Abstract
Minimal residual disease (MRD) is considered the strongest relevant predictor of prognosis and an effective decision-making factor during the treatment of hematological malignancies. Remarkable breakthroughs brought about by new strategies, such as epigenetic therapy and chimeric antigen receptor-T (CAR-T) therapy, have led to considerably deeper responses in patients than ever, which presents difficulties with the widely applied gold-standard techniques of MRD monitoring. Urgent demands for novel approaches that are ultrasensitive and provide sufficient information have put a spotlight on high-throughput technologies. Recently, advances in methodology, represented by next-generation sequencing (NGS)-based clonality assays, have proven robust and suggestive in numerous high-quality studies and have been recommended by some international expert groups as disease-monitoring modalities. This review demonstrates the applicability of NGS-based clonality assessment for MRD monitoring of B-cell malignancies by summarizing the oncogenesis of neoplasms and the corresponding status of immunoglobulin (IG) rearrangements. Furthermore, we focused on the performance of NGS-based assays compared with conventional approaches and the interpretation of results, revealing directions for improvement and prospects in clinical practice.
Similar content being viewed by others
Introduction
The minimal residual disease (MRD) level achieved at the end of induction/consolidation therapy is a recognized important factor for risk stratification and relapse prediction in several hematological malignancies, such as acute lymphoblastic leukemia (ALL) [1, 2], multiple myeloma (MM) [3, 4], mantle cell lymphoma (MCL) [5, 6], follicular lymphoma (FL) [7], diffuse large B-cell lymphoma (DLBCL) [8], and chronic lymphocytic leukemia (CLL) [9]. Significantly better recovery and survival outcomes in patients with negative MRD at certain time points were observed in multiple studies [10,11,12]. Nevertheless, relapses among patients with negative MRD confirmed by conventional methods occur, especially among patients treated with novel strategies such as CAR-T therapies and lack preliminary MRD data [13], indicating the importance of assessing the required depth, ideal test sensitivity, and proper definition of MRD negativity. MRD negativity is usually defined as less than 1 tumor cell in 100,000 bone marrow (BM) cells (1 × 10–5); however, recent evidence suggests that prolonged progression-free survival (PFS) and/or overall survival (OS) outcomes are observed when the threshold for MRD negativity is changed to 1 × 10–6 [14].
The traditional, widely applied methods used for MRD monitoring mainly include polymerase chain reaction (PCR)-based strategies (e.g., allele-specific oligonucleotide-real time quantitative PCR, ASO-RQ-PCR) and immunophenotype-based strategies (e.g., multicolor flow cytometry, MFC) with sensitivities of approximately 1 × 10–5 [11] and 1 × 10–4 [15], respectively. Imaging techniques (e.g., positron emission tomography-computed tomography, PET-CT) are generally less sensitive than MRD-based approach [16]. The advent of next-generation flow cytometry (NGF), which outperforms conventional MFC by the optimized combination of fluorochromes and antibody reagents, further improves the sensitivity of MRD detection to 2 × 10–6 [17]. Another alternative approach developed on the basis of clonality assessment through IG V(D)J rearrangements, NGS, emerged in parallel, exhibiting even higher sensitivity up to 1 × 10–6 and providing more substantial genetic-level information [18, 19].
Compared with other MRD monitoring methods, the NGS-based IG clonality approach possesses substantial potential for a wide range of applications due to the distinct features of the technique, such as the comprehensiveness of the information gained, the ability to recognize clonal evolution, and the ability to standardize the workflow [20,21,22,23]. Furthermore, NGS-based is recommended in the NCCN Guidelines for MRD monitoring for ALL, CLL, and MM, indicating considerable market prospects. However, the relatively insufficient data from clinical trials and the lack of systematic summaries on the working principles and the scope of application have seriously impeded the promotion of IG NGS-based MRD monitoring. This review demonstrates the feasibility and reasonability of the IG NGS approach applied for MRD detection of B-cell malignancies from multiple perspectives, including the distinct features of IG rearrangements in different neoplasms and the interpretation of corresponding IG NGS results, a comparison of the performance of present MRD methods and the unique advantages of NGS-based methods, and, most importantly, a summary of the current clinical studies involving NGS-based MRD monitoring, highlighting translational medicine applicability and the use of high-throughput technology in clinical practice.
The rearrangement of Ig genes in normal B cells
During the process of maturation, the immunoglobulin genes in normal B cells undergo a process referred to as V(D)J recombination to produce a unique receptor (B-cell receptor, BCR) for combination with its specific antigen (Additional file 1 Fig. S1). At chromosome 14 (14q32.33), the IGHV, IGHD, and IGHJ segments of the immunoglobulin heavy chain (IGH) gene are rearranged in an ordered fashion, while at chromosome 2 (2p11.2), IGKV and IGKJ or IGLV and IGLJ (chromosome 22:22q11) undergo the same phenomenon but at a later time [24]. The whole process is completed through by precisely controlled enzymatic machinery mediated by the interaction of rag (coded by recombination activating gene, RAG) with recombination signal sequence (RSS) motifs located near the V(D)J segments and the subsequent splice site [25]. During the pro-B-cell stage, D to JH recombination precedes VH to DJH with deletion of the intervening gene segments, ultimately producing an intact and unique variable (V) region. The rearrangement events that occur at the light chain locus are much more sophisticated, involving both deletions and inversions due to the participation of IGKV(D) clusters, which are located upstream of IGKV clusters and have similar sequences and opposite-orientation RSSs [26,27,28].
Based on previous studies [29], the principles of V(D)J rearrangements, also designated allelic exclusion, were analyzed and summarized (Fig. 1). The rules are implemented as follows: (1) the IGH gene segments must rearrange to produce a functional V region that can successfully pass the in-frame selection. (2) The nonproductive IGH rearrangements are inactivated in parallel with the initiation of the second rearrangement at another allele. (3) Rearrangements of IGK gene segments occur after the appropriate IGH rearrangement, while failed IGH rearrangements occurring in both alleles result in apoptosis. (4) A second rearrangement can occur in either allele when the first IGK fails to produce a functional result. (5) The nonproductive IGK rearrangements are inactivated by the deletion of intervening DNA sequences through either Kde-IGKJ or intron-Kde recombination [30]. (6) Rearrangements of IGL gene segments occur after failed IGK rearrangements in both alleles, while productive IGK recombination tends to leave the IGL gene in its germline configuration [31, 32]. The successful expression of IgH marks the transition from pro-B cells to pre-B cells, in which IgH combines with a surrogate light chain to form a pre-BCR, activating in-frame selection events and light chain rearrangements. Following somatic hypermutation (SHM) and class-switch recombination (CSR) in the germinal center (GC), immature B cells from the BM are converted into mature B cells equipped with antigen specific, high-affinity, and unique BCRs [33] and then differentiate into either plasma cells (PCs) or memory B cells [34, 35] (Fig. 1).
Summary of the allelic exclusion theory and the normal B cell differentiation process. A Heavy chain rearrangement precedes light chain rearrangement, and recombination of the IGK segment precedes IGL. SHM and CSR occur in GC after successful Ig rearrangement to produce mature BCR. B Maturation of B cells from hematopoietic stem cells to mature B cells with class-switched BCR (IgA/IgG) through Ig rearrangement and BCR signaling
Targets utilized for MRD monitoring by NGS
The high diversity of the BCR repertoire results from nearly infinite combinations of V(D)J gene segments. The 123–129 VH gene segments, of which 38–46 segments are functional and 36 are considered pseudogenes, can be approximately grouped into 7 families (VH1–VH7) [36]. A total of 27 D gene segments and 9 JH segments comprise 23 and 6 functional components, respectively [37]. The possible VH/D/JH combinations and SHM that occur in hotspots endow the complementarity determining regions 3 (CDR3) of BCRs with the ability to interact with distinct antigen epitopes and serve as a fingerprint when recognizing and tracking the specific B-cell [12]. Similar processes occur at the IGK or IGL locus, within which 34–38 and 29–33 functional segments can be selected from a total of 76 Vκ and 73–74 Vλ genes, respectively [38]. Recombination in the constant region of the IG gene initiates during CSR in the GC environment [39]. By simply deleting the intervening DNA sequence between the S region of Cμ and another constant component, the IgM isotype of BCR is converted into a mature IgA or IgG isotype [39].
During adaptive immune responses, B cells react to antigenic stimulation and rapidly proliferate, forming clones with the same V(D)J pattern and possible intraclonal diversity at the nucleotide level attributed to SHM. Similarly, after malignant transformation at a certain time point during B cell differentiation triggered by either activation of oncogenes or inactivation of tumor suppressor genes, B-lineage cancer cells carrying the same complete V(D)J or incomplete DJ rearrangement unlimitedly multiply with possible subclone characteristics caused by ongoing SHM or ongoing V(D)J recombination, respectively [40,41,42]. Given that the rearranged IG gene is unique and the quantification of these specific sequences will dramatically increase to a level far beyond the background of the normal IG gene repertoire when malignant cells proliferate, it is convincing and feasible to consider the IG V(D)J rearrangement pattern as an alternative for both clonality assessment of diagnosis and targets of MRD monitoring.
The oncogenesis of B-lineage malignancies and the corresponding status of IG rearrangements
The deletion–recombination reactions of V(D)J rearrangement, SHM, and CSR require a double-strand break (DSB) at a specific locus, introducing potential aberrant translocation events that can serve as distinguishing signs in fluorescence in situ hybridization or IG-based clonality assessment [43,44,45,46]. Characteristics associated with the abnormal IG repertoire, such as biased V-J usage [40, 47], stereotyped CDR3 [48,49,50,51,52], the tendency to mutate frequently [18, 45, 53] or retain germline configuration [40, 54, 55], ongoing SHM [56,57,58] or lack of intraclonal diversification [46], were further confirmed in many hematology studies based on the sequencing of IG genes. Detailed information is exhibited in Table 1 and Fig. 2 to demonstrate the interpretation of sequence analysis of different B-linage malignancies.
Schematic representation of the oncogenesis of B-lineage malignancies. The t(14;18)(q32;q21) rearrangement caused by aberrant D-JH recombination during the pro-B-cell stage plus the acquisition of N-gly sites during the SHM period ultimately leads to FL. The blockade at the pre-B-cell stage to the immature B-cell stage in parallel with the ongoing recombination events promotes the development of ALL. MCL originates from immature B cells with t(11;14)(q13;q32). GCB-DLBCL is transformed from B cells under continuing antigenic pressures in GC characterized by ongoing SHM or is transformed from FL, while the non-GCB subtype originates from plasma cells or memory-like B cells that have completed the GC reaction. MM is caused by an aberrant translocation involving the IGH locus (14q32), which occurs during V(D)J recombination, SHM or CSR. HL derives from surviving cells that escape from apoptosis caused by unfavorable mutations by the activation of oncogenes. N-gly sites, asparagine-X-serine/threonine sites
IG gene rearrangement pattern in ALL
ALL is considered to originate from pre-B cells, which are aberrantly blocked at the transition to immature B cells. This mechanism explains the unmutated or low-mutated status due to lack of SHM, the high frequency of unproductive IGH rearrangements due to continuously active recombinase enzyme, and the initiation of IGK/IGL rearrangements that go against allelic exclusion rules due to improper in-frame selection [40, 41, 47, 59]. Clonal evolution can’t be ignored in ALL and likely occurs by continuing rearrangement processes (successive VH to DJH or secondary rearrangements) [40, 41] and selection pressure mediated by treatments [60]. Measurements of the IG gene repertoire exhibited biased VH usage toward VH3 and VH1 families, most frequently involving the VH6-1, VH1-2, VH3-11, VH3-13, and VH3-15 segments. D2 and D3 families were overrepresented, in which the D2-21 segment was the most frequent. JH4 and JH6 were more frequently selected in JH families. In the Vκ family, Vκ1 and Vκ2 are preferentially used [40, 47]. Unfortunately, there is no obvious evidence indicating an association between IG gene characteristics and prognosis, but this conclusion is not yet validated due to the lack of large-scale studies.
IG gene rearrangement pattern in MCL
The origins of conventional MCL (cMCL) cells and nonnodal MCL (nnMCL) cells are believed to be naïve mature B cells and memory-like B cells, respectively [44, 55]. The cMCL subtype generally exhibits an unmutated or minimally mutated CDR3 region [54]. The core mechanism in the malignant transformation of MCL has been demonstrated to be a translocation involving chromosomes 11 and 14 (t(11;14)(q13,32), CCND1/IGH), leading to the overexpression of CCND1 protein [5, 61, 62], while other cases may be driven by a CCND2 or CCND3 translocation with IGK or IGL [63]. Aberrant translocation events in MCL can occur in V(D)J recombination, SHM, or CSR based on the DSBs formed during these processes [44]. Similarly, a biased VH-D-JH repertoire has been observed, marked by the preferential use of the VH3, VH4, D3, D6, JH4, and JH6 families. In the VH repertoire, VH3-21, VH3-23, VH4-34, VH1-8, and VH4-59 were most frequently used, while in the D repertoire, D3-22 and D3-3 were overrepresented. The Vλ1, Vλ2, Vλ3, and Vκ3 families account for the highest proportion of the Vλ and Vκ repertoires, in which the Vλ2-14 and Vκ3-20 were the most frequent components. Stereotyped CDR3 regions of both heavy chain (HCDR3) and light chain (LCDR3) were addressed in MCL. The phenomenon of the narrowing of the VH repertoire and the occurrence quasi-identical receptors due to the involvement of a limited set of antigens in the development of lymphomas is referred to as “stereotyped” [51]. Several stereotyped forms of V(D)J combinations have been discovered and described, including VH4-34/D2-2/JH6, VH3-21/D3-9/JH6, VH3-21/D6-6/JH6 + Vλ3-19/Jλ2, Vλ3-19/Jλ2-1, Vλ2-14/Jλ2-1, and Vκ3-10/Jκ2-1 [51, 54, 55]. These features endow the MCL group with good suitability for IG-based clonality assessment.
IG gene rearrangement pattern in CLL
Opinions on the origin of CLL cells are widely divided due to the existence of unmutated (U-CLL) and mutated (M-CLL) subtypes, classified by the level of SHM with a cutoff value of 2% [48, 49]. Currently accepted theories include transformation from immature B cells and B cells at an early stage of SHM, possibly resulting in U-CLL with more aggressive behavior, B cells exhibiting SHM in M-CLL with more benign features, or transformation from marginal zone B cells in both subtypes [64, 65]. SHM in CLL is concentrated in hotspots with limited intraclonal diversification, indicating the absence of an ongoing SHM process [52]. The VH1, VH3, and VH4 gene families were more preferentially used, in which the VH1-69, VH4-34, VH3-23, VH3-30, and VH1-2 segments were observed to be the most frequent [51]. The most striking characteristic of CLL is the high proportion of clones with stereotyped BCRs represented by VH1-69/D3-16/JH3 + VκA27, VH1-69/D3-3/JH6 + Vλ3-9, VH3-21/D3-3/JH6 + Vλ2-14, VH3-21 + Vλ3-21, VH4-39/D6-13/JH5 + Vκ(D)1–39, etc. BCRs involved with VH1-69 possess longer and unmutated CDR3, while BCRs involved in VH3-21 possess a shorter and less mutated CDR3 [48,49,50,51,52]. CLL cases with stereotyped and nonstereotyped BCRs may undergo different oncogenetic trajectories. Most major subsets of stereotyped BCR in CLL were unmutated with high conservation across the entire HCDR3. Moreover, studies have confirmed the coexistence of satellite subsets, defined by a small quantity and high similarity with consistent clinical profiles with major subsets [66]. Measurements of the IG gene repertoire of CLL have been demonstrated to have a certain value in diagnosis and prognosis prediction.
IG gene rearrangement pattern in DLBCL
DLBCL, not otherwise specified (DLBCL, NOS), is classified into two distinct groups, the germinal center B-cell-like (GCB) subtype and non-GCB subtype, characterized by different cells-of-origin (COO) and responses to chemotherapies or targeted therapies [67]. Both subtypes of DLBCL display established features of canonical SHM, while GCB cases exhibit ongoing SHM associated with poorer survival, and non-GCB were considered mutated [48, 53, 56, 68]. High rates of SHM in DLBCL were verified in both the HCDR3 region and the LCDR3 region of BCR, with a worse prognosis in the former case and a better prognosis in the latter case [56]. SHM can also occur in the framework regions (FR), requiring complexity in the design of sequencing primers [69]. Moreover, Kikuchi et al. demonstrated that the overexpression of BACH2 was critical for ongoing SHM of HCDR3, and this phenomenon occurred more frequently in the GCB subtype, which further helped to decipher the molecular mechanism and its link to clinical behaviors [70]. Assessment of the IG gene repertoire showed more frequent use of VH1 and VH3, followed by VH4 and VH2. VH1-2, VH4-34, VH3-23, VH4-39, VH1-69, VH5-51, and VH3-21 segments were preferentially selected in DLBCL with a biased distribution in two COO subtypes, highlighted by the clustered highly mutated VH4-34 segments in the non-GCB subtype and VH3-21 segments more frequently used in unmutated cases [53, 69]. The D2, D3, JH4, and JH6 gene families were overrepresented, and D3-22 and D3-10 outnumbered the other segments. The D2 gene family and D2-2 segment were more frequently used in incomplete D-JH rearrangements. Evidence supports a stereotyped HCDR3 region in DLBCL, including VH1-69/D3-10/JH6, VH1-69/D3-3/JH6, and VH4-34/D3-22/JH2, summarized by Sebastián et al. [53, 56]. Clonally related relapses resulting from clonal evolution in DLBCL can be intricate, operating in either early-divergent mode or late-divergent mode, both proven clonally related as evidenced by the same V(D)J rearrangement. The early-divergent mode was named according to the behavior of the preexisting, chemoresistant subclones diverging early and developing in parallel with the major clone, characterized by more SHM sites in the relapse sample than in the diagnostic sample. The late-divergent mode is considered to occur at a later period of oncogenesis with subclones derived directly from the major clone that have fewer differences in SHM sites and number. However, there was no significant correlation between DLBCL subtypes and evolution-relapse mode. Several studies have also noted the selection pressure between remission and relapse, such as that resulting from treatments and antigens [68, 71,72,73]. Other IG repertoire-associated factors influencing the prognosis included monoclonality, the length of the HCDR3, and the abnormal ratio of functional IGK/IGL rearrangements [56, 69, 74, 75].
IG gene rearrangement pattern in FL
FL cells originate from GC B cells, marked by t(14;18) (q32;q21) (IGH/BCL2), resulting in the overexpression of BCL2 [76]. Recently, several studies have stressed the concept of in situ FL, an intermediate stage between FL and normal t(14;18) B cells, as an origin of FL [45]. The acquisition of asparagine-X-serine/threonine (N-gly) sites in the IGHV region has been recognized as one of the early initiating events of FL pathogenesis and a stable, conserved, and essential hallmark for the survival, proliferation, and dissemination of FL cells [45, 77]. The CDR3 of either IGH or IGL in FL cells is highly mutated, with significant intraclonal diversity caused by continuous exposure to GC [45, 57, 76, 78, 79]. Similar to the other B-lineage malignancies mentioned above, the VH3, VH4, and VH1 gene families were more frequently used in FL, in which VH1-18, VH3-48, VH3-15, VH3-34, VH3-23, VH3-30, and VH3-21 were preferentially selected. D3-10, D3-22, and D3-3 accounted for most of the D2 and D3 gene families used in FL. JH4 was the most frequent JH component [58, 69, 80]. Interestingly, FL can transform into other more aggressive malignancies, usually DLBCL. The transformed FL exhibits a clonal relationship with the original FL and changes through histological transformation, involving continuous BCR signaling, possibly associated with the overrepresented VH3 gene family, especially the VH3-48 segment [80]. Additionally, FL-transformed DLBCL tends to occur through a divergent pattern from the common progenitor rather than via direct linear evolution [73].
IG gene rearrangement pattern in MM
Compared with other B-lineage lymphomas, MM possesses more stable properties at the molecular level due to its origin from plasma cells, which have completed the GC reaction and consequently exhibit more mature features. CDR3 regions of either heavy chain or light chain in MM cells are highly mutated without intraclonal variation, while most MM secretes class-switched Igs, indicating the initiation of pathogenesis at the relatively late phase of the GC reaction [46, 79, 81, 82]. A higher level of SHM was associated with better OS outcomes [18]. The ontogeny of MM was also demonstrated to possibly result from translocation events involving the IGH gene (14q32) caused by aberrant V(D)J recombination, CSR, or SHM [43]. The IG repertoire, the relationship between Ig secretion, and the corresponding V(D)J recombination patterns in MM resemble normal cells [18, 83]. The VH3, VH4, and VH1 gene families are more frequently used, and VH3-30, VH3-23, VH3-9, VH4-31, VH1-69, and VH5-51 are the most frequent. Interestingly, the autoreactivity-associated component, the VH4-34 segment, is completely excluded from the IG repertoire of MM, indicating an intrinsic anti-autoimmunity quality. The D3-10 segment in the overrepresented D2 and D3 families occurs most frequently. JH4 and JH6 are again preferentially used in MM [79, 83]. In the IGK/IGL repertoire, Vκ1, Vκ2, Vκ3, Jκ4, Jκ2, Jλ2, and Jλ3 are more frequently chosen, while no clear preference for Vλ segments was observed. Vκ2-30 and Vκ1(D)-33 account for a sizeable portion of the Vκ repertoire [46, 83]. More IGK rearrangements, including Kde-mediated deletions, and less SHM in the IGKV regions, were demonstrated in λ-restricted cases than in κ-restricted cases, consistent with normal allelic exclusion [14, 84]. Clonal evolution in MM is rare, indicated by the stability of dominant sequences identified at diagnosis over time [18]. MRD monitoring of MM through IGH-based clonality assessment is feasible due to disease progression without variation at the molecular level, and the sensitivity could be further improved by the addition of IGK panels [83].
MRD monitoring through clonality assessment by NGS
Because almost all B-lineage malignancies have distinct and stable V(D)J recombination patterns, the BIOMED-2 protocol was first designed by a European BIOMED-2 collaborative study as a PCR-based technology for routine clonality diagnostics [85]. This classical method relies on multiplex PCR with 97 standardized primers designed for amplification of different Ig/TCR gene segments, and the PCR products can be analyzed for clonality assessment by heteroduplex analysis or GeneScanning [29]. High-throughput sequencing techniques have been rapidly developed and upgraded. The Lymphotrack assay was established by combining the basic strategy of BIOMED-2 and NGS technology. After input, the compatible FASTQ files can be processed into fully analyzed data by the corresponding application Lymphotrack DataAnalysis [86]. Productive rearrangements were further analyzed for parse, re-organization, and exportation using algorithms. In this process, a clonotype was established in the tumor sample using locus-specific primer sets for IGH-V, -D, and -J rearrangements and the Miseq Illumina platform. The output form of results was then further analyzed based on the international ImMunoGeneTics (IMGT) information system to identify the exact V–D–J sequence and the corresponding frequency. Information was ultimately reported as Ig gene repertoires, VH CDR3 length, exact amino acid sequence and frequency of SHM. Generally, a clonotype with a frequency of higher than 5% of all rearranged V(D)J sequences were identified as a malignant clone. The malignant clone with the highest frequency in the baseline sample was named the “index” clone, and was tracked in the follow-up samples for the MRD measurement. Standard and automated data processing can be performed easily, locally and securely, making it feasible in most laboratories. In addition, a similar product, ClonoSeq from Adaptive Biotechnologies [87], has already been approved by the FDA for MRD tracking in ALL and MM.
Considering the abundance of information produced by the NGS IG method, it is plausible and practical to apply this strategy for clonality assessment in diagnosis at baseline and MRD monitoring follow-up assessments.
Importance of MRD monitoring in clinical practice
The variance of clinical remission (CR) among different regimens and diseases has revealed the limitation of the current definition by clinical manifestation and imaging tests, raising a claim for a more stringent version. MRD is generally acknowledged as one of the most powerful approaches for the prediction of relapse and prognosis. MRD-positive patients have far less favorable event-free survival outcomes than MRD-negative patients [10,11,12, 15, 22, 88,89,90,91]. The prognostic value of MRD among patients undergoing specific treatments is mainly reflected in the dynamic risk-stratification ability. Moreover, by serially monitoring the clearance of tumor cells in the BM or PB during and after chemotherapy or novel immunotherapies, the modulation of treatment duration and intensity can be prompted and executed by either early termination/intensification of treatment in patients who remain MRD-positive or after interruption of continuous treatment in patients who become MRD-negative. Measurement of MRD at the end of therapy also assisted in identifying cured or optimally treated patients, in turn providing an evaluation of specific therapeutic effects [61, 92]. Methods for the effective application and integration of MRD monitoring in clinical practice for relapse prediction were established on the essential premise that (1) molecular relapse precedes clinical relapse by a time interval long enough for intervention and (2) instant intervention initiated during the lead time influences the outcome and results in a better prognosis [61].
However, MRD monitoring is not widely available in lymphoma care despite the benefits shown in therapeutic outcomes and scientific research. Several obstacles have impeded the improvement of MRD and the ability to obtain feedback from real experiences, including the lack of incorporation of MRD monitoring in prospective trials involving novel treatment, the relatively high cost of high-throughput sequencing per sample, the long delays between technical evolution and the ultimate outcome (e.g., OS), the labor and special care required in the trial and the complex statistics required for analysis [61]. Furthermore, the conclusion inferred from MRD monitoring can be obscure due to the nonuniform standard of MRD negativity across laboratories. MRD negativity is typically defined as the absence of clonal malignant cells in BM aspirates with a minimum sensitivity of 1-cell in ≥ 105 nucleated cells [10, 22]. However, recent studies provided evidence for more OS and PFS benefits and better relapse prediction capabilities when a threshold of 1 × 10–6 is used [14, 93, 94]. A more sensitive, repeatable, and multifunctional method for MRD monitoring is urgently needed.
The process of IG NGS-based clonality assessment in MRD monitoring
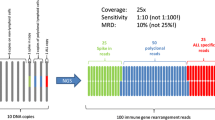
The complete process of IG-based diagnosis and MRD monitoring by NGS in lymphoma is shown in Fig. 3 B. After the initial diagnosis of B-lineage malignancy was confirmed by clinical symptoms, imaging manifestations, and histopathological examinations, the BM aspirate samples were preserved and subjected to high-throughput sequencing. Index clones were identified in these samples by the following criteria: (1) the proportion of index clones needs to be at least 3% of all sequences at the specific locus, (2) the frequency of cells that carry index clones needs to be at least 0.2% of all nucleated cells [5], and (3) other criteria in kits designed by different companies [14]. By using the algorithm of exact match and up to 2-bp mismatches, the disease clones in follow-up samples were compared with the initial index clones in diagnostic samples, based on which the presence or absence of MRD was identified, and the quantity of MRD was calculated [18]. By analyzing the IG repertoire in the sequenced sample, including V–J usage and SHM levels, the disease of a specific patient can be further classified at a molecular level and ranked in order of the degree of risk. The index clone was tracked in a series of BM aspirations during and after treatment for the dynamic evaluation of therapeutic effects and prognostication, including relapse prediction. When tracking multiple clonotype sequences, it is important to consider the type of gene rearrangement being tracked. A check of the tracking sequence in a negative control is also necessary to ensure that it is not a part of the polyclonal background, which could lead to a false-positive result. It should also be noted that the adequacy and tumor infiltration level of the specimen required for the identification of an index clone at baseline is relatively high for sufficient DNA input to reach a sensitivity of at least 1 × 10–5. The functionality of MRD monitoring based on this assay can be influenced by several technical limitations of sampling and sequencing, including the amount of input DNA, the cellularity of a BM sample, and possible significant overestimation of residual tumor cells due to the calculations (detectable index clonal sequences/total IGH/IGK sequencing reads) [5, 18].
Strategy of the IG-based NGS method. A Pairs of primers for IGH and IGK sequencing. The forward primers target FR1, FR2 and FR3, while the inverse primer targets the JH region in IGH. 3 pairs of primers were designed for IGK sequencing, including the forward primer targeting Vκ or introns and the inverse primers targeting Jκ or Kde. B The workflow of NGS MRD monitoring. After diagnosis by the gold-standard, the samples of patients are collected (BM or PB) and sequenced to identify index clones. Information acquired during sequencing can also be used in risk stratification and prediction of prognosis. By tracking the index clone, the MRD level is measured continuously during and after the treatment. The major clones in samples from patients who experience relapse are compared with the index clone at diagnosis to study clonal evolution
The strategy designed to amplify specific regions in the IGH/IGK gene is based on a set of consensus primers targeted at the conserved FR region (Fig. 3A). For IGH sequencing, the forward primers target FR 1 to 3 (FR1, FR2, and FR3) in the VH region, while several consensus reverse primers target the JH region. For IGK sequencing, primers target the VK-JK, VK-Kde, and INTR-Kde gene rearrangements. IGL sequencing is rarely applied [14, 19]. Targeted sequences are amplified and purified, based on which the DNA library is established and optimized by intense quality control. The output of sequencing and alignment, usually formatted as a FASTQ file, is analyzed and sorted to identify index clones by predetermined criteria.
For some cases, the IGHV mutational status plays an important role in prognosis prediction, such as in CLL. The matched bioinformatics software Lymphotrack IGH FR1 Assay Master Mixes can be used to meet the needs for evaluating the SHM rate of the IGHV region. The IGHV mutational status will be determined based on the percent of mismatch between the clonal amplicons and the corresponding germline reference genes, the prediction of in-frame or out-of-frame translation, the possibility of a premature codon introduced by the mutation, and the percentage of VH gene coverage in the region targeted. SHM, in turn, may hamper the identification of clonal immunoglobulin rearrangements due to the possible prevention of primer annealing during multiplex PCR. Nonetheless, to identify distinct IGHV genes in the IGHV subgroup, commercial kit software will require a match using the IgBLAST or IMGT database and list the candidate IGHV genes in order of identity. The best match is preferentially chosen for subsequent analyses. The result will be considered dubious and excluded when a stop codon or frameshift mutation is produced. Although the bias resulting from SHM is hard to avoid, the comparison step ensures the validity of the interpretation as much as possible.
The information presented in the analysis report is as follows: (1) a summary of a sorted list of unique sequences, including the best-matched V–J family recombination event, length of the read, the actual and cumulative rate of the unique read in total reads, coverage of the identified V-gene by the unique read, the mutation rate to partial V gene and the prediction of in-frame mutation and stop codon; (2) graphs of V–J usage/sequence frequency and (3) a FASTA file of the unique sequences sorted by count.
Comparison of the performance of techniques applied in clonality assessments and MRD monitoring
The advantages and drawbacks of the methods for clonality assessment are presented in Table 2 in detail [5, 29, 60, 72, 78, 93, 95,96,97,98,99,100].
The gold standard for clonality assessment is the BIOMED-2 multiplex PCR-based protocol for IG/TCR targets, but it is not suitable for MRD monitoring [101]. The workflow, experimental conditions, and analysis methods were standardized in the BIOMED-2 protocols. Monoclonality, which represents malignant proliferation, is displayed as 1–2 peaks in the background, while polyclonality, which indicates physiological immune reactions, has a Gaussian distribution.
ASO-RQ-PCR can be applied for diagnosis and MRD monitoring of lymphoma with a sensitivity of 10–4 ~ 10–5. In this approach, IG regions are targeted and amplified by consensus primers and then sequenced to design more precise, patient-specific primers and probes [95]. ASO-RQ-PCR provides quantitative results recorded by fluorescence signals, making it suitable for MRD measurement. Given that the design of patient-specific primers and probes is complex, labor-intensive, and cannot be standardized, ASO-RQ-PCR is difficult to apply widely in multiple centers.
Compared with the BIOMED-2 protocol and ASO-RQ-PCR method, the IG-based NGS approach performed better on many levels, such as higher sensitivity, the ability to obtain more information about the IG repertoire, and the ability to track clonal evolution; additionally, the workflow is well-standardized (Table 2). Since the sensitivity of the BIOMED-2 method was 0.1%, which partially hampered its utilization for MRD tracking, the comparison between BIOMED-2 and NGS mostly focused on the concordance of the types of clonality or the sequences detected. The EuroClonality-NGS working group tested the suitability of NGS-based IG rearrangement detection in frozen and formalin-fixed paraffin-embedded specimens (low-quality DNA) using the ARResT/Interrogate platform for data analysis. Accurate clonotypes in all healthy samples were successfully identified by NGS, while dominant IG gene rearrangements identified by NGS were identical to those identified by BIOMED-2. The NGS-based method also identified 22% more clonal rearrangements that were not detected by the conventional method, possibly due to the new design of shorter amplicons [101]. A multicenter study compared these two technologies based on 209 specimens of reactive and malignant lymphoproliferation and validated the high interlaboratory concordance (99%) of NGS-IG detection and its high concordance (98%) with the BIOMED-2 method for the exact sequences and clonality. An even higher sensitivity was obtained with the NGS-IG method than the gold standard, inferred from a higher detection rate in diagnosed lymphoma samples. Satisfactory resolution of reactive and malignant samples was also achieved by NGS-based clonality tests [102]. Similarly, several other studies established for testing the NGS-based IG clonality method in routine clinical practice unveiled superior performance of NGS, marked by a clonality detection rate of 97% and high concordance (96%) with capillary electrophoresis assays at diagnosis [103] and a much higher positive rate obtained by NGS compared with fragment analysis in follow-up samples for MRD detection [84]. Less efficient identification of clonal IG rearrangements by NGS in HL compared with NHL was observed in a study with a small sample size; however, the NGS IG method performed better than BIOMED-2 [104]. Overall, the NGS-based method is superior to BIOMED-2 for clonality detection.
FC-based methods, including MFC and NGF, are considered the gold standard of MRD measurement in clinical practice with a wide applicable range, short turnaround time, reliable results, and relatively low cost. Malignant cells in samples are identified and quantified based on aberrant immunophenotypes labeled with different colored antibody signals. The sensitivity reaches 1 × 10–4 in MFC and 2 × 10–6 in NGF [15, 20, 21]. However, FC-based approaches are limited by technical defects and expertise requirements [105]. First, the analysis of FC results requires a high level of expertise to avoid possible subjective interpretation, while variations in instrument settings and reagents (antibodies and fluorescein) are unavoidable. Second, FC is complicated by the change or loss of the surface markers selected during monitoring resulting from clonal evolution and targeted therapies such as CAR-T cells. Low tumor burdens, sample hypocellularity, and diseases lacking specific markers, such as DLBCL, also limit the application of FC. The relatively low sensitivity of MFC limits MRD detection after intensive treatments (false-negative results), while false-positive results may arise after the induction of immune reconstruction. Third, the fast turnaround time was paralleled with the requirement of fresh instead of cryopreserved samples, which are not available for retrospective studies. Last, FC results lack stringent quality control. These limitations were partially overcome by the advent of NGF, in which the sensitivity was significantly improved, the workflow was standardized following the EuroFlow guidelines, and the results were controlled based on the detection of hemodilution [17, 88].
Another PCR-based method, the droplet digital PCR (ddPCR) assay, can also be applied for MRD monitoring. ddPCR was proven to be an efficient method for MRD monitoring in malignancies with distinct translocation/fusion transcripts/recurrently mutated gene markers, such as BCL/IGH rearrangements in FL [106], BCR-ABL fusion transcript in chronic myeloid leukemia [107, 108], MYD88 L265 mutation in Waldenstrom macroglobulinemia[109] and NPM1 mutation [110] or IDH1/2 mutation [111] in AML. ddPCR solves some of the disadvantages of quantitative PCR, including establishing a standard curve and positive cases with unquantifiable results while maintaining a sensitivity of 1 × 10–5. Furthermore, several studies demonstrated that ddPCR had a good concordance with RT–qPCR, but its ability to quantify the level of markers was more precise [108, 112]. Based on water–oil emulsion technology, ddPCR fractionates the sample into nanoliter-sized 20,000 droplets and amplifies the template molecules in each droplet [113]. The high partitioning endows ddPCR with highly sensitive and reliable absolute quantification capability [107, 114]. However, as an approach developed to quantify specific leukemic aberrations, ddPCR is still strictly dependent on qualitative nested PCR as the marker screening tool. The prevalence of the specific hallmarks (absent in 35–40% of patients) significantly limits the utilization of ddPCR in most hematological diseases, making this PCR-based method suitable for only a minority of patients [114, 115]. High mutation rates and translocations or mutations unrelated to tumors can also influence the final interpretation of results. The ddPCR method can be time-saving, cheaper, and easier to perform when compared with NGS-based methods, but it only detects genetic alterations that have already been identified, while NGS provides more comprehensive information [116]. Additionally, the primers used for ddPCR need to be specifically designed for patients based on sequencing results.
The comparison of FC-based, NGS-based, and PCR-based approaches is shown in Table 3 [18, 20, 100, 106, 109, 115, 117,118,119,120,121,122]. The unique advantages of NGS-based techniques stand out from the other approaches to MRD monitoring. The NGS method exhibited superior detection for cases with lower tumor burdens, minor subclones, and a high level of SHM at diagnosis. MRD can be better defined by tracking the behavior of specific clones through NGS, providing a more accurate prediction of relapse and more evidence of clonal evolution. Moreover, biclonalities, oligo-clonalities, and uncommon rearrangements can also be identified with reasonable confidence. The IG NGS approach provided a common picture of not only the MRD of malignancies but also the immune repertoire. Similar to PCR-based approaches, the IG NGS method can be used in cryopreserved and fixed samples. The objective interpretation and the automated analysis of subsequent samples based on pipelines and predefined thresholds further expanded the scope of its application. Limitations of the IG NGS approach exist, such as the higher standard for accessibility caused by requirements for better computing and high-quality DNA input at diagnosis, longer turnaround time of approximately 5–7 days, failures of detection due to the indistinguishable background of reactive B cells, V deletions and incomplete DJ rearrangements or hemodilution, and the lack of well-established, widely accepted protocols. NGS technology is based on an initial PCR step, which could be influenced by annealing and SHM in primer-binding regions. The high cost per sample is also a constraint, preventing the use of NGS-based MRD monitoring in most clinical trials.
The superiority of NGS-MRD in clinical practice
Statistics from clinical trials in which treatment decisions were made based on MRD are scarce due to the obstacles to practically applying MRD monitoring. However, several studies have focused on the functionality of the NGS-based MRD method by comparing it with other gold-standard approaches in registered clinical trials evaluating different B-lineage malignancies (Table 4). In summary, NGS-based MRD exhibited excellent performance in sensitivity, precision, reproductivity, and prediction of relapse. This method showed at least a comparable ability to identify the accurate sequence compared with Sanger sequencing [18, 98, 123] and could define and track the index clone compared with ASO-PCR [11, 22, 93, 124], MFC [15, 125], NGF [18, 20, 21, 123] and mass spectrometry [118]. With further optimization for satisfactory quantification and higher economical efficiency in the future, NGS-based MRD monitoring has the potential for wider application in routine clinical practice.
Current challenges in IG NGS-based MRD monitoring
MRD monitoring has already become a relatively mature and widely acceptable technology despite insufficient reliability when independently guiding treatment-associated decisions. However, the answers to several questions remain obscure or controversial. The first is the significance of sensitivity in real-world utilization. Theoretically, the deeper the detection is, the more accurate the results will be, which was emphasized in some articles that demonstrated a higher relapse rate of NGS or NGF MRD-positive patients compared with those assessed with other techniques with lower sensitivity [11, 125]. However, those MRD-positive patients with stable and nearly disease-free status with no evidence of relapse, common in clinical trials evaluating novel treatments, were ignored in most studies, raising the question of whether the presence of MRD detected by the instruments themselves or the quantity of MRD above a predefined threshold influences the prognosis. Another factor that should be considered is the sample requirement. A substantial concentration of DNA input is needed to reach a sensitivity of 10–6, which is usually unavailable from patients who have experienced intensive treatment elsewhere and is also time-and labor-consuming. Although it is difficult to find and achieve the right balance of economic benefits and optimal results, the combination of higher sensitivity for detection and risk stratification based on the number of residual tumor cells can be an inspiration to develop next-generation techniques for MRD monitoring. The second question is associated with the sample types. Diagnosis and MRD monitoring are generally performed by analyzing the infiltration of tumor cells in the BM. It should be noted that BM aspiration and biopsy are invasive procedures with potential risks, limiting the ability of doctors to take repeated samples.
Future prospects of IG NGS-based MRD monitoring
Compared with those for examining BM aspirations, tests for peripheral blood are more convenient and accessible. Recently, liquid biopsy technology has emerged to capture information about SHM, V(D)J rearrangements, amplification and gene copy variations. By using circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), cell-free DNA (cfDNA), or other cell-free nucleic acids (mRNA, microRNA), liquid biopsy can be conducted in a noninvasive manner [126]. The results of several studies have suggested that ctDNA and cfDNA alone are practical for most lymphomas [5]. The rapid clearance of cfDNA allows tracking of the dynamic changes in MRD [61]. Unfortunately, the cfDNA level was proven insufficient as an independent prognostic factor in some studies [127]. The IG NGS-based method can also be applied to detect ctDNA and peripheral blood mononuclear cells, which is particularly promising for application in DLBCL [57, 128]. However, this approach is limited by the very low concentration of ctDNA or CTCs early in the disease, more localized infiltration of tumors, and MRD monitoring after intensive treatment; thus, liquid technologies inevitably present considerable and ongoing challenges requiring the development of ultrasensitive techniques [129].
Conclusion
This review provides a comprehensive evaluation of IG NGS-based MRD monitoring, including the necessity of MRD monitoring, the scope of application of the IG NGS method, the superiority of the IG NGS method for diagnosing and tracking MRD, the existing limitations, future trends, and potential development directions. With the continuing increasing sensitivity and affordability of HTS technology, the routine use of IG NGS-based MRD monitoring in clinical practice is expected within the near future, with robust performance and reasonable per-sample cost.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- ASO:
-
Allele-specific oligonucleotide
- BCR:
-
B cell antigen receptor
- BM:
-
Bone marrow
- CAR-T:
-
Chimeric antigen receptor-T cell
- CDR3:
-
Complementarity determining regions 3
- cfDNA:
-
Cell free DNA
- CLL:
-
Chronic lymphocytic leukemia
- CR:
-
Clinical remission
- COO:
-
Cell of origin
- CSR:
-
Class switch recombination
- CTC:
-
Circulating tumor cell
- ctDNA:
-
Circulating tumor DNA
- ddPCR:
-
Digital droplet PCR
- DSB:
-
Double strand break
- DLBCL:
-
Diffuse large B cell lymphoma
- FL:
-
Follicular lymphoma
- GC:
-
Germinal center
- GCB:
-
Germinal center B cell
- HCDR3:
-
Heavy chain CDR3
- IG:
-
Immunoglobulin
- IGH:
-
Immunoglobulin heavy chain
- LCDR3:
-
Light-chain CDR3
- MCL:
-
Mantle cell lymphoma
- MFC:
-
Multi-color flow cytometry
- MM:
-
Multiple myeloma
- MRD:
-
Minimal residual disease
- NGF:
-
Next-generation flow cytometry
- NGS:
-
Next-generation sequencing
- N-gly:
-
Asparagine-X-serine/threonine
- OS:
-
Overall survival
- PC:
-
Plasma cell
- PCR:
-
Polymerase Chain Reaction
- PET-CT:
-
Positron emission tomography-computed tomography
- PFS:
-
Progress free survival
- RAG:
-
Recombination activating gene
- RSS:
-
Recombination signal sequence
- SHM:
-
Somatic hypermutation
- V:
-
Variable
References
van der Velden VH, Willemse MJ, van der Schoot CE, Hählen K, van Wering ER, van Dongen JJ. Immunoglobulin kappa deleting element rearrangements in precursor-B acute lymphoblastic leukemia are stable targets for detection of minimal residual disease by real-time quantitative PCR. Leukemia. 2002;16:5.
Szczepański T, Langerak AW, Wolvers-Tettero IL, Ossenkoppele GJ, Verhoef G, Stul M, et al. Immunoglobulin and T cell receptor gene rearrangement patterns in acute lymphoblastic leukemia are less mature in adults than in children: implications for selection of PCR targets for detection of minimal residual disease. Leukemia. 1998;12:7.
Martinez-Lopez J, Alonso R, Wong SW, Rios R, Shah N, Ruiz-Heredia Y, et al. Making clinical decisions based on measurable residual disease improves the outcome in multiple myeloma. J Hematol Oncol. 2021;14:1.
Burgos L, Puig N, Cedena MT, Mateos MV, Lahuerta JJ, Paiva B, et al. Measurable residual disease in multiple myeloma: ready for clinical practice? J Hematol Oncol. 2020;13:1.
Jung D, Jain P, Yao Y, Wang M. Advances in the assessment of minimal residual disease in mantle cell lymphoma. J Hematol Oncol. 2020;13:1.
Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J Hematol Oncol. 2020;13:1.
Lee MS, Chang KS, Cabanillas F, Freireich EJ, Trujillo JM, Stass SA. Detection of minimal residual cells carrying the t(14;18) by DNA sequence amplification. Science. 1987;237:4811.
Wang L, Li LR, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. 2020;13:1.
Hou JZ, Ye JC, Pu JJ, Liu H, Ding W, Zheng H, et al. Novel agents and regimens for hematological malignancies: recent updates from 2020 ASH annual meeting. J Hematol Oncol. 2021;14:1.
Press RD, Eickelberg G, Froman A, Yang F, Stentz A, Flatley EM, et al. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol. 2019;94:8.
Takamatsu H, Takezako N, Zheng J, Moorhead M, Carlton VEH, Kong KA, et al. Prognostic value of sequencing-based minimal residual disease detection in patients with multiple myeloma who underwent autologous stem-cell transplantation. Ann Oncol. 2017;28:10.
Fries C, Burack WR. A clinical perspective on immunoglobulin heavy chain clonal heterogeneity in B cell acute lymphoblastic leukemia. Leuk Res. 2018. https://doi.org/10.1016/j.leukres.2018.10.018.
Zhao H, Wei J, Wei G, Luo Y, Shi J, Cui Q, et al. Pre-transplant MRD negativity predicts favorable outcomes of CAR-T therapy followed by haploidentical HSCT for relapsed/refractory acute lymphoblastic leukemia: a multi-center retrospective study. J Hematol Oncol. 2020;13:1.
Rustad EH, Hultcrantz M, Yellapantula VD, Akhlaghi T, Ho C, Arcila ME, et al. Baseline identification of clonal V(D)J sequences for DNA-based minimal residual disease detection in multiple myeloma. PLoS ONE. 2019;14:3.
Kriegsmann K, Hundemer M, Hofmeister-Mielke N, Reichert P, Manta CP, Awwad MHS, et al. Comparison of NGS and MFC methods: key metrics in multiple myeloma MRD assessment. Cancers (Basel). 2020;12:8.
Zhao D, Yue P, Wang T, Wang P, Song Q, Wang J, et al. Personalized analysis of minimal residual cancer cells in peritoneal lavage fluid predicts peritoneal dissemination of gastric cancer. J Hematol Oncol. 2021;14:1.
Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, García-Sánchez O, Böttcher S, et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:10.
Ho C, Syed M, Roshal M, Petrova-Drus K, Moung C, Yao J, et al. Routine evaluation of minimal residual disease in myeloma using next-generation sequencing clonality testing: feasibility, challenges, and direct comparison with high-sensitivity flow cytometry. J Mol Diagn. 2021;23:2.
Visentin A, Facco M, Gurrieri C, Pagnin E, Martini V, Imbergamo S, et al. Prognostic and Predictive Effect of IGHV mutational status and load in chronic lymphocytic leukemia: focus on FCR and BR treatments. Clin Lymphoma Myeloma Leuk. 2019;19:10.
Medina A, Puig N, Flores-Montero J, Jimenez C, Sarasquete ME, Garcia-Alvarez M, et al. Comparison of next-generation sequencing (NGS) and next-generation flow (NGF) for minimal residual disease (MRD) assessment in multiple myeloma. Blood Cancer J. 2020;10:10.
Avet-Loiseau H, Bene MC, Wuilleme S, Corre J, Attal M, Arnulf B, et al. Concordance of post-consolidation minimal residual disease rates by multiparametric flow cytometry and next-generation sequencing in CASSIOPEIA. Clin Lymphoma Myeloma and Leukemia. 2019;19:e3.
Yao Q, Bai Y, Orfao A, Chim CS. Standardized minimal residual disease detection by next-generation sequencing in multiple myeloma. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00449.
Medina A, Jiménez C, Puig N, Sarasquete ME, Flores-Montero J, García-Álvarez M, et al. Interlaboratory analytical validation of a next-generation sequencing strategy for clonotypic assessment and minimal residual disease monitoring in multiple myeloma. Arch Pathol Lab Med. 2022;146:7.
Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:5909.
Davis MM, Kim SK, Hood LE. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980;209:4463.
Beishuizen A, Verhoeven MA, Mol EJ, van Dongen JJ. Detection of immunoglobulin kappa light-chain gene rearrangement patterns by Southern blot analysis. Leukemia. 1994;8:12.
Tümkaya T, Beishuizen A, Wolvers-Tettero IL, van Dongen JJ. Identification of immunoglobulin lambda isotype gene rearrangements by Southern blot analysis. Leukemia. 1996;10:11.
Weichhold GM, Ohnheiser R, Zachau HG. The human immunoglobulin kappa locus consists of two copies that are organized in opposite polarity. Genomics. 1993;16:2.
van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted Action BMH4-CT98-3936. Leukemia. 2003;17:12.
Siminovitch KA, Bakhshi A, Goldman P, Korsmeyer SJ. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 1985;316:6025.
van der Burg M, Tümkaya T, Boerma M, de Bruin-Versteeg S, Langerak AW, van Dongen JJ. Ordered recombination of immunoglobulin light chain genes occurs at the IGK locus but seems less strict at the IGL locus. Blood. 2001;97:4.
Hieter PA, Korsmeyer SJ, Waldmann TA, Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981;290:5805.
Küppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:20.
Ghia P, ten Boekel E, Sanz E, de la Hera A, Rolink A, Melchers F. Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain gene loci. J Exp Med. 1996;184:6.
Davi F, Faili A, Gritti C, Blanc C, Laurent C, Sutton L, et al. Early onset of immunoglobulin heavy chain gene rearrangements in normal human bone marrow CD34+ cells. Blood. 1997;90:10.
Matsuda F, Ishii K, Bourvagnet P, Kuma K, Hayashida H, Miyata T, et al. The complete nucleotide sequence of the human immunoglobulin heavy chain variable region locus. J Exp Med. 1998;188:11.
Corbett SJ, Tomlinson IM, Sonnhammer EL, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J Mol Biol. 1997;270:4.
Lefranc MP, Lefranc G. Immunoglobulins or antibodies: IMGT(®) bridging genes. Struct Funct Biomed. 2020;8:9.
Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12:7.
Sudhakar N, Rajkumar T, Rajalekshmy KR, Nancy NK. Characterization of clonal immunoglobulin heavy (IGH) V-D-J gene rearrangements and the complementarity-determining region in South Indian patients with precursor B-cell acute lymphoblastic leukemia. Blood Res. 2017;52:1.
Katsibardi K, Braoudaki M, Papathanasiou C, Karamolegou K, Tzortzatou-Stathopoulou F. Clinical significance of productive immunoglobulin heavy chain gene rearrangements in childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52:9.
Theunissen PMJ, de Bie M, van Zessen D, de Haas V, Stubbs AP, van der Velden VHJ. Next-generation antigen receptor sequencing of paired diagnosis and relapse samples of B-cell acute lymphoblastic leukemia: clonal evolution and implications for minimal residual disease target selection. Leuk Res. 2019. https://doi.org/10.1016/j.leukres.2018.10.009.
Nishio Y, Sakai H, Saiki Y, Uchida A, Uemura Y, Matsunawa M, et al. Light-chain plasma cell myeloma caused by 14q32/IGH translocation and loss of the other allele. Int J Hematol. 2019;109:5.
Nadeu F, Martin-Garcia D, Clot G, Díaz-Navarro A, Duran-Ferrer M, Navarro A, et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood. 2020;136:12.
Kosmidis P, Bonzheim I, Dufke C, Colak S, Hentrich T, Schroeder C, et al. Next generation sequencing of the clonal IGH rearrangement detects ongoing mutations and interfollicular trafficking in in situ follicular neoplasia. PLoS ONE. 2017;12:6.
González D, van der Burg M, García-Sanz R, Fenton JA, Langerak AW, González M, et al. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood. 2007;110:9.
Klein F, Feldhahn N, Mooster JL, Sprangers M, Hofmann WK, Wernet P, et al. Tracing the pre-B to immature B cell transition in human leukemia cells reveals a coordinated sequence of primary and secondary IGK gene rearrangement, IGK deletion, and IGL gene rearrangement. J Immunol. 2005;174:1.
Tobin G. The immunoglobulin genes and chronic lymphocytic leukemia (CLL). Ups J Med Sci. 2005;110:2.
Tobin G. The immunoglobulin genes: structure and specificity in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:6.
Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, Smilevska T, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: pathogenetic implications and clinical correlations. Blood. 2007;109:1.
Biderman BV, Likold EB, Smirnova SY, Nikitin EA, Koroleva DA, Zvonkov EE, et al. Repertoire of rearranged immunoglobulin heavy chain genes in russian patients with B-cell lymphoproliferative diseases. Clin Lymphoma Myeloma Leuk. 2021;21:12.
Darzentas N, Stamatopoulos K. The significance of stereotyped B-cell receptors in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2013;27:2.
Sebastián E, Alcoceba M, Balanzategui A, Marín L, Montes-Moreno S, Flores T, et al. Molecular characterization of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma: antigen-driven origin and IGHV4-34 as a particular subgroup of the non-GCB subtype. Am J Pathol. 2012;181:5.
Pighi C, Barbi S, Bertolaso A, Zamò A. Mantle cell lymphoma cell lines show no evident immunoglobulin heavy chain stereotypy but frequent light chain stereotypy. Leuk Lymphoma. 2013;54:8.
Hadzidimitriou A, Agathangelidis A, Darzentas N, Murray F, Delfau-Larue MH, Pedersen LB, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118:11.
Xu-Monette ZY, Li J, Xia Y, Crossley B, Bremel RD, Miao Y, et al. Immunoglobulin somatic hypermutation has clinical impact in DLBCL and potential implications for immune checkpoint blockade and neoantigen-based immunotherapies. J Immunother Cancer. 2019;7:1.
Sarkozy C, Huet S, Carlton VE, Fabiani B, Delmer A, Jardin F, et al. The prognostic value of clonal heterogeneity and quantitative assessment of plasma circulating clonal IG-VDJ sequences at diagnosis in patients with follicular lymphoma. Oncotarget. 2017;8:5.
Berget E, Molven A, Løkeland T, Helgeland L, Vintermyr OK. IGHV gene usage and mutational status in follicular lymphoma: correlations with prognosis and patient age. Leuk Res. 2015;39:7.
van der Burg M, Barendregt BH, Szczepañski T, van Wering ER, Langerak AW, van Dongen JJ. Immunoglobulin light chain gene rearrangements display hierarchy in absence of selection for functionality in precursor-B-ALL. Leukemia. 2002;16:8.
Thörn I, Forestier E, Thuresson B, Wasslavik C, Malec M, Li A, et al. Applicability of IG/TCR gene rearrangements as targets for minimal residual disease assessment in a population-based cohort of Swedish childhood acute lymphoblastic leukaemia diagnosed 2002–2006. Eur J Haematol. 2010;84:2.
Chase ML, Armand P. Minimal residual disease in non-Hodgkin lymphoma—current applications and future directions. Br J Haematol. 2018;180:2.
Simonsen AT, Schou M, Sørensen CD, Bentzen HH, Nyvold CG. SOX11, CCND1, BCL1/IgH and IgH-VDJ: a battle of minimal residual disease markers in mantle cell lymphoma? Leuk Lymphoma. 2015;56:9.
Martín-Garcia D, Navarro A, Valdés-Mas R, Clot G, Gutiérrez-Abril J, Prieto M, et al. CCND2 and CCND3 hijack immunoglobulin light-chain enhancers in cyclin D1(-) mantle cell lymphoma. Blood. 2019;133:9.
Gemenetzi K, Agathangelidis A, Zaragoza-Infante L, Sofou E, Papaioannou M, Chatzidimitriou A, et al. B cell receptor immunogenetics in B cell lymphomas: immunoglobulin genes as key to ontogeny and clinical decision making. Front Oncol. 2020. https://doi.org/10.3389/fonc.2020.00067.
Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117:6.
Agathangelidis A, Chatzidimitriou A, Gemenetzi K, Giudicelli V, Karypidou M, Plevova K, et al. Higher-order connections between stereotyped subsets: implications for improved patient classification in CLL. Blood. 2021;137:10.
Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and Pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:15.
Rizzo D, Viailly PJ, Mareschal S, Bohers E, Picquenot JM, Penther D, et al. Oncogenic events rather than antigen selection pressure may be the main driving forces for relapse in diffuse large B-cell lymphomas. Am J Hematol. 2017;92:1.
Catherwood MA, Gonzalez D, Patton C, Dobbin E, Venkatraman L, Alexander HD. Improved clonality assessment in germinal centre/post-germinal centre non-Hodgkin’s lymphomas with high rates of somatic hypermutation. J Clin Pathol. 2007;60:5.
Kikuchi T, Tokunaka M, Kikuti YY, Carreras J, Ogura G, Takekoshi S, et al. Over-expression of BACH2 is related to ongoing somatic hypermutation of the immunoglobulin heavy chain gene variable region of de novo diffuse large B-cell lymphoma. Pathol Int. 2013;63:7.
Jiang Y, Redmond D, Nie K, Eng KW, Clozel T, Martin P, et al. Deep sequencing reveals clonal evolution patterns and mutation events associated with relapse in B-cell lymphomas. Genome Biol. 2014;15:8.
Jiang Y, Nie K, Redmond D, Melnick AM, Tam W, Elemento O. VDJ-seq: deep sequencing analysis of rearranged immunoglobulin heavy chain gene to reveal clonal evolution patterns of b cell lymphoma. J Vis Exp. 2015. https://doi.org/10.3791/53215.
Juskevicius D, Lorber T, Gsponer J, Perrina V, Ruiz C, Stenner-Liewen F, et al. Distinct genetic evolution patterns of relapsing diffuse large B-cell lymphoma revealed by genome-wide copy number aberration and targeted sequencing analysis. Leukemia. 2016;30:12.
Cho YA, Yang WI, Song JW, Min YH, Yoon SO. The prognostic significance of monoclonal immunoglobulin gene rearrangement in conjunction with histologic B-cell aggregates in the bone marrow of patients with diffuse large B-cell lymphoma. Cancer Med. 2016;5:6.
Jardin F, Delfau-Larue MH, Molina TJ, Copie-Bergman C, Brière J, Petrella T, et al. Immunoglobulin heavy chain/light chain pair measurement is associated with survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:9.
Pulsoni A, Della Starza I, Cappelli LV, Tosti ME, Annechini G, Cavalli M, et al. Minimal residual disease monitoring in early stage follicular lymphoma can predict prognosis and drive treatment with rituximab after radiotherapy. Br J Haematol. 2020;188:2.
Odabashian M, Carlotti E, Araf S, Okosun J, Spada F, Gribben JG, et al. IGHV sequencing reveals acquired N-glycosylation sites as a clonal and stable event during follicular lymphoma evolution. Blood. 2020;135:11.
Matynia AP, Li KD, Szankasi P, Schumacher J, Liew M, Salama ME, et al. Molecular fingerprinting of anatomically and temporally distinct B-Cell lymphoma samples by next-generation sequencing to establish clonal relatedness. Arch Pathol Lab Med. 2019;143:1.
Kosmas C, Stamatopoulos K, Stavroyianni N, Zoi K, Belessi C, Viniou N, et al. Origin and diversification of the clonogenic cell in multiple myeloma: lessons from the immunoglobulin repertoire. Leukemia. 2000;14:10.
García-Álvarez M, Alonso-Álvarez S, Prieto-Conde I, Jiménez C, Sarasquete ME, Chillón MC, et al. Immunoglobulin gene rearrangement IGHV3-48 is a predictive marker of histological transformation into aggressive lymphoma in follicular lymphomas. Blood Cancer J. 2019;9:7.
Rustad EH, Misund K, Bernard E, Coward E, Yellapantula VD, Hultcrantz M, et al. Stability and uniqueness of clonal immunoglobulin CDR3 sequences for MRD tracking in multiple myeloma. Am J Hematol. 2019;94:12.
Medina A, Jiménez C, Sarasquete ME, González M, Chillón MC, Balanzategui A, et al. Molecular profiling of immunoglobulin heavy-chain gene rearrangements unveils new potential prognostic markers for multiple myeloma patients. Blood Cancer J. 2020;10:2.
Kim M, Jeon K, Hutt K, Zlotnicki AM, Kim HJ, Lee J, et al. Immunoglobulin gene rearrangement in Koreans with multiple myeloma: clonality assessment and repertoire analysis using next-generation sequencing. PLoS ONE. 2021;16:6.
Ha J, Lee H, Shin S, Cho H, Chung H, Jang JE, et al. Ig Gene clonality analysis using next-generation sequencing for improved minimal residual disease detection with significant prognostic value in multiple myeloma patients. J Mol Diagn. 2022;24:1.
Langerak AW, Groenen PJ, Brüggemann M, Beldjord K, Bellan C, Bonello L, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26:10.
Cho H, Shin S, Chung H, Jang JE, Kim YR, Cheong JW, et al. Real-world data on prognostic value of measurable residual disease assessment by fragment analysis or next-generation sequencing in multiple myeloma. Br J Haematol. 2022;198:3.
Ryan CE, Sahaf B, Logan AC, O’Brien S, Byrd JC, Hillmen P, et al. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128:25.
Kostopoulos IV, Ntanasis-Stathopoulos I, Gavriatopoulou M, Tsitsilonis OE, Terpos E. Minimal residual disease in multiple myeloma: current landscape and future applications with immunotherapeutic approaches. Front Oncol. 2020;10:860.
Yee AJ, Raje N. Minimal residual disease in multiple myeloma: why, when, where. Hematology Am Soc Hematol Educ Program. 2021;2021:1.
Silvennoinen R, Lundan T, Kairisto V, Pelliniemi TT, Putkonen M, Anttila P, et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood Cancer J. 2014;4:10.
Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the medical research council myeloma IX study. J Clin Oncol. 2013;31:20.
Lobetti-Bodoni C, Mantoan B, Monitillo L, Genuardi E, Drandi D, Barbero D, et al. Clinical implications and prognostic role of minimal residual disease detection in follicular lymphoma. Ther Adv Hematol. 2013;4:3.
Ladetto M, Brüggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2014;28:6.
Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:26.
García-Castillo H, Barros-Núñez P. Detection of clonal immunoglobulin and T-cell receptor gene recombination in hematological malignancies: monitoring minimal residual disease. Cardiovasc Hematol Disord Drug Targets. 2009;9:2.
Smirnova SY, Sidorova YV, Ryzhikova NV, Sychevskaya KA, Parovichnikova EN, Sudarikov AB. Evolution of tumor clones in adult acute lymphoblastic leukemia. Acta Naturae. 2016;8:4.
Brüggemann M, Kotrová M, Knecht H, Bartram J, Boudjogrha M, Bystry V, et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia. 2019;33:9.
Genuardi E, Romano G, Beccuti M, Alessandria B, Mannina D, Califano C, et al. Application of the Euro Clonality next-generation sequencing-based marker screening approach to detect immunoglobulin heavy chain rearrangements in mantle cell lymphoma patients: first data from the fondazione Italiana linfomi MCL0208 trial. Br J Haematol. 2021;194:2.
Kumar A, Bantilan KS, Jacob AP, Park A, Schoninger SF, Sauter C, et al. Noninvasive monitoring of mantle cell lymphoma by immunoglobulin gene next-generation sequencing in a phase 2 study of sequential chemoradioimmunotherapy followed by autologous stem-cell rescue. Clin Lymphoma Myeloma Leuk. 2021;21:4.
Della Starza I, Chiaretti S, De Propris MS, Elia L, Cavalli M, De Novi LA, et al. Minimal residual disease in acute lymphoblastic leukemia: technical and clinical advances. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00726.
Scheijen B, Meijers RWJ, Rijntjes J, van der Klift MY, Möbs M, Steinhilber J, et al. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: a technical feasibility study by euroclonality-NGS. Leukemia. 2019;33:9.
van den Brand M, Rijntjes J, Möbs M, Steinhilber J, van der Klift MY, Heezen KC, et al. Next-generation sequencing-based clonality assessment of ig gene rearrangements: a multicenter validation study by euroclonality-NGS. J Mol Diagn. 2021;23:9.
Arcila ME, Yu W, Syed M, Kim H, Maciag L, Yao J, et al. Establishment of immunoglobulin heavy (IGH) chain clonality testing by next-generation sequencing for routine characterization of B-cell and plasma cell neoplasms. J Mol Diagn. 2019;21:2.
van Bladel DAG, van den Brand M, Rijntjes J, Pamidimarri Naga S, Haacke D, Luijks J, et al. Clonality assessment and detection of clonal diversity in classic Hodgkin lymphoma by next-generation sequencing of immunoglobulin gene rearrangements. Mod Pathol. 2022;35:6.
Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125:20.
Cavalli M, De Novi LA, Della Starza I, Cappelli LV, Nunes V, Pulsoni A, et al. Comparative analysis between RQ-PCR and digital droplet PCR of BCL2/IGH gene rearrangement in the peripheral blood and bone marrow of early stage follicular lymphoma. Br J Haematol. 2017;177:4.
Alikian M, Whale AS, Akiki S, Piechocki K, Torrado C, Myint T, et al. RT-qPCR and RT-Digital PCR: a comparison of different platforms for the evaluation of residual disease in chronic myeloid leukemia. Clin Chem. 2017;63:2.
Bochicchio MT, Petiti J, Berchialla P, Izzo B, Giugliano E, Ottaviani E, et al. Droplet digital PCR for BCR-ABL1 monitoring in diagnostic routine: ready to start? Cancers (Basel). 2021;13:21.
Drandi D, Genuardi E, Dogliotti I, Ferrante M, Jiménez C, Guerrini F, et al. Highly sensitive MYD88(L265P) mutation detection by droplet digital polymerase chain reaction in Waldenström macroglobulinemia. Haematologica. 2018;103:6.
Forghieri F, Comoli P, Marasca R, Potenza L, Luppi M. Minimal/measurable residual disease monitoring in npm1-mutated acute myeloid leukemia: a clinical viewpoint and perspectives. Int J Mol Sci. 2018;19:11.
Brambati C, Galbiati S, Xue E, Toffalori C, Crucitti L, Greco R, et al. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101:4.
Della Starza I, Nunes V, Cavalli M, De Novi LA, Ilari C, Apicella V, et al. Comparative analysis between RQ-PCR and digital-droplet-PCR of immunoglobulin/T-cell receptor gene rearrangements to monitor minimal residual disease in acute lymphoblastic leukaemia. Br J Haematol. 2016;174:4.
Grassi S, Guerrini F, Ciabatti E, Puccetti R, Salehzadeh S, Metelli MR, et al. Digital droplet PCR is a specific and sensitive tool for detecting IDH2 mutations in acute myeloid leukemia patients. Cancers (Basel). 2020;12:7.
Cilloni D, Petiti J, Rosso V, Andreani G, Dragani M, Fava C, et al. Digital PCR in myeloid malignancies: ready to replace quantitative PCR? Int J Mol Sci. 2019;20:9.
Grimaldi D, Genuardi E, Ferrante M, Ferrero S, Ladetto M. Minimal residual disease in indolent lymphomas: a critical assessment. Curr Treat Options Oncol. 2018;19:12.
Coccaro N, Tota G, Anelli L, Zagaria A, Specchia G, Albano F. Digital PCR: a reliable tool for analyzing and monitoring hematologic malignancies. Int J Mol Sci. 2020;21:9.
Gimenez E, Chauvet M, Rabin L, Puteaud I, Duley S, Hamaidia S, et al. Cloned IGH VDJ targets as tools for personalized minimal residual disease monitoring in mature lymphoid malignancies; a feasibility study in mantle cell lymphoma by the groupe ouest est d’etude des leucémies et autres maladies du sang. Br J Haematol. 2012;158:2.
Langerhorst P, Noori S, Zajec M, De Rijke YB, Gloerich J, van Gool AJ, et al. Multiple myeloma minimal residual disease detection: targeted mass spectrometry in blood vs next-generation sequencing in bone marrow. Clin Chem. 2021;67:12.
Ching T, Duncan ME, Newman-Eerkes T, McWhorter MME, Tracy JM, Steen MS, et al. Analytical evaluation of the clonoSEQ Assay for establishing measurable (minimal) residual disease in acute lymphoblastic leukemia, chronic lymphocytic leukemia, and multiple myeloma. BMC Cancer. 2020;20:1.
Buccisano F, Palmieri R, Piciocchi A, Arena V, Maurillo L, Del Principe MI, et al. Clinical relevance of an objective—limit of detection—limit of quantification—based flow cytometry approach for measurable residual disease assessment in acute myeloid leukemia a post-hoc analysis of the GIMEMA AML1310 trial. Haematologica. 2022. https://doi.org/10.3324/haematol.2021.279777.
de Boer EN, Johansson LF, de Lange K, Bosga-Brouwer AG, van den Berg E, Sikkema-Raddatz B, et al. Detection of fusion genes to determine minimal residual disease in leukemia using next-generation sequencing. Clin Chem. 2020;66:8.
Paiva B, Puig N, Cedena MT, Rosiñol L, Cordón L, Vidriales MB, et al. Measurable residual disease by next-generation flow cytometry in multiple myeloma. J Clin Oncol. 2020;38:8.
Medina A, Jiménez C, Puig N, Sarasquete ME, Flores-Montero J, García-Álvarez M, et al. Interlaboratory analytical validation of a next-generation sequencing strategy for clonotypic assessment and minimal residual disease monitoring in multiple myeloma. Arch Pathol Lab Med. 2021;146:182.
Li Z, Jiang N, Lim EH, Chin WHN, Lu Y, Chiew KH, et al. Identifying IGH disease clones for MRD monitoring in childhood B-cell acute lymphoblastic leukemia using RNA-Seq. Leukemia. 2020;34:9.
Pulsipher MA, Carlson C, Langholz B, Wall DA, Schultz KR, Bunin N, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk all patients. Blood. 2015;125:22.
Decruyenaere P, Offner F, Vandesompele J. Circulating RNA biomarkers in diffuse large B-cell lymphoma: a systematic review. Exp Hematol Oncol. 2021;10:1.
Rossi D, Diop F, Spaccarotella E, Monti S, Zanni M, Rasi S, et al. Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood. 2017;129:14.
Roschewski M, Dunleavy K, Pittaluga S, Moorhead M, Pepin F, Kong K, et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: a correlative biomarker study. Lancet Oncol. 2015;16:5.
Darrah JM, Herrera AF. Updates on circulating tumor DNA assessment in lymphoma. Curr Hematol Malig Rep. 2018;13:5.
Acknowledgements
We thank all the faculty and staff in the Clinical and Laboratory Unit of the Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their clinical and technical support.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81770211 to Min Xiao) and the National Natural Science Foundation of China (No.81830008 to Jianfeng Zhou).
Author information
Authors and Affiliations
Contributions
XD conceived, drafted the manuscript and drew the figures. MZ discussed and revised the manuscript. MX and JZ provided guidance and approved the version to be submitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1:
Fig. S1 Schematic representation of Ig heavy and light chain rearrangements in normal B cells. (A) Complete VH-D-JH recombination in the IGH locus (14q32). (B) The classical Vκ-Jκ recombination in the IGK locus (2p11) (up), the inversion Vκ(D)-Jκ rearrangement (middle), and Kde-mediated deletions by RSS-intron or Cκ (below). V, variable. D, diversity. J, joining. C, constant.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, X., Zhang, M., Zhou, J. et al. Next-generation sequencing for MRD monitoring in B-lineage malignancies: from bench to bedside. Exp Hematol Oncol 11, 50 (2022). https://doi.org/10.1186/s40164-022-00300-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-022-00300-2