Abstract
Objective
To explore the correlation of mutation landscape with clinical outcomes in patients with peripheral T-cell lymphoma (PTCL).
Methods
We retrospectively analyzed the clinicopathological and prognosis data of 53 patients with PTCL from November 2011 to December 2017. Targeted next-generation sequencing of a 659-gene panel was performed for tissues from 53 patients with PTCLs. The correlation of mutation landscape with clinical outcomes was analyzed.
Results
TET2 was the most frequently mutated gene (64%), followed by RHOA (43%), PCLO (23%), DNMT3A (19%), IDH2 (17%), PIEZO1 (17%) and TP53 (15%). When mutated genes were categorized into functional groups, the most common mutations were those involved in epigenetic/chromatin modification (75%), T-cell activation (74%), and the DNA repair/TP53 pathway (64%). TET2/TP53 mutations were significantly associated with positive B symptoms (P = 0.045), and elevated lactate dehydrogenase (LDH) level (P = 0.011). Moreover, TET2/TP53 mutation was a risk factor for PTCL patient survival (HR 3.574, 95% CI 1.069 − 11.941, P = 0.039). The occurrence of JAK/STAT pathway mutations in angioimmunoblastic T-cell lymphoma (AITL) patients conferred a worse progression-free survival (HR 2.366, 95% CI 0.9130–6.129, P = 0.0334).
Conclusions
Heterogeneous gene mutations occur in PTCL, some of which have a negative impact on the survival outcome.
Similar content being viewed by others
Background
Peripheral T-cell lymphoma (PTCL) is a group of heterogeneous clinicopathological entities of non-Hodgkin lymphoma (NHL) and is often characterized by its aggressive disease course and poor clinical outcome, representing 10% to 15% of all lymphoid malignancies [1]. Recent high-throughput genomic and molecular profiling researches have provided a great deal of information that has prominently improved our understanding of the pathogenesis and pathobiology of these malignant diseases. Mutations within PTCLs recurrently occur in different classes of epigenetic modifiers [2] including ten-eleven translocation 2 (TET2) [3], isocitrate dehydrogenase 1/2 (IDH1/2) [4], and DNA methyltransferase 3A (DNMT3A) [5], as well as in the T-cell receptor (TCR) and coreceptor signaling pathways [6], and components or regulators of the JAK/STAT signaling pathway [7]. Although multiple genetic alterations are shared among different PTCL subtypes, the frequency is significantly different and certain alterations are unique to a specific subtype. For example, RHOA and TET2 mutations are frequently detected in angioimmunoblastic T-cell lymphoma (AITL) [8, 9] and IDH2R172 variant appears to be a unique mutation in AITL [4].
Given the limited value of chemotherapy for PTCLs, the clinical need of improving the treatment and outcomes of PTCL is still unmet. Better description of the mutational landscape may help advance our understanding of the value of the existing treatment options and development of novel therapeutic approaches. We hypothesized that differences may exist in the baseline clinical features and prognosis between different subgroups of patients according to their mutation spectrum. Therefore, to further explore the mutation profile of PTCL patients and correlate the molecular spectrum with baseline clinical characteristics and outcomes, we retrospectively applied targeted sequencing analysis of patients with PTCLs.
Methods
Patients and samples
A total of 53 patients with PTCLs diagnosed in Peking University Cancer Hospital (China) between November 2011 and December 2017 were enrolled for next-generation sequencing. Among these patients, 49 with sufficient clinical information were included in the clinical relevance analysis. The diagnosis of PTCL was based on the criteria of the 2008 World Health Organization (WHO) classification [10]. Tumor tissue biopsies were collected from the patients before the initial therapy and guardians of all the patients provided informed consent in accordance with the Declaration of Helsinki. Medical records were reviewed for clinical and pathological data. All procedures were approved by the Institutional Review Board and the Ethical Committee of Peking University Cancer Hospital and Institute.
DNA extraction
The genomic DNA (gDNA) of formalin-fixed, paraffin-embedded (FFPE) samples and fresh-frozen tissue samples was isolated by using the Maxwell® 16 FFPE Plus LEV DNA Purification Kit (Qiagen, Hilden, Germany; catalog: AS1135) and the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), respectively. The DNA concentration was quantified using a Qubit fluorometer and the Qubit dsDNA HS (High Sensitivity) Assay Kit (Invitrogen, Carlsbad, CA, USA).
Library preparation and target enrichment
One μg each of genomic DNA sample was sheared into fragments of 300 bp in length by sonication (Covaris S2 ultrasonicator, Covaris, Woburn, MA, USA) before library construction. The KAPA DNA Library Preparation Kit (Kapa Biosystems, Wilmington, MA, USA) was used for the preparation of indexed Illumina next-generation sequencing (NGS) libraries of gDNA. A custom SeqCap EZ Library (Roche NimbleGen, Madison, WI, USA) was applied for target enrichment. To investigate the genetic properties of PTCL, we designed a capture probe basing on genomic regions of approximately 2.4 Mb from 659 genes (see Additional file 1: Table S1) that are frequently mutated in PTCLs and other common hematological malignancies. Barcoded libraries were hybridized according to the manufacturer’s protocol. DNA fragments captured from the hybrid selection were then amplified and combined to generate various libraries.
Next-generation sequencing and sequence data analysis
The gDNA libraries were subjected to high-throughput sequencing with 150-bp pair-end reads on the NovaSeq 60,000 Sequencing System (Illumina, San Diego, CA) supported by a commercial vendor (Geneplus-Beijing, China). The average sequencing depth of tissues was 506× (136× to 1003×). Sequence reads were aligned using BWA version 0.5.9 (Broad Institute). Single nucleotide variants (SNVs) were called using MuTect (version 1.1.4) and NChot [11, 12]. Small insertions and deletions (Indels) were determined by GATK [13]. All final candidate variants were manually reviewed by using the IGV browser.
Statistical analysis
Overall survival (OS) was considered from disease confirmation to the end of follow-up or death. The date of diagnosis to the date of disease progression, death or last follow-up was determined as progression-free survival (PFS). We performed group comparisons by using Fisher’s exact test and Student’s t-test for categorical and continuous variables, respectively. Survival was assessed by Kaplan–Meier and log-rank methods. The Cox regression model was adopted to evaluate the prognostic factors. Statistical analyses were performed with Graphpad Prism version 8.0.1 and SPSS version 26.0. P < 0.05 was considered statistically significant.
Results
Patient characteristics
All patients had histopathologically confirmed PTCLs, comprising angioimmunoblastic T-cell lymphoma (AITL; n = 33, 62%), peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS; n = 10, 19%), anaplastic lymphoma kinase (ALK) positive anaplastic large cell lymphoma (ALK + ALCL; n = 4, 8%) and ALK negative ALCL (ALK − ALCL, n = 6, 11%). The general characteristics of the 49 PTCLs patients with sufficient clinical information for inclusion in the clinical relevance analysis are summarized in Table 1. The median age was 54 years (range 26–80 years). Most (34 of 49, 69%) patients were males and diagnosed at stage 3 or stage 4 (40 of 49, 82%), and 15 (31%) patients had international prognostic index (IPI) scores of 3 to 5. Elevated serum lactate dehydrogenase (LDH) was detected in 31 (63%) patients, and 24 (50%) patients presented with positive B symptoms.
The mutational landscape of PTCL
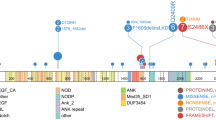
We identified 856 non-dbSNP variants (750 SNVs and 106 Indels) within the coding regions of 334 genes, including 176 genes mutated in ≥ 2 cases (see Additional file 2: Table S2). In addition, mutations in 10 genes (including driver genes such as TET2, RHOA, DNMT3A, IDH2, and TP53, which are well-known PTCL hotspot panels) were found in the specimens of more than 15% patients (Fig. 1, 2a). The most frequent mutation in the whole cohort was observed in TET2 (n = 34, 64%; including 17 with double mutations and 10 with ≥ 3 mutations), followed by RHOA (n = 23, 43%), PCLO (n = 12, 23%; including 3 double mutations) and DNMT3A (n = 10, 19%, including 1 double mutation) (Fig. 2a). In our study, a total of 15 PCLO mutations were observed in 12 patients, comprising seven AITL patients (21%), four PTCL-NOS patients (40%) and one ALK- ALCL patient (17%) (Fig. 1). Heterogeneous genetic mutations were detected in different histological types. The most frequent mutation was TET2 (85%) in AITL patients, PCLO (40%) in PTCL-NOS patients, TP53 (25%) in ALK + ALCL patients, and TP53 (50%) in ALK- ALCL patients (Fig. 1). We further identified the distribution of gene mutations in different histological subtypes. As shown in Additional file 3: Fig. S1a, TET2, RHOA and DNMT3A mutations tended to cluster in AITL and PTCL-NOS patients, while none in ALK + or ALK- ALCL. And IDH2 showed a subtype-specific mutation pattern, which only observed in AITL patients (27.2%). Mutations in PCLO were enriched in PTCL-NOS (40%), but TP53 mutations observed at a relatively higher frequency in ALK- ALCL (50%).
Gene mutation profile in 53 patients with newly diagnosed PTCL. Each column represents an individual patient. The block color represents the type of mutation. The percentages on the left of the table represent percent of study patients with a mutation in each gene. The bar graphs to the top of the plot indicate the number of mutations in each case
When mutated genes were categorized into functional groups, the most common mutations were those involved in epigenetic/chromatin modifications (n = 40, 75%), T-cell activation (n = 39, 74%), the DNA repair/TP53 pathway (n = 34, 64%), the RTK pathway (n = 33, 62%), the PI3K/AKT signaling/mTOR pathways (n = 23, 43%), transcriptional regulation (n = 23, 43%), the JAK-STAT pathway (n = 22, 42%), the APC (Wnt) pathway (n = 16, 30%), the MAPK pathway (n = 15, 28%), the TCR pathway (n = 12, 23%), and the NF-κB pathway (n = 11, 21%) (Fig. 1, 2b). Mutations in epigenetic pathway and T-cell activation were mainly observed in AITL and PTCL-NOS, while mutated genes of DNA repair/ TP53 pathway in AITL and ALK- ALCL (Additional file 3: Fig. S1b).
In our study, TET2 mutations were frequently detected in 28 (85%) AITL patients. Of note, 22 of the 28 AITL patients harbored more than two TET2 mutations. Five of the 10 (50%) PTCL-NOS patients also had TET2 mutations. The 75 TET2 mutations comprised 26 missense mutations, 22 nonsense mutations, 22 frameshifts, four splices and one coding sequence insertion-deletion (CDS-Indel) (Additional file 3: Fig. S1c). TP53 mutations occurred mainly in ALK- ALCL patients (n = 3, 50%), followed by PCTL-NOS patients (n = 3, 30%), ALK + ALCL patients (n = 1, 25%), and AITL patients (n = 1, 3%), comprising four missense mutations, one nonsense mutation, one frameshift, one splice and one CDS-Indel (Additional file 3: Fig. S1d). We further surveyed mutation sites in TET2 and TP53 in mutated samples. The TET2 mutations were scattered throughout the genes, while TP53 mutations were present mainly in the DNA binding domain (Fig. 2c).
Clinical implications of gene mutations
We analyzed the clinical influence of the most common recurrent mutations (Table 1 and Additional file 4: Table S3). There were significant differences in the mutational status of TET2, RHOA, IDH2, and TP53 among the pathological subgroups, and AITL patients were more likely to harbor mutations in TET2 (88%; P < 0.001), RHOA (65%, P = 0.001), and IDH2 (28%; P = 0.024) than in other types of PTCLs. We also observed correlations between several laboratory variables and the presence of TET2 or TP53 mutations (Table 1). Patients with TET2 mutations tended to have positive B symptoms (P = 0.025), elevated LDH level (P = 0.037) and advanced disease (P = 0.001), while PTCL patients with TP53 mutations were more likely to presented with higher Ki-67 expression (P = 0.029). TET2/TP53 mutations were significantly associated with positive B symptoms (P = 0.045) and elevated LDH level (P = 0.011).
Impact of mutations on clinical outcome
In our cohort, 46 patients received CHOP-like regimens in the frontline, and we analyzed the association between genetic mutations and clinical outcome. Among the 46 patients evaluated for response to CHOP-like therapy, 37 patients received the CHOP plus etoposide regimen, while the other nine patients received CHOP alone therapy. Seven patients who responded to CHOP-like treatment received autologous stem cell transplant (ASCT) consolidation therapy. And 9 patients were administrated with epigenetic therapy. The overall response (OR) rate of the 46 patients was 65.2% [30 of 46 patients], with 54.3% [25 of 46] patients achieving complete response (CR) and 10.9% (5 of 46) patients achieving partial response (PR). However, as shown in Additional file 5: Table S4, there was no correlation between the mutations identified in this study and the efficacy of CHOP-like chemotherapy.
The median follow-up time was 28.0 months (range 5.0–84.9 months). Thirty-seven (80.4%) patients progressed or relapsed and 26 (56.5%) patients died. The median OS was 26.3 months (range 4.2–85.2 months). The 3-year OS and PFS rates for the cohort were 46.9% and 25.7%, respectively. Patients with ASCT consolidation therapy tended to have a better OS (HR 0.1532, 95%CI 0.059–0.396, P = 0.0329, Additional file 6: Fig. S2a). No significant correlation between individual mutations and the OS of the cohort was observed. Patients carrying TET2 and IDH2 co-mutations showed a better PFS compared with only TET2 mutated group (HR 0.3004, 95% CI 0.129–0.700, P = 0.0347, Fig. 3a).
We further analyzed the prognostic effect of epigenetic modification, T-cell activation and DNA repair pathway mutation in PTCL patients and found no significant differences in OS or PFS between the wild-type (WT) group and the mutated group (P > 0.05; Fig. 3b–d). However, patients with DNA repair pathway mutations demonstrated worse survival. The combination of TET2 and TP53 mutations provided much better prognostic capacity for distinguishing patients with a poor prognosis from those with a good prognosis (23.9 months vs. 52.8 months, HR 3.535, 95 CI 1.546–8.083, P = 0.0272, Fig. 3d). Patients with TET2 and TP53 co-mutations showed a superior OS compared with those with TP53 mutations alone. In the univariate analysis, adverse prognosis factors were abnormal LDH level (HR 2.703, 95% CI 1.079–6.772, P = 0.034), and TET2/TP53 mutations (HR 3.535, 95 CI 1.546–8.083, P = 0.0272). Multivariate analysis showed that TET2/TP53 mutations (HR 3.574 95% CI 1.069 − 11.941, P = 0.039) was an independent adverse risk factor for OS (Table 2).
Epigenetic/chromatin modifiers were reported to be frequently mutated in AITL patients. Given that our study cohort was comprised mainly AITL patients (28/46), we further analyzed survival in this subtype of patients. After we excluded 7 patients who have received epigenetic therapy, AITL patients with IDH2 mutations had a superior PFS compared to patients without mutations (HR 0.3167, 95% CI 0.1227–0.8177, P = 0.0483, Fig. 4a). In addition, we also found a significant correlation between the JAK/STAT pathway mutations and the PFS of ATIL patients. The occurrence of JAK/STAT pathway mutations in AITL patients was shown to confer an inferior PFS (HR 2.366, 95% CI 0.9130–6.129, P = 0.0334, Fig. 4c).
Discussion
PTCL patient outcomes are generally dismal and their long-term survival prospects have not been improved by the existing treatment regimens or even novel drugs [14]. Therefore, a better understanding of the pathogenesis and biology of PTCL is needed to help develop novel therapeutic approaches and improve patient outcomes. By analyzing a series of PTCL cases, we have refined the information related to previously reported genetic alterations and oncogenic pathways. Furthermore, we explored the prognostic impact of these mutations in PTCL patients.
In our study, we found that the most common mutations occurred in epigenetic/chromatin remodeling, followed by T-cell activation, DNA repair/TP53 pathway, the RTK pathway, the PI3K/AKT pathway and the JAK/STAT signaling pathway. Frequent alterations in epigenetic modifiers, including TET2, DNMT3A and IDH2, the TCR signaling pathway, and the JAK/STAT pathway, have been reported previously [4, 6, 15,16,17]. Overall, our sequencing results showed that different subtypes of PTCLs have their unique characteristics with a high level of heterogeneity among them. According to Heavican and other researchers’ studies, T follicular helper (TFH) cells could be identified as the cell of origin of AITLs [18, 19]. AITL has been characterized by recurrent mutations in genes of epigenome regulators (TET2, DNMT3A, and IDH2R172) as well as the proximal TCR and costimulatory signaling pathways [6]. Inactivating TET2 mutations have been found in up to 85% of AITL patients [8]. In accordance with previous studies, the AITL patients in our cohort were more likely to harbor mutations in genes of epigenetic modifiers and T-cell activation pathway (TET2 85%, RHOA 64% and IDH2 27%). We also found that the frequency of TET2 mutations is significantly higher in patients with positive B symptom or elevated LDH level, as confirmed in the study by Zhifang Xu’s team [20]. High LDH level and positive B symptom is known as an indicator of tumor burden and plays a crucial role in tumor maintenance [21].
Most of the RHOA mutations (56/68, 82%) detected in our study were proved to be RHOAG17V variants (c.50G > T), which results in a glycine to valine substitution at residue 17 of the RHOA protein [22]. Furthermore, 95% of AITL patients with RHOA mutations also harbored TET2 mutations. Cortes et al. reported that RHOAG17V expression combined with TET2 mutation results in the development of AITL in mice, suggesting the existence of crosstalk between these mutations in AITL [9]. However, there was no combinational effect of TET2 and RHOA mutations on patient survival in this study. We found that the co-occurrence of TET2 and IDH2 mutations conferred a better PFS, compared with TET2 alone mutation, which also highlighting the importance of epigenetic modifications in PTCL patients.
We also detected TET2 mutations in PTCL-NOS patients, but at a lower frequency than the other PTCL entities, which may due to the TFH-like subtype of PTCL-NOS. Apart from TET2 alterations, mutations in PTCL-NOS patients were predominantly observed in PCLO and TP53. Regarding genomic alterations, TP53 mutations were detected at a much lower frequency in PTCLs compared with B-cell lymphoma [23] and have not been extensively investigated. In our study, we detected frequent TP53 mutations (15%), which occurred mainly in ALK- ALCL and PTCL-NOS patients. A recent genomic study showed that TP53 mutations were associated with PTCL-GATA3 [24]. TP53 alterations are reported more commonly in ALK- ALCL patients than in ALK + patients and may contribute to a more aggressive disease course [25]. A study by Vasmatzis et al. also suggested that a series of genetic mutations and epigenetic abnormalities may conduce destruction of p53-associated tumor suppressor function in PTCL [26].
Of note, we observed frequent mutations in PCLO (23%), which is reported to participate in modulating neurotransmitter release and critical for the recycling and maintenance of synaptic vesicles [27]. Recurrent mutations in PCLO were also discovered in diffuse large B-cell lymphoma [28] and several solid tumors, including glioblastoma, liver cancers, and stomach adenocarcinoma [29,30,31,32]. Qiu et al. have reported that PCLO mutations could precisely predict etoposide sensitivity in small cell lung cancer [29]. PCLO alterations have not yet been reported in PTCL and further researches are warranted to clarify the influence of PCLO mutations in PTCL.
We found no difference in the outcome of PTCL patients with any individual mutation. In AITL patients, the only individual mutation which confers a significant inferior survival was IDH2. The adverse impact of TET2, RHOA, and DNMT3A mutations in AITL reported by other groups [2, 8] were not observed in our study. In contrast to our findings, previous studies in PTCL-NOS showed that mutations in histone methyltransferase genes (KDM6A, MLL, or MLL2) were corelated with an adverse clinical outcome [17]. The reasons for this discrepancy are still undetermined but may be due to a relatively small patient cohort size. In our study, the combination of TET2 and TP53 mutations provided a much better prognostic capacity and TET2/TP53 mutation was proved to be an independent prognostic factor for worse overall survival. A recent study revealed a functional interaction between p53 and TET2 during anti-cancer treatment [33]. In p53 knockout cells, TET2 accumulates in the nucleus and protects the genome from DNA damage, which promotes cell proliferation and ultimately leads to chemotherapy resistance.
The standard treatment for PTCL remains undefined. In spite of their significant differences in clinical manifestation and pathologic appearance, the most common entities of PTCL, including AITL, PTCL-NOS and ALCL (ALK positive and negative), tend to be treated similarly [34]. And approximately 85% PTCL patients received CHOP or CHOP-like regimens within frontline treatment [14]. In our study, we analyzed the efficacy and outcome of CHOP-like therapy in PTCL patients. Our results showed that the overall response rate (ORR) to CHOP-like regimens was 65.2% and the CR rate was 54.3%, which is consistent with previous research data [35,36,37]. Several prospective and retrospective autologous stem cell transplant (ASCT) researches have revealed an improved outcome in PTCL patients with an OS rate of 60–70% [38, 39]. We also observed superior OS among patients treated ASCT consolidation therapy, compared with those receiving CHOP-like therapy alone.
The limitations of our study should be noted. We identified an association of TET2 and TP53 mutations with inferior survival in PTCL; however, the number of patients recruited in our cohorts was relatively small. Thus, our results should be interpreted with caution and further prospective studies of TET2/TP53 mutations and PTCL are warranted. The mechanisms underlying the association of TET2 and TP53 mutations with an adverse prognosis for PTCL patients are still unclear. In future research, a large cohort of PTCL patients will be enrolled to validate the initial findings of this study. We will also conduct further investigations to determine the transcriptome profiles of TET2/TP53 mutations in PTCLs to gain additional insights into their consequences.
Conclusion
Our findings suggest that heterogeneous gene mutations occur in PTCL patients. TET2/IDH2 co-mutation conferred a superior PFS, compared with TET2 mutation, and TET2/TP53 mutations are associated with an adverse prognosis. The JAK/STAT pathway had a negative impact on survival outcomes in AITL patients, thus implicating this as a potential target for this malignant subtype.
Availability of data and materials
All relevant data of the study are included within the article and its additional files. Further inquiries of the original data can be directed to the corresponding authors.
Abbreviations
- PTCL:
-
Peripheral T-cell lymphoma
- LDH:
-
Lactate dehydrogenase
- AITL:
-
Angioimmunoblastic T-cell lymphoma
- NHL:
-
Non-Hodgkin lymphoma
- TET2:
-
Ten-eleven translocation 2
- IDH1/2:
-
Isocitrate dehydrogenase 1/2
- DNMT3A:
-
DNA methyltransferase 3A
- TCR:
-
T-cell receptor
- gDNA:
-
Genomic DNA
- FFPE:
-
Formalin-fixed, paraffin-embedded
- NGS:
-
Next-generation sequencing
- SNVs:
-
Single nucleotide variants
- Indels:
-
Insertions and deletions
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- ALK:
-
Anaplastic lymphoma kinase
- ALCL:
-
Anaplastic large cell lymphoma
- PTCL-NOS:
-
Peripheral T-cell lymphoma, not otherwise specified
- CDS-Indel:
-
Coding sequence insertion-deletion
- CHOP:
-
Cyclophosphamide, doxorubicin, vincristine, prednisone
- ASCT:
-
Autologous stem cell transplant
- OR:
-
Overall response
- CR:
-
Complete response
- PR:
-
Partial response
- WT:
-
Wild-type
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- TFH:
-
T follicular helper
- ORR:
-
Overall response rate
References
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89(11):3909–18.
Palomero T, Couronne L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46(2):166–70.
Sakata-Yanagimoto M. Multistep tumorigenesis in peripheral T cell lymphoma. Int J Hematol. 2015;102(5):523–7.
Wang C, McKeithan TW, Gong Q, Zhang W, Bouska A, Rosenwald A, et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood. 2015;126(15):1741–52.
Haney SL, Upchurch GM, Opavska J, Klinkebiel D, Appiah AK, Smith LM, et al. Loss of Dnmt3a induces CLL and PTCL with distinct methylomes and transcriptomes in mice. Sci Rep. 2016;6:34222.
Vallois D, Dobay MP, Morin RD, Lemonnier F, Missiaglia E, Juilland M, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood. 2016;128(11):1490–502.
Manso R, Sanchez-Beato M, Gonzalez-Rincon J, Gomez S, Rojo F, Mollejo M, et al. Mutations in the JAK/STAT pathway genes and activation of the pathway, a relevant finding in nodal Peripheral T-cell lymphoma. Br J Haematol. 2018;183(3):497–501.
Lemonnier F, Couronné L, Parrens M, Jaïs JP, Travert M, Lamant L, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120(7):1466–9.
Cortes JR, Ambesi-Impiombato A, Couronne L, Quinn SA, Kim CS, da Silva Almeida AC, et al. RHOA G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell. 2018;33(2):259-73.e7.
Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: Press I; 2008.
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–9.
Yang X, Chu Y, Zhang R, Han Y, Zhang L, Fu Y, et al. Technical validation of a next-generation sequencing assay for detecting clinically relevant levels of breast cancer-related single-nucleotide variants and copy number variants using simulated cell-free DNA. JMD. 2017;19(4):525–36.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30.
Iqbal J, Weisenburger DD, Greiner TC, Vose JM, McKeithan T, Kucuk C, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115(5):1026–36.
Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123(9):1293–6.
Schatz JH, Horwitz SM, Teruya-Feldstein J, Lunning MA, Viale A, Huberman K, et al. Targeted mutational profiling of peripheral T-cell lymphoma not otherwise specified highlights new mechanisms in a heterogeneous pathogenesis. Leukemia. 2015;29(1):237–41.
de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109(11):4952–63.
Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915–23.
Hu J, Xu J, Tian T, Xie J, Fan L, Zhu G, et al. TET2 rs2454206, TET2 rs12498609 and ASXL1 rs3746609 single nucleotide polymorphisms in patients with myelodysplastic syndromes. Blood Cells Mol Dis. 2019;74:44–50.
Bouafia F, Drai J, Bienvenu J, Thieblemont C, Espinouse D, Salles G, et al. Profiles and prognostic values of serum LDH isoenzymes in patients with haematopoietic malignancies. Bull Cancer. 2004;91(7–8):E229–40.
Yoo HY, Sung MK, Lee SH, Kim S, Lee H, Park S, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(4):371–5.
Hatta Y, Koeffler HP. Role of tumor suppressor genes in the development of adult T cell leukemia/lymphoma (ATLL). Leukemia. 2002;16(6):1069–85.
Heavican TB, Bouska A, Yu J, Lone W, Amador C, Gong Q, et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood. 2019;133(15):1664–76.
Parrilla Castellar ER, Jaffe ES, Said JW, Swerdlow SH, Ketterling RP, Knudson RA, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–80.
Vasmatzis G, Johnson SH, Knudson RA, Ketterling RP, Braggio E, Fonseca R, et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood. 2012;120(11):2280–9.
Ackermann F, Schink KO, Bruns C, Izsvák Z, Hamra FK, Rosenmund C, et al. Critical role for Piccolo in synaptic vesicle retrieval. eLife. 2019;8:12.
Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109(10):3879–84.
Qiu Z, Lin A, Li K, Lin W, Wang Q, Wei T, et al. A novel mutation panel for predicting etoposide resistance in small-cell lung cancer. Drug Design Dev Ther. 2019;13:2021–41.
Park AK, Kim P, Ballester LY, Esquenazi Y, Zhao Z. Subtype-specific signaling pathways and genomic aberrations associated with prognosis of glioblastoma. Neuro-oncology. 2019;21(1):59–70.
Wang H, Shen L, Li Y, Lv J. Integrated characterisation of cancer genes identifies key molecular biomarkers in stomach adenocarcinoma. J Clin Pathol. 2020;12:8.
Fujimoto A, Furuta M, Shiraishi Y, Gotoh K, Kawakami Y, Arihiro K, et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120.
Zhang J, Tan P, Guo L, Gong J, Ma J, Li J, et al. p53-dependent autophagic degradation of TET2 modulates cancer therapeutic resistance. Oncogene. 2019;38(11):1905–19.
Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123(17):2636–44.
Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151(2):159–66.
Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–25.
Liu X, Yang M, Wu M, Zheng W, Xie Y, Zhu J, et al. A retrospective study of the CHOP, CHOPE, and CHOPE/G regimens as the first-line treatment of peripheral T-cell lymphomas. Cancer Chemother Pharmacol. 2019;83(3):443–9.
Gkotzamanidou M, Papadimitriou CA. Peripheral T-cell lymphoma: the role of hematopoietic stem cell transplantation. Critical Rev Oncol Hematol. 2014;89(2):248–61.
Wu M, Wang X, Xie Y, Liu W, Zhang C, Ping L, et al. Outcome and prospective factor analysis of high-dose therapy combined with autologous peripheral blood stem cell transplantation in patients with peripheral T-cell lymphomas. Int J Med Sci. 2018;15(9):867–74.
Acknowledgements
We would like to thank all patients included in this study and Geneplus-Beijing for technical support.
Funding
This study was supported by the Capital’s Funds for Health Improvement and Research (No. 2018–1-2151), the National Nature Science Foundation of China (Nos. 82070205, 81870154, 81972807, 81670187, 81970179 and 81700197); the Beijing Natural Science Foundation (Nos. 7202025 and 7202026), Beijing Municipal Science & Technology Commission (Z181100001918019) and Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20151001).
Author information
Authors and Affiliations
Contributions
YY collected and processed the data, reviewed literature, wrote and edited the manuscript. LM performed statistical analysis and edited the manuscript. YS and WL collected the data and edited the manuscript. ND and YS helped with literature review, and edited the manuscript. SS and JZ designed the study, reviewed literature, and wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of Peking University Cancer Hospital and guardians of all the patients provided informed consent for their inclusion in this study.
Consent for publication
The participants’ legal guardians provided informed consent for the publication of the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Gene list.
Additional file 2: Table S2.
Somatic mutations detected in patients with PTCL.
Additional file 3: Figure S1.
(a) The distribution of gene mutations in different histological subtypes of PTCL: The bars indicate mutation frequencies in each subtype. (b) The distribution of mutated functional groups in different histological subtypes. Gene mutation function proportion of TET2 (c) and TP53 (d) in newly diagnosed PTCL patients.
Additional file 4: Table S3.
Patients' characteristics based on gene mutational status.
Additional file 5: Table S4.
Impact of mutations on the efficacy of CHOP‐like chemotherapy.
Additional file 6: Figure S2.
Kaplan–Meier curves for univariate analysis. Stratified by (a) ASCT, (b) TET2 mutation, (c) IDH2 mutation. (d) TET2/IDH2 co-mutation vs TET2 alone mutation or IDH2 alone mutation. (e) TET2/RHOA co-mutation vs TET2 alone mutation or RHOA alone mutation. (f) TET2/DNMT3A co-mutation vs TET2 alone mutation or DNMT3A alone mutation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, Y., Ding, N., Mi, L. et al. Correlation of mutational landscape and survival outcome of peripheral T-cell lymphomas. Exp Hematol Oncol 10, 9 (2021). https://doi.org/10.1186/s40164-021-00200-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-021-00200-x