phyla (Fig. 3A). Notably, the phylum Chloroflexi became dominant in enrichment cultures amended with CO as the electron donor and carbon source, regardless of the buffering agent used (bicarbonate or HEPES). At the genus level, Thermincola emerged as the predominant bacteria (53.0%) in enrichment cultures supplemented with both CO and acetate. Nonetheless, its presence was entirely absent in cultures lacking acetate amendment. This observation aligns with the known physiological capabilities of Thermincola carboxydiphila strain 2204, a described alkalitolerant, CO-utilizing, H2-producing, thermophilic anaerobe [51], which possesses the ability for chemolithotrophic growth via anaerobic CO oxidation coupled to H2 and CO2 production [51, 52]. The second most abundant genus in CO plus acetate enrichment cultures was Sporomusa (11.9%), capable of growth on either CO or H2/CO2 [53]. Methanogenic archaea Methanosarcina (2.5%) was exclusively detected in CO plus acetate enrichment cultures, with ~ 450 µmol/bottle of methane detected. In the third transferred cultures amended with CO only, the most abundant genus was Acetobacterium (64.6%), whereas its relative abundance in the third transfer cultures amended with CO plus acetate was only 1.3%. Given the capacity of several Acetobacterium species to convert CO to acetate [54], it is hypothesized that Acetobacterium in the CO-fueled enrichment cultures serves as the primary producer and provides carbon source acetate for other populations within the community. It is worth noting that the model acetogen Acetobacterium woodii cannot grow on CO as a sole carbon and energy source, suggesting that the Acetobacterium identified in this study may differ from Acetobacterium woodii [10]. Meanwhile, Acetobacterium wieringae strain JM has the ability to grow with CO as both carbon and energy source and was isolated recently [55]. Dehalococcoides (1.1–39.3%) was the only OHRB phylotype detected in all the enrichment cultures. Acetobacterium and Dehalococcoides were the top two most abundant genera in the fifth transfers cultivated with CO only (Fig. 3B). Subsequently, we obtained a nearly complete (i.e., ~ 1300 bp) 16S rRNA gene from the CO-fed consortium using the Dehalococcoides-specific primers. As shown in Fig. 3C, the amplicon shared 98.5–100% sequence similarities with the 16S rRNA gene of known Dehalococcoides isolates (e.g., 195, BAV1), which provides additional evidence for the presence of a Dehalococcoides population in the CO-fed TCE-dechlorinating community. We hypothesized that certain genera employ CO as a precursor to generate CO2 and H2. Acetobacterium, in turn, harnesses these products to synthesize acetate. The symbiotic interaction involving H2 and acetate facilitates the survival of Dehalococcoides and enhances its capability to reductively dechlorinate TCE to ethene within the microbial community.
Microbial community structures at both the phylum (A) and genus (B) levels in the enrichment cultures following complete TCE depletion. Maximum-likelihood phylogenetic tree of Dehalococcoides (including its three subgroups) and Dehalogenimonas based on 16S rRNA gene sequences (C). Bootstrap values (1000 replicates) are indicated at branch points, and the scale bar represents nucleotide substitutions per site. GenBank accession numbers are provided in parentheses
Dehalococcoides growth coupled with TCE reductive dechlorination fueled by CO
To elucidate the crucial role of CO in facilitating Dehalococcoides growth and TCE dechlorination, we conducted an experiment involving the transfer of CO enrichment cultures (in a bicarbonate buffer) with varying CO supplement doses, resulting in total added CO of 14 mL (571.4 µmol) or 35 mL (1428.6 µmol), respectively. Our findings revealed that the initial 38.1 ± 0.3 µmol TCE was dechlorinated to stoichiometric amounts of ethene within approximately 90 and 65 days in cultures supplemented with 2 mL CO/dose and 5 mL CO/dose, respectively. A clear correlation was observed between the amount and frequency of CO supplementation and the rate of TCE dechlorination (Fig. 4A, B). Concomitant with TCE dechlorination, Dehalococcoides cell numbers in the cultures increased significantly from (3.6 ± 0.3) × 105 to (1.5 ± 0.2) × 108 cells per mL cultures (416.9-fold increase) and (1.3 ± 0.1) × 108 cells per mL cultures (346.4-fold increase), respectively (Fig. 4E). Concomitant with the supplementation of CO, a noteworthy increase in H2 production was observed. However, despite significant CO additions, H2 generation during the TCE-to-VC dechlorination phase remained exceptionally low (maximum amounts measured were 33.7 ± 1.4 nmol/bottle and 158.1 ± 6.9 nmol/bottle for 2 and 5 mL CO doses, respectively) over approximately 40 days. Intriguingly, the accumulation of H2 was exclusively evident during the VC-to-ethene transition, with the maximum amounts measured at 631.8 ± 172.5 nmol/bottle and 334.7 ± 158.2 nmol/bottle, respectively (Fig. 4D). This stage-specific pattern suggests a potential shift in metabolic pathways or microbial community dynamics during the dechlorination process. Additionally, CO-fueled acetogenesis by Acetobacterium was evident in the enrichment cultures, as depicted by the final acetate concentrations of 2.4 ± 0.01 mM and 4.4 ± 0.4 mM in the 2 mL and 5 mL CO/dose cultures, respectively (Fig. 4C). This acetate production potentially serves as a substrate for other microbial populations within the community, further influencing the observed H2 dynamics.
Reductive dechlorination of TCE by enrichment cultures exclusively amended with 5 mL CO/dose (A) and 2 mL CO/dose (B) coupled with acetate formation (C), H2 formation (D), and Dehalococcoides growth (E). Concurrently, the figures detail the growth of Dehalococcoides (C), the formation of H2 (D), and the production of acetate (E). Error bars, reflecting standard deviations from triplicate samples, are omitted when their magnitude is below the symbol. The red arrows in the figures indicate the specific points of CO supplementation
Draft genome of the TCE-dechlorinating Dehalococcoides
The binning of metagenomic contigs resulted in the assembly of a draft Dehalococcoides genome, designated as strain CO. This genome comprised 4 contigs with a total size of 1,360,741 bp, a G + C content of 47.2% and N50 = 781,482 bp. CheckM analysis indicated that the genome was nearly 95.1% complete with 1.5% contamination [49]. PATRIC and RAST annotation of the draft genome predicted a total of 1497 genes including 1440 coding sequences (CDS) and 57 non-coding RNA sequences. Dehalococcoides strain CO exhibited a high genome-aggregate average nucleotide identity (ANI) with strains CBDB1 (99.3%), FL2 (99.7%), 11a5 (99.4%), and KS (99.4%) (Table S1), exceeding the 95% ANI threshold for species demarcation [56]. However, strain CO showed lower ANI compared to strains 195 (86.3%), VS (86.8%), and CG3 (87.7%). A total of 20 rdhA were annotated in the draft genome of strain CO. Two identical RDases RdhA1 (NCBI Accession #PP060998) and RdhA2 (NCBI Accession #PP060999) were annotated in the draft genome of strain CO, sharing amino acid identities of 96.5%, 96.9%, 97.9%, and 96.7% with the VcrA protein sequences in the KB-1 consortium, Dehalococcoides strain VS, strain WBC-2, strain GT, respectively (Fig. S3). Another RDase RdhA3(NCBI Accession #PP061000) with a full length of 500 amino acids shared 94.4% and 99.6% amino acid identities when compared with TceA in Dehalococcoides strain 195 and the KB-1 consortium, respectively (Fig. S4). Other putative RDases exhibited relatively lower similarities to the charactered RDases.
Draft genomes of the CO-oxidating anaerobes
Binning of the metagenomic contigs enabled the assembly of a draft Acetobacterium genome, designated as strain Z1. This genome consisted of 46 contigs with a total size of 3,373,213 bp, a G + C content of 44.5%, and N50 = 781,482 bp. CheckM analysis indicated that the genome was almost 100% complete with 0.3% contamination [49]. PATRIC and RAST annotation of the draft genome predicted a total of 3298 genes, including 3255 coding sequences (CDS) and 43 non-coding RNA sequences. Strain Z1 exhibited relatively lower ANI (< 90%) and dDDH (< 70%) compared to other Acetobacterium species (Table S3). The comparison and phylogenetic tree based on the genomes (Fig. S5) indicated that strain Z1 represented a new species within the genus Acetobacterium. No RDase genes were annotated in the draft genome of strain Z1, suggesting it is not capable of organohalide respiration. The CODH annotated in the draft genome of strain Z1 shared 97.7%, 95.3%, 94.8%, 92.7%, and 90.0% amino acid identities with the CODH protein sequences in Acetobacterium woodii, Acetobacterium malicum, Acetobacterium wieringae, Acetobacterium dehalogenans, and Acetobacterium tundrae, respectively (Fig. S6). Enrichment cultures harbored a diverse microbial community, with several prominent genera—Acetobacterium, Methanosarcina, Desulfomicrobium, and Desulfocurvibacter—exhibiting the genomic potential for CO utilization via the presence of CODH. The draft genome of strain Z1 encompasses the complete WLP, with the key enzyme ACS likely playing a crucial role in survival and growth within the CO-fed cultures. H2 formation from CO oxidation serves as a central reaction, driving electron flow throughout the community. This highlights the increasing recognition of hydrogenogenic carboxydotrophs, identified in diverse environments [57]. While H2 production by Acetobacterium remains unreported, Acetobacterium wieringae and Acetobacterium woodii are known to generate CO2 and acetate as CO oxidation end products [55, 58]. The genes encoding the CO oxidation system (Coo) and energy-converting hydrogenase (Ech) serve as marker genes for hydrogenogenic carboxydotrophy [59, 60]. Genomic annotations revealed that Desulfomicrobium, Desulfocurvibacter, Methanosarcina, Methanoculleus, Methanosarcina, and Methanofollis were annotated with both Coo and Ech, establishing them as potential H2 producers in the CO-fed cultures.
Discussion
To date, the complete detoxification of chlorinated ethenes into the environmentally benign product ethene relies exclusively on a specific subset of OHRB within the class Dehalococcoidia (e.g., Dehalococcoides, Dehalogenimonas) [17, 40]. It is noteworthy that all Dehalococcoides isolates exhibit a strict requirement for H2 as electron donor, an indispensable role that cannot be substituted [17]. Various pathways for H2 production have been demonstrated, including organic acids fermentation, phosphite oxidation, acetate oxidation, nitrogen fixation, and anaerobic carbon monoxide oxidation [61]. The established knowledge regarding the evolution of H2 supporting OHRB has been well documented. Here, we demonstrate that CO can serve as an alternative electron donor for the dechlorination of chlorinated ethenes, ultimately yielding the environmentally friendly end product ethene.
A total of 1428.6 µmol of CO (i.e., 35 mL CO) were meticulously introduced into the 5 mL CO/dose supplemented enrichment cultures. Following the principles delineated in Table 1, it can be deduced that each molecule of CO can be converted into one molecule of H2, as depicted by equation 2. Thus, the maximum theoretical H2 production would amount to 1428.6 µmol. In accordance with equation 1, the complete dechlorination of 33.3 µmol of TCE to ethene would theoretically consume 100.0 µmoles of H2. The remaining H2, if exclusively utilized for acetate production, would reach a substantial quantity of 332.1 µmol, equivalent to 4.2 mM in an 80 mL medium, as indicated by equation 3. Remarkably, the measured final concentration of acetate was determined to be 4.4 ± 0.4 mM, underscoring the intricate metabolic dynamics orchestrated within the microbial community.
The analyses of carbon and electron balance revealed that the principal outcomes of CO oxidation in our study were the production of acetate, CO2, and H2 (Table 2). These findings underscored the intricate interplays among CO oxidation, TCE dechlorination, and WLP in the metabolic processes occurring within the enrichment cultures. Within the enrichment cultures, a remarkable division of labor emerged concerning CO utilization. Acetobacterium assumed the primary role in converting CO into acetate, likely via the WLP. Concurrently, several hydrogenogenic carboxydotrophs (e.g., Desulfomicrobium, Desulfocurvibacter, Methanosarcina) potentially drove the alternative pathway, transforming CO into CO2 and H2. This strategic cooperation provided a dual benefit: H2 and acetate, generated by the initial CO oxidation, were subsequently channeled to Dehalococcoides for fueling its reductive dechlorination of TCE. CO functioned as an indirect yet crucial source of both energy and carbon for Dehalococcoides, enabling its vital role in the overall dechlorination process.
Previous research had indicated the detrimental effect of CO, inhibiting the reductive dechlorination of TCE and hexachlorobenzene driven by Dehalococcoides strain 195 or CBDB1, respectively [27, 63]. Even a modest concentration of 6 µmol per bottle for strain 195 or 1 µmol per bottle for strain CBDB1 of CO could severely impede the growth of Dehalococcoides [27]. Additionally, CO accumulation as a metabolic by-product in dechlorinating cultures dominated by Dehalogenimonas etheniformans strain GP has been shown to negatively impact reductive dechlorination activity. Externally amended CO at 4 µmol (~ 880 ppmv in the culture vessel) strongly inhibited vinyl chloride (VC) degradation by strain GP, indicating Dehalogenimonas strains, like Dehalococcoides, are sensitive to CO. These findings underscore the need for strategies (e.g., syntrophy) to mitigate CO toxicity in dechlorinating systems comprising obligate OHRB like Dehalococcoides and Dehalogenimonas [64, 65]. Surprisingly, in our study, we amended a maximum CO (5 mL) concentration of up to 204.1 µmol per bottle. Contrary to expectations, not only did Dehalococcoides endure under such elevated CO concentrations, but it also thrived and proliferated by harnessing CO, a specific interaction mechanism hitherto undocumented in the literature. It is important to note that due to the dynamic nature of CO dissolution and consumption in the liquid phase, the actual CO concentration experienced by Dehalococcoides in our enrichment culture was likely substantially lower than the calculated equilibrium concentration. Therefore, the true CO tolerance of Dehalococcoides in our system could be lower than the levels supplemented in the culture vessels.
While CO offers advantages in terms of energy conservation compared to H2, its utilization by microorganisms has been restricted by issues related to microbial tolerance [8]. Nevertheless, certain anaerobes have demonstrated the capacity to utilize CO for the production of carboxylates and alcohols. Other than that, the coupling of CO oxidation with various respiratory processes, such as desulfurication, hydrogenesis, acetogenesis, and methanogenesis, has been established [58]. For instance, Clostridium ljungdahlii can produce acetate and ethanol through WLP using CO [66]. Methanogens, especially Methanosarcina acetivorans, have been extensively studied for their ability to grow on CO as the sole substrate, with the concomitant formation of acetate [58, 67]. However, only a limited number of anaerobes capable of utilizing CO as their sole source of energy and carbon have been documented to date. Acetobacterium, a well-studied anaerobic microorganism possessing a complete WLP, is also known for its ability to utilize CO. However, the capacity to grow solely on CO as the carbon and energy source has only been observed in one strain, JM, to date [10, 55, 68]. In our study, we demonstrated that CO could effectively function as the sole carbon and energy source, thereby maintaining the stability of the microbial community. Acetobacterium spp. are frequently co-cultured with OHRB, playing a significant role in their activities: (1) they provide essential metabolites like acetate, vitamin B12, and other cofactors to support the growth and dehalogenation capabilities of OHRB such as Dehalococcoides, Trichlorobacter (formerly Geobacter), and Sulfurospirillum; (2) they can mitigate the toxicity of CO, a common inhibitor of OHRB; and (3) certain strains, like Acetobacterium strain AG, possess the ability to directly debrominate polybrominated diphenyl ethers (PBDEs), suggesting facultative organohalide respiration within the genus [35,36,37,38, 69,70,71,72]. Additionally, our findings regarding CO-dependent H2 production hold significant implications, offering a promising alternative in the context of diminishing fossil fuel resources [58]. It is crucial to acknowledge that H2 production coupled with CO oxidation has been infrequently observed, possibly due to technological limitations. The exploration of CO-dependent energy conservation presents an exciting avenue for future research.
H2 is produced in anoxic environments through the oxidation of organic matter [73, 74]. In addition to anaerobic fermentation, H2 can also be generated directly or indirectly through bio-photolysis, photo-fermentation, CO gas-fermentation, and nitrogen fixation [61, 75, 76]. For instance, nitrogen fixation, despite its energy consumption, results in the annual production of 2.4–4.9 Tg H2 per year [75]. These H2 can be rapidly consumed in microbial-mediated terminal electron-accepting processes, such as iron reduction, sulfate reduction, denitrification, methanogenesis, and organohalide respiration [73, 74]. By comparison, the organohalide respiration process can compete with other electron-accepting processes, possessing a thermodynamic advantage. This advantage arises from the ability of organohalide-respiring bacteria to utilize a relatively low threshold H2 concentration [77]. The H2 threshold concentrations for the reduction of various chlorinated compounds differ. For instance, the mean H2 concentrations during the reductive dehalogenation of 2,4-dichlorophenol (2,4-CP), 2,3,4-trichlorophenol (2,3,4-CP), pentachlorophenol (PCP), and tetrachloroethene (PCE) were 3.6 nM, 4.1 nM, 0.3 nM, and 0.8 nM, respectively [77]. H2 threshold concentrations range from 0.6 to 0.9 nM for PCE and TCE reduction, 0.1–2.5 nM for cDCE reduction, and 2–24 nM for VC reduction [78, 79]. In our study, a noticeable production of H2 from CO oxidation was observed only between approximately day 60 and day 75. We speculate that during the reductive dechlorination process from TCE to VC, H2 produced from CO oxidation was promptly utilized for dechlorination. Low concentrations of H2 may have failed to fuel VC dechlorination, resulting in H2 accumulation (Fig. 4D). These findings align with previous reports indicating that electron donor (e.g., H2) limitation can inhibit the growth of VC-dechlorinating Dehalococcoides populations [80]. The dynamic interplay between hydrogenogenic carboxydotrophy and Dehalococcoides, involving H2 transfer, indicates a microbial ecological collaboration with advantages. Further investigation is warranted to confirm the underlying mechanisms governing this intricate microbial interaction.
In this study, in addition to Dehalococcoides and Acetobacterium, draft genomes for several other genera, including Youngiibacter, Desulfocurvibacter, Gudongella, Methanofollis, Aminivibrio, and Petrimonas (Table S4), were successfully assembled. Youngiibacter, a strictly anaerobic microorganism, ferments various carbohydrates into ethanol, formate, acetate, and CO2 [81]. It likely engages in the fermentation of unidentified carbohydrates in CO-supplemented enrichment cultures. Desulfocurvibacter, a sulfate-reducing bacterium, typically thrives through pyruvate fermentation [82]; by comparison, it may function as a CO consumer in this study. Gudongella strain W6T exhibits N2-fixing capability and utilizes amino acids while refraining from growth on acetate [83]. Methanofollis, a strictly anaerobic archaeon, oxidizes CO to produce H2 and CO2, and employs H2 and acetate to produce methane in CO-fed cultures [84]. Aminivibrio and Petrimonas are anaerobic fermentative bacteria with the capacity to ferment organic acids [85, 86]. Nevertheless, elucidating the precise functions of these anaerobes within CO-oxidizing and dechlorinating microbial communities remains challenging and speculative at this juncture (Fig. 5). Top-down approaches, involving the reduction of microbial community complexity through serial dilution or the isolation of specific microorganisms, and bottom-up strategies, integrating and synthesizing co-cultures or tri-cultures, hold promise for providing insights into the potential ecological functions of these anaerobes.
Carbon recovery and electron recovery, acetate, ethene, and methane were the main three products (A). Proposed interaction networks within CO-fed TCE-dechlorinating cultures based on assembled draft genomes of various genera suggest diverse metabolic pathways. CO oxidation to CO2 and H2 is hypothesized to occur in Desulfocurvibacter, Desulfomicrobium, and Methanosarcina, facilitated by the presence of CODH and Ech. Acetobacterium is identified as capable of exclusively oxidizing CO to acetate, attributed to the presence of complete WLP genes. Acetate and H2 collectively support the reductive dechlorination of TCE to ethene by Dehalococcoides. Methanosarcina and Methanofollis utilize acetate, H2, and CO2 to produce methane. Genera such as Youngiibacter, Gudongella, and Petrimonas, identified as fermentation specialists, demonstrate the ability to metabolize carbohydrates and some organic acids, while Aminivibrio, a fermentation bacterium, exhibits a preference for acetate utilization. These proposed interactions outline a complex web of metabolic relationships in the CO-enriched TCE-dechlorinating cultures (B)
In summary, the biologically mediated “water-gas shift reaction” (CO + H2O ⇌ CO2 + H2) is predominantly catalyzed by CODH in potential hydrogenogenic carboxydotrophy Desulfomicrobium, Desulfocurvibacter, Methanosarcina, Methanoculleus, Methanosarcina, and Methanofollis. This enzymatic process results in the formation of H2 and CO2, which are subsequently utilized by Acetobacterium and Methanosarcina in acetogenesis and methanogenesis, respectively. The produced acetate and H2 are accessible for Dehalococcoides, enabling the reductive dechlorination of TCE to ethene. Additionally, acetate serves as a versatile carbon source, potentially harnessed by Aminivibrio, Petrimonas, Methanofollis, Methanosarcina, and other microbial groups. H2, acting as a ubiquitous energy currency in anoxic environments, underscores its essential role in interspecies H2 transfer (IHT), crucial for maintaining the community structure and function of CO-fed enrichment cultures (Fig. 5).
Availability of data and materials
All sequencing data generated and analyzed in this study have been deposited in the NCBI Sequence Read Archive (SRA) under the accession numbers provided in the Data availability section. Additional data or materials relevant to the study are available from the corresponding author upon reasonable request.
References
Olson KR, Donald JA, Dombkowski RA, Perry SF. Evolutionary and comparative aspects of nitric oxide, carbon monoxide and hydrogen sulfide. Respir Physiol Neurobiol. 2012;184(2):117–29.
Kharecha P, Kasting J, Siefert J. A coupled atmosphere–ecosystem model of the early Archean Earth. Geobiology. 2005;3(2):53–76.
Martin W, Baross J, Kelley D, Russell MJ. Hydrothermal vents and the origin of life. Nat Rev Microbiol. 2008;6(11):805–14.
Huber C, Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe, Ni)s under primordial conditions. Science. 1997;276(5310):245–7.
Ducat DC, Silver PA. Improving carbon fixation pathways. Curr Opin Chem Biol. 2012;16(3):337–44.
Gong F, Cai Z, Li Y. Synthetic biology for CO2 fixation. Sci China Life Sci. 2016;59(11):1106–14.
Ragsdale SW. Life with carbon monoxide. Crit Rev Biochem. 2004;39(3):165–95.
Diender M, Stams AJM, Sousa DZ. Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front Microbiol. 2015;6:1275.
Cordero PRF, Bayly K, Leung PM, Huang C, Islam ZF, Schittenhelm RB, et al. Carbon monoxide dehydrogenases enhance bacterial survival by respiring atmospheric CO. bioRxiv. 2019:628081. https://doi.org/10.1101/628081.
Bertsch J, Müller V, Lovell CR. CO metabolism in the acetogen Acetobacterium woodii. Appl Environ Microbiol. 2015;81(17):5949–56.
Uffen RL. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. PNAS. 1976;73(9):3298–302.
DePoy AN, King GM, Ohta H. Anaerobic carbon monoxide uptake by microbial communities in volcanic deposits at different stages of successional development on O-yama volcano, Miyake-jima, Japan. Microorganisms. 2020;9(1):12.
Hug LA, Maphosa F, Leys D, Löffler FE, Smidt H, Edwards EA, et al. Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Philos Trans R Soc Lond B Biol Sci. 2013;368(1616):20120322.
Yohda M, Ikegami K, Aita Y, Kitajima M, Takechi A, Iwamoto M, et al. Isolation and genomic characterization of a Dehalococcoides strain suggests genomic rearrangement during culture. Sci Rep. 2017;7(1):2230.
Asai M, Morita Y, Meng L, Miyazaki H, Yoshida N. Dehalococcoides mccartyi strain NIT01 grows more stably in vessels made of pure titanium rather than the stainless alloy SUS304. Environ Microbiol Rep. 2023;15(6):557–67.
Saiyari DM, Chuang H, Senoro DB, Lin T, Whang L, Chiu Y, et al. A review in the current developments of genus Dehalococcoides, its consortia and kinetics for bioremediation options of contaminated groundwater. Sustain Environ Res. 2018;28(4):149–57.
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, et al. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol. 2015;65(6):2015–15.
Maymó-Gatell X, Anguish T, Zinder SH. Reductive dechlorination of chlorinated ethenes and 1,2-dichloroethane by “Dehalococcoides ethenogenes” 195. Appl Environ Microbiol. 1999;65(7):3108–13.
Magnuson Jon K, Romine Margaret F, Burris David R, Kingsley MT. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: sequence of tceA and substrate range characterization. Appl Environ Microbiol. 2000;66(12):5141–7.
He J, Ritalahti KM, Yang K, Koenlgsberg SS, Löffler FE. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature. 2003;424(6944):62–5.
Krajmalnik-Brown R, Hölscher T, Thomson IN, Saunders FM, Ritalahti KM, Löffler FE. Genetic identification of a putative vinyl chloride reductase in Dehalococcoides sp. strain BAV1. Appl Environ Microbiol. 2004;70(10):6347–51.
Lee PKH, Cheng D, West KA, Alvarez-Cohen L, He J. Isolation of two new Dehalococcoides mccartyi strains with dissimilar dechlorination functions and their characterization by comparative genomics via microarray analysis. Environ Microbiol. 2013;15(8):2293–305.
Parthasarathy A, Stich TA, Lohner ST, Lesnefsky A, Britt RD, Spormann AM. Biochemical and EPR-spectroscopic investigation into heterologously expressed vinyl chloride reductive dehalogenase (VcrA) from Dehalococcoides mccartyi strain VS. J Am Chem Soc. 2015;137(10):3525–32.
Müller Jochen A, Rosner Bettina M, von Abendroth G, Meshulam-Simon G, McCarty Perry L, Spormann Alfred M. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Appl Environ Microbiol. 2004;70(8):4880–8.
Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L. Genome sequence of the chlorinated compound–respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol. 2005;23(10):1269–73.
Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe BA, et al. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science. 2005;307(5706):105–8.
Zhuang W-Q, Yi S, Bill M, Brisson VL, Feng X, Men Y, et al. Incomplete Wood-Ljungdahl pathway facilitates one-carbon metabolism in organohalide-respiring Dehalococcoides mccartyi. PNAS. 2014;111(17):6419–24.
Chin BY, Otterbein LE. Carbon monoxide is a poison … to microbes! CO as a bactericidal molecule. Curr Opin Pharmacol. 2009;9(4):490–500.
Fukuyama Y, Inoue M, Omae K, Yoshida T, Sako Y. Chapter three - Anaerobic and hydrogenogenic carbon monoxide-oxidizing prokaryotes: versatile microbial conversion of a toxic gas into an available energy. In: Gadd GM, Sariaslani S, editors. Advances in applied microbiology, Volume 110. New York: Academic Press; 2020. p. 99–148.
Chen C, Xu G, He J. Substrate-dependent strategies to mitigate sulfate inhibition on microbial reductive dechlorination of polychlorinated biphenyls. Chemosphere. 2023;342:140063.
Ferry JG, House CH. The stepwise evolution of early life driven by energy conservation. Mol Biol Evol. 2006;23(6):1286–92.
Smidt H, de Vos WM. Anaerobic microbial dehalogenation. Annu Rev Microbiol. 2004;58:43–73.
Meßmer M, Wohlfarth G, Diekert G. Methyl chloride metabolism of the strictly anaerobic, methyl chloride-utilizing homoacetogen strain MC. Arch Microbiol. 1993;160(5):383–7.
Traunecker J, Preuß A, Diekert G. Isolation and characterization of a methyl chloride utilizing, strictly anaerobic bacterium. Arch Microbiol. 1991;156(5):416–21.
Ziv-El M, Popat SC, Parameswaran P, Kang D-W, Polasko A, Halden RU, et al. Using electron balances and molecular techniques to assess trichoroethene-induced shifts to a dechlorinating microbial community. Biotechnol Bioeng. 2012;109(9):2230–9.
Trueba-Santiso A, Fernandez-Verdejo D, Marco-Rius I, Soder-Walz JM, Casabella O, Vicent T, et al. Interspecies interaction and effect of co-contaminants in an anaerobic dichloromethane-degrading culture. Chemosphere. 2020;240:124877.
Ding C, Chow Wai L, He J. Isolation of Acetobacterium sp. strain AG, which reductively debrominates octa- and pentabrominated diphenyl ether technical mixtures. Appl Environ Microbiol. 2013;79(4):1110–7.
Lim ML, Brooks MD, Boothe MA, Krzmarzick MJ. Novel bacterial diversity is enriched with chloroperoxidase-reacted organic matter under anaerobic conditions. FEMS Microbiol Ecol. 2018;94(5):fiy050.
Yang Y, Capiro NL, Marcet TF, Yan J, Pennell KD, Löffler FE. Organohalide respiration with chlorinated ethenes under low pH conditions. Environ Sci Technol. 2017;51(15):8579–88.
Yang Y, Higgins SA, Yan J, Simsir B, Chourey K, Iyer R, et al. Grape pomace compost harbors organohalide-respiring Dehalogenimonas species with novel reductive dehalogenase genes. ISME J. 2017;11(12):2767–80.
Löffler FE, Sanford RA, Ritalahti KM. Enrichment, cultivation, and detection of reductively dechlorinating bacteria. In: Methods in enzymology, volume 397. New York: Academic Press; 2005. p. 77–111.
Jiang L, Yang Y, Jin H, Wang H, Swift CM, Xie Y, et al. Geobacter sp. strain IAE dihaloeliminates 1,1,2-trichloroethane and 1,2-dichloroethane. Environ Sci Technol. 2022;56(6):3430–40.
Yang Y, Sanford R, Yan J, Chen G, Capiro NL, Li X, et al. Roles of organohalide-respiring Dehalococcoidia in carbon cycling. mSystems. 2020;5(3):e00757-19.
Wolin EA, Wolin MJ, Wolfe RS. Formation of methane by bacterial extracts. J Biol Chem. 1963;238(8):2882–6.
Kalendar R, Boronnikova S, Seppänen M. Isolation and purification of DNA from complicated biological samples. In: Besse P, editor. Molecular plant taxonomy: methods and protocols. New York: Springer, US; 2021. p. 57–67.
Arkin AP, Cottingham RW, Henry CS, Harris NL, Stevens RL, Maslov S, et al. KBase: the United States department of energy systems biology knowledgebase. Nat Biotechnol. 2018;36(7):566–9.
Menzel P, Ng KL, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun. 2016;7:11257.
Wu YW, Simmons BA, Singer SW. Maxbin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32(4):605–7.
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–55.
Yan J, Im J, Yang Y, Löffler FE. Guided cobalamin biosynthesis supports Dehalococcoides mccartyi reductive dechlorination activity. Philos Trans R Soc Lond B Biol Sci. 2013;368(1616):20120320.
Sokolova TG, Kostrikina NA, Chernyh NA, Kolganova TV, Tourova TP, Bonch-Osmolovskaya EA. Thermincola carboxydiphila gen. nov., sp. nov., a novel anaerobic, carboxydotrophic, hydrogenogenic bacterium from a hot spring of the Lake Baikal area. Int J Syst Evol Microbiol. 2005;55(Pt 5):2069–73.
Zavarzina DG, Sokolova TG, Tourova TP, Chernyh NA, Kostrikina NA, Bonch-Osmolovskaya EA. Thermincola ferriacetica sp. nov., a new anaerobic, thermophilic, facultatively chemolithoautotrophic bacterium capable of dissimilatory Fe(III) reduction. Extremophiles. 2007;11(1):1–7.
Breznak JA. The genus Sporomusa. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes: volume 4: Bacteria: Firmicutes, Cyanobacteria. New York: Springer, US; 2006. p. 991–1001.
Najafpour GD. Chapter 3 - Gas and liquid system (aeration and agitation). In: Najafpour GD, editor. Biochemical engineering and biotechnology. Amsterdam: Elsevier; 2007. p. 22–68.
Arantes AL, Moreira JPC, Diender M, Parshina SN, Stams AJM, Alves MM, et al. Enrichment of anaerobic syngas-converting communities and isolation of a novel carboxydotrophic Acetobacterium wieringae strain JM. Front Microbiol. 2020;11:58.
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57(1):81–91.
Robb F, Techtmann S. Life on the fringe: microbial adaptation to growth on carbon monoxide [version 1; peer review: 3 approved]. F1000Res. 2018;7:1981.
Oelgeschläger E, Rother M. Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch Microbiol. 2008;190(3):257–69.
Esquivel-Elizondo S, Maldonado J, Krajmalnik-Brown R. Anaerobic carbon monoxide metabolism by Pleomorphomonas carboxyditropha sp nov, a new mesophilic hydrogenogenic carboxydotroph. FEMS Microbiol Ecol. 2018;94(6). https://doi.org/10.1093/femsec/fiy056.
Esquivel-Elizondo S, Delgado AG, Krajmalnik-Brown R. Evolution of microbial communities growing with carbon monoxide, hydrogen, and carbon dioxide. FEMS Microbiol Ecol. 2017;93(6). https://doi.org/10.1093/femsec/fix076.
Akhlaghi N, Najafpour-Darzi G. A comprehensive review on biological hydrogen production. Int J Hydrogen Energ. 2020;45(43):22492–512.
Lide DR. Handbook of chemistry and physics. 84th ed. Boca Raton: LLC: CRC Press; 2004. p. 2616.
Chau ATT, Lee M, Adrian L, Manefield MJ. Syntrophic partners enhance growth and respiratory dehalogenation of hexachlorobenzene by Dehalococcoides mccartyi strain CBDB1. Front Microbiol. 2018;9:1927.
Chen G, Kara Murdoch F, Xie Y, Murdoch Robert W, Cui Y, Yang Y, et al. Dehalogenation of chlorinated ethenes to ethene by a novel isolate, “Candidatus Dehalogenimonas etheniformans”. Appl Environ Microbiol. 2022;88(12):e00443-e522.
Cui Y, Li X, Yan J, Lv Y, Jin H, Wang J, et al. Dehalogenimonas etheniformans sp. nov., a formate-oxidizing, organohalide-respiring bacterium isolated from grape pomace. Int J Syst Evol Micr. 2023;73(5). https://doi.org/10.1099/ijsem.0.005881.
Köpke M, Held C, Hujer S, Liesegang H, Wiezer A, Wollherr A, et al. Clostridium ljungdahlii represents a microbial production platform based on syngas. PNAS. 2010;107(29):13087–92.
Diender M, Pereira R, Wessels HJ, Stams AJ, Sousa DZ. Proteomic analysis of the hydrogen and carbon monoxide metabolism of Methanothermobacter marburgensis. Front Microbiol. 2016;7:1049.
Ragsdale SW, Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta Gen Subj. 2008;1784(12):1873–98.
Qiao W, Liu G, Li M, Su X, Lu L, Ye S, et al. Complete reductive dechlorination of 4-hydroxy-chlorothalonil by Dehalogenimonas populations. Environ Sci Technol. 2022;56(17):12237–46.
Duhamel M, Wehr SD, Yu L, Rizvi H, Seepersad D, Dworatzek S, et al. Comparison of anaerobic dechlorinating enrichment cultures maintained on tetrachloroethene, trichloroethene, cis-dichloroethene and vinyl chloride. Water Res. 2002;36(17):4193–202.
Duhamel M, Edwards EA. Microbial composition of chlorinated ethene-degrading cultures dominated by Dehalococcoides. FEMS Microbiol Ecol. 2006;58(3):538–49.
Zhao S, Ding C, Xu G, Rogers MJ, Ramaswamy R, He J. Diversity of organohalide respiring bacteria and reductive dehalogenases that detoxify polybrominated diphenyl ethers in E-waste recycling sites. ISME J. 2022;16(9):2123–31.
Shin H-S, Youn J-H, Kim S-H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int J Hydrogen Energ. 2004;29(13):1355–63.
Hoehler TM, Alperin MJ, Albert DB, Martens CS. Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim Cosmochim Acta. 1998;62(10):1745–56.
Conrad R, Seiler W. Contribution of hydrogen production by biological nitrogen fixation to the global hydrogen budget. J Geophys Res Oceans. 1980;85(C10):5493–8.
Bellenger JP, Darnajoux R, Zhang X, Kraepiel AML. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: a review. Biogeochemistry. 2020;149(1):53–73.
Mazur CS, Jones WJ, Tebes-Stevens C. H2 consumption during the microbial reductive dehalogenation of chlorinated phenols and tetrachloroethene. Biodegradation. 2003;14(4):285–95.
Luijten ML, Roelofsen W, Langenhoff AA, Schraa G, Stams AJ. Hydrogen threshold concentrations in pure cultures of halorespiring bacteria and at a site polluted with chlorinated ethenes. Environ Microbiol. 2004;6(6):646–50.
Lu XX, Tao S, Bosma T, Gerritse J. Characteristic hydrogen concentrations for various redox processes in batch study. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2001;36(9):1725–34.
Mayer-Blackwell K, Azizian MF, Green JK, Spormann AM, Semprini L. Survival of vinyl chloride respiring Dehalococcoides mccartyi under long-term electron donor limitation. Environ Sci Technol. 2017;51(3):1635–42.
Wawrik CB, Callaghan AV, Stamps BW, Wawrik B. Genome sequence of Youngiibacter fragilis, the type strain of the genus Youngiibacter. Genome Announc. 2014;2(1):e01183-13. https://doi.org/10.1128/genomea.01183-13.
Galushko A, Kuever J. Desulfocurvibacter. In; Bergey’s manual of systematics of archaea and bacteria. 2020. p. 1–6.
Wu K, Dai L, Tu B, Zhang X, Zhang H, Deng Y, et al. Gudongella oleilytica gen. nov., sp. nov., an aerotorelant bacterium isolated from Shengli oilfield and validation of family Tissierellaceae. Int J Syst Evol Microbiol. 2020;70(2):951–7.
Imachi H, Sakai S, Nagai H, Yamaguchi T, Takai K. Methanofollis ethanolicus sp. nov., an ethanol-utilizing methanogen isolated from a lotus field. Int J Syst Evol Microbiol. 2009;59(4):800–5.
Honda T, Fujita T, Tonouchi A. Aminivibrio pyruvatiphilus gen. nov., sp. nov., an anaerobic, amino-acid-degrading bacterium from soil of a Japanese rice field. Int J Syst Evol Microbiol. 2013;63(Pt_10):3679–86.
Grabowski A, Tindall BJ, Bardin V, Blanchet D, Jeanthon C. Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int J Syst Evol Microbiol. 2005;55(3):1113–21.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2023YFE0122000), National Natural Science Foundation of China (Grant No. 42177220 and 42377133), Key Research Program of Frontier Sciences, Chinese Academy of Sciences (Grant No. ZDBS-LY-DQC038), Natural Science Foundation of Liaoning Province of China (Grant No. 2021-MS-026), Zhiyuan Science Foundation of BIPT (Grant No.2023004), and Major Program of Institute of Applied Ecology, Chinese Academy of Sciences (Grant No. IAEMP202201).
Author information
Authors and Affiliations
Contributions
JJW designed and conducted the experiments, analyzed and interpreted the data, and wrote the initial draft and revisions of the manuscript. XYL conducted experiments, analyzed and interpreted data, acquired funding, and contributed to manuscript revisions. HJJ analyzed and interpreted data and contributed to manuscript revisions. SJY, LY, HYW, SQH, HYL, and XHW conducted experiments and contributed to data analysis and interpretation. JY provided resources and supervised experiments, data analysis, and interpretation, as well as reviewed and edited the manuscript. YY provided resources, oversaw experiments, acquired funding, analyzed and interpreted data, and revised and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
40168_2024_1869_MOESM1_ESM.docx
Additional file 1: Figure S1. Microcosm setup and transferred enrichment cultures. Trichloroethylene (TCE) was dechlorinated in enrichment cultures fed with acetate and CO (represented by the royal blue bottles). Similarly, TCE dechlorination occurred in CO-fed enrichment cultures with bicarbonate-buffered medium (represented by the green bottles) and HEPES-buffered medium (represented by the orange bottles). Conversely, in acetate-fed enrichment cultures without CO or H2 supplementation (represented by the yellow bottles), the TCE dechlorination process did not occur, denoted by the symbol “X”, signifying the inability to dechlorinate. Figure S2. Reductive dechlorination of TCE in CO plus acetate enrichment cultures from transfer 1 to transfer 3. Approximately 33 μmol of TCE was dechlorinated to ethene over 100 days in transfer 1 cultures fed with acetate and CO (A). In transfer 2 cultures, 33 μmol of TCE was dechlorinated to ethene within 30 days, and an additional 33 μmol of TCE was dechlorinated within 25 days (B). In transfer 3 cultures, 33 μmol of TCE was dechlorinated to ethene over 50 days (C). Red arrows indicate CO additions, with each dose amounting to 2 mL. Figure S3. Reductive dechlorination of TCE in CO-fed enrichment cultures from transfer 2 to transfer 4. Approximately 33 μmol of TCE was dechlorinated to VC over 120 days in transfer 2 cultures (A). In transfer 3 cultures, 33 μmol of TCE was dechlorinated to VC with a small amount of ethene in 80 days (B). In transfer 4 cultures, 33 μmol of TCE was dechlorinated to ethene over 160 days (C). Red arrows indicate CO additions, with each dose amounting to 2 mL. Figure S4. Phylogenetic tree constructed based on the amino acid sequences of 43 RDases, with branches calculated from 500 bootstrap iterations. Functional assignments of these RDases were determined through biochemical characterization, expression analysis, or phylogenetic inference, representing a diverse array of OHRB. The tree also includes 20 RDases annotated from the draft genome of strain CO, highlighted in blue branches. Figure S5. Phylogeny of Acetobacterium based on genome sequences. The tree was constructed using the maximum-likelihood method, with GenBank accession numbers provided in parentheses. Bootstrap values, derived from 1,000 resamplings, are indicated at branching points. Figure S6. Phylogenetic tree based on protein sequences of 63 carbon monoxide dehydrogenases (CODHs) from diverse bacteria and methanogens (archaea). CODHs from methanogens are indicated with a blue background, those from Acetobacterium with a green background, and CODHs identified in CO enrichment cultures are highlighted in blue. Figure S7. Proposed model for interspecies interactions supporting TCE-to-ethene dechlorination by Dehalococcoides with CO as electron donor and carbon source. Hydrogenogenic carboxydotrophs (e.g., Methanosarcina) oxidize CO, generating H2. This H2 is then transferred to Acetobacterium, which in turn produces acetate. Both acetate and H2 are subsequently utilized by Dehalococcoides for reductive dechlorination of TCE to ethene. Additionally, Methanosarcina can use acetate and H2 to produce methane. Table S1. The average nucleotide identity (%) and digital DNA-DNA hybridization (%) of strain CO with other strains in the genus of Dehalococcoides. Table S2. RDases with assigned functions and their host OHRB used in Figure S4. Table S3. The average nucleotide identity (%) and digital DNA-DNA hybridization (%) of strain Z1 with other strains in the genus of Acetobacterium. Table S4 Metagenome-assembled genomes recovered from CO-fed TCE-dechlorinating enrichment cultures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Li, X., Jin, H. et al. CO-driven electron and carbon flux fuels synergistic microbial reductive dechlorination. Microbiome 12, 154 (2024). https://doi.org/10.1186/s40168-024-01869-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40168-024-01869-y