Abstract
Background
The quality and yield of cashmere fibre are closely related to the differentiation and development of secondary hair follicles in the skin of cashmere goats. The higher the density of secondary hair follicles, the higher the quality and yield of cashmere from the fleece. Development of secondary hair follicles commences in the embryonic stage of life and is completed 6 months after birth. Preliminary experimental results from our laboratory showed that melatonin (MT) treatment of goat kids after their birth could increase the density of secondary hair follicles and, thus, improve the subsequent yield and quality of cashmere. These changes in the secondary hair follicles resulted from increases in levels of antioxidant and expression of anti-apoptotic protein, and from a reduction in apoptosis. The present study was conducted to explore the molecular mechanism of MT-induced secondary hair follicle differentiation and development by using whole-genome analysis.
Results
MT had no adverse effect on the growth performance of cashmere kids but significantly improved the character of the secondary hair follicles and the quality of cashmere, and this dominant effect continued to the second year. Melatonin promotes the proliferation of secondary hair follicle cells at an early age. The formation of secondary hair follicles in the MT group was earlier than that in the control group in the second year. The genome-wide data results involved KEGG analysis of 1044 DEmRNAs, 91 DElncRNAs, 1054 DEcircRNAs, and 61 DEmiRNAs which revealed that the mitogen-activated protein kinase (MAPK) signaling pathway is involved in the development of secondary hair follicles, with key genes (FGF2, FGF21, FGFR3, MAPK3 (ERK1)) being up-regulated and expressed. We also found that the circMPP5 could sponged miR-211 and regulate the expression of MAPK3.
Conclusions
We conclude that MT achieves its effects by regulating the MAPK pathway through the circMPP5 sponged the miR-211, regulating the expression of MAPK3, to induce the differentiation and proliferation of secondary hair follicle cells.
In addition there is up-regulation of expression of the anti-apoptotic protein causing reduced apoptosis of hair follicle cells. Collectively, these events increase the numbers of secondary hair follicles, thus improving the production of cashmere from these goats.
Similar content being viewed by others
Introduction
Cashmere fibres form the fleece of cashmere goats and produce a high-grade textile material with considerable economic value. Internationally, China, Mongolia and India are the main producers of cashmere [1]. The quality of cashmere is closely related to the diameter of individual fibres: the finer their diameter the higher the quality of the material. Hair follicles are subsidiary structures of the skin, categorised as either primary or secondary hair follicles. Primary hair follicles of cashmere goats produce coarse hair fibres of no commercial value, whereas secondary hair follicles produce the valuable cashmere fibres [2, 3]. Growth of cashmere fibre follows a seasonal pattern with growth from the secondary hair follicles commencing after the summer solstice but slowing down and eventually ceasing when daily photoperiods increase following the winter solstice. Differentiation and development of primary and secondary hair follicles commences during the embryonic stages of development of goats. The primary hair follicles are differentiated from epithelial cells, whereas secondary hair follicles branch off from the epidermal cell layer of the skin near the primary hair follicles. Primary hair follicles are fully developed at birth of the goats and the secondary hair follicles reach their full development at about 6 months of age (Fig. 1) [4, 5]. Formation of cashmere fibre ensues when the secondary hair follicles commence their cyclical pattern of activity [6]. For cashmere goats in Inner Mongolia, this means that formation of cashmere commences each year in July, stops growing from February to March in the following year, and is naturally shed from the skin by the end of April. Thereafter, from May to July, there is a non-growing cashmere period.
Proliferation and development of hair follicles in fetal and newborn cashmere goats. 45–75 d post conception: proliferation and development of primary hair follicles. 75–125 d post conception: adjacent structures appear; proliferation and development of secondary (cashmere) hair follicles, initiation of primary fibre production. 125 d post conception to birth: secondary hair follicles continue to proliferate; some cashmere fibre production. Birth to 6 months of age: the key period of secondary hair follicle growth
Melatonin (MT) is a neuroendocrine hormone involved in seasonal regulation of events such as the hair follicle growth cycle and has been applied to improve fur production in species such as fox, mink and rabbits [7,8,9]. MT has been used in goats to promote growth of cashmere fibre, but this has been complicated by alteration of the shedding process [10, 11]. Studies with administration of MT have been conducted by the cashmere goat team at China Agricultural University for nearly 20 years. They have used MT on adult cashmere goats successfully to: induce growth of secondary hair follicles, advance the onset of cashmere fibre growth, reduce the non-growing cashmere period, solve the problem of cashmere shedding, and improve cashmere yield and quality [12]. The greater the density of secondary hair follicles and the finer the fibre diameter, the better the yield and quality of the cashmere fleece. For goat kids, the period from birth to 6 months of age is critical for development of secondary hair follicles, and the number of fixed secondary hair follicles produced during this time determines the final number of secondary hair follicles, and thus the magnitude of the cashmere yield in adulthood. Preliminary results from our laboratory showed that treatment of young goats with MT shortly after birth: enhanced antioxidant levels in blood and skin, increased expression of the anti-apoptotic protein, and reduced apoptosis of hair follicle cells, thus increasing the number of secondary hair follicles and improving the quality and yield of cashmere [13]. We propose that MT increases the number of secondary hair follicles in goat kids in two ways: by promoting differentiation and proliferation of secondary hair follicle cells and by reducing the occurrence of apoptosis in these cells.
With the development of genomics, more studies show that non-coding RNA plays an important role in the development of hair follicle, and miRNA/lncRNA can directly or indirectly regulate the occurrence of hair follicle through targeted signaling pathways [14, 15]. However, there are few studies on circRNA in hair follicle development. Reports show that circRNA has a sponge effect on miRNA, and then regulates the expression of target genes [16, 17]. Therefore, based on the previous research, this study used genome-wide technology to explore the expression of non-coding genes and coding genes under MT intervention, and revealed the potential signaling pathways in which MT may be involved in secondary hair follicle growth and development. In addition, we developed corresponding tracking plans to study the long-term effects of MT on the growth of hair follicles and production of cashmere fibre in these cashmere goats.
Materials and methods
Animals and management
Thirty-two newborn cashmere goat kids with similar live weights (mean 3.00 ± 0.2 kg) were randomly allocated to two groups (n = 16); melatonin (MT) and control. Goats in the MT group were implanted s.c. with melatonin pellets to provide a dose of 2 mg/kg on d 1 from birth and at 2 and 4 months of age. The sustained release period of these pellets lasts for 60 d and the dosage is based on results of our previous studies [13]. Goats in the control group did not receive any treatment. From birth the goat kids were suckled by their dams that had lucerne and water freely available with supplementary starter feed (200 g/d, see Additional file 1: Table S1) provided as required. This study was conducted on the Yiwei white cashmere goat breeding farm at Ordos, Inner Mongolia, China (39°11′ N,107°16′ E).
Live weight and collection of cashmere and skin samples
Live weight of the animals was recorded at birth, during the MT controlled-release period at 3 and 6 months of age, and at 1 and 2 years of age. Cashmere was harvested by combing at 3 and 6 months of age and after the first and second year of growth. Measurements of cashmere performances were conducted as described by Duan et al. [12]. Quadruplicate samples of skin were collected from the upper flank and right shoulder at 1 day of age, at 2, 4, and 6 months of age, and in the second year. Duplicate samples were placed immediately in liquid nitrogen for subsequent storage at −80 ℃ until they were subjected to transcriptome and biomolecular analyses. Microscopy with Sacpic staining was employed with the other duplicate samples to examine the morphology of hair follicles and to count their numbers. The main indices determined from the microscopic examination using procedures described by Yang et al. [13].
Immunofluorescence analysis
Paraffin sections of skin samples underwent antigen repair using EDTA antigen repair buffer (pH 8.0) after deparaffinization and rehydration. Blocking was performed with hydrogen peroxide and rabbit serum, the corresponding primary and secondary antibodies were added (Ki-67—an indicator of mitotic activity (1:100, Abcam, Cambridge, UK), K-14—a keratin (1:500, Covance, Princeton, NJ, USA), Fn1 (1:500, Servicebio, Wuhan, China), Wnt-10a (1:500, Sanying, Wuhan, China)), then DAPI dye solution was added for DAPI re-staining of cell nuclei, and the preparations were sealed. Fluorescence of the stained sections was analyzed with a TCS SPE confocal microscope (Leica Microsystems, Bannockburn, IL, USA).
Transcriptome analysis
The skin samples collected at 4 months of age underwent high-throughput sequencing analysis. RNA isolation, library construction, RNA sequencing, and analysis accomplished by Majorbio Co., Ltd. (Shanghai, China). The Majorbio cloud platform and software were used for analysis the items according to relevant literature and instructions [18,19,20,21,22].
RT-qPCR and western blotting
The RT-qPCR was performed using a Fluorescence Quantitative PCR Kit (Takara Bio, Kusatsu, Shiga, Japan). For analysis of RNA expression, RT-qPCR was carried out using the primer (Tsingke Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s instructions. Levels of mRNA, microRNA and circulating RNA expression were calculated by 2−ΔΔCt method. The proteins extracted from skin tissue blocks were quantified using the bicinchoninic acid method (BCA protein assay) and adjusted to the same concentration as the candidate proteins. Proteins were loaded onto the SDS-PAGE gel, electrophoresed, and analyzed by Western blotting using antibodies against the protein under test. The Western blotting results were visualized using a high-sensitivity chemiluminescence detection kit (Beyotime, Shanghai, China), and the X-ray film was exposed for developing and photographing.
Luciferase gene reporter assay
Primers for amplifying target genes and target gene 3′-untranslated regions (UTRs) were based on gene sequences in Gen Bank, and 3′-UTR sequences were amplified by PCR using cashmere goat genomic DNA as template. PCR products were cloned into the dual-luciferase reporter gene vector to construct the wild-type plasmid. The target sequence of chi-miR-211 in the mitogen-activated protein kinase (MAPK3) gene was mutated to construct mutant plasmids. Finally, expression of the luciferase reporter was measured, and the target sites of miRNAs in the transfected 3′-UTRs were analyzed. The plasmid and chi-miR-211 mimics were synthesized by Hanheng Biotechnology Co., Ltd. (Shanghai, China). For the circRNA test, 293 T cells were co-transfected with wild type (WT) or mutant circMPP5 and miR-211-mimics or negative control-mimics (NC-mimics) using Lipofectamine 3000. Renilla luciferase activity was normalized to Firefly luciferase. After transfection for 48 h, cells were subjected to dual-luciferase analysis.
Statistical analyses
The SPSS27.0 and the GraphPad 9.0 were employed in this study. Results were expressed as mean value ± SD. Data on the cashmere performances and parameters related to hair follicle by Student’s t-test. Difference was considered significant at P < 0.05.
Results
Growth performance
There was no effect of the melatonin treatment on live weight at any of the times the animals were weighed (Fig. 2). At the end of the MT treatment period (6 months of age) mean live weights were 15.93 ± 0.96 and 15.70 ± 1.08 kg for treated and control groups, respectively (P > 0.05, Fig. 2).
Cashmere production
Treatment of the goat kids with MT generated an improvement in cashmere growth that was visible in relation to that in the control animals (Fig. 3A). Following treatment with MT, cashmere fibre diameter was reduced by 1.73 μm at the first year (P < 0.01) and by 0.68 μm in the second year (P < 0.05) (Fig. 3B). The staple length was increased on all four occasions and differing by 1.46 cm in the second year (P < 0.05) (Fig. 3C). The pattern of onset of cashmere fibre growth, which was monitored by counting the numbers of goats with growing fleeces throughout the second year after the MT treatment (Fig. 3D), showed an advance of onset of about one month in the treated goats. As well as effects on fibre quality and onset of growth, MT increased the yield of cashmere in both years following treatment, by about 252 g in the first year’s combing (P < 0.01) (Fig. 3E). The increase was about 115 g in the second year (P < 0.05), when the overall yield was lower (Fig. 3E).
Effects of melatonin on cashmere growth. A Photographs of selected animal’s fleeces (CK—control, MT—melatonin treated) at 1st (1) and 2nd (2) combing. B Cashmere fibre diameter of MT-treated and control goats at different times following treatment. C Cashmere fibre length of MT-treated and control goats at different times following treatment. D Number of control and MT-treated goats initiating fibre growth at different times during the second year following treatment. E Cashmere yield of MT-treated and control goats at each of the first 2 years following treatment. Values represent means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 MT-treated vs. controls
Primary and secondary hair follicles
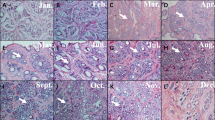
Representative photomicrographs of hair follicles are provided in Fig. 4. Consecutive representative photomicrographs from skins of MT-treated and control goats taken at each of the seven sampling stages are shown in Fig. 5 and 6. These figures provide visual impressions of the effects of MT treatment that are summarized graphically in Fig. 7. In the case of primary skin follicle density, there was no difference between treated and controls at any stage (Fig. 7A), the value being higher at 1 day of age for both groups than thereafter. However, in the case of secondary skin follicles, MT treatment of the goats led to improvements in the parameters recorded here (Fig. 7B–F). Apart from the day when the MT implants were applied (day 1 of age), MT treatment increased the measures of density of secondary skin follicles at almost all other sampling times (Fig. 7B–E). The ratio of secondary to primary skin follicles was increased invariably by treatment with MT (Fig. 7F).
Hair follicle density, activity and ratio of secondary:primary hair follicles of MT-treated and control goats recorded from birth to 18 months of age. A Primary hair follicle density. B Secondary hair follicle density. C Secondary hair follicle total number. D Secondary hair follicle density index. E Mature or active hair follicle density. F The ratio (S:P) of secondary hair follicles to primary hair follicles.Values represent means ± SD. Different symbols (*, **) above the bars indicate level of significance of differences (MT-treated vs. controls, *P < 0.05, **P < 0.01)
Proliferation of hair follicle cells
The results of Ki-67, K-14, Wnt-10a and Fn1 genes in the CK groups showed significant differences. Based on these gene results, protein expression was analyzed. The optical signal of immunofluorescence arising from Ki-67, an indicator of mitotic activity, was much stronger in paraffin sections from skin of the MT-treated animals than in those from controls. Likewise, the keratin, K-14, produced a stronger signal in MT-treated animals indicating enhanced cell differentiation in this group, and similar result were observed for Wnt-10a, while Fn1 shows the opposite result. The results of western blot (WB) also verified the protein expression again (Fig. 8B, C).
Melatonin promoting the proliferation and differentiation of secondary hair follicle cells. A The q-PCR and RNA-seq of Ki-67, K-14, Wnt-10a and Fn1. B and C The fluorescence signal DAPI (blue), Ki-67 and Fn1 (red), K-14 and Wnt-10a (green), merge in the transection ( CK-1 and MT-2) and longitudinal section ( CK-3 and MT-4), Scale bar = 100 μm, and another is WB. *P < 0.05, **P < 0.01
MAPK signaling pathway
Data for the various RNAs investigated in this study are listed in Additional file 1: Table S2 and S3. The results showed that there were 1044 DEmRNAs (609 upregulation and 435 downregulation), 91 DElncRNAs (46 upregulation and 45 downregulation), 1054 DEcircRNAs (484 upregulation and 570 downregulation), and 61 DEmiRNAs (33 upregulation and 28 downregulation) in the control group compared with the MT group, respectively. The volcano plots and the heat maps for these are presented in Fig. 9. Using the KEGG enrichment analysis to determine the signaling pathways that involve the DEmRNAs, specific enrichment of genes was observed for signaling pathways including: regulating pluripotency of stem cells, MAPK signaling pathway, calcium signaling pathway, endocrine resistance, PI3k-Akt signaling pathway, protein digestion and absorption, hippo signaling pathway, melanogenesis, pathways of neurodegeneration-multiple diseases, Alzheimer’s disease (Fig. 10A). In the MAPK pathway, key genes such as fibroblast growth factor-2 (FGF2), FGF21, FGFR3 and MAPK3 were up-regulated (Fig. 10B). The results of q-PCR and western blotting (Fig. 10C and D) showed that the protein expression of several essential genes in the MAPK pathway in the MT group was higher than those in the controls.
MAPK3, a key gene in the MAPK pathway, proved to be a target gene of miR-211 (Fig. 11A). Levels of gene expression (determined from q-PCR) of miR-211 were higher for controls than for MT-treated goats whereas in the case of MAPK3, gene expression was greater in the MT group (Fig. 11B and C) and this accounts for the negative correlation between expression levels of these genes (Fig. 11D). Sequence alignment of miR-211 with MAPK3 revealed that miR-211 possessed binding sites for MAPK3-3′-UTR, and MAPK3 and these can be confirmed as potential target genes of miR-211. From the dual-luciferase reporter gene assay system, it was shown that miR-211 downregulated (P < 0.01) expression of the wild-type MAPK3-3′-UTR, indicating binding between the two molecules, and this downregulation effect disappeared after two mutations (P > 0.05), indicating that the mutation was successful (Fig. 11E). In addition, analysis of the DEcircRNA data to explore the interaction between circRNA and miR-211 under the intervention of MT showed that circRNAs such as circ_15_6990054_6994382, circMPP5, circ_5_26960151, circATE1 and circPI3R4, had targeting ability with miR-211 in the MT-treated animals (Fig. 12A, B). q-PCR data showed that levels of circRNA differed between MT-treated and control groups (Fig. 12C) and there was a negative correlation between circMPP5 and miR-211 (Fig. 12D). The dual luciferase reporter gene results showed that miR-211 reduced circMPP5 luciferase intensity in comparison with the mutated (Mut) vector (Fig. 12E). The circMPP5 was positively correlated with MAPK3 (Fig. 12F).
The miR-211 regulates the MAPK3. A Schematic illustration showing the target MAPK3 of DEmiRNA from MT and CK group. B and C Quantitative real-time PCR assays of miR-211 and MAPK3 in MT and control group. D Correlation analysis of miR-211 and MAPK3 expression. E Predicted complementary binding sites between miR-211 and MAPK3, and verified by luciferase activity assay. **P < 0.001, ***P < 0.01
CircMPP5 serves as a sponge for miR-211. A Potential circRNAs that can regulate miR-211. B circMPP5. C Quantitative real-time PCR assays of circMPP5 in MT and control group. D Correlation analysis of circMPP5 and miR-211. E Predicted complementary binding sites between circMPP5 and miR-211, and verified by luciferase activity assay. F Correlation analysis of circMPP5 and MAPK3 expression. **P <0.01, ***P <0.001
Discussion
This study was conducted to investigate the potential mechanisms via which treatment of young goats with MT is able to promote the differentiation and development of secondary hair follicles in their skin. The results show that MT regulates the MAPK pathway in goats by activating the circMPP5/miR-211/MAPK3 to promote the differentiation and proliferation of secondary hair follicles, thereby improving the quality and yield of cashmere. Furthermore, this MT-treatment effect occurred in both the current and subsequent cashmere growing seasons indicating a potential enhancement of lifetime cashmere production from these goats.
As in other studies where MT has been used to enhance fibre production in goats and rabbits [23,24,25] the present results confirm this is achieved without effect on live weight or growth performance of the animals. As a textile fibre, the quality of cashmere is improved by reducing fibre diameter and increasing its length [26, 27] and the improvements in these parameters generated here by the treatment with MT has also been recorded in our earlier studies and by others [28, 29]. These effects of MT are achieved by advancing the onset of secondary follicle activity and by increasing the total number of active secondary hair follicles [30, 31] as can be deduced from the various indices of secondary follicle numbers and activity recorded here. Persistence of the fibre growth improvement into the second growth season following MT did not occur in our earlier study [32], so this finding may not be a consistent outcome of the treatment. Likewise, there is no published evidence of the treatment affecting fibre production by offspring of treated animals [33, 34].
Release of endogenous MT from the pineal gland of adult animals is regulated by changes in daily photoperiod [35] which explains why the naturally-occurring changes in fibre production are linked to seasons of the year. However, differentiation and development of hair follicles in goats begins before birth and is largely completed by about 6 months of age, prior to entering the annual hair growth cycle [8, 10, 36]. This explains why treatment of the goats with MT at birth is able to influence the subsequent cashmere growth cycles. Specific protein markers (biomarkers) of proliferation of secondary hair follicle cells such as keratin 14 (K-14) [37, 38] and Wnt10a [39,40,41,42,43] and their genes were elevated in the MT group whereas expression and protein levels of fibrinolectin (Fn1), an indicator of cell maturation [44,45,46], were lowered.
Whole transcriptome sequencing of extracts from the skin involving epithelial regions where actively growing hair follicles are located [47, 48] may provide markers for breed selection [49] and, importantly for the present study, can reveal gene expression activated by the MT treatment applied in this study, especially those relating to secondary hair follicles [50, 51]. The MAPK signaling pathway is one of the aggregation pathways of cellular information transmission induced by extracellular signals, including extracellular growth factor, extracellular growth factor receptor, and intracellular factors [52, 53]. As well as MT, insulin, platelet growth factor receptor and fibroblast growth factors (FGFs) can affect hair follicle growth by regulating the MAPK signaling pathway [54,55,56]. FGF20 controls the formation of secondary hair follicles and there is a high expression of FGF2, FGF21 and FGF-receptor genes during growth of secondary hair follicles [57, 58]. Extracellular signal-regulated kinases (ERKs, also known as MAP kinases) are vital proteins in the differentiation, proliferation and survival of epidermal stem cells [59,60,61]. In the present study, genes for the key extracellular and intracellular factors, FGF2, FGF21, FGFR, and MAPK3 (ERK1) in the MAPK pathway showed an up-regulation trend following MT treatment, providing strong evidence that MT exerts its influence on secondary hair follicles via effects on the MAPK pathway.
There is an increasing body of evidence for miRNA having a key role in the early development of hair follicles [62, 63]. Ocu-miR-205 can promote hair follicles from the anagen to catagen stage by regulating the expression of genes for proteins in the notch and bone BMP signaling pathways [64]. Other studies showed that miRNA-203, miRNA-214 and miRNA-195-5p express the genes which regulate the development of hair follicles in cashmere goats [65, 66] and that MT can influence these miRNAs in the hair follicles of cashmere goats [67, 68]. miRNA-211 can regulate the MAPK3 pathway negatively, so it is in keeping with its role in this pathway that MT reduced miR-211 in the present study. CircRNA can compete with miRNA to regulate gene expression [69] and is a component of circRNA/miRNA/mRNA involved in regulatory networks as diverse as hepatocellular carcinoma (HCC), contraction of skeletal muscle [70] and proliferation of myoblasts [71]. Differentially expressed circRNAs have been studied in hair follicles of cashmere goats [72] and Angora rabbits [73]. The targeted binding relationship between miRNA-27b-3p and circRNA3236, miRNA-16b-3p and miR-16b-3p revealed that circRNA regulates gene expression by binding miRNA, thereby controlling hair follicle proliferation and fibre production in cashmere goats [74]. The present study adds to this body of knowledge by its examination of the differentially expressed circRNAs under the stimulatory influence of MT. The results show that MT in goats can competitively bind miR-211 through circMPP5 thus enhancing expression of the target gene MAPK3 and promoting differentiation of secondary hair follicle cells.
Conclusion
In this study, the results showed that MT can mediate regulation of the MAPK pathway via the circMPP5 sponged the miR-211, regulating the expression of MAPK3, to promote the proliferation and differentiation of secondary hair follicle cells in goat kids (Fig. 13). In conjunction with findings of our earlier studies, results of the current study indicate that MT increases the number of secondary hair follicles in goat kids in two ways: (a) by promoting their differentiation and proliferation, and (b) by reducing the occurrence of apoptosis. The improvement in cashmere fibre quality and yield resulting from treatment of newborn goat kids with MT occurred in the cashmere growth season following insertion of the MT implants and persisted into the following year’s growth period.
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- circRNA:
-
CircularRNA
- DAPI:
-
2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride
- DE RNA:
-
Different express RNA
- ERK:
-
Extracellular regulated protein kinase
- FGF:
-
Fibroblast growth factor
- FGFR:
-
Fibroblast growth factor receptor
- Fn-1:
-
Fibronectin-1
- GO:
-
Gene Ontology
- K-14:
-
Keratin 14
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- lncRNA:
-
Longnon-codingRNA
- mRNA:
-
MessengerRNA
- miRNA:
-
MicroRNA
- MT:
-
Melatonin
- MAPK:
-
Mitogen-activated protein kinase
- PI3K-Akt:
-
Phosphatidylinositol 3-kinase-protein kinase
- SD:
-
Standard Deviation
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- WT:
-
Wild type
- Wnt-10a:
-
Wingless-type MMTV integration site family, member 10a
References
Berger J, Buuveibaatar B, Mishra C. Globalization of the cashmere market and the decline of large mammals in central asia. Conserv Biol. 2013;27(4):679–89. https://doi.org/10.1111/cobi.12100.
Hu S, Zhai P, Chen Y, Zhao B, Yang N, Wang M, et al. Morphological characterization and gene expression patterns for melatonin pigmentation in Rex rabbit. Biochem Genet. 2019;57(5):734–44. https://doi.org/10.1007/s10528-019-09929-x.
Han W, Li X, Wang L, Wang H, Yang K, Wang Z, et al. Expression of fox-related genes in the skin follicles of inner mongolia cashmere goat. Asian Australas J Anim Sci. 2018;31(3):316–26. https://doi.org/10.5713/ajas.17.0115.
Lv X, Chen L, He S, Liu S, Liu M. Effect of nutritional restriction on the hair follicles development and skin transcriptome of Chinese merino sheep. Animals(Basel). 2020;10(6):1058. https://doi.org/10.3390/ani10061058.
Schinckel PG. The post-natal development of the skin follicle population of a strain of Merino sheep. Aust J Agr Res. 1955;6(1):68–76. https://doi.org/10.1071/AR9550068.
Parry AL, Norton BW, Restall BJ. Skin follicle development in the Australian cashmere goat. Aust J Agr Res. 1992;43(4):857–70. https://doi.org/10.1071/AR9920857.
Allain D, Malpaux B, Puechal F, Thebault RG, De Rochambeau H, Chemineau P, et al. Genetic variability of the pattern of night melatonin blood levels in relation to coat changes development in rabbits. Genet Sel Evol. 2004;36(2):207–16. https://doi.org/10.1186/1297-9686-36-2-207.
Ntoumi F, Martinet L, Mondain-Monval M. Effects of melatonin treatment on the gonadotropin-releasing hormone neuronal system and on gonadotropin secretion in male mink, Mustela vison, in the presence or absence of testosterone feed back. J Pineal Res. 1994;16(1):18–25. https://doi.org/10.1111/j.1600-079X.1994.tb00077.x.
Sergina S, Baishnikova I, Ilyukha V, Lis M, Lapinski S, Niedbala P, et al. Comparison of the antioxidant system response to melatonin implant in raccoon dog (Nyctereutes procyonoides) and silver fox (Vulpes vulpes). Turk J Vet Anim Sci. 2013;37(6):641–6. https://doi.org/10.3906/vet-1302-48.
Mitchell RJ, Betteridge K, Gurnsey MP, Welch RAS. Fibre growth of cashmere-bearing goats given melatonin in late autumn and winter. New Zeal J Agr Res. 1991;34(4):419–25. https://doi.org/10.1080/00288233.1991.10417686.
Cong Y, Deng H, Feng Y, Qian C, Yu S. Melatonin implantation from winter solstice could extend the cashmere growth phase effectively. Small Ruminant Res. 2011;99(1):48–53. https://doi.org/10.1016/j.smallrumres.2011.03.055.
Duan CH, Xu JH, Zhang Y, Jia ZH, Zhang W, Plaizier J. Melatonin and cashmere growth in Inner Mongolian cashmere goats. Can J Anim Sci. 2016;96(2):108–13. https://doi.org/10.1139/cjas-2015-0018.
Yang CH, Xu JH, Ren QC, Duan T, Mo F, Zhang W, et al. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J Pineal Res. 2019;67(1):e12569. https://doi.org/10.1111/jpi.12569.
Zhao B, Chen Y, Yang N, Chen Q, Bao Z, Liu M, et al. Mir-218-5p regulates skin and hair follicle development through wnt/beta-catenin signaling pathway by targeting sfrp2. J Cell Physiol. 2019;234(11):0329–41. https://doi.org/10.1002/jcp.28633.
Zheng Y, Wang Z, Zhu Y, Wang W, Bai M, Jiao Q, et al. Lncrna-000133 from secondary hair follicle of cashmere goat: identification, regulatory network and its effects on inductive property of dermal papilla cells. Anim Biotechnol. 2020;31(2):122–34. https://doi.org/10.1080/10495398.2018.1553788.
Zhang J, Liu H, Hou L, Wang G, Zhang R, Huang Y, et al. Circular rna_larp4 inhibits cell proliferation and invasion of gastric cancer by sponging mir-424-5p and regulating lats1 expression. Mol Cancer. 2017;16(1):151. https://doi.org/10.1186/s12943-017-0719-3.
Sand M, Bechara FG, Gambichler T, Sand D, Bromba M, Hahn SA, et al. Circular rna expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2016;83(3):210–8. https://doi.org/10.1016/j.jdermsci.2016.05.012.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. https://doi.org/10.1038/nmeth.3317.
Li B, Dewey CN. Rsem: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. https://doi.org/10.1186/1471-2105-12-323.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with deseq2. Genome Biol. 2014;15(12):550. https://doi.org/10.1186/s13059-014-0550-8.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. Stringtie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5. https://doi.org/10.1038/nbt.3122.
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, et al. Kobas 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39(Issue suppl_2):W316–22. https://doi.org/10.1093/nar/gkr483.
Zhu Z, Li R, Lv Y, Zeng W. Melatonin protects rabbit spermatozoa from cryo-damage via decreasing oxidative stress. Cryobiology. 2019;88:1–8. https://doi.org/10.1016/j.cryobiol.2019.04.009.
Shamsalddini S, Mohammadabadi MR, Esmailizadeh AK. Polymorphism of the prolactin gene and its effect on fiber traits in goat. Russ J Genet. 2016;52(4):405–8. https://doi.org/10.1134/S1022795416040098.
Klören WRL, Norton BW. Melatonin and fleece growth in australian cashmere goats. Small Ruminant Res. 1995;2(17):179–85. https://doi.org/10.1016/0921-4488(95)00659-9.
Pattie WA, Restall BJ. The inheritance of cashmere in Australian goats. 2. Genetic parameters and breeding values. Livest Prod Sci. 1989;3(21):251–61. https://doi.org/10.1016/0301-6226(89)90054-7.
Ardeshir M, Mahnaz S, Zeynab KB. Effect of melatonin and bromocryptine administration on cashmere production and fibre length growth of raeini goats. Anim Sci J. 2017;30(116):69–78. https://doi.org/10.22092/ASJ.2017.113949.
Gao Y, Wang X, Yan H, Zeng J, Ma S, Niu Y, et al. Comparative transcriptome analysis of fetal skin reveals key genes related to hair follicle morphogenesis in cashmere goats. Plos One. 2016;11(3):e151118. https://doi.org/10.1371/journal.pone.0151118.
Yuan C, Wang X, Geng R, He X, Qu L, Chen Y. Discovery of cashmere goat (capra hircus) microRNAs in skin and hair follicles by solexa sequencing. Bmc Genomics. 2013;14:511. https://doi.org/10.1186/1471-2164-14-511.
Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44(1):1–15. https://doi.org/10.1111/j.1600-079X.2007.00512.x.
Foldes A, Hoskinson RM, Baker P, McDonald BJ, Maxwell CA, Restall BJ. Effect of immunization against melatonin on seasonal fleece growth in feral goats. J Pineal Res. 1992;13(2):85–94. https://doi.org/10.1111/j.1600-079X.1992.tb00059.x.
Plikus MV, Van Spyk EN, Pham K, Geyfman M, Kumar V, Takahashi JS, et al. The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms. 2015;30(3):163–82. https://doi.org/10.1177/0748730414563537.
Zhang Y, Wu K, Wang L, Wang Z, Han W, Chen D, et al. Comparative study on seasonal hair follicle cycling by analysis of the transcriptomes from cashmere and milk goats. Genomics. 2020;112(1):332–45. https://doi.org/10.1016/j.ygeno.2019.02.013.
Feng Y, Gun S. Melatonin supplement induced the hair follicle development in offspring Rex rabbits. J Anim Physiol Anim Nutr (Berl). 2021;105(1):167–74. https://doi.org/10.1111/jpn.13417.
Nixon AJ, Ashby MG, Saywell DP, Pearson AJ. Seasonal fiber growth cycles of ferrets (mustela putorius furo) and long-term effects of melatonin treatment. J Exp Zool. 1995;272(6):435–45. https://doi.org/10.1002/jez.1402720605.
Holst PJ, Clarke WH, Maddocks IG. Skin and fleece characteristics of two groups of feral goats. Aust J Exp Agr. 1982;116(22):173–6. https://doi.org/10.1071/EA9820173.
Matalon S, Remme CA, Samson WK. Physiological reviews: the past, the present, and the future. Physiol Rev. 2021;101(2):733–8. https://doi.org/10.1152/physrev.00001.2021.
Guo Y, Redmond CJ, Leacock KA, Brovkina MV, Ji S, Jaskula-Ranga V, et al. Keratin 14-dependent disulfides regulate epidermal homeostasis and barrier function via 14-3-3sigma and yap1. Elife. 2020;9:e53165. https://doi.org/10.1101/824219.
Lee J, Jang H, Park S, Myung H, Shim S. Platelet-rich plasma activates AKT signaling to promote wound healing in a mouse model of radiation-induced skin injury. J Transl Med. 2019;1(17):1–10. https://doi.org/10.1186/s12967-019-2044-7.
Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2(5):643–53. https://doi.org/10.1016/S1534-5807(02)00167-3.
Zhang Z, Guo Q, Tian H, Lv P, Zhou C, Gao X. Effects of wnt10a on proliferation and differentiation of human dental pulp cells. J Endod. 2014;40(10):1593–9. https://doi.org/10.1016/j.joen.2014.07.009.
Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. BioEssays. 2005;27(3):247–61. https://doi.org/10.1002/bies.20184.
Yue Z, Li C, Liu Y, Liu M, Zhao M, Li F, et al. Vitamin a alleviates heat stress-induced damage to hair follicle development in Rex rabbits. J Sci Food Agric. 2022;102(6):2291–9. https://doi.org/10.1002/bies.20184.
Zhang Y, Yin J, Li C, LI J. Study on development of skin and hair follicle from fetal inner mongolian arbas cashmere goats. Acta Vet Zootech Sinica. 2006;8(37):761. https://doi.org/10.3321/j.issn:0366-6964.2006.08.006.
Estrach S, Tosello L, Tissot FS, Cailleteau L, Cervera L, Jensen Kb, et al. Fibronectin meshwork controls epithelial stem cell fate. bioRxiv. 2021; https://doi.org/10.1101/2021.05.26.445735.
Lukjanenko L, Jung MJ, Hegde N, Perruisseau-Carrier C, Migliavacca E, Rozo M, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22(8):897–905. https://doi.org/10.1038/nm.4126.
Wilhelm K-P. Skin aging. Arch Dermatol. 1991;127(12):1806. https://doi.org/10.1016/j.jtv.2016.03.002.
Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132–42. https://doi.org/10.1016/j.cub.2008.12.005.
Ahmed NS, Ghatak S, El MM, Gnyawali SC, Roy S, Amer M, et al. Epidermal E-cadherin dependent β-catenin pathway is phytochemical inducible and accelerates anagen hair cycling. Mol Ther. 2017;25(11):2502–12. https://doi.org/10.1016/j.ymthe.2017.07.010.
Zhang G, Xu J, Zhang Y, Yang S, Jiang H. Expression of miRNA-1–3p and its target gene in hair follicle cycle development of Liaoning cashmere goat. Anim Biotechnol. 2022; https://doi.org/10.1080/10495398.2022.2058519.
Kurek D, Garinis GA, Van Doorninck JH, Van der Wees J, Grosveld FG. Transcriptome and phenotypic analysis reveals gata3-dependent signalling pathways in murine hair follicles. Development. 2007;134(2):261–72. https://doi.org/10.1242/dev.02721.
Zhou HM, Allain D, Li JQ, Zhang WG, Yu XC. Effects of non-genetic factors on production traits of inner mongolia cashmere goats in China. Small Ruminant Res. 2003;1(47):85–9. https://doi.org/10.1016/S0921-4488(02)00246-8.
Bazley L, Gullick W. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12:S17–27. https://doi.org/10.1677/erc.1.01032.
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. BBA-Mol Basis Dis. 1802;2010(4):396–405. https://doi.org/10.1016/j.bbadis.2009.12.009.
Ozasa A, Komatsu Y, Yasoda A, Miura M, Sakuma Y, Nakatsuru Y, et al. Complementary antagonistic actions between C-type natriuretic peptide and the MAPK pathway through FGFR-3 in ATDC5 cells. Bone. 2005;36(6):1056–64. https://doi.org/10.1016/j.bone.2005.03.006.
Pelegrinelli FF, Thirone AC, Gasparetti AL, Araujo EP, Velloso LA, Saad MJ. Early steps of insulin action in the skin of intact rats. J Invest Dermatol. 2001;117(4):971–6. https://doi.org/10.1046/j.0022-202x.2001.01473.x.
Singh B, Goldberg LJ. Autologous platelet-rich plasma for the treatment of pattern hair loss. Am J Clin Dermatol. 2016;17(4):359–67. https://doi.org/10.1007/s40257-016-0196-2.
Kawano M, Komi-Kuramochi A, Asada M, Suzuki M, Oki J, Jiang J, et al. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol. 2005;124(5):877–85. https://doi.org/10.1111/j.0022-202X.2005.23693.x.
Saxena N, Mok KW, Rendl M. An updated classification of hair follicle morphogenesis. Exp Dermatol. 2019;28(4):332–44. https://doi.org/10.1111/exd.13913.
Bai T, Liu F, Zou F, Zhao G, Jiang Y, Liu L, et al. Epidermal growth factor induces proliferation of hair follicle-derived mesenchymal stem cells through epidermal growth factor receptor-mediated activation of ERK and AKT signaling pathways associated with upregulation of cyclin d1 and downregulation of p16. Stem Cells Dev. 2017;26(2):113–22. https://doi.org/10.1089/scd.2016.0234.
Bing L, Wang JA, Dermatology DO. Role of the ERK pathway in the proliferation and differentiation of human epidermal stem cells. Chin J Tissue Eng Res. 2016;45(20):6807 (https://www.cjter.com/EN/Y2016/V20/I45/6807).
Panni S, Lovering RC, Porras P, Orchard S. Non-coding RNA regulatory networks. BBA-Gene Regul Mech. 2020;1863(6):194417. https://doi.org/10.1016/j.bbagrm.2019.194417.
Li L. The role of microRNAs in vitiligo: regulators and therapeutic targets. Ann Dermatol. 2020;32(6):441–51. https://doi.org/10.1016/j.bbagrm.2019.194417.
Liu G, Li S, Liu H, Zhu Y, Bai L, Sun H, et al. The functions of ocu-mir-205 in regulating hair follicle development in rex rabbits. BMC Dev Biol. 2020;20(1):8. https://doi.org/10.1186/s12861-020-00213-5.
Jin F, Li M, Li X, Zheng Y, Zhang K, Liu X, et al. Dnmt1-mediated methylation inhibits microRNA-214-3p and promotes hair follicle stem cell differentiate into adipogenic lineages. Stem Cell Res Ther. 2020;11(1):444. https://doi.org/10.1186/s13287-020-01864-8.
Heo JS, Lim JY, Yoon DW, Pyo S, Kim J. Exosome and melatonin additively attenuates inflammation by transferring miR-34a, miR-124, and miR-135b. Biomed Res Int. 2020;2020:1621394. https://doi.org/10.1155/2020/1621394.
Wu X, Xia T, Cao M, Zhang P, Shi G, Chen L, et al. LncRNA bancr promotes pancreatic cancer tumorigenesis via modulating miR-195-5p/Wnt/beta-catenin signaling pathway. Technol Cancer Res Treat. 2019;18:1078155610. https://doi.org/10.1177/1533033819887962.
Fu S, Zhao H, Zheng Z, Li J, Zhang W. Melatonin regulating the expression of miRNAs involved in hair follicle cycle of cashmere goats skin. Hereditas. 2014;36(12):1235–42. https://doi.org/10.3724/sp.j.1005.2014.1235.
Spiegelman VS, Elcheva IA. Metabo-mir: miR-211 regulates mitochondrial energy metabolism in vitiligo. J Invest Dermatol. 2017;137(9):1828–30. https://doi.org/10.1016/j.jid.2017.06.012.
Zhou D, Dong L, Yang L, Ma Q, Liu F, Li Y, et al. Identification and analysis of circRNA-miRNA-mRNA regulatory network in hepatocellular carcinoma. IET Syst Biol. 2020;14(6):391–8. https://doi.org/10.1049/iet-syb.2020.0061.
Li B, Yin D, Li P, Zhang Z, Zhang X, Li H, et al. Profiling and functional analysis of circular RNAs in porcine fast and slow muscles. Front Cell Dev Biol. 2020;8:322. https://doi.org/10.3389/fcell.2020.00322.
Wei X, Li H, Yang J, Hao D, Dong D, Huang Y, et al. Circular rna profiling reveals an abundant circlmo7 that regulates myoblasts differentiation and survival by sponging mir-378a-3p. Cell Death Dis. 2017;8(10):e3153. https://doi.org/10.1038/cddis.2017.541.
Hang F, Wang Y, Ma R, Di Z, Wu Z, Hai E, et al. Expression profiling and functional analysis of circular RNAs in inner mongolian cashmere goat hair follicles. Front Genet. 2021;12:8825. https://doi.org/10.3389/fgene.2021.678825.
Zhao B, Chen Y, Hu S, Yang N, Wang M, Liu M, et al. Systematic analysis of non-coding RNAs involved in the Angora rabbit (oryctolagus cuniculus) hair follicle cycle by RNA sequencing. Front Genet. 2019;10:407. https://doi.org/10.3389/fgene.2019.00407.
Acknowledgements
We would like to thank Yiwei cashmere goat farming Co., Ltd. for the providing the cashmere goats this study.
Funding
This work was supported by the China Agriculture Research System (CARS-39).
Author information
Authors and Affiliations
Contributions
XGD conceived of the study, carried out the experiments and drafted the manuscript. LYY and TD collected the sample and performed the research, analyzed data. JXQ and LWH assisted with the sample analysis. WZ participated in the studys design and coordination. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal management and experimental procedures followed the animal care protocols approved by the China Agricultural University Animal Care and Use Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors claim that there are no conflicts of interest.
Supplementary Information
Additional file 1: Table S1.
Nutrient levels of the basal diet (air dry basis) for goat kid. Table S2. Summary of identified transcripts. Table S3. Differentially expressed mRNA, lncRNA, circRNA and miRNA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Diao, X., Yao, L., Duan, T. et al. Melatonin promotes the development of the secondary hair follicles by regulating circMPP5. J Animal Sci Biotechnol 14, 51 (2023). https://doi.org/10.1186/s40104-023-00849-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-023-00849-w