Abstract
Background
Genome editing has been considered as powerful tool in agricultural fields. However, genome editing progress in cattle has not been fast as in other mammal species, for some disadvantages including long gestational periods, single pregnancy, and high raising cost. Furthermore, technically demanding methods such as microinjection and somatic cell nuclear transfer (SCNT) are needed for gene editing in cattle. In this point of view, electroporation in embryos has been risen as an alternative.
Results
First, editing efficiency of our electroporation methods were tested for embryos. Presence of mutation on embryo was confirmed by T7E1 assay. With first combination, mutation rates for MSTN and PRNP were 57.6% ± 13.7% and 54.6% ± 13.5%, respectively. In case of MSTN/BLG, mutation rates were 83.9% ± 23.6% for MSTN, 84.5% ± 18.0% for BLG. Afterwards, the double-KO embryos were transferred to surrogates and mutation rate was identified in resultant calves by targeted deep sequencing. Thirteen recipients were transferred for MSTN/PRNP, 4 calves were delivered, and one calf underwent an induction for double KO. Ten surrogates were given double-KO embryos for MSTN/BLG, and four of the six calves that were born had mutations in both genes.
Conclusions
These data demonstrated that production of genome edited cattle via electroporation of RNP could be effectively applied. Finally, MSTN and PRNP from beef cattle and MSTN and BLG from dairy cattle have been born and they will be valuable resources for future precision breeding.
Similar content being viewed by others
Introduction
Genome editing has played a powerful role in various fields. In the same line with other fields, in livestock, genome editing tools such as ZFN, TALENs, and CRISPR/Cas9, has been applied to disease resistance, climate response, allergy free and increasing productivity [1,2,3,4,5,6,7,8]. Recently, several countries including Japan, Brazil and Australia announced that genome edited livestock with simple indel mutation was not categorized into genetically modified organisms (GMOs) because of no integration of the exogenous DNA. In USA, FDA announce that genome edited cattle will be approved to be low level risk [9]. It is believed that in response to these changes, the development of gene-editing cattle is predicted to be accelerated for improving the traits.
However, genome editing progress in cattle hasn’t been fast compared to other mammal species in that there are some issues including long gestational periods, single pregnancy, and high cost. And high skilled person with microinjection and somatic cell nuclear transfer (SCNT) technologies are needed to produce the genetically engineered cattle to date. Particularly, while SCNT with high frequency mutated somatic cells have contributed to similar embryonic developmental competence with in vitro fertilized embryos, abnormal reprogramming issues such as embryonic absorption and sudden death enable us to be hard for progress [10, 11]. Thus, to produce a live cloned offspring, many surrogate mothers are needed [3] compared to using in vitro fertilization combined with microinjection, which are reported in our paper [1, 12].
And some studies, as an alternative for microinjection, electroporation showed that it can be used for knockout animal with efficient and simple way [13,14,15]. However, although there have been in vitro studies on electroporation in cattle [13, 14], in vivo results have not yet been demonstrated. Here, we proved that double gene edited cattle were efficiently born by electroporation via ribonucleoprotein (RNP).
Methods
In vitro embryo production
In vitro oocytes were collected in the ovaries from beef or dairy slaughterhouses. Immature oocytes were matured in TCM based medium as previously reported [1]. Motile spermatozoa were selected using the Percoll gradient method as previous described. Briefly, frozen-thawed semen from F0 bull at 35 ºC was filtered by centrifugation on a Percoll discontinuous gradient (45%–90%) at 366 × g for 15 min. To produce the 45% Percoll solution, 1 mL of capacitation-Tyrode's albumin lactate pyruvate (TALP) medium was added to 1 mL of 90% Percoll. The sperm pellet was washed two times by the addition of 3 mL of the capacitation-TALP medium and was subsequently centrifuged at 366 × g for 5 min. Washed motile spermatozoa were used for IVF. Spermatozoa (1–2 × 106 sperm/mL) were incubated with mature oocytes for 18 h in 50 μL microdrops of IVF-TALP medium covered with mineral oil (Nidacon, Cat. no. NO-100) in a humidified atmosphere of 5% CO2 at 38.5 ºC. After 18 h of co-incubation, cumulus cells were removed from presumptive zygotes. The zygotes were cultured in a two-step chemically defined culture media [1, 12] that was covered in mineral oil in an atmosphere of 5% O2, 5% CO2, and 90% N2 at 38.5 °C.
Designing sgRNA and gene mutation assay
Single guide RNA (sgRNA) targeting bovine PRNP (exon3), MSTN (exon2) and BLG (exon3) was designed by Cas-Designer software (http://www.rgenome.net/cas-designer/) that showed sgRNA candidates for the target genome (Additional file 1) [1, 13]. Following the details of kit manual, the sgRNA was synthesized using Precision gRNA synthesis kit (ThermoFisher, A29377).
Gene mutation was confirmed through the T7 endonuclease 1 (T7E1) assay. For this, genome DNA was extracted by kit (Qiagen, 69504). The PCR primers (Additional file 1) for target loci (PRNP, MSTN, and BLG) was designed using PRIMER3 software (http://bioinfo.ut.ee/primer3-0.4.0), and the target sequence was amplified by polymerase chain reaction (PCR) at 94 ºC for 5 min, 35–40 cycles at 94 ºC for 20 s, at 57 ºC for 30 s, at 72 ºC for 35 s, and 72 ºC for 5 min. The PCR product from each sample was treated with T7E1 enzyme (NEB, Cat. No. M0302L) to detect gene mutations. In case of single-cell analysis, wildtype PCR product was added into all samples to detect homozygous mutation. Digested and undigested mixes were observed on a 1% agarose gel. The estimated gene modification was calculated as described previously [16].
Electroporation of RNP
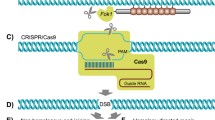
Genome Editor electroporator (BEX, GEB 15) and electrode (gap: 1.0 mm, volume: 40 µL) (BTX, 45–0104) were used for electroporation. The electrode was connected to the electroporator and was set under a stereoscopic microscope. Before electroporation, bovine zygotes were washed with Opti-MeM l (ThermoFisher, 31985062). At one time, 30–40 bovine zygotes were electroporated. Zygotes 18 h after IVF were washed with Opti-MEM I three times to remove the serum in the medium, placed in a line in the electrode gap filled with 10 µL of Opti-MEM I which is containing 200 ng/ µL of Cas9 protein (ThermoFisher, A36499) and 100 ng/µL each sgRNA, and subjected to electroporation. The electroporation condition was 15 V (3 ms ON + 97 ms OFF) × 7 times. After electroporation, the zygotes were immediately collected from the electrode chamber and subjected to four washes with TCM-199 based medium followed. The embryos were then cultured in chemically defined medium at 38.5 °C, 5% CO2, and 5% O2 in an incubator.
Primary cell culture and genomic DNA extraction
Primary cells were obtained by biopsy of the ear skin of calves. The ear skin was chopped into small pieces with a sterile scalpel and then washed several times and incubated at 38.5 °C for 4–18 h in Hank's Balanced Salt Solution (Gibco, 14175095) supplemented with collagenase (Collagenase type I, Gibco, Cat. no. 17–100–017). The following day the dispersed cells were washed several times in DMEM (Gibco, 21068028) and cultured in DMEM supplemented with 10% fetal calf serum (Gibco, GIB-11150–059), 1% penicillin/streptomycin (Gibco, Cat. no. 15140148), 1% non-essential amino acids (Gibco, 11140050), and 100 mmol/L β-mercaptoethanol (Sigma–Aldrich, M3418). Genomic DNA from primary cells was extracted using a DNA extraction kit (Qiagen, 69504). Extracted DNA was used for PCR-T7E1 assay, Cloning-Sanger sequencing and deep sequencing.
Deep sequencing
Target sites were first amplified to a size of ~1000 bp from extracted genomic DNA using Maxime™ PCR PreMix i-StarTaq (Intron biotechnology, Cat. 25167). The 1st PCR amplicons were amplified again to a size of ~220 bp through 2nd primers having custom index sequence. And 2nd PCR products also were amplified for adding adaptor sequence to NGS (Mini-seq, Illumina). Primers used in this study are listed in Additional file 2.
Then, 3rd PCR amplicons were pooling and purified using a PCR purification kit (Macherey-Nagel™ NucleoSpin®, Gel and PCR clean up, Cat. 740609). This purified library was used for NGS according to the illumina manual. The sequencing results of Mini-seq was saved as fastaq files and it could be analyzed through Cas-analyzer (www.rgenome.net).
Results
In vitro production of double gene edited embryos via RNP electroporation
MSTN/PRNP double knockout was performed three times using 320 embryos, and the development competence of blastocysts was 16.2% ± 2.8%. To analyze the mutation of the target genes, individual blastocysts were analyzed by T7E1 assay. MSTN mutation was observed in 13 blastocysts (57.6% ± 13.7%) and PRNP mutation was observed in 12 blastocysts (54.6% ± 13.5%) (Fig. 1A and B). Among them, 45.1% ± 19.5% of the embryos showed positive results for both targets. In the case of MSTN/BLG, which was tested four times, and 232 in vitro fertilized embryos were used, and the formation rate of blastocysts was 14.0% ± 4.2%. Among them, 16 blastocysts showed MSTN mutation (83.9% ± 23.6%), and 17 blastocysts showed BLG mutation (84.5% ± 18.0%) (Fig. 1C and D). In MSTN/BLG, 80.4% ± 24.3% were double positive.
In vitro production of double gene edited embryo via RNP electroporation. A MSTN/PRNP double gene edited embryos. B T7E1 assay results for mutation of MSTN (a) and PRNP (b) in MSTN/PRNP double gene edited embryos (lane 2–7). C MSTN/BLG double gene edited embryos. D T7E1 assay results for mutation of MSTN (a) and BLG (b) in MSTN/BLG double gene edited embryos (lane 2–6). M = 1 kb ladder, lane 1 = Wild type, N = negative control, P = positive control, red asterisk = lane with T7E1 positive results
Production of double gene edited cattle
Based on mutation in vitro produced embryos, one blastocyst per a recipient was transferred, and presumptive MSTN/PRNP knockout embryos were transplanted into a total of 13 recipients, and presumptive MSTN/BLG knockout embryos were transplanted into 10 recipients. When the 60-day evaluation was carried out, pregnancy was confirmed in 14 out of 23. A total of 10 calves were born from 14 pregnant individuals. Finally, four calves from MSTN/PRNP group and six calves from MSTN/BLG group were born.
To evaluate the mutation of the target genes (MSTN, PRNP and BLG), genomic DNA derived from skin tissue were analyzed by targeted deep sequencing. MSTN/PRNP mutation was observed in one calf and MSTN/BLG mutation were observed in 4 calves (Table 1). Types of alleles edited for each target loci are summarized in Fig. 2. Additionally, to clarify the efficiency of double knock-out for target genes, we checked mutation rate of each target genes in single cell level by T7E1 assay, using primary cells from 5 calves in which we confirmed presence of mutation (Table 2). In MSTN/PRNP mutated cattle, 78.38% of cells showed MSTN mutation and 24.32% of cells showed PRNP mutation with only 2.7% of cells showed MSTN/PRNP double mutation. In case of MSTN/BLG mutated calves, all tested cells showed BLG mutation with variable rate of MSTN mutation (Table 2).
To figure out off-target effect of RNP on genome, the candidate sites were analyzed for presence of mutation on the sites using targeted deep sequencing and the results showed in Additional file 3.
And blood analysis was conducted to monitor if any health problems were caused by environmental factors and/or genome editing. There were not any significant changes in blood analysis and no clinical symptoms were identified up to date (Additional file 4). Current pictures of calves are showed in Fig. 3.
Discussion
Since genome editing have been applied to eukaryotic organisms, there were big changes in biological system [17]. In agriculture fields, genome editing rapidly has been applied to plants and livestock [18]. Particularly, cattle in livestock have been important species [19]. Genome edited cattle will be used for productivity, diseases resistance, and bioreactors [20]. In this study, to improve the genetic traits, genome editing technologies may be selected, over than one genetic trait. Among methods to produce genome-edited live animal, we selected electroporation method for its simplicity. Although there are always concerns about mosaicism when editing embryos, we tried to minimize mosaicism by using RNP complex, i.e., Cas9 protein and guide RNA complex according to previous report [7, 21].Three targeted loci were selected, that is, MSTN for productivity, PRNP for disease resistance, and BLG for allergy free milk. For beef cattle, MSTN/PRNP mutated calves were born and for dairy cattle, MSTN/BLG mutated calves were born via RNP electroporation. Currently, we analyzed genotyping from all the double knockout calves (Table 1) and in MSTN, where its phenotyping can be easily detected, it began to be observed (Fig. 3). Other phenotyping will be analyzed later because BLG should be analyzed after pregnancy and for PRNP, its test should be evaluated in the continuously follow up.
As summarized in Fig. 2, there was a unique fact in the genotyping analysis. In previous study, the MSTN mutated cattle showed only −12 bps [1]. However, in this study, although another gene delivery was applied into zygotes via RNP, −12 bps genotyping was dominantly identified as well, and new genotyping (1, 25 bps insertion and 3 bps deletion) was observed. In more details, no more than 2 genotyping was identified in calves with MSTN mutations (Fig. 2). However, three genotyping were observed in PRNP locus, and 3 or more mutations were observed in BLG except for one mutated calf. According to our results so far, −12 bp is dominantly observed in MSTN knockout cattle through microinjection or electroporation on IVF embryos. In the future, we will continue to monitor the results of knockout genotyping in vivo results through various gene loci. And we found very low percentage of off-target mutation in #5 (Additional file 3). After the offspring become at over than 12 months old, the genotyping and off-target analysis in their germ cells will be followed.
One interesting point is about double knockout event. In dairy breed calves, in which MSTN and BLG were targeted, there were high double knockout efficiency in randomly selected single cell colony analysis. However, in beef breed MSTN/PRNP KO calf, double knockout event in the offspring was very low while its event was comparable with that of MSTN/BLG in blastocyst stage. Because there is only one offspring with MSTN/PRNP KO, it is difficult to determine whether this occurrence was caused by specific target gene locus or breed/individual predisposition. We intend to conduct relevant research in the near future.
Recently, a genome edited organism without any recombinant DNA integration can be classified as non-GMO in many countries [9]. For instance, in Japan, MSTN edited fish is approved to be edible as a food chain [21] and in USA, low-risk determination was made for marketing genome-edited cattle [22]. In the case of animals, safety analysis is not necessary, and their health can be considered an indicator of animal product safety [23]. We were able to find no special complications or significant changes in blood analysis when we assessed the health of animals born as double knockouts, indicating that they are currently growing healthily. As a result, it is expected that the safety of meat and milk from these sources will be unaffected in the future.
Conclusions
In conclusion, these data demonstrated that genome editing on bovine embryos via electroporation of RNP could be effectively applied and proved. Finally, beef cattle with MSTN and PRNP mutation and dairy cattle with MSTN and BLG mutation have been born and they will be valuable resources for future precise breeding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BLG:
-
Beta-lactoglobulin
- Cas9:
-
CRISPR associated protein 9
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- GMO:
-
Genetically modified organism
- IVF:
-
In vitro fertilization
- KO:
-
Knock-out
- MSTN:
-
Myostatin gene
- PRNP:
-
Prion protein gene
- RNP:
-
Ribonucleoprotein
- SCNT:
-
Somatic cell nuclear transfer
- sgRNA:
-
Single guide RNA
- T7E1:
-
T7 endonuclease I
- TALEN:
-
Transcription activator-like effector nucleases
- TALP:
-
Tyrode’s albumin lactate pyruvate
- ZFN:
-
Zinc-finger nuclease
References
Gim GM, Kwon DH, Eom KH, Moon JH, Park JH, Lee WW, et al. Production of MSTN-mutated cattle without exogenous gene integration using CRISPR-Cas9. Biotechnol J. 2022;17(7):e2100198.
Lee K, Uh K, Farrell K. Current progress of genome editing in livestock. Theriogenology. 2020;150:229–35.
Luo J, Song Z, Yu S, Cui D, Wang B, Ding F, et al. Efficient generation of myostatin (MSTN) biallelic mutations in cattle using zinc finger nucleases. PLoS ONE. 2014;9(4):e95225.
Proudfoot C, Carlson DF, Huddart R, Long CR, Pryor JH, King TJ, et al. Genome edited sheep and cattle. Transgenic Res. 2015;24:147–53.
Su F, Wang Y, Liu G, Ru K, Liu X, Yu Y, et al. Generation of transgenic cattle expressing human beta-defensin 3 as an approach to reducing susceptibility to Mycobacterium bovis infection. FEBS J. 2016;283(5):776–90.
Wang K, Ouyang H, Xie Z, Yao C, Guo N, Li M, et al. Efficient generation of myostatin mutations in pigs using the CRISPR/Cas9 system. Sci Rep. 2015;5:16623.
Whitworth KM, Rowland RRR, Ewen CL, Trible BR, Kerrigan MA, Cino-Ozuna AG, et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat Biotechnol. 2016;34(1):20–2.
Wu H, Wang Y, Zhang Y, Yang M, Lv J, Liu J, et al. TALE nickase-mediated SP110 knockin endows cattle with increased resistance to tuberculosis. Proc Natl Acad Sci U S A. 2015;112(13):E1530–9.
Van Eenennaam AL, Wells KD, Murray JD. Proposed U.S. regulation of gene-edited food animals is not fit for purpose. npj Sci Food. 2019;3:3.
Gouveia C, Huyser C, Egli D, Pepper MS. Lessons learned from somatic cell nuclear transfer. Int J Mol Sci. 2020;21(7):2314.
Thuan NV, Kishigami S, Wakayama T. How to improve the success rate of mouse cloning technology. J Reprod Dev. 2010;56(1):20–30.
Yum SY, Lee SJ, Kim HM, Choi WJ, Park JH, Lee WW, et al. Efficient generation of transgenic cattle using the DNA transposon and their analysis by next-generation sequencing. Sci Rep. 2016;6:27185.
Gim GM, Uhm KH, Kwon DH, Kim MJ, Jung DJ, Kim H, et al. Germline transmission of MSTN knockout cattle via CRISPR-Cas9. Theriogenology. 2022;192:22–7.
Camargo LSA, Owen JR, Van Eenennaam AL, Ross PJ. Efficient one-step knockout by electroporation of ribonucleoproteins into zona-intact bovine embryos. Front Genet. 2020;11:570069.
Chen S, Lee B, Lee AYF, Modzelewski AJ, He L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein rlectroporation of zygotes. J Biol Chem. 2016;291(28):14457–67.
Guschin DY, Waite AJ, Katibah GE, Miller JC, Holmes MC, Rebar EJ. A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol. 2010;649:247–56.
Wang JY, Doudna JA. CRISPR technology: A decade of genome editing is only the beginning. Science. 2023;379(6629):eadd8643.
Platani M, Sokefun O, Bassil E, Apidianakis Y. Genetic engineering and genome editing in plants, animals and humans: Facts and myths. Gene. 2023;856:147141.
Van Eenennaam AL. Application of genome editing in farm animals: cattle. Transgenic Res. 2019;28(Suppl 2):93–100.
Yum SY, Youn KY, Choi WJ, Jang G. Development of genome engineering technologies in cattle: from random to specific. J Anim Sci Biotechnol. 2018;9:16.
Japan embraces CRISPR-edited fish. Nat Biotechnol. 2022;40:10. https://doi.org/10.1038/s41587-021-01197-8.
FDA news. FDA makes low-risk determination for marketing of products from genome-edited beef cattle after safety review. 2022. https://www.fda.gov/news-events/press-announcements/fda-makes-low-risk-determination-marketing-products-genome-edited-beef-cattle-after-safety-review.
Trott JF, Young AE, McNabb BR, Yang X, Bishop TF, Van Eenennaam AL. Animal health and food safety analyses of six offspring of a genome-edited Hornless bull. GEN Biotechnology. 2022;1(2):192–206.
Acknowledgements
We thank the members of the Goo Jang lab and Gyeonggi-do Animal Hygiene Testing Center for their valuable comments and the technical support.
Funding
This study was financially supported by the National Research Foundation of Korea (NRF-2021R1A5A1033157 for SRC program: 382 Comparative medicine Disease Research Center; NRF-2021R1F1A105195313), the Research Institute of Veterinary Science, the BK21 Four for Future Veterinary Medicine Leading Education and Research Center, and a Seoul National University (SNU) grant (#550e2020005).
Author information
Authors and Affiliations
Contributions
GJ devised the experiment. GMG and JHL produced and analyzed embryos for mutation. DJJ, DHK and JKY prepared surrogates and WWL transferred embryos. GMG, KHE and DHK collected the samples and analyzed mutation with WJS, which was supervised over by SYY. GMG and EKH merged whole data and prepared the manuscript which was edited by GJ. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee (SNU-180403–1) and performed under the guideline of Seoul National University. All animal practices such as blood collection or embryo transfer was carried out by a veterinarian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1
. List of guide RNA and detecting PCR primer sequences for each target genes.
Additional file 2
. List of primer sequences for deep sequencing of each target genes and off-target sites.
Additional file 3.
Off-target effect detection for gene edited calves.
Additional file 4.
Blood analysis of calves with gene mutation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gim, GM., Eom, KH., Kwon, DH. et al. Generation of double knockout cattle via CRISPR-Cas9 ribonucleoprotein (RNP) electroporation. J Animal Sci Biotechnol 14, 103 (2023). https://doi.org/10.1186/s40104-023-00902-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-023-00902-8