Abstract
Background
Intestinal barrier plays key roles in maintaining intestinal homeostasis. Inflammation damage can severely destroy the intestinal integrity of mammals. This study was conducted to investigate the protective effects of embelin and its molecular mechanisms on intestinal inflammation in a porcine model. One hundred sixty 21-day-old castrated weaned pigs (Duroc × Landrace × Yorkshire, average initial body weight was 7.05 ± 0.28 kg, equal numbers of castrated males and females) were allotted to four groups and fed with a basal diet or a basal diet containing 200, 400, or 600 mg embelin/kg for 28 d. The growth performance, intestinal inflammatory cytokines, morphology of jejunum and ileum, tight junctions in the intestinal mucosa of piglets were tested. IPEC-1 cells with overexpression of P300/CBP associating factor (PCAF) were treated with embelin, the activity of PCAF and acetylation of nuclear factor-κB (NF-κB) were analyzed to determine the effect of embelin on PCAF/NF-κB pathway in vitro.
Results
The results showed that embelin decreased (P < 0.05) serum D-lactate and diamine oxidase (DAO) levels, and enhanced the expression of ZO-1, occludin and claudin-1 protein in jejunum and ileum. Moreover, the expression levels of critical inflammation molecules (interleukin-1β, interleukin-6, tumor necrosis factor-α, and NF-κB) were down-regulated (P < 0.05) by embelin in jejunal and ileal mucosa. Meanwhile, the activity of PCAF were down-regulated (P < 0.05) by embelin. Importantly, transfection of PCAF siRNAs to IPEC-1 cell decreased NF-κB activities; embelin treatment downregulated (P < 0.05) the acetylation and activities of NF-κB by 31.7%–74.6% in IPEC-1 cells with overexpression of PCAF.
Conclusions
These results suggested that embelin ameliorates intestinal inflammation in weaned pigs, which might be mediated by suppressing the PCAF/NF-κB signaling pathway.
Similar content being viewed by others
Introduction
Breast feeding and weaning are important physiologically significant luminal events that influence the growth of the small intestine in humans [1]. Previous study indicated that breast fed infants have smaller villi and crypts than bottle fed infants, suggesting that crypt fission may be the predominant mechanism of epithelial growth during milk feeding whereas crypt hyperplasia predominates later during weaning (2–6 months of age) [2]. As known that weaning process is commonly associated with intestinal inflammation and barrier dysfunction, and this phenomenon is particularly evident in piglets, which is responsible for the stunted growth and diarrhoea observed in the first 2 weeks after weaning [3, 4], therefore, weaned piglets are regarded as an important laboratory animal for studying the intestinal health of infants. Intestinal barrier is mainly formed by a single layer of enterocytes and intercellular tight junctions of enterocytes [5]. It serves as a selective permeable membrane which not only permits the absorption of nutrients from the intestinal lumen into circulation but also provides an innate defense barrier to resist the passage of luminal pathogens and toxins into systemic circulation [6]. But many stimuli factors including infection, inflammation can damage intestinal barrier function by inducing overproduction of pro-inflammatory cytokines [7, 8]. Therefore, inhibition of intestinal inflammation during the weaning stage may play an important role in alleviating intestinal damage in infants.
The transition of weaned piglets from breast milk to solid feed will cause intestinal inflammation, and then lead to diarrhea, growth performance decline, and even death of piglets, and finally bring huge economic losses to animal husbandry [9]. Numerous studies showed that the expression of inflammatory factor signaling pathways was elevated in the intestine of weaned piglets [10, 11]. Among them, the inhibition of the activity of NF-κB, the initiator of the inflammatory pathway, is considered to be the most effective way to alleviate intestinal inflammation [12]. Moreover, PCAF has been reported to promote the inflammatory signaling pathways by acetylating the NF-κb [13]. Many studies have pointed out that plants and their extracts play an important role in promoting animal growth, enhancing immunity and maintaining animal health [14,15,16]. As another excellent anti-inflammatory plant extract, embelin, the major component of Embelia ribes Burm plant (specifically, its fruit), has been considered to be an acetylation inhibitor of PCAF [17]. Due to this efficacy, embelin may be beneficial in improving the intestinal inflammation, growth performance, and diarrhea of weaning pigs.
Currently, most of the studies of embelin have focused on the anti-inflammatory and antioxidant activities in vitro studies or some in vivo studies [18, 19]. However, the beneficial effects of embelin on the alleviation of the intestinal inflammation and gut barrier as well as the improvement of diarrhea in weaned pigs have not been reported. The purpose of this study was to investigate the effects of embelin on diarrhea, intestinal barrier function and inflammation in weaned piglets.
Material and methods
Animals and experimental design
All animal protocols used in this study were in accordance with the Guidelines for the Care and Use of Animals for Research and Teaching and approved by the Animal Care and Use Committee of Huazhong Agricultural University.
A total of 160 weaned pigs (Duroc × Landrace × Yorkshire) were randomly assigned to 4 dietary treatments with 4 replicate pens per treatment. Their initial body weight (BW) was 7.05 ± 0.28 kg. Control pigs (CON) were fed a basal diet, and the other animals were fed a basal diet supplemented with 200, 400, or 600 mg/kg embelin (E200, E400, E600) for 2 weeks. The basal diets were formulated to meet the NRC (2012) specifications for weaned pigs [20]. And the ingredients and composition of the basal diet are listed in Additional file 1: Table S1. The embelin was purchased from Xin Lu Biotechnology Company (Xi’an, China), and the purity was 98.1%, as measured by HPLC. All pigs were allowed ad libitum access to feed and water throughout the experimental period. All the pigs were housed in pens in an environmentally controlled room at 24 ~ 25 ℃ and alternating light and dark cycles with 12 h intervals. All experimental pigs were healthy during the feeding period. Weights were obtained on every pig and feed disappearance was recorded on day 0, 7, 14 and prior to slaughter to calculate average daily feed intake (ADFI), average daily gain (ADG), and feed/gain ratio (F/G) at 24 ~ 25 ℃.
Sample collections
At the end of the trial, blood samples were collected via jugular venipuncture at 08:00 after 12 h of fasting. Then, the samples were centrifuged at 3500 × g at 4 °C for 10 min. After centrifugation, the serum samples were collected and frozen at –20 °C until analysed. After blood collection, pigs were euthanised with an intravenous injection of chlorpromazine hydrochloride (3 mg/kg body weight, MedChemExpress, Monmouth Junction, NJ, United States) and then slaughtered by exsanguination protocols [21]. Immediately, a piece (2-cm length) of the middle jejunum and distal ileum (2-cm length) were collected, rinsed with cold saline (NaCl 9 g/L, 4 °C), and fixed in paraformaldehyde. Other segments of the middle jejunum and distal ileum (5-cm length) of the piglets were opened and thoroughly rinsed with sterile normal saline, and then the mucosa was collected by scraping with glass slides and immediately snap-frozen in liquid nitrogen and stored at − 80 °C. Fecal scores were monitored during the feeding trial and quantified using a scale ranging from 0 to 3 with 0 = normally shaped feces, 1 = shapeless (loose) feces, 2 = thick, liquid (soft) feces, and 3 = thin, liquid feces and watery diarrhea. A piglet with a score greater than 1 was regarded as having diarrhea. The incidence of diarrhea (%) was calculated as a percentage of the number of diarrheal piglets during the period divided by the total number of piglets.
Intestinal cytokines determined by ELISA
After grinding in liquid nitrogen, total protein was extracted from the jejunal and ileal mucosa samples with lysis buffer (KeyGEN, Nanjing, China), followed by clarification by centrifugation (3500 × g for 10 min at 4 °C). The concentrations of IL-1β, IL-6, IL-10, and tumor necrosis factor-α (TNF-α) were determined by using ELISA kits (Nanjing Jiancheng Bioengineering company, Jiangsu, China).
Analysis of intestinal morphology and permeability
Histological examination of the intestinal morphology was conducted according to Wang et al. [19]. Briefly, after 24 h of fixation, the intestinal segments were dehydrated in ethanol concentration gradient and cleared up with xylene, embedded in paraffin, cut incross sections with a thickness of approximately 5 μm with a microtome and then stained with haematoxylin and eosin. Villous height and crypt depth of two intestinal segments were measured and villous height/crypt depth ratio (VCR) was calculated. The contents of DAO and D-lactate and ET-1 and NO of serum were measured using an instrument (Biochemical Analytical Instrument, Beckman CX4, Beckman Coulter Inc., Brea, CA, USA).
Cell culture and treatment
Intestinal porcine epithelial cells (IPEC-1) were isolated from the jejunum of unsuckled newborn pigs according to Wang et al. [22]. The cells were grown in serial passage in uncoated plastic cell culture flasks (75 cm2) with a vent cap in DMEM-F12 containing 17.5 mmol/L D-glucose, 15 mmol/L HEPES (pH 7.4), 5% FBS, epidermal growth factor (5 μg/L), insulin (5 μg/mL), transferrin (5 μg/mL), selenium (5 ng/mL), penicillin (50 μg/mL), streptomycin (4 μg/mL) and 0.25 μg/mL amphotericin B (Fungizone). Medium was changed every 2 days. All cell cultures were carried out at 37 °C in a 5% CO2 incubator. At confluence, cells were passaged using trypsinization according to Dekaney et al. [23]. Cells in 6-well plates were pre-treated with embelin (2.5 and 5 μmol/L) for 2 h and incubated further for 48 h. Besides, cells were transfected by Lipofectamine 2000 (Thermo Fisher, Waltham, MA, USA) with a mixture of small interfering RNA (siRNA) directed isolation of cells and cell culture towards PCAF (Qiagen, Valencia, CA, USA) for 4 h (5’-GCAGATACCAAA-CAAGTTT-3’). To confirm PCAF knock-down, Western blot was used to analyse PCAF expression.
Total RNA isolation and reverse transcription
Total RNA was isolated from frozen jejunal or ileal samples using TRIzol (Takara Biotechnology, Beijing, China). All the procedures were guided by the manufacturer’s manual. Briefly, 100 mg tissues were put into a mortar and grinded with 1 mL TRIzol reagent. The proteins in the grinded samples were precipitated by chloroform. After centrifugation, the supernatant was transferred into a new tube and isopropanol was added and mixed for 10 min. The total RNA has settled by centrifugation. The integrity of RNA was checked by electrophoresis on a 1.5% agarose gel, and the concentration and quality were verified by UV spectrophotometry using a NanoDrop 2000 (Thermo Fisher, Waltham, MA, USA). After RNA isolation, 1 μg of total RNA was reverse-transcribed into cDNA using a PrimeScript™ RT reagent reverse-transcribed into cDNA using a PrimeScript™ RT reagent kit with cDNA Eraser (Takara Biotechnology, Beijing, China). The following conditions were used: 42 °C for 2 min, then 37 °C for 15 min, followed by 85 °C for 5 s.
Real-time quantitative PCR was performed in an Option Monitor 3 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using the SYBR Green Supermix (Takara Biotechnology, Beijing, China). Expression levels of β-actin (housekeeper genes), TNF-α, IL-6, IL-10, IL-1β, ZO-1, occludin and claudin-1 in the small intestinal were analysed using SYBR Premix Ex Taq II (Tli RNaseH Plus) reagents (Takara Biotechnology, Beijing, China) and the QuanStudio 6 Flex Real-Time PCR detection system (Applied Biosystems, Carlsbad, CA, USA). All primers were commercially synthesised and purified by Sangon Biotech Co. Ltd and are shown in Additional file 1: Table S2. The reaction was performed in a volume of 10 μL consisting of 5 μL of SYBR Premix ExTaq (2 ×), 1 μL of reverse primers, 1 μL of forward primers, 2 μL of doubled-distilled water and 1 μL of cDNA template. Cycling conditions were as follows: 5 °C for 30 s, followed by forty cycles at 95 °C for 5 s, 60 °C for 34 s, under melt curve conditions at 95 °C for 15 s, 60 °C for 1 min and then 95 °C for 15 s (temperature change velocity 0.5 °C/s). The target gene mRNA expression level was calculated using the 2–ΔΔCt method [24]. Each sample was repeated in triplicate.
Western blotting and immunoprecipitation
Western blot analyses were performed as described previously [25]. For the analyses, the major antibodies, including ZO-1, occludin, claudin-1, NFκB and β-actin (ACTB), are submitted in online Additional file 1: Table S3. The mucosal scrapings were homogenized on ice in RIPA lysis buffer (Upstate; Temecula, CA, USA) containing protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and phosphathase inhibitor cocktail 1 (Sigma, St. Louis, MO, USA). After centrifugation at 4 °C and 14,000 × g, the supernatants were collected for the assay. Detection of concentrations of protein in the ileum was performed by the bicinchoninic acid assay. Western blotting was performed for acetylation detection. For blocking, 50 mmol/L Tris (pH 7.5) with 1% peptone (Amresco, Solon, OH, USA) and 10% (v/v) Tween-20 were used. For the primary and secondary antibodies, 50 mmol/L Tris (pH 7.5) with 0.1% peptone was prepared [26].
Statistical analysis
Data were analyzed using the the one-way ANOVA with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The results in the tables are shown with the mean ± SEM, and other figure results are presented with mean ± SD. Means are considered significantly different at P < 0.05.
Results
Growth performance of weaned piglets
The effects of embelin on the growth performance of weaned piglets were shown in Table 1. Piglets dietary administered embelin increased the final weight and ADG (P < 0.05), and reduced the feeding efficiency (P < 0.05), and had no significant effect on ADFI (P = 0.49). In addition, dietary embelin significantly decreased the diarrhea rate compared with that in control group (P < 0.05). Piglets dietary administered embelin reduced the diarrhea rate as much as 45%. The F/G and diarrhea rates in the E400 and E600 embelin groups were significantly lower than those in the E200 group.
Intestinal inflammatory cytokines of weaned piglets
As shown in Fig. 1, dietary supplementation of embelin increased expression of jejunum and ileum IL-10 (P < 0.05, Fig. 1A, F), where E400 and E600 embelin groups were significantly higher relative to the control and E200 groups. Piglets dietary administered embelin had significantly reduced the expression IL-6, IL-1β, TNF-α in jejunum and ileum (P < 0.05, Fig. 1B-D, G-I). Relative to the control group, E400 and E600 embelin group significantly reduced jejunum and ileum NF-κB expression (P < 0.05, Fig. 1E, J).
Effects of dietary embelin on the IL-10 (A, F), IL-6 (B, G), IL-1β (C, H), TNF-α (D, I) and NF-κB (E, J) in jejunum and ileum of weaned piglets. Data are shown as the mean ± SD, n = 10/group. Means without a common letter differ, P < 0.05. IL-1β: interleukin-1β; IL-6: interleukin-6; IL-10; NF-κB: nuclear factor-κB; TNF-α: tumor necrosis factor-α
Morphology and permeability of jejunum and ileum in weaned piglets
As shown in Table 2, diets supplemented with E400 and E600 of embelin significantly increased the villus height in the jejunum (P < 0.05). Although there was no significant effect on the villus height in ileum (P = 0.46). All three dose addition groups had no significant effect on jejunal (P = 0.35) and ileal crypt depths (P = 0.38). Both E400 and E600 embelin significantly improved the villus height to crypt depth ratios relative to the control and E200 embelin groups (P < 0.05). For intestinal permeability (Table 3), compared to the control group, dietary embelin significantly decreased the diamine oxidase (DAO) and D-lactate contents (P < 0.05). The addition of embelin had no significant effect on the content of both ET-1 (P = 0.37) and NO (P = 0.45).
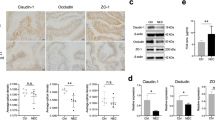
Tight junctions in the intestinal mucosa of weaned piglets
As shown in Fig. 2, for tight junction-related components, compared with the control group, dietary embelin significantly increased (P < 0.05) ZO-1 and claudin-1 protein expression in both the jejunal and ileal mucosa and occludin in the ileum (Fig. 2A-B, E–F). There was no significant effect for jejunal occludin protein levels (P > 0.05, Fig. 2C). The expression of ZO-1 in both jejunal and ileal mucosa of the E400 and E600 embelin groups was significantly higher than those in the control and E200 groups (P < 0.05). In addition, the expression of jejunal claudin-1 and ileal occludin of E600 embelin groups was significantly higher than that in the other groups (P < 0.05).
Effects of dietary embelin on the expression of ZO-1 (A, E), claudin-1 (B, F) and occludin (C, G) in jejunum and ileum of weaned piglets. The protein expression in the intestine tissues in each group was determined by Western blotting (D, H), and each blot represents at least three independent samples. Data are shown as the mean ± SD, n = 10/group. Means for a given protein without a common letter differ, P < 0.05
Dietary embelin decreases the PCAF activity and acetylation of NF-κB and in weaned piglets
Addition of E600 embelin significantly reduced PCAF and NF-κB activity in the jejunum compare to the other three groups (P < 0.05, Fig. 3 A-B, D-E). The activity of PCAF and NF-κB in the ileum of E400 and E600 embelin groups was significantly lower than that in the control and E200 embelin groups (P < 0.05). The acetylation level of NF-κB in the E400 and E600 embelin groups was significantly lower (P < 0.05) than those of the E200 embelin group (Fig. 3C). Compared with the control group, all dosages of the embelin groups significantly reduced (P < 0.05) the NF-κB acetylation levels in ileum (Fig. 3F).
Effects of dietary embelin on the PCAF (A, D) and NF-κB (B, E) activity, acetylation level of NF-κB (C, F) in jejunum and ileum of weaned piglets. Data are shown as the mean ± SD, n = 5–10/group. Means for a given protein without a common letter differ, P < 0.05. NF-κB: nuclear factor-κB; PCAF, P300/CBP associating factor
Embelin inhibits the PCAF-dependent acetylation of NF-κB in IPEC-1 cell
All three doses of embelin in IPEC-1 cell significantly reduced PCAF and NF-κB activity (Fig. 4A, B, P < 0.05). IPEC-1 cell were transfected with the indicated K (lysine) acetyltransferase (KAT) constructs in combination with expression vectors containing PCAF. As expected, PCAF expression was increased (P < 0.05) by 65%, while transfection with siRNAs targeting PCAF in IPEC-1 cell decreased the protein expression by ∼75% (P < 0.05, Fig. 4C). Both interference with PCAF and addition of embelin significantly reduced NF-κB activity (P < 0.05). Overexpression of PCAF significantly increased NF-κB activity, accompanied by increased acetylation levels of NF-κB (P < 0.05). Interestingly, only upon stimulation with embelin could we detect a decrease (P < 0.05) in the levels of activity and acetylation of NF-κB in IPEC-1 cell overexpressing PCAF (Fig. 4D, E).
Effects and mechanisms of dietary embelin on the the activity of PCAF (A) and NF-κB (B, D), protein expression of PCAF (C), acetylation level of NF-κB (E) in IPEC-1 cells. Data are shown as the mean ± SD, n = 10/group. Means for a given protein without a common letter differ, P < 0.05; *P < 0.05. NF-κB: nuclear factor-κB; PCAF, P300/CBP associating factor
Discussion
In this study, we demonstrated for the first time that as a natural ingredient, embelin efficiently improved growth performance and diarrhea, ameliorated intestinal barrier dysfunction and inflammation of weaning piglets. First, the effect of dietary embelin on growth performance and diarrhea was measured. The median lethal dose of embelin is reported to be 2000 mg/kg body weight in mice and rats without any mortality or adverse effects indicating its safety [27]. So, we chose 200, 400 and 600 mg/kg embelin as the additive dosage. At the beginning of the study, we collected blood samples and verified that there were no differences between piglets and that they were healthy at the beginning of the study. However, after a 28-day period trial, the control group had a higher incidence of diarrhea, which may be related to weaning events. Dietary embelin increased ADG and decreased F/G compared to the control group, indicating improved growth performance. Moreover, dietary embelin significantly reduced the rate of diarrhea in weaned piglets. Previous studies have shown that weight gain and nutrient absorption as well as diarrhea rates in piglets are associated with oxidative stability, intestinal morphology, and intestinal inflammation [28]. We therefore tested the intestinal barrier function in weaned piglets and the improvement of the intestinal barrier by embelin.
The integrity of the intestinal epithelial barrier acts a key role in the digestion and absorption of nutrients and the inhibition of invasion by pathogenic bacteria [29, 30]. However, numerous mammalian studies have shown that weaning stress can severely disrupt small intestinal barrier function and exacerbate intestinal inflammation within a short period of time after weaning [31,32,33]. It is well known that intestinal permeability is usually assessed by some blood indicators. D-lactic acid is a specific end product of intestinal bacteria and is released into the blood when the intestinal mucosa is damaged. DAO is an intracellular enzyme synthesized mainly by mammalian intestinal epithelial cells and is mainly found in the cytoplasm. Once the intestinal epithelial barrier is damaged, intestinal DAO is released into the circulation [34]. Therefore, blood D-lactic acid concentration and DAO activity can be used as circulating markers to monitor the extent of intestinal barrier damage. In the present study, the addition of embelin to the diet reduced serum D-lactic acid concentration and DAO activity in weaned piglets, suggesting that embelin has a beneficial effect on reducing intestinal permeability in weaned piglets. Furthermore, tight junctions between intestinal epithelial cells maintain and regulate the intestinal epithelial barrier [35]. The formation of tight junctions requires several unique proteins, such as the claudin family, occludin, and intracellular linker proteins, such as ZOs, which anchor directly or indirectly to the actin-based cytoskeleton and then form a selective permeability barrier [36]. Thus, the expression level and distribution of tight junction proteins are closely related to intestinal barrier function. However, weaning stress can disrupt intestinal tight junctions by down-regulating the expression of intestinal barrier-related genes in pigs [37, 38]. The results of this study showed that the addition of embelin to the diet significantly upregulated the expression of claudin-1, occludin and ZO-1 in the small intestine of weaned piglets. Thus, embelin can improve intestinal barrier function by maintaining the expression of tight junction proteins, which may be partly responsible for embelin's ability to reduce intestinal permeability and inflammatory response to weaning stress injury in piglets.
Accumulating evidence has confirmed that inflammation is an important inducing factor in intestinal barrier disruption [39, 40]. Some cytokines are thought to be critical in the predisposition to and exacerbation of some gastrointestinal dysfunctions. NF-κB is a central mediator of intestinal inflammatory diseases [41] and has been shown to play a role in the control of intestinal permeability [42]. Moreover, pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 have been shown to exert negative roles in maintaining the tight junction and cytoskeleton structure and function. In the present study, embelin feed inhibited NF-κB activity and the pro-inflammatory factors TNF-α, IL-1β, and IL-6, which is consistent with previous studies [43]. Evidence to date demonstrates that the functions of NF-κB are modulated by diverse post-transcriptional modifications [44]. Among these modifications, acetylation has been shown to enhance the nuclear localization of NF-κB and lead to the transcription of NF-κB target genes [45]. NF-κB is mainly acetylated by PCAF. PCAF acetylates multiple lysine residues of NF-κB, including Lys-122, -123, -218, -221 and -310, activating its transcriptional activity [46]. Interestingly, embelin is regarded as an extremely potent natural inhibitor of p300/CBP—associating factor (PCAF), which has been observed to activate the inflammatory pathway through acetylation of the NF-Κb [47]. Therefore, embelin may have a predictable potential ability to attenuate the intestinal inflammation of weaned animals, especially in piglets, which has been proved to be a suitable animal model for human infants [48]. Indeed, in the present study, dietary supplementation with embelin at 3 dosages decreased PCAF activity, suggesting that embelin may attenuate NF-κB activity by inhibiting the acetylation activity of PCAF.
To further explore the molecular mechanism of embelin in attenuating intestinal inflammatory response,we investigated the effects of embelin on the activation of PCAF/NF-κB signaling pathway which has been considered as a key inducer of inflammation. It was reported that embelin rapidly inactivates PCAF activity and has a strong apoptosis-inducing effect on leukemia cell lines and on prostate and pancreatic cancer cells through the inhibition of DNA binding [49]. In our study, embelin decreased PCAF activity and NF-κB acetylation suggesting that embelin-induced decreased PCAF activity may reduce inflammatory cytokines in weaned piglets. Furthermore, the upregulation of PCAF and NF-κB in weaned piglets was reversed by embelin treatment, so we propose that the PCAF-NF-κB pathway may be involved in the embelin-mediated alleviation the inflammatory response after weaning, siRNAs inhibits PCAF in cultured IPEC-1 cell, indicating that nonembelin-mediated inhibition of PCAF has similar effects. Activated PCAF can stimulate the activation of NF-κB signal pathway and then increase the expression of various inflammatory cytokines' genes, including IL-6, TNF-α and IL-1β [50]. Therefore, reducing the inflammatory response mediated by the PCAF/NF-κB signaling pathway may play a protective effect in the intestine of weaned pigs.
In conclusion, the present study indicated for the first time that dietary embelin supplementation exerts beneficial effects on ameliorating the intestinal inflammation in weaned pigs. The mechanisms of action might be closely related to improving tight junction protein expression, suppressing intestinal inflammatory mediators released, alleviating intestinal inflammation via downregulation of acetylation of NF-κB by PCAF.
Nevertheless, there were some limitations in this study. In this experiment, due to the imperfect experimental plan, the optimal concentration of embelin in the piglet diet was not further clarified. In addition, there may be other potential mechanisms that have not been explored in this study. For example, embelin itself has a certain antibacterial effect, which may be an important factor in improving the gut mircobiota and intestinal health. Future experiments could focus on evaluating the anti-inflammatory and antibacterial properties of embelin, and further clarification of its role in piglet diet production and human infant health.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADFI:
-
Average daily feed intake
- ADG:
-
Average daily gain
- DAO:
-
Diamine oxidase
- ET-1:
-
Endothelin-1
- F/G:
-
Feed/gain ratio
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- NF-κB:
-
Nuclear factor-κB
- TNF-α:
-
Tumor necrosis factor-α
- ZO1:
-
Zonula occludens protein
- PCAF:
-
P300/CBP associating factor
References
Cummins AG, Thompson FM. Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut. 2002;51(5):748–54. https://doi.org/10.1136/gut.51.5.748.
Cummins AG, Jones BJ, Thompson FM. Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig Dis Sci. 2006;51(4):718–23. https://doi.org/10.1007/s10620-006-3197-9.
Zeng Y, Wang Z, Zou T, Chen J, Li G, Zheng L, et al. Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front Vet Sci. 2021;8:623899. https://doi.org/10.3389/fvets.2021.623899.
Zou TD, Deng CX, Wang ZR, Ye YL, You JM. Dietary alanyl-glutamine improves growth performance of weaned piglets through maintaining intestinal morphology and digestion-absorption function. Animal. 2019;13(9):1826–33. https://doi.org/10.1017/s1751731119000223.
Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physio. 2016;120(6):692–701. https://doi.org/10.1152/japplphysiol.00536.2015.
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. https://doi.org/10.1038/nri2653.
Oz HS, Chen TS, Nagasawa H. Comparative efficacies of 2 cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl Res. 2007;150(2):122–9. https://doi.org/10.1016/j.trsl.2006.12.010.
Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16(5):297–304. https://doi.org/10.1016/j.jnutbio.2004.09.007.
Rhouma M, Fairbrother JM, Beaudry F, Letellier A. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet Scand. 2017;59(1):31. https://doi.org/10.1186/s13028-017-0299-7.
Liao P, Li Y, Li M, Chen X, Yuan D, Tang M, et al. Baicalin alleviates deoxynivalenol-induced intestinal inflammation and oxidative stress damage by inhibiting NF-κB and increasing mTOR signaling pathways in piglets. Food Chem Toxicol. 2020;140:111326. https://doi.org/10.1016/j.fct.2020.111326.
Song ZH, Xiao K, Ke YL, le Jiao F, Hu CH. Zinc oxide influences mitogen-activated protein kinase and TGF-β1 signaling pathways, and enhances intestinal barrier integrity in weaned pigs. Innate Immun. 2015;21(4):341–8. https://doi.org/10.1177/1753425914536450.
Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43(6):856–60. https://doi.org/10.1136/gut.43.6.856.
Park SY, Lee YH, Seong AR, Lee J, Jun W, Yoon HG. Selective inhibition of PCAF suppresses microglial-mediated β-amyloid neurotoxicity. Int J Mol Med. 2013;32(2):469–75. https://doi.org/10.3892/ijmm.2013.1407.
Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49(1):76. https://doi.org/10.1186/s13567-018-0562-6.
Callaway TR, Lillehoj H, Chuanchuen R, Gay CG. Alternatives to antibiotics: A symposium on the challenges and solutions for animal health and production. Antibiotics. 2021;10(5):471. https://doi.org/10.3390/antibiotics10050471.
Yadav AS, Kolluri G, Gopi M, Karthik K, Malik YS, Dhama K. Exploring alternatives to antibiotics as health promoting agents in poultry- A review. J Exp Biol Agric Sci. 2016;4:368–83. https://doi.org/10.18006/2016.4(3S).368.383.
Ko JH, Lee SG, Yang WM, Um JY, Sethi G, Mishra S, et al. The application of embelin for cancer prevention and therapy. Molecules. 2018;23(3):621. https://doi.org/10.3390/molecules23030621.
Schaible AM, Traber H, Temml V, Noha SM, Filosa R, Peduto A, et al. Potent inhibition of human 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 by the anti-carcinogenic and anti-inflammatory agent embelin. Biochem Pharmacol. 2013;86(4):476–86. https://doi.org/10.1016/j.bcp.2013.04.015.
Wang H, Zhang H, Wang Y, Yang L, Wang D. Embelin can protect mice from thioacetamide-induced acute liver injury. Biomed Pharmacother. 2019;118:109360. https://doi.org/10.1016/j.biopha.2019.109360.
NRC. Nutrient requirements of swine: Eleventh. Revised. Washington, DC: The National Academies Press; 2012.
Gan Z, Wei W, Li Y, Wu J, Zhao Y, Zhang L, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. 2019;24(7):1220. https://doi.org/10.3390/molecules24071220.
Wang T, Yao W, Li J, Shao Y, He Q, Xia J, et al. Dietary garcinol supplementation improves diarrhea and intestinal barrier function associated with its modulation of gut microbiota in weaned piglets. J Anim Sci Biotechnol. 2020;11:12. https://doi.org/10.1186/s40104-020-0426-6.
Dekaney CM, Wu G, Yin YL, Jaeger LA. Regulation of ornithine aminotransferase gene expression and activity by all-transretinoic acid in Caco-2 intestinal epithelial cells. J Nutr Biochem. 2008;19(10):674–81. https://doi.org/10.1016/j.jnutbio.2007.09.002.
Yao W, Xia J, Wang T, Li J, Huang L, Huang F. Garcinol promotes hepatic gluconeogenesis by inhibiting P300/CBP-associated factor in late-pregnant sows. Br J Nutr. 2021;126(1):1–8. https://doi.org/10.1017/s000711452000375x.
Yan X, Pepper MP, Vatamaniuk MZ, Roneker CA, Li L, Lei XG. Dietary selenium deficiency partially rescues type 2 diabetes-like phenotypes of glutathione peroxidase-1-overexpressing male mice. J Nutr. 2012;142(11):1975–82. https://doi.org/10.3945/jn.112.164764.
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–4. https://doi.org/10.1126/science.1179689.
Mahendran S, Badami S, Maithili V. WITHDRAWN: Evaluation of antidiabetic effect of embelin from Embelia ribes in alloxan induced diabetes in rats. Biomed Pharmacother. 2010. https://doi.org/10.1016/j.biopha.2010.08.003
Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135(5):969–72. https://doi.org/10.1093/jn/135.5.969.
Magalhaes JG, Tattoli I, Girardin SE. The intestinal epithelial barrier: how to distinguish between the microbial flora and pathogens. Semin Immunol. 2007;19(2):106–15. https://doi.org/10.1016/j.smim.2006.12.006.
Lee B, Moon KM, Kim CY. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J Immunol Res. 2018;2018:2645465. https://doi.org/10.1155/2018/2645465.
Wan J, Zhang J, Chen D, Yu B, Mao X, Zheng P, et al. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J Anim Sci Biotechnol. 2018;9:58. https://doi.org/10.1186/s40104-018-0273-x.
Xiao K, Song ZH, Jiao LF, Ke YL, Hu CH. Developmental changes of TGF-β1 and Smads signaling pathway in intestinal adaption of weaned pigs. PLoS ONE. 2014;9(8):e104589. https://doi.org/10.1371/journal.pone.0104589.
Feng J, Wang L, Xie Y, Chen Y, Yi H, He D. Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int Immunopharmacol. 2020;85:106658. https://doi.org/10.1016/j.intimp.2020.106658.
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. https://doi.org/10.1186/s12876-014-0189-7.
Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann N Y Acad Sci. 2012;1258:9–18. https://doi.org/10.1111/j.1749-6632.2012.06613.x.
Gao Y, Mruk DD, Lui WY, Lee WM, Cheng CY. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget. 2016;7(39):64203–20. https://doi.org/10.18632/oncotarget.11887.
Li C, Wang W, Liu T, Zhang Q, Wang G, Li F, et al. Effect of early weaning on the intestinal microbiota and expression of genes related to barrier function in lambs. Front Microbiol. 2018;9:1431. https://doi.org/10.3389/fmicb.2018.01431.
Chen H, Mao X, He J, Yu B, Huang Z, Yu J, et al. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br J Nutr. 2013;110(10):1837–48. https://doi.org/10.1017/s0007114513001293.
Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):362–81. https://doi.org/10.1002/ibd.21403.
Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132(4):1344–58. https://doi.org/10.1053/j.gastro.2007.01.051.
Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. https://doi.org/10.1038/sigtrans.2017.23.
Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-kappa B activation in the intestinal epithelia. J Immunol. 2003;171(6):3194–201. https://doi.org/10.4049/jimmunol.171.6.3194.
Park SY, Lim SL, Jang HJ, Lee JH, Um JY, Kim SH, et al. Embelin induces apoptosis in human glioma cells through inactivating NF-κB. J Pharmacol Sci. 2013;121(3):192–9. https://doi.org/10.1254/jphs.12137fp.
Baud V, Collares D. Post-translational modifications of RelB NF-κB subunit and associated functions. Cells. 2016;5(2):22. https://doi.org/10.3390/cells5020022.
Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. Embo j. 2002;21(23):6539–48. https://doi.org/10.1093/emboj/cdf660.
Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293(5535):1653–7. https://doi.org/10.1126/science.1062374.
Modak R, Basha J, Bharathy N, Maity K, Mizar P, Bhat AV, et al. Probing p300/CBP associated factor (PCAF)-dependent pathways with a small molecule inhibitor. ACS Chem Biol. 2013;8(6):1311–23. https://doi.org/10.1021/cb4000597.
Caballero Valcárcel AM, Martínez Graciá C, Martínez Miró S, Madrid Sánchez J, González Bermúdez CA, Domenech Asensi G, et al. Iron bioavailability of four iron sources used to fortify infant cereals, using anemic weaning pigs as a model. Eur J Nutr. 2019;58(5):1911–22. https://doi.org/10.1007/s00394-018-1742-x.
Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. https://doi.org/10.1186/1750-2187-5-10.
Chen D, Lu D, Liu H, Xue E, Zhang Y, Shang P, et al. Pharmacological blockade of PCAF ameliorates osteoarthritis development via dual inhibition of TNF-α-driven inflammation and ER stress. EBioMedicine. 2019;50:395–407. https://doi.org/10.1016/j.ebiom.2019.10.054.
Acknowledgements
We sincerely thank each of the authors for their contributions during the conduct of the experiments and for the facilities provided by the experimental platform at the College of Animal Science and Technology, Huazhong Agricultural University.
Funding
The research is supported by the National Natural Science Foundation of China (Grant no. 32072742), National Key Research and Development Program (Grant no. 2021YFD1300300) and the Fellowship of China Postdoctoral Science Foundation (grant no. 2022M711274).
Author information
Authors and Affiliations
Contributions
WY, TW, and FH designed the experiment; ZB, and SW conducted the experiment. WY, and LH analyzed the data; WY wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal protocols used in this study were in accordance with the Guidelines for the Care and Use of Animals for Research and Teaching and approved by the Animal Care and Use Committee of Huazhong Agricultural University. The animal handling protocol permit number is HZAUSW-2017–0006.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
WY, TW, LH, ZB, SW, and FH, no conflicts of interest.
Supplementary Information
Additional file 1: Table S1
The ingredients and nutritional levels of diets. Table S2 Primers used for real-time quantitative PCR. Table S3 Name, type, dilution, and source of primary antibodies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yao, W., Wang, T., Huang, L. et al. Embelin alleviates weaned piglets intestinal inflammation and barrier dysfunction via PCAF/NF-κB signaling pathway in intestinal epithelial cells. J Animal Sci Biotechnol 13, 139 (2022). https://doi.org/10.1186/s40104-022-00787-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-022-00787-z