Abstract
Background
High-altitude pulmonary hypertension (HAPH) is a life-threating condition for animals in high altitude, and disturbance of endothelial nitric oxide (NO) synthesis contributes to its pathogenesis. N-carbamylglutamate (NCG), which enhances arginine synthesis, promotes endogenous synthesis of NO. In this study, we determined the effects of NCG on alleviating HAPH in Holstein heifers that ascended to Tibet (Lhasa, 3,658 m).
Methods
Exp. 1, 2,000 Holstein heifers were transported from low elevation (1,027 m) to Lhasa. After being exposed to hypoxia for 1 yr, Holstein heifers were assigned to a healthy group (Control, n = 6) with mean pulmonary hypertension (mPAP) < 41 mmHg, and an HAPH affected group (HAPH, n = 6) with mPAP > 49 mmHg. Lung tissues were collected to evaluate histopathological changes and the expression of endothelial nitric oxide synthase (eNOS). Exp. 2, ten healthy heifers and 10 HAPH affected heifers were supplemented with NCG (20 g/d per heifer) for 4 wk. Physiological parameters were determined and blood samples were collected on d − 1 and d 28 of the feeding trial.
Results
Expression of eNOS in small pulmonary arteriole intima was higher in the healthy than HAPH group (P = 0.006), whereas HAPH group had significantly thicker media and adventitia than healthy group (all P < 0.05). The mRNA of eNOS and protein level of eNOS were higher in the lungs of heifers in the healthy group than in the HAPH group (both P < 0.001), whereas endothelin-1 protein levels were higher in HAPH group than in the healthy group (P = 0.025). NCG supplementation decreased mPAP and ammonia (both P = 0.001), whereas it increased the expression of eNOS, arginine, and plasma NO (all P < 0.05).
Conclusions
The expression of eNOS was decreased in Holstein heifers with HAPH. NCG supplementation decreased mPAP through the restoration of eNOS and endogenous NO synthesis.
Similar content being viewed by others
Background
Bovine brisket disease, which occurs in high altitude areas (altitude > 1,524 m) was first defined by Glover and Newsom in 1915, which is initiated by high altitude pulmonary hypertension (HAPH) [1, 2]. Compared with the other mammalian species, cattle exhibit the most severe chronic hypoxic pulmonary hypertension responses [3]. Over 2 million heads of cattle reside at high altitude locations in the United States of America [4], and HAPH affects 3%–25% of some herds transported from low to high altitudes [1]. Acute altitude exposure results in marked reduction of arterial oxygen saturation and oxygen supply to the cardiovascular system [5], whereas it increases mPAP in unadapted individuals. This disease commonly occurs in beef and dairy cattle grown at high altitudes, and there is currently no effective therapy [6,7,8].
Nitric oxide (NO) is a vasodilator of pulmonary circulation [9], which relaxes vascular smooth muscle tone, and plays a key role in decreasing pulmonary artery resistance and maintaining dilation of the pulmonary vasculature [10]. A reduction in the synthesis of NO by endothelial nitric oxide synthase (eNOS) in the lungs can result in pulmonary vasoconstriction [9], and pulmonary arterial pressure is increased by deleting the eNOS gene [11]. Augmented endogenous NO production contributes to suppressing vasoconstriction in Yaks [12]. Furthermore, Tibetans have high NO levels, which confers higher blood flow as a central adaptive mechanism to high-altitude hypoxia [13]. However, eNOS expression and NO production are reduced in rats with pulmonary hypertension [14]. Inhaled NO attenuates pulmonary vasoconstriction [15], and has been shown to improve survival rates in a rat model [16]. However, chronic therapy with inhaled NO has been restricted by its high expense [17]. L-arginine, a substrate for eNOS [18], has been used to attenuate pulmonary hypertension in broiler chickens grown at high altitudes [19]. N-carbamylglutamate (NCG), which is a structural analog of N-acetylglutamate [20], promotes urea cycling, and increases the endogenous synthesis of arginine [21], eNOS [22], and NO [23]. Although the effects of L-arginine in increasing NO synthesis and eNOS expression, and attenuating pulmonary hypertension have been previously studied [19, 24], the effects of NCG on alleviating HAPH remain unknown.
With the rapid worldwide growth in dairy and beef consumption, large numbers of Holstein and Angus cattle have been moved from low-to high-altitude ranches both in the Qinghai-Tibet plateau of China, and the Rocky Mountains region of the United States of America. However, despite the high incidence rate of HAPH in cattle transported to high altitudes, there is currently no effective treatment available. Therefore, the aim of the current study was to determine 1) pulmonary vascular reconstruction and expression of eNOS in lung tissues of Holstein heifers; 2) effects of NCG on restoring arginine, eNOS and NO synthesis, and alleviating HAPH in Holstein heifers.
Methods
Animals and experimental design
The study was conducted in Lhasa, Tibet, China (3,658 m), 2,000 Holstein heifers were transported from Xi’an, Shaanxi Province, China (1,027 m) to Lhasa in 2015 and they were exposed to the hypoxic environment for one year. Diagnosis of HAPH in heifers was based on mean pulmonary artery pressure (mPAP), heifers with mPAP > 49 mmHg were considered to have HAPH, whereas mPAP < 41 mmHg were considered to be healthy heifers [1].
Exp. 1, six healthy Holstein heifers (mPAP: 38.13 ± 1.50 mmHg) with body weight of 557 ± 14 kg, and six HAPH affected Holstein heifers (mPAP: 72.28 ± 1.91 mmHg) with body weight of 480 ± 18 kg, with an average age of 18 ± 2 months were selected. Heifers from each group were euthanized to collect lung tissue for detecting pulmonary vascular reconstruction, and for analyses of mRNA and protein expression of eNOS and endothelin-1 (ET-1).
Exp. 2, ten heifers exhibiting HAPH (460 ± 36.5 kg body weight), and 10 healthy Holstein heifers (497 ± 10.2 kg body weight) with a mean age of 18 ± 2 months, were selected for a feeding trial. The heifers were housed in individual tie-stalls. Heifers were fed three times daily with a total mixed ration (TMR; Additional file 1: Table S1). Physiological parameters were determined, and blood samples were collected after a 30-day adaptation period. Thereafter, heifers were individually supplemented with 20 g of NCG (Asia Pacific Xingmu Technology Co., Ltd., Beijing, China; 98% purity) per day [23] at 07:00 a.m. bytop-dressed feeding onto the total mixed ration (TMR, Additional file 1: Table S1). The physiological and blood parameters were determined after a 4-week feeding period.
Sample collection
In Exp. 1, heifers were euthanized followed by exsanguination. Lung tissue samples were collected (one dorsally and one ventrally) near the tip of the right lobe. Lung tissue was fixed in 10% neutral buffered formalin and processed for histopathological examination. In Exp. 2, 2 mL of blood was collected from the jugular vein into a 3-mL tube containing 0.25 mL of lithium heparin (1,000 IU/mL). Blood samples were centrifuged at 1,500×g for 15 min at 4 °C and plasma was stored at − 20 °C. Samples of the TMR and orts for individual cows were collected daily and pooled weekly during Exp. 2 for analyzing TMR ingredients and chemical composition.
Physiological parameters measurements
To determine mPAP, the right external jugular vein was pricked using a needle, and a Swan-Ganz catheter (7F) was passed through during heifer awake. A three-way stop cock was used to connect a pressure transducer (Millar Instruments, Houston, TX, USA) to a physiological recorder (Powerlab ML786), and pressure waves were viewed using Chart 5 computer software (AD Instruments, Colorado Springs, CO, USA), as previously described [1]. Measurements were repeated three times and the average of 20 pressure cycles was calculated as the pressure value. Body weights were measured at the beginning of the trial. Peripheral blood pressure and heart rate were measured. Peripheral blood pressure and heart rate were measured using the 9401BP Cardell Veterinary Monitor (Sharn Veterinary, Inc., Tampa, FL), as previously described [25]. Two veterinarians counted the breathing rates at the same time, and measurements were taken from each animal on three consecutive occasions.
Blood sample analysis
The plasma concentration of eNOS was measured by an enzyme-linked immunosorbnent assay using a primary rabbit polyclonal eNOS antibody (ab5589; Abcam, Cambridge, UK), and a secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (cat. no. 2004; Santa Cruz Biotechnology, CA, USA) as described previously [26]. The intra- and- inter- assay coefficients of variation were 6.0% and 11%, respectively. Plasma NO was measured using a commercial NO kit (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) [23]. Plasma urea was measured using a urea analysis kit (ab83362; Abcam, Cambridge, UK), and ammonia was measured using an ammonia analysis kit (ab83362; Abcam, Cambridge, UK). Plasma arginine was determined by fluoro-metric high-performance liquid chromatography methods as described previously [21].
Histological analysis for pulmonary arteries reconstruction
Lung tissues were sectioned at 8 μm. Verhoeff–Van Gieson staining (VVG, HT25A; Sigma Aldrich, St. Louis, MO, USA) was conducted, and the ratio of staining area to artery area was calculated in both pulmonary arterioles (diameter < 100 μm and diameter ≥ 100 μm) [27]. The media of pulmonary arteries was measured using a primary antibody against smooth muscle-specific alpha-actin (anti α-SMA; Affinity, OH, USA) [28], as described previously [27]. Slides were incubated with a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (sc-2005; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at 37 °C. After adding 3, 3′-diaminobenzidine chromogenic, slides were examined and assessed for the ratio of media/(media + lumen + intima). The immunostaining density of eNOS in the endothelium was measured using an eNOS antibody (ab5589; Abcam, Cambridge, UK) [26], following the manufacturer’s protocol [14]. Six slides (five arteriole within each section) were selected for taking pictures using Leica microscope (DM2500, Leica Camera AG, Solms, German) and quantification using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Reverse-transcription polymerase chain reaction (RT-PCR) for eNOS and ET-1 in lung tissues
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA), and 2 μg of DNAse-treated total RNA was reverse transcribed using a reverse transcriptase kit (Promega, Madison, WI, USA). Reverse-transcription polymerase chain reaction was performed using Biosystems 7300 System (Foster, CA, USA) with a master mix (4367659; SYBR Green; Foster, CA, USA). The primer pairs used for endothelin-1 (ET-1), eNOS and β-actin amplification and the reaction conditions were as follows as described previously [29, 30]. The reaction efficiency for each primer assay was (ET-1 = 94.54%, eNOS = 96.84%, β-actin = 98.44%, respectively). Data obtained from RT-PCR were normalized against β-actin. The relative quantification of gene amplification by RT-PCR was performed using cycle threshold (Ct) values. The comparative Ct value method was employed to quantify expression levels for eNOS and ET-1, as described previously [22].
Western blot for eNOS and ET-1 in lung tissues
Total protein (100 mg) was extracted using a protein extraction kit (Beyotime, Beijing, China). Protein concentrations were measured using a bicinchoninic protein assay kit (CoWin Biotech, Beijing, China). Protein extracts were resolved on 5.0% SDS polyacrylamide gels. Proteins were transferred onto a polyvinylidene fluoride membrane (Millipore, Temecula, CA, USA), and incubated with 5% bovine serum albumin (BSA) at 4 °C overnight. The membranes were subsequently incubated with primary ET-1 antibody (ab2786, Abcam, Cambridge, UK, 1:500 dilution), eNOS antibody (ab5589, Abcam, Cambridge, UK, 1:1,000 dilution) [26] or GAPDH antibody (Sigma-Aldrich, St. Louis, MO, USA, 1:10,000 dilution). After incubation, the membrane was washed three times with phosphate buffer solution containing tween (1 × phosphate buffer solution + 0.01%Tween-20). A horseradish peroxidase conjugated goat anti mouse secondary antibody (Jackson, PA, USA) was applied for ET-1 (1:10,000 dilution);horseradish peroxidase-conjugated goat anti rabbit secondary antibody (1:10,000 dilution) was used for eNOS; horseradish peroxidase-conjugated rabbit anti mouse secondary antibody (1:10,000 dilution) was used for GAPDH (G9545, Sigma-Aldrich, St. Louis, MO, USA,). Gel images were scanned using a Gel Image system (ver.4.00; Tanon, Shanghai, China). Target bands were quantified by densitometry using Image-Pro Plus (Media Cybernetics, Rockville, MD, USA).
TMR ingredients and chemical composition analyses
All TMR and orts samples were dried at 65 °C in a forced-air oven (Model 2000; Experimental Mill, China) for 48 h to a constant weight, ground through a 1-mm screen using a Wiley mill (standard model 4; Arthur H. Thomas Co., Philadelphia, PA), and analyzed for dry matter (DM), crude protein (CP; method 4.2.08; AOAC 1990), ether extract (method 920.85; AOAC 1990), ash (942.05; AOAC 1990), calcium and phosphorus (method 945.46; AOAC 1990), acid detergent fiber (ADF; expressed exclusive ash) (method 973.18; AOAC 1990) [31], and neutral detergent fiber (NDF) [32]. The NDF was determined using sodium sulfite without α-amylase and was expressed exclusive to ash using the Ankom 200 fiber Analyzer (Ankom Technology, Fairport, NY, USA). The chemical composition of the TMR is presented in Additional file 1: Table S1.
Data analyses
Data are expressed as means ± SEM or pooled SEM. Statistical comparisons were performed using unpaired Student’s t-test. The effects of NCG were analyzed using two-way repeated-measures ANOVA, with Bonferroni post hoc correction for significant interactions, NCG treatment and Holstein heifers’ state as main factors. Differences among individual treatment within a column were tested by Duncan’s multiple comparison when a significant interaction between the main effects was observed. Statistical analyses were performed using Graph Pad software (Graph Pad Software, Inc., La Jolla, CA). Statistical significance was declared at P ≤ 0.05.
Results
Differences in pulmonary vascular reconstruction
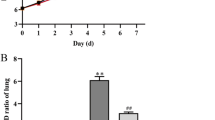
The adventitia of pulmonary arteries were significantly thicker in the HAPH group compared with healthy group; [(diameter < 100 μm; P = 0.033) and (diameter ≥ 100 μm; P = 0.001)] (Fig. 1a-c). A significantly higher percentage of vascular media area was observed in the HAPH group compared with the healthy group [(diameter < 100 μm; P = 0.006) and (diameter ≥ 100 μm; P < 0.001)] (Fig. 1d-f). Immunostaining densities of eNOS were significantly lower (P = 0.006; Fig. 1g-i) in the HAPH group than in the healthy group. Moreover, the intima of pulmonary arteries in the healthy group were significantly thicker compared with the HAPH group (Fig. 1g-h).
Immunohistochemical staining of small pulmonary arteries in lung tissue sections. (Control = healthy Holstein heifers, HAPH = Holstein heifers with high altitude pulmonary hypertension. Means ± SEM (n = 6), *P < 0.05; **P < 0.01). (a) Verhoeff–Van Gieson staining (VVG) for elastin in small pulmonary arteries from healthy heifers. (b) Representative images of lung histological sections of heifers in HAPH group. Arrows indicate the proportional volume of elastin-positive adventitia. (c) Bar graphs representing the percentage area of vascular elastin in the small (diameter < 100 μm) and other (diameter ≥ 100 μm) pulmonary arteries. (d) Immunohistochemical staining of alpha smooth muscle actin (α-SMA) in small pulmonary arteries of Healthy heifer. (e) Representative images of histological lung sections from Holstein heifers with HAPH. Arrows indicate the proportional volume of the media layer. (f) Bar graph representing the percentage area of vascular media in small (diameter < 100 μm) and other (diameter ≥ 100 μm) pulmonary arteries. (g) Immunohistochemical staining of endothelial nitric oxide synthases (eNOS) in the healthy heifers. (h) Representative images of lung histological sections from heifers in the HAPH group. Arrows indicate immunostaining of eNOS. Scale bar = 50 μm. (i) Bar graph representing the relative immunostaining density of eNOS in pulmonary arterial endothelium (75 μm ≤ diameter < 100 μm). Scale bars = 100 μm
Expression of eNOS and ET-1 in healthy and HAPH affected heifers
In lung tissues, eNOS mRNA and protein levels of eNOS were higher in the healthy group than in the HAPH group (P < 0.001 and P = 0.008; Fig. 2a and b, respectively). Furthermore, HAPH group exhibited higher ET-1 protein levels compared with the healthy group (P = 0.025; Fig. 2c).
Expression of endotheline-1 and endothelial nitric oxide synthases mRNA and protein in lung tissues. Control = healthy Holstein heifers, HAPH = Holstein heifers with high altitude pulmonary hypertension. Means ± SEM (n = 3), *P < 0.05; **P < 0.01. Relative mRNA levels of endotheline-1 (ET-1) and endothelial nitric oxide synthases (eNOS) in lung tissues. The expression of β-actin served as an internal control. Bar graph representing the relative ET-1 and eNOS protein levels. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein expression served as an internal control. Western blot showing ET-1 and eNOS protein levels in lung tissues
Effect of NCG supplementation on physiological parameters
Heifers in the HAPH group had greater mPAP than heifers in the healthy group (P = 0.001; Table 1). Supplementation of NCG significantly reduced mPAP in both groups (P = 0.001). Supplementation of NCG had a tendency to decrease systolic blood pressure (P = 0.068), and a tendency to increase breath rate (P = 0.065) in HAPH group. For systolic blood pressure the state × treatment interaction was significant (P = 0.005) and supplementation with NCG significantly reduced systolic blood pressure only in the HAPH group.
Effect of NCG supplementation on NO synthesis parameters
Plasma eNOS, arginine, and NO were significantly lower in the HAPH group compared with the healthy group (P = 0.020, P = 0.018, P = 0.001; respectively, Table 2). However, supplementation with NCG significantly decreased the plasma ammonia concentration (P = 0.001), while it increased the plasma eNOS, NO, and arginine concentrations (P = 0.001, P = 0.002, P = 0.001; respectively) in both groups. The state × treatment interaction (P = 0.022) significantly affected the plasma ammonia concentration.
Discussion
The effects of supplementation with NCG in Holstein heifers with HAPH were studied for the first time. Pulmonary hypertension is associated with a disorder of endothelial cell proliferation in humans [33], and reduced expression of NO enzymes in the endothelium of pulmonary arteries with abnormal wall morphology [14]. Furthermore, endothelial NO synthase plays an important role in the maintenance of normal blood pressure and pulmonary vascular wall structure [14]. In the present study, the vascular intima was relatively thinner in heifers in the HAPH group than in the healthy group, which was accompanied by fewer endothelial cells and reduced eNOS expression. It has been reported that the degree of intimal thickening is negatively correlated with the severity of lung disease [34]. In human patients, an inverse correlation was observed between the intimal thickness of blood vessels and impaired NO release [35]. These findings indicate that in heifers with HAPH, the vascular intimal structure was impaired, which in turn decreased the expression of eNOS in the pulmonary arteries.
In the present study, the lower NO production in HAPH heifers could not be attributed to arginine deficiency. This is because L-arginine, the substrate for eNOS, is the major determinant of NO synthesis [36], which was higher in heifers in the HAPH group than in the healthy group. A previous study reported similar results, indicating that the impaired NO production is not due to a deficiency in L-arginine availability and/or transport [35]. It was further demonstrated that, in the lungs of patients with pulmonary hypertension, and an abnormal wall morphology in the endothelium of pulmonary arteries, eNOS expression was substantially reduced [14].We also found that the expression of eNOS was reduced in HAPH affected heifers, and this reduces the efficiency of plasma arginine utilization. Previous studies have reported that the expression of the eNOS gene tends to increase in allantochorion tissue of sow placentas after NCG supplementation [22]. We also found that NCG supplementation increased the plasma concentration of L-arginine, eNOS, and NO. We speculated that NCG enhances L-arginine synthesis, which may contribute to the restoration of eNOS-coupled activity by reducing the generation of superoxide anions, and increasing NO synthesis [24]. We conclude that a reduction in NO expression in heifers with HAPH was mainly attributed to a decrease in eNOS expression, rather than to a deficiency in plasma L-arginine.
Urea cycle disorders are attributed to a deficiency in N-acetylglutamate synthase, which can be successfully treated with NCG and arginine hydrochloride [20]. In Holstein cattle, dietary supplementation with NCG was shown to decrease plasma ammonia and urea concentrations [23]. In the present study, NCG supplementation decreased plasma ammonia concentrations, which suggests that NCG is a potent agent for accelerating ammonia reduction and arginine synthesis.
Endothelial produced NO is a potent vasodilator, and induces vascular smooth muscle relaxation [17]. In the present study, we found that NCG supplementation decreased mPAP in both group. Because mPAP measurement is actually a measure of pulmonary blood flow resistance [34], these results indicate that NCG supplementation reduces pulmonary vascular resistance by generating NO. In cattle, cor pulmonale occurs when pulmonary hypertension, caused by increased pulmonary vascular resistance, which increases the cardiac workload [37]. These results indicate that pulmonary arterial blood pressure can be decreased in Holstein heifers by NCG supplementation, which has beneficial effects in terms of reducing vascular resistance. Increased endogenous NO production, is responsible for the low pulmonary arterial pressure found in high-altitude adapted yaks [12]. Tibetans had > 10-fold higher circulating concentrations of bioactive NO products, which contribute to adaptation to high-altitude hypoxia by increasing blood flow [13]. Actually, augmented NO expression maintain normal levels of oxygen achieve and normal oxygen delivery by promoting blood flow and decreasing vascular resistance in Tibetans [13]. Present study also observed that NCG improved NO synthesis and decreased mPAP. Further, NO induces pulmonary vasodilation not only through increasing the production of cyclic guanosine monophosphate but also indirectly by inhibiting ET-mediated pulmonary vasoconstriction [38]. These findings indicate that supplementation of NCG increase NO expression in heifers ascended to high altitude, which accounts for adaptation to high-altitude hypoxia.
Conclusions
In conclusion, the findings indicate that eNOS synthesis decreased in Holstein heifers with HAPH, which likely contributed to a deterioration of HAPH. Dietary supplementation with NCG reduced mPAP, peripheral systolic blood pressure, through the restoration of eNOS and endogenous NO synthesis in Holstein heifers with HAPH. Because NCG has a lower rumen degradability compared with arginine, it could be a potential alternative to arginine for attenuation of bovine HAPH, and could also be used to alleviate pulmonary hypertension in human patients.
Abbreviations
- eNOS:
-
Endothelial nitric oxide synthase
- ET-1:
-
Endothelin-1
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HAPH:
-
High-altitude pulmonary hypertension
- mPAP:
-
Mean pulmonary hypertension
- NCG:
-
N-carbamylglutamate
- NO:
-
Nitric oxide
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
- TMR:
-
Total mixed ration
- VVG:
-
Verhoeff–Van Gieson staining
- α-SMA:
-
Alpha smooth muscle actin
References
Holt TN, Callan RJ. Pulmonary arterial pressure testing for high mountain disease in cattle. Vet Clin North Am Food Anim Pract. 2007;23:575–96.
Glover GH, Newsom IE. Brisket disease: dropsy of high altitudes. Colo Agric Exp Station. 1914;
Rhodes J. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol. 2005;98:1092–100.
Newman JH, Holt TN, Cogan JD, Womack B, Phillips JA, Li C, et al. Increased prevalence of EPAS1 variant in cattle with high-altitude pulmonary hypertension. Nat Commun. 2015;6:6863.
Heinonen I, Luotolahti M, Vuolteenaho O, Nikinmaa M, Saraste A, Hartiala J, et al. Circulating N-terminal brain natriuretic peptide and cardiac function in response to acute systemic hypoxia in healthy humans. J Transl Med. 2014;12:189.
Gulick A, Garry F, Holt T, Retallick-Trennepohl K, Enns R, Thomas M, Neary J. Angus calves born and raised at high altitude adapt to hypobaric hypoxia by increasing alveolar ventilation rate but not hematocrit. J Anim Sci. 2016;94:4167–71.
Malherbe CR, Marquard J, Legg DE, Cammack KM, O’Toole D. Right ventricular hypertrophy with heart failure in Holstein heifers at elevation of 1,600 meters. J Vet Diagn Investig. 2012;24:867–77.
Neary MT, Neary JM, Lund GK, Holt TN, Garry FB, Mohun TJ, et al. Myosin heavy chain 15 is associated with bovine pulmonary arterial pressure. Pulm Circ. 2014;4:496–503.
Fagan KA, Morrissey B, Fouty BW, Sato K, Harral JW, Morris KG, et al. Upregulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir Res. 2001;2:1.
Tonelli AR, Haserodt S, Aytekin M, Dweik RA. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ. 2013;3:20–30.
Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, et al. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest. 1998;101:2468–77.
Ishizaki T, Koizumi T, Ruan Z, Wang Z, Chen Q, Sakai A. Nitric oxide inhibitor altitude-dependently elevates pulmonary arterial pressure in high-altitude adapted yaks. Resp Physiol Neurobiol. 2005;146:225–30.
Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci U S A. 2007;104:17593–8.
Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–21.
Scherrer U, Vollenweider L, Delabays A, Savcic M, Eichenberger U, Kleger GR, et al. Inhaled nitric oxide for high-altitude pulmonary edema. N Engl J Med. 1996;334:624–9.
Omura A, Roy R, Jennings T. Inhaled nitric oxide improves survival in the rat model of high-altitude pulmonary edema. Wilderness Environ Med. 2000;11:251–6.
Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–85.
Marletta MA. Nitric-oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–4.
Khajali F, Tahmasebi M, Hassanpour H, Akbari MR, Qujeq D, Wideman RF. Effects of supplementation of canola meal-based diets with arginine on performance, plasma nitric oxide, and carcass characteristics of broiler chickens grown at high altitude. Poult Sci. 2011;90:2287–94.
Gessler P, Buchal P, Schwenk HU, Wermuth B. Favourable long-term outcome after immediate treatment of neonatal hyperammonemia due to N-acetylglutamate synthase deficiency. Eur J Pediatr. 2010;169:197–9.
Wu X, Ruan Z, Gao Y, Yin Y, Zhou X, Wang L, et al. Dietary supplementation with l-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–9.
Wu X, Yin Y, Liu Y, Liu X, Liu Z, Li T, et al. Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Animl Reprod Sci. 2012;132:187–92.
Chacher B, Zhu W, Ye JA, Wang DM, Liu JX. Effect of dietary N-carbamoylglutamate on milk production and nitrogen utilization in high-yielding dairy cows. J Dairy Sci. 2014;97:2338–45.
Ou Z-J, Wei W, Huang D-D, Luo W, Luo D, Wang Z-P, et al. L-arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol-Endoc M. 2010;298:E1131–9.
Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals part 2: blood pressure measurement in experimental animals. A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;25:e22–33.
Ootaki C, Yamashita M, Ootaki Y, Kamohara K, Weber S, Klatte RS, Emancipator SN, Golding LA, Fukamachi K. Reduced pulsatility induces periarteritis in kidney: role of the local renin-angiotensin system. J Thorac Cardiovasc Surg 2008;, et al. 136:150–158.
Xu XF, Gu WZ, Wu XL, Li RY, Du LZ. Fetal pulmonary vascular remodeling in a rat model induced by hypoxia and indomethacin. J Matern Fetal Neonatal Med. 2011;24:172–82.
Zhao S, Gao YY, Xia XJ, Che YY, Wang YP, Liu HT, et al. TGF-beta 1 promotes Staphylococcus aureus adhesion to and invasion into bovine mammary fibroblasts via the ERK pathway. Microb Pathog. 2017;106:25–9.
Pires PR, Santos NP, Adona PR, Natori MM, Schwarz KR, de Bem TH, et al. Endothelial and inducible nitric oxide synthases in oocytes of cattle. Anim Reprod Sci. 2009;116:233–43.
Korzekwa AJ, Bodek G, Bukowska J, Blitek A, Skarzynski DJ. Characterization of bovine immortalized luteal endothelial cells: action of cytokines on production and content of arachidonic acid metabolites. Reprod Biol Endocrinol. 2011;9:27.
AOAC. Official methods of analysis. 15th edition ed. Washington, DC: Association of Official Analytical Chemists; 1990.
Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97.
Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–65.
Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, et al. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–47.
Dinh-Xuan A, Pepke-Zaba J, Butt A, Cremona G, Higenbottam T. Impairment of pulmonary-artery endothelium-dependent relaxation in chronic obstructive lung disease is not due to dysfunction of endothelial cell membrane receptors nor to L-arginine deficiency. Br J Pharmacol. 1993;109:587–91.
Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2001;98:4202–8.
Angel KL, Tyler JW. Pulmonary hypertension and cardiac insufficiency in three cows with primary lung disease. J Vet Intern Med. 1992;6:214–9.
Zhou Z, de Beer VJ, de Wijs-Meijler D, Bender SB, Hoekstra M, Laughlin MH, et al. Pulmonary vasoconstrictor influence of endothelin in exercising swine depends critically on phosphodiesterase 5 activity. Am J Physiol Lung Cell Mol Physiol 2014; 306:442–452.
Acknowledgments
Thanks to Zhen. La and Sang. Zhu for their help with farm work.
Funding
Funding for the study was provided by the National Dairy Industry and Technology System (Beijing, P. R. China).
Availability of data and materials
The datasets analyzed are not publically available due to ownership by the funding partners, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
SL conception and design of research. SW performed experiments. AA was a contributor in revised manuscript. SW prepared figures. SW drafted manuscript. Yajing Wang and Zhijun Cao edited and revised manuscript; Shengli Li approved final version of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All protocols for sampling and handling of animals were approved by the Institutional Animal Care and Use Committee at China Agricultural University (Beijing, P. R. China). The experiment was conducted according to the regulations and guidelines established by this committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional file
Additional files 1:
Table S1. Ingredients and chemical composition of the total mixed ration. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, S., Azarfar, A., Wang, Y. et al. N-carbamylglutamate restores nitric oxide synthesis and attenuates high altitude-induced pulmonary hypertension in Holstein heifers ascended to high altitude. J Animal Sci Biotechnol 9, 63 (2018). https://doi.org/10.1186/s40104-018-0277-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-018-0277-6