Abstract
Background
The histone code is an established epigenetic regulator of early embryonic development in mammals. The lysine residue K9 of histone H3 (H3K9) is a prime target of SIRT1, a member of NAD+-dependent histone deacetylase family of enzymes targeting both histone and non-histone substrates. At present, little is known about SIRT1-modulation of H3K9 in zygotic pronuclei and its association with the success of preimplantation embryo development. Therefore, we evaluated the effect of SIRT1 activity on H3K9 methylation and acetylation in porcine zygotes and the significance of H3K9 modifications for early embryonic development.
Results
Our results show that SIRT1 activators resveratrol and BML-278 increased H3K9 methylation and suppressed H3K9 acetylation in both the paternal and maternal pronucleus. Inversely, SIRT1 inhibitors nicotinamide and sirtinol suppressed methylation and increased acetylation of pronuclear H3K9. Evaluation of early embryonic development confirmed positive effect of selective SIRT1 activation on blastocyst formation rate (5.2 ± 2.9% versus 32.9 ± 8.1% in vehicle control and BML-278 group, respectively; P ≤ 0.05). Stimulation of SIRT1 activity coincided with fluorometric signal intensity of ooplasmic ubiquitin ligase MDM2, a known substrate of SIRT1 and known limiting factor of epigenome remodeling.
Conclusions
We conclude that SIRT1 modulates zygotic histone code, obviously through direct deacetylation and via non-histone targets resulting in increased H3K9me3. These changes in zygotes lead to more successful pre-implantation embryonic development and, indeed, the specific SIRT1 activation due to BML-278 is beneficial for in vitro embryo production and blastocyst achievement.
Similar content being viewed by others
Background
Correct formation of maternal and paternal pronuclei in the fertilized mammalian oocyte, the zygote, is required for the first mitotic cell cycle, subsequent zygotic genome activation and successful development of early embryo [1, 2]. Many events, such as protamine-histone replacement [3, 4], protein recycling through ubiquitin-proteasome system (UPS) [5, 6] and correct establishment of euchromatin and heterochromatin [7, 8], lead to genome-wide alterations required for the biogenesis of pronuclei. In addition to these essential genomic and cellular events, pronuclei undergo epigenetic changes, i.e. DNA methylation as well as histone methylation and acetylation, collectively termed the histone code establishment [9,10,11,12,13]. Epigenetic changes in the early zygote include DNA demethylation in both the maternal and paternal pronucleus [14] as well as parent-of-origin specific modifications of pronuclear histone code [9]. However, up-stream factors of histone code in zygote and their influence on embryo development and blastocyst quality are poorly understood.
Sirtuins (SIRTs) are a family of NADP+-dependent histone-deacetylases including 7 isoforms with specific subcellular localization patterns [15]. Among them, SIRT1 is the most potent regulator of histone code, present notably in the nucleus and it enhances cell viability by regulating epigenome remodeling [16, 17]. The expression of SIRTs in mammalian oocytes and embryos have been observed [18,19,20,21,22], and the essential role of SIRT1 in oocyte maturation and early embryonic development has been established [19, 23]. Accordingly, beneficial effect of red grape flavonoid resveratrol, a cell protectant/antioxidant substance and a strong activator of SIRT1, on oocyte quality and success of embryonic development is well-known [24,25,26,27]; however, we lack the understanding of mechanisms by which SIRT1 enhances oocyte maturation, fertilization and early embryonic development.
Based on somatic cell studies, SIRT1 is able to remove the acetyl group from lysine residues of several histones, resulting in deacetylation of histone H1 on lysine K26 [28, 29], H3 on K9, K14 and K56 [28, 30], and H4 on K8, K12 and K16 [28, 31]. Acetylation of H3K9 is an established marker of translational activity, but it is also frequently associated with DNA damage [32]. Deacetylation of H3K9 makes it available for methyl group addition by histone methyltransferases [33,34,35,36]. The involvement of UPS, through the participation of Mouse double minute 2 homolog (MDM2), an E3-type ubiquitin ligase, in SIRT1-mediated H3K9 methylation is indicated [37] and remains the lone consideration of SIRT1 mechanism in the nucleus.
Based on the above knowledge, we hypothesized that SIRT1 affects acetylation-methylation pattern of H3K9 in formatting porcine zygote pronuclei. We also predicted that the SIRT1-modulated H3K9 zygotic histone code establishment will enhance early embryonic development measured by development to blastocyst and blastocyst quality.
Methods
Collection and in vitro maturation (IVM) of porcine oocytes
Porcine ovaries were obtained from 6- to 8-month-old non-cycling gilts (a crossbreed of Landrace x Large White) at the local slaughterhouse (Jatky Plzen a.s., Plzen, Czech Republic) and transported to laboratory at 39 °C. Cumulus-oocyte complexes (COCs) were collected from ovarian follicles with a diameter of 2–5 mm by aspiration with a 20-gauge needle and handled in HEPES-buffered Tyrode lactate medium containing 0.01% (w/v) polyvinyl alcohol (TL-HEPES-PVA). Only fully grown oocytes with evenly dense cytoplasm, surrounded by compact cumuli, were selected for IVM and washed in maturation medium. The medium used for IVM was modified tissue culture medium (mTCM) 199 (Gibco, Life Technologies, UK) supplemented with 0.1% PVA, 3.05 mmol/L D-glucose, 0.91 mmol/L sodium pyruvate, 0.57 L-cysteine, 0.5 μg/mL LH (Sigma-Aldrich, USA), 0.5 μg/mL FSH (Sigma), 10 ng/mL epidermal growth factor (EGF; Sigma), 10% porcine follicular fluid, 75 μg/mL penicillin G and 50 μg/mL streptomycin. After 22 h of culture, the COCs were cultured in TCM199 without LH and FSH for an additional 22 h. The COCs were cultured in 500 μL of the medium covered by mineral oil in a four-well Petri dish (Nunc, Denmark), at 39 °C and 5% CO2 in air [38].
In vitro fertilization (IVF) and culture (IVC) of porcine oocytes and zygotes
After 44 h of IVM, cumulus cells were removed with 0.1% hyaluronidase in TL-HEPES-PVA and the metaphase II (MII) oocytes with extruded first polar body were selected for IVF. The oocytes were washed three-times with modified Tris-buffered medium (mTBM) [38] with 0.2% bovine serum albumin (BSA; A7888; Sigma) and placed into 100 μL drops of mTBM, covered with mineral oil in a 35 mm Petri dish. The dishes were allowed to equilibrate at 38.5 °C and 5% CO2 for 30 min before spermatozoa were added for fertilization. Spermatozoa were prepared as follows: 1 mL liquid semen preserved in BTS-based extender was washed twice in phosphate buffered saline (PBS) with 0.1% PVA (PBS-PVA) at 1,500 rpm for 5 min. The last wash was supplemented with MitoTracker CMTRos (400 nmol/L; M7510, Invitrogen) for 10 min at 39 °C, used to tag sperm mitochondria that associate with the paternal pronucleus inside the fertilized oocytes. Labeled spermatozoa were resuspended in mTBM (2.5–5 × 107 spermatozoa per mL) and 1 μL of this sperm suspension was added to the medium containing the oocytes to give a final sperm concentration of 2.5 or 5 × 105 spermatozoa per mL. Oocytes were co-incubated with spermatozoa for 5 to 6 h at 38.5 °C and 5% CO2 in air. For zygote acquisition, oocytes were thereafter washed and transferred into 100 μL PZM3 medium [39] containing 0.4% BSA (A6003; Sigma), for further culture for 22 h. Simultaneously, presumed zygotes were cultured in 500 μL of PZM3 medium for 144 h to reach blastocyst stage. PZM3 medium contained different concentrations of SIRT1 activators or inhibitors dissolved in DMSO (in its final concentration 0.1%) as described below. The IVF and IVC studies were repeated three to five times for each treatment regimen.
Sirtuin activation and inhibition
Activation and inhibition of SIRT1 was performed in PZM3 during IVC of early zygote development. Activators of SIRT1 included resveratrol (3.0, 6.25, 12.5 μmol/L; non-selective sirtuin activator, Abcam, ab120726) and BML-278 (3.0 μmol/L; selective SIRT1 activator, Abcam, ab144536). Inhibitors of SIRT1 included nicotinamide (2.5, 5.0, 7.5 mmol/L; non-selective sirtuin inhibitor, Abcam, ab120864) and sirtinol (10 μmol/L; selective SIRT1 and SIRT2 deacetylase inhibitor, Abcam, ab141263). The effective concentrations of BML-278 (N-Benzyl-3,5-dicarbethoxy-4-phenyl-1,4-dihydropyridine) and sirtinol were chosen based on preliminary experiments conducted to optimize the concentrations of resveratrol and nicotinamide (data not shown). All compounds were dissolved in DMSO, the concentration of which never bypassed 0.5%, as also used for vehicle controls.
Immunofluorescence and imaging of zygotes
After 22 h of IVC, presumed zygotes were treated with 0.5% pronase for zona pellucida removal and processed as described by Yi et al. [40], with modifications. Briefly, zygotes were fixed in 2% formaldehyde and permeabilized in 0.1% Triton-X-100 in PBS (PBS-TX) for 40 min. Thereafter, zygotes were blocked in 5% normal goat serum (NGS) in PBS-TX for 25 min. A mixture of mouse monoclonal anti-histone H3 tri-methylated K9 antibody (H3K9me3; ab71604, Abcam, UK; 1:200) and rabbit monoclonal anti-histone H3 acetylated K9 antibody (H3K9ac; ab32129, Abcam, Cambridge, UK; 1:200) was applied overnight at 4 °C. Subsequently, the oocytes were washed twice in 1% NGS in BPS-TX before being incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti mouse (GAM) IgG (cat. #62–6511; Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA; 1:200) and cyanine dye (Cy5)-conjugated goat anti-rabbit (GAR) IgG (111–175-144; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), for 40 min at room temperature. Thereafter, the oocytes were washed twice and mounted into slides in a Vectashield medium with 4′6’-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA). SIRT1 was detected by adapting the above protocol for mouse monoclonal antibody clone 1F3 (ab104833, Abcam). Images were acquired using the Ti-U microscope (Nikon Co., Tokyo, Japan) with Clara Interline CCD camera (Andor Technology PLC, Belfast, Northern Ireland) operated by NIS Elements Ar software (Nikon Co., Tokyo, Japan). Negative controls were performed by omitting specific antibodies and these slides were processed at comparable settings. The image analysis was performed by NIS Elements. Signal intensities of pronuclear H3K9ac and H3K9me3 were scaled by a basal signal intensity of corresponding zygote cytoplasm and expressed as a relative acetylation/methylation ratio.
Evaluation of fertilization and embryonic development
Within the imaging of 22 h zygotes, an assessment of penetration rate, creation of paternal pronucleus/pronuclei and monospermic fertilization was performed. Embryo cleavage was assessed by microscopy at 48 h of IVC; after 144 h of IVC, embryos were fixed in 2% formaldehyde for 40 min at room temperature (RT), washed three times with PBS, permeabilized with PBS-Triton X-100 for 30 min, and stained with 2.5 μg/mL DAPI (DNA staining; Molecular Probes, Eugene, OR, USA) for 40 min. Embryo cleavage, blastocyst formation, and cell number per blastocyst were assessed under an Eclipse Ci fluorescence microscope (Nikon Co., Tokyo, Japan).
Quantification of fluorescent immunolabeling intensity of the ooplasmic ubiquitin ligase MDM2
Presumed zygotes were subjected to immunofluorescence protocol described above. A mixture of rabbit polyclonal anti-MDM2 antibody (ab137413, Abcam, UK; 1:200) and mouse monoclonal anti-Glyceraldehyde 3-phosphate dehydrogenase antibody (GAPDH; ab8245, Abcam, UK; 1:200) was used. Only zygotes with visible pronuclei were used for further analysis. Images were acquired and analysed as was described. The MDM2 signal intensity was normalized to signal intensity of GAPDH, considered a housekeeping factor with constant expression in pig oocytes [41, 42].
Western blotting
In vitro matured MII oocytes, cells undergoing to in vitro fertilization assay, and their cumulus cells were used for western blot analysis. Samples were prepared and processed using the method set out by Nevoral et al. [42], with slight modifications. In brief, denuded oocytes and their cumulus cells were precipitated in 80% aceton for at least 60 min and then lysed in 20 μL of Laemmli buffer containing Triton-X-100 (0.003%, v/v) and SDS (0.001%, v/v), enriched with Complete Mini Protease Inhibitor Cocktail (Roche, Switzerland). Samples were boiled and subjected to SDS-PAGE electrophoresis in 12.5% separating gels and blotted using Trans-Blot TurboTM Transfer System (Biorad Laboratories, Steenvoorde, France) onto a PVDF membrane (GE Healthcare Life Sciences, Amersham, UK). After blocking in 5% non-fat milk in TBS with 0.5% Tween-20 (TBS-T) for 60 min at room temperature, the membrane was incubated with mouse monoclonal anti-SIRT1 (ab110304, Abcam, 1:1,000) or rabbit polyclonal anti-MDM2 (ab137413, Abcam, UK; 1:1,000) diluted in TBS-T for 60 min at room temperature. Mouse monoclonal anti-GAPDH loading control antibody (ab8245, Abcam, UK; 1:2,000) was used under same conditions. Subsequently, the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit IgG in TBS (1:3,000; Invitrogen, USA) for 60 min at room temperature. Proteins with adequate molecular weight were detected using the ECL Select Western Blotting Detection Reagent (GE Healthcare Life Sciences, Amersham, UK) and visualised by ChemiDocTM MP System (Biorad, France).
Statistics
Immunofluorescence data are presented as the mean ± S.E.M. of at least 20 zygotes per experimental group. All analysis were performed in at least three independent repetitions. The general linear models (GLM) procedure in SAS package 9.3 (SAS Institute Inc., Cary, NC, USA) was used, followed by Sheffe’s test and Duncan’s multiple range test for zygote analysis and evaluation of early embryonic development, respectively. P ≤ 0.05 was considered to be statistically significant.
Results
SIRT1 is accumulated in zygotic pronuclei
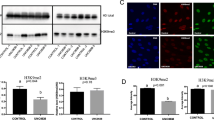
The aim of this experiment was to demonstrate the presence of SIRT1 and verify its subcellular localization in porcine zygotes. Immunocytochemical analysis demonstrated the presence of SIRT1 in both paternal and maternal pronuclei (Fig. 1a) while the intensity of cytoplasmic SIRT1 fluorescence was weak. Such localization was not observed in negative control samples where the primary antibody was omitted. A band of anticipated mass (80.9 kDa; UniProtKB (A7LKB1)) was detected with the same anti-SIRT1 antibody by Western blotting (see Additional file 1).
Representative image of SIRT1 immunofluorescence in a 22-h zygote. a SIRT1 is exclusively localized in zygote pronuclei. b Yellow arrows indicate the level of signal intensity profiles in the respective maternal and paternal pronuclei. NC: negative control where the primary antibody was omitted. Scale bar represents 50 μm
Resveratrol increases H3K9 methylation in zygotic pronuclei
To evaluate the effect of SIRT1 activation on pronuclear H3K9 acetylation and methylation, the presumed zygotes were treated by resveratrol, a non-selective activator of SIRTs, and examined by epifluorescence microscopy and relative fluorescence intensity measurement (Fig. 2a, b). Specific antibodies against modified H3K9 modification, validated for zygote imaging by a number of previous studies [43,44,45], and epifluorescence microscopy followed by image analysis were used. Fertilization rate, paternal pronucleus (PPN) formation and monospermic fertilization rate were simultaneously examined.
The effect of resveratrol on H3K9 methylation and acetylation in zygotic pronuclei. a Fluorescent signal intensities relative to signal intensity of vehicle control (= 1) and b representative images of H3K9me3 and H3K9ac in both pronuclei. a,b,c;1,2,3Significant differences in H3K9me3 and H3K9ac, respectively, among experimental groups (P ≤ 0.05). Asterisks indicate paternal pronucleus. Scalebar represents 25 μm
A significantly increased intensity of H3K9me3 in both pronuclei was observed in zygotes treated with 3 μmol/L and 12.5 μmol/L resveratrol (1.39 and 1.32 fold, respectively, compared to the vehicle control). Interestingly, the 6.25 μmol/L resveratrol did not show a significant difference from control. Contrary to H3K9 methylation, H3K9 acetylation decreased after resveratrol treatment. Although the H3K9 methylation was affected, resveratrol treatments had no effect on IVF indicators (see Additional file 2).
Nicotinamide protects pronuclear H3K9 from deacetylation
The effect of the inhibition of SIRTs on H3K9 acetylation and methylation in the presumed zygotes treated with nicotinamide (non-selective SIRTs’ inhibitor) was assessed by epifluorescence microscopy and pixel intensity measurement (Fig. 3a, b). In contrast to resveratrol, nicotinamide protected acetylation of H3K9 (1.42 and 2.28-fold increase with 5.0 and 7.5 mmol/L nicotinamide, respectively, compared to vehicle control) and decreased H3K9me3 at 7.5 mmol/L concentration (relative H3K9me3 pixel intensity of 1.00 ± 0.00 versus 0.51 ± 0.05), differences were statistically significant. Similar to resveratrol, nicotinamide did not influence the outcomes of IVF (see Additional file 2).
The effect of nicotinamide on H3K9 methylation and acetylation in zygotic pronuclei. a Fluorescent signal intensities relative to vehicle control (= 1) and b representative images of H3K9me3 and H3K9ac in both pronuclei. a,b,c;1,2,3Significant differences in H3K9me3 and H3K9ac, respectively, among experimental groups (P ≤ 0.05). Asterisk indicates paternal pronucleus. Scalebar represents 25 μm
Specific modulation of SIRT1 activity amplifies the modification of H3K9
Selective SIRT1 activator and inhibitor, BML-278 and sirtinol, respectively, were used for modulation of H3K9 methylation and acetylation in the treated and control zygotes (Figs. 4 and 5). Treatment with BML-278 increased the H3K9me3 and reciprocally decreased H3K9ac (change of 1.87 ± 0.18-fold and 0.58 ± 0.05-fold in H3K9me3 and H3K9ac, respectively, compared with control; P ≤ 0.05), (Fig. 4). In reverse, sirtinol significantly decreased H3K9me3 and increased H3K9ac to 0.70 ± 0.08 and 1.28 ± 0.12-fold change over control, respectively (Fig. 5). Similar to resveratrol and nicotinamide, the fertilization and monospermy/polyspermy rates were not affected (see Additional file 2).
The effect of BML-278 on H3K9 methylation and acetylation in the zygotic pronuclei. a Fluorescent signal intensities relative to vehicle control (= 1) and b representative images of H3K9me3 and H3K9ac in both pronuclei. *#Significant difference between control and treated group (P ≤ 0.05). Asterisk indicates paternal pronucleus. Scalebar represents 25 μm
The effect of sirtinol on H3K9 methylation and acetylation in zygotic pronuclei. a Fluorescent signal intensities relative to vehicle control (= 1) and b representative images of H3K9me3 and H3K9ac in both pronuclei. *#Significant differences between control and treated group (P ≤ 0.05). Asterisk indicates paternal pronucleus. Scalebar represents 25 μm
SIRT1-modulation of H3K9 at the zygote stage improves subsequent embryonic development
To assess the effect of SIRT1-modulating treatments on preimplantation embryo development and blastocyst quality, fertilized oocytes were cultured in PZM3 medium with varying concentrations of SIRT1 activator or inhibitor for 144 h.
A significantly lower percentage (39.2 ± 5.2%) of cleaved zygotes was observed in embryos cultured with 12.5 μmol/L resveratrol compared to vehicle control treatment (65.9 ± 8.3%; P ≤ 0.05) or to other activator/inhibitor treatments (Table 1). The inhibition of SIRT1 by sirtinol did not impact blastocyst formation rate. However, the rate of blastocyst formation was significantly increased when embryos were cultured in the presence of 3 μmol/L BML-278 (32.9 ± 8.1; P ≤ 0.05). There were no significant differences in average cell number per blastocyst among treatment groups (Additional file 3). Representative blastocyst of each treated group is shown in Additional file 3.
Specific activation of SIRT1 reduces the fluorescent immunolabeling intensity of the ooplasmic ubiquitin ligase MDM2
Based on published evidence of SIRT1 mediating the regulation of MDM2 and vice versa [37, 46], we detected MDM2 in matured MII oocytes due to western blotting (see Additional file 1) and measured the fluorescent signal intensity of in situ immunolabeled MDM2 in the zygotes cultured for 22 h under control and SIRT1 stimulating conditions. The signal intensity was normalized to GAPDH. A significant reduction of GAPDH-normalized MDM2 signal intensity (1.13 ± 7.4 versus 0.62 ± 7.7 in vehicle control and treated zygotes; P ≤ 0.05) was observed after SIRT1 activation with BML-278 (3 μmol/L) (Fig. 6), indicating significant decrease of MDM2 protein amount.
Quantification of MDM2 signal intensity in 22 h zygotes after treatment with BML-278. a Fluorescent signal intensity of MDM2 was normalized against housekeeping protein GAPDH. b Representative images show MDM2 and GADPH in control and treated zygotes. *Significant difference between control and treated group (P ≤ 0.05). Scalebar represents 50 μm
Discussion
Histone deacetylase SIRT1 has been shown to deacetylate a number of histone protein Lys-residues including but not limited to H1K26 [28, 29], H3 K9, H3K14 and H3K56 [28, 30], and H4K8, H4K12 and H4K16 [28, 31]. Moreover, SIRT1 activity leads to methylation of H3K9, and this lysine residuum represents a dual target of SIRT1 through both direct histone targeting and indirect non-histone targeting of other enzymes involved in the modifications of H3K9 (reviewed in [47]). Our study is the first to show that the SIRT1 accumulates in the pronuclei of a mammalian zygote and favors methylation of pronuclear H3K9. Our observations are in accordance with predominant SIRT1 presence in cell nucleus and an overall positive effect of SIRT1 activity on cell lifespan, accompanied with histone methylation, as described [28, 31]. Furthermore, we increased the percentage of blastocyst formation by embryo treatment with a specific, SIRT1 activating synthetic compound, BML-278 [48]. This observation agrees with previously documented beneficial effect of resveratrol, a naturally occurring general sirtuin activator, on preimplantation embryo development [24,25,26]. Our study thus solidifies previously anecdotal evidence of SIRT1 involvement in the establishment and remodeling of zygotic epigenome. In particular, we show that pharmacological modulation of SIRT1 alters pronuclear histone code in a manner affecting the developmental potential of the preimplantation embryo.
Treatment of porcine zygotes with resveratrol, a non-selective, SIRT-family wide activator increased H3K9me3 methylation after 22 h of IVC. Inversely, such treatment decreased H3K9 acetylation supporting the assumption that histone methylation and acetylation replace each other [49, 50]. Indeed, nicotinamide, a non-selective sirtuin inhibitor, had an opposite effect on pronuclear H3K9 modification, wherein H3K9me3 was decreased and H3K9ac was protected in the treated zygotes. While the involvement of SIRT1 in aforementioned modifications of pronuclear H3K9 is plausible, such findings are based on wide-spectrum activation or inhibition of SIRT family, while other effects should be considered as well, such as the antioxidant/reactive oxygen-scavenger action of resveratrol [51]. Hence, BML-278 and sirtinol, the selective SIRT1 activator and inhibitor, respectively, were used. Favoring SIRT1 over SIRT2 or SIRT3 as a substrate [48], BML-278 treatment lead to higher H3K9 methylation and reduced H3K9 acetylation; sirtinol had an opposite, complementary effect. These observations agree with previous studies on sirtinol [19]. Compared to naturally occurring compounds, synthetic small molecules such as BML-278 and sirtinol have a more specific and more prominent effect on SIRT1 activity without secondary targets. Notably, BML-278 can be used for pharmacological modulation of SIRT1 signaling. On the other hand, molecular mechanism of BML-278 remains unknown and there are few alternative SIRT1 activators with suggested resveratrol-like allosteric mechanism mediating interactions between SIRT1 and its substrates [52, 53]. Activating effect of BML-278 is substrate-selective at a level similar to resveratrol [54, 55].
Early zygote features partially demethylated chromatin, resulting from pronuclear histone asymmetry [56, 57] and ongoing, active DNA demethylation [58,59,60, 14]. Based on the differential tagging of pronuclei with MitoTracker, we found the hyperacetylation of paternal pronucleus on H3K9 and higher H3K9me3 in maternal pronucleus, but no-detectable SIRT1 effect on the asymmetry of pronuclear histone code. The pattern of post-translationally modified histones in pronuclei mirrors DNA methylation status and points out the positive correlation of DNA- and histone H3K9 methylation, indicated earlier [61, 62]. Accordingly, DNA methyltransferase DNMT1 is regulated by H3K9me3-heterochromatin protein 1α complex and crosstalk between DNMTs and histone methyltransferases is obvious [63,64,65]. Although histone acetylation in general accompanies gene expression and in some cases causes DNA fragmentation [66], H3K9me3 is specifically associated with gene silencing and heterochromatin establishment [67, 68]. These changes are beneficial for chromatin stability and cell longevity; however, they suppress gene expression [67, 69, 70]. With respect to major zygotic genome activation (MZGA) when gene transcriptional activity is desired, SIRT1-derived histone methylation and gene silencing seems to be strictly selective in not affecting promoters and genes of which the expression is essential for early embryonic development.
In addition to histone targets of SIRT1, non-histone substrates may be involved in SIRT1 action in the zygote [71, 72]. Suppressor of variegation 3–9 homolog 1 (SUV39H1) is one of non-histone targets of SIRT1, responsible for SIRT1-mediated increase of H3K9me3 [33, 35]. SIRT1-regulated modification of SUV39H1 activity occurs by two mechanisms: SUV39H1 deacetylation on K266 [35] and indirect protection of SUV39H1 protein via suppression of its polyubiquitination by Mouse double minute 2 homolog (MDM2), which is an E3-type ubiquitin ligase responsible for proteasomal degradation of SUV39H1 [37]. The second mechanism points to a crosstalk of SIRT1 and UPS [73, 74] during SIRT1-modulation of the histone code. However, current knowledge of SIRT1 involvement in ubiquitin ligation and UPS-mediated proteolysis remains incomplete. Therefore, we performed the MDM2 fluorescent signal intensity measurement after BML-278 treatment. Our findings of decreased MDM2 signal intensity support the role of SIRT1 in MDM2-SUV39H1-H3K9me3 signaling, indicated earlier [37]. Based on such evidence, we can consider SIRT1 as a negative regulator of MDM2, presumably affecting MDM2 via lysine deacetylation and thus exposure of lysine residues for ubiquitination-mediated autocatalytic loop [37, 75, 76]. Such a scenario agrees with observation of SIRT-promoted protein degradation of β-TrPC E3 ligase [77].
Our study thus provides the partial understanding of how resveratrol improves embryonic development in vitro via SIRT1 activity modulation. The effect of resveratrol was compared with sirtuin inhibitors and BML-278, a specific SIRT1 activator. Surprisingly, resveratrol showed no positive effect on development to blastocyst, while nicotinamide, a non-selective sirtuin family inhibitor actually increased the blastocyst formation rate. These results indicate non-specific effects of nicotinamide and are in agreement with the observations of improved embryonic development after treatment with trichostatine, an inhibitor of histone deacetylases [78,79,80]. Compared to resveratrol, BML-278, a specific SIRT1 activator, significantly improved early embryonic development. The wide scale modification of histone code favoring methylation of specific loci is a plausible epigenetic mechanism of SIRT1 action on early embryonic development. Moreover, SIRT1 factors in the sperm-derived transgenerational inheritance [81] and further experiments focused on specific SIRT1-affected loci during embryogenesis and SIRT1-modulated epigenetic memory are needed.
Improving blastocyst formation rate through SIRT1 stimulation could be applied to optimization of assisted reproductive technologies such as somatic cell nuclear transfer (SCNT) in animals and assisted fertilization in human infertility patients, wherein the maximizing of embryo developmental competence is paramount to the success of subsequent embryo transfer. Based on our observation, the adequate modulation of SIRT1 in the in vitro fertilized oocytes and embryos could also benefit the production of genetically modified pigs for biomedical research and production agriculture [82, 83]. This work thus offers a new approach to increasing the efficiency of reproductive biotechnologies for creation of biomedical animal models as well as for human assisted reproduction.
Further experiments will be needed to more precisely understand molecular mechanisms, including SIRT1 signal pathways, leading to successful pre-implanted embryonic development.
Conclusions
Specific activation of SIRT1 via BML-278 modulated zygotic histone code, expressed by methylated and acetylated H3K9 in zygote pronuclei. In addition to increased pronucleic H3K9me3, activity of MDM2, a non-histone target of SIRT1, has been described after BML-278 treatment. The positive effect of 3.0 μmol/L BML-278 on blastocyst achievement is obvious and, assumably, is a result of described changes on histone code and non-histone targets in one-cell zygote. BML-278 as a novel compound and its molecular action through SIRT1 signalization in porcine embryo represents a promising tool utilizable in biotechnology and assisted reproduction of animals and human.
Abbreviations
- DNMT:
-
DNA methyltransferase
- EGF:
-
Epidermal growth factor
- FSH:
-
Follicle stimulating hormone
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- H3K9ac:
-
Acetylation of histone H3 on lysine K9
- H3K9me3:
-
Trimethylation of histone H3 on lysine K9
- IVC:
-
In vitro culture of embryos
- IVF:
-
In vitro fertilization
- LH:
-
Luteinizing hormone
- MDM2:
-
Mouse double minute 2 homolog, E3-type ubiquitin ligase
- MII:
-
Metaphase II
- mTBM:
-
Modified Tris-buffered medium
- mTCM:
-
Modified tissue culture medium
- MZGA:
-
Major zygotic genome activation
- PPN:
-
Paternal pronucleus
- PZM3:
-
Porcine zygote medium
- SCNT:
-
Somatic cell nuclear transfer
- SIRT1:
-
Silent mating type information regulator 2 homolog 1, NADP + −dependent histone-deacetylase
- SUV39H1:
-
Suppressor of variegation 3–9 homolog 1, histone –lysine N-methyltransferase
- TL-HEPES-PVA:
-
HEPES-buffered Tyrode lactate medium with polyvinyl alcohol
- UPS:
-
Ubiquitin-proteasome system
- β-TrPC E3 ligase:
-
β-Transducin repeat-containing protein
References
Li L, Lu X, Dean J. The maternal to zygotic transition in mammals. Mol Asp Med. 2013;34:919–38.
Langley AR, Smith JC, Stemple DL, Harvey SA. New insights into the maternal to zygotic transition. Development. 2014;141:3834–41.
Nakazawa Y, Shimada A, Noguchi J, Domeki I, Kaneko H, Kikuchi K. Replacement of nuclear protein by histone in pig sperm nuclei during in vitro fertilization. Reproduction. 2002;124:565–72.
Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod. 2006;75:442–51.
Huo LJ, Fan HY, Liang CG, LZ Y, Zhong ZS, Chen DY, et al. Regulation of ubiquitin-proteasome pathway on pig oocyte meiotic maturation and fertilization. Biol Reprod. 2004;71:853–62.
Sutovsky P, Manandhar G, McCauley TC, Caamaño JN, Sutovsky M, Thompson WE, et al. Proteasomal interference prevents zona pellucida penetration and fertilization in mammals. Biol Reprod. 2004;71:1625–37.
Winking H, Gerdes J, Traut W. Expression of the proliferation marker Ki-67 during early mouse development. Cytogenet Genome Res. 2004;105:251–6.
van der Heijden GW, Derijck AA, Ramos L, Giele M, van der Vlag J, de Boer P. Transmission of modified nucleosomes from the mouse male germline to the zygote and subsequent remodeling of paternal chromatin. Dev Biol. 2006;298:458–69.
Adenot PG, Mercier Y, Renard JP, Thompson EM. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development. 1997;124:4615–25.
Rouquier S, Taviaux S, Trask BJ, Brand-Arpon V, van den Engh G, Demaille J, et al. Distribution of olfactory receptor genes in the human genome. Nat Genet. 1998;18:243–50.
Torres-Padilla ME, Bannister AJ, Hurd PJ, Kouzarides T, Zernicka-Goetz M. Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol. 2006;50:455–61.
Lindeman LC, Andersen IS, Reiner AH, Li N, Aanes H, Østrup O, et al. Prepatterning of developmental gene expression by modified histones before zygotic genome activation. Dev Cell. 2011;21:993–1004.
Van De Werken C, Van Der Heijden GW, Eleveld C, Teeuwssen M, Albert M, Baarends WM, et al. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat Commun. 2014;5:5868.
Guo F, Li X, Liang D, Li T, Zhu P, Guo H, et al. Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell Stem Cell. 2014;15:447–58.
Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S Jr, D’Alessandro AM, et al. Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging. Oxidative Med Cell Longev. 2015;2015:659687.
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–2.
Hayakawa T, Iwai M, Aoki S, Takimoto K, Maruyama M, Maruyama W, et al. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS One. 2015;10:e0116480.
Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, et al. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest. 2010;120:2817–28.
Kwak SS, Cheong SA, Yoon JD, Jeon Y, Hyun SH. Expression patterns of sirtuin genes in porcine preimplantation embryos and effects of sirtuin inhibitors on in vitro embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology. 2012a;78:1597–610.
Di Emidio G, Falone S, Vitti M, D'Alessandro AM, Vento M, Di Pietro C, et al. SIRT1 signaling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29:2006–17.
Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One. 2014;9:e94488.
Zhao HC, Ding T, Ren Y, Li TJ, Li R, Fan Y, et al. Role of Sirt3 in mitochondrial biogenesis and developmental competence of human in vitro matured oocytes. Hum Reprod. 2016;31:607–22.
Riepsamen A, Wu L, Lau L, Listijono D, Ledger W, Sinclair DA, et al. Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One. 2015;10:e0126194.
Lee K, Wang C, Chaille JM, Machaty Z. Effect of resveratrol on the development of porcine embryos produced in vitro. J Reprod Dev. 2010;56:330–5.
Kwak SS, Cheong SA, Jeon Y, Lee E, Choi KC, Jeung EB, et al. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology. 2012b;78:86–101.
Takeo S, Sato D, Kimura K, Monji Y, Kuwayama T, Kawahara-Miki R, et al. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J Reprod Dev. 2014;60:92–9.
Itami N, Shirasuna K, Kuwayama T, Iwata H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology. 2015;83:1360–7.
Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105.
Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–18.
Das C, Lucia MS, Hansen KC, Tyler JK, et al. Nature. 2009;459:113–7.
Vaquero A, Sternglanz R, Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007b;26:5505–20.
Khobta A, Anderhub S, Kitsera N, Epe B. Gene silencing induced by oxidative DNA base damage: association with local decrease of histone H4 acetylation in the promoter region. Nucleic Acids Res. 2010;38:4285–95.
Peters AH, Kubicek S, Mechtler K, O'Sullivan RJ, Derijck AA, Perez-Burgos L, et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–89.
Ait-Si-Ali S, Guasconi V, Fritsch L, Yahi H, Sekhri R, Naguibneva I, et al. A Suv39h-dependent mechanism for silencing S-phase genes in differentiating but not in cycling cells. EMBO J. 2004;23:605–15.
Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007a;450:440–4.
Park KE, Johnson CM, Wang X, Cabot RA. Differential developmental requirements for individual histone H3K9 methyltransferases in cleavage-stage porcine embryos. Reprod Fertil Dev. 2011;23:551–60.
Bosch-Presegué L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J, et al. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol Cell. 2011;42:210–23.
Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN. Presence of beta-mercaptoethanol can increase the glutathione content of pig oocytes matured in vitro and the rate of blastocyst development after in vitro fertilization. Theriogenology. 1998;50:747–56.
Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod. 2002;66:112–9.
Yi YJ, Sutovsky M, Song WH, Sutovsky P. Protein deubiquitination during oocyte maturation influences sperm function during fertilization, antipolyspermy defense and embryo development. Reprod Fertil Dev. 2015;27:1154–67.
Kuijk EW, du Puy L, van Tol HT, Haagsman HP, Colenbrander B, Roelen BA. Validation of reference genes for quantitative RT-PCR studies in porcine oocytes and preimplantation embryos. BMC Dev Biol. 2007;7:58.
Nevoral J, Žalmanová T, Zámostná K, Kott T, Kučerová-Chrpová V, Bodart JF, et al. Endogenously produced hydrogen sulfide is involved in porcine oocyte maturation in vitro. Nitric Oxide. 2015;51:24–35.
Hou J, Liu L, Zhang J, Cui XH, Yan FX, Guan H, et al. Epigenetic modification of histone 3 at lysine 9 in sheep zygotes and its relationship with DNA methylation. BMC Dev Biol. 2008;8:60.
Kan R, Jin M, Subramanian V, Causey CP, Thompson PR, Coonrod SA, et al. Potential role for PADI-mediated histone citrullination in preimplantation development. BMC Dev Biol. 2012;12:19.
Oliveira CS, Saraiva NZ, de Souza MM, Tetzner TA, de Lima MR, Garcia JM, et al. Effects of histone hyperacetylation on the preimplantation development of male and female bovine embryos. Reprod Fertil Dev. 2010;22:1041–8.
Peng L, Yuan Z, Li Y, Ling H, Izumi V, Fang B, et al. Ubiquitinated sirtuin 1 (SIRT1) function is modulated during DNA damage-induced cell death and survival. J Biol Chem. 2015;290:8904–12.
Martínez-Redondo P, Vaquero A. The diversity of histone versus nonhistone sirtuin substrates. Genes Cancer. 2013;4(3–4):148–63.
Mai A, Valente S, Meade S, Carafa V, Tardugno M, Nebbioso A, et al. Study of 1,4-dihydropyridine structural scaffold: discovery of novel sirtuin activators and inhibitors. J Med Chem. 2009;52:5496–504.
Nicolas E, Roumillac C, Trouche D. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol Cell Biol. 2003;23:1614–22.
Stewart MD, Li J, Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol Cell Biol. 2005;25:2525–38.
Kong Q, Ren X, Hu R, Yin X, Jiang G, Pan Y. Isolation and purification of two antioxidant isomers of resveratrol dimer from the wine grape by counter-current chromatography. J Sep Sci. 2016;39:2374–9.
Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–54.
Cao D, Wang M, Qiu X, Liu D, Jiang H, Yang N, et al. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015;29:1316–25.
Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–34.
Lakshminarasimhan M, Rauh D, Schutkowski M, Steegborn C. Sirt1 activation by resveratrol is substrate sequence-selective. Aging (Albany NY). 2013;5:151–4.
Lepikhov K, Walter J. Differential dynamics of histone H3 methylation at positions K4 and K9 in the mouse zygote. BMC Dev Biol. 2004;4:12.
Ma XS, Chao SB, Huang XJ, Lin F, Qin L, Wang XG, et al. The Dynamics and Regulatory Mechanism of Pronuclear H3k9me2 Asymmetry in Mouse Zygotes. Sci Rep. 2015;5:17924.
Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–8.
Park JS, Jeong YS, Shin ST, Lee KK, Kang YK, Dynamic DNA. methylation reprogramming: active demethylation and immediate remethylation in the male pronucleus of bovine zygotes. Dev Dyn. 2007;236:2523–33.
Reis Silva AR, Adenot P, Daniel N, Archilla C, Peynot N, Lucci CM, et al. Dynamics of DNA methylation levels in maternal and paternal rabbit genomes after fertilization. Epigenetics. 2011;6:987–93.
Liu H, Kim JM, Aoki F. Regulation of histone H3 lysine 9 methylation in oocytes and early pre-implantation embryos. Development. 2004;131:2269–80.
Timoshevskiy VA, Herdy JR, Keinath MC, Smith JJ. Cellular and Molecular Features of Developmentally Programmed Genome Rearrangement in a Vertebrate (Sea Lamprey: Petromyzon marinus). PLOS Genet. 2016;12:e1006103.
Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S, et al. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol. 2003;13:1192–200.
Sun L, Huang L, Nguyen P, Bisht KS, Bar-Sela G, Ho AS, et al. DNA methyltransferase 1 and 3B activate BAG-1 expression via recruitment of CTCFL/BORIS and modulation of promoter histone methylation. Cancer Res. 2008;68:2726–35.
Rai K, Jafri IF, Chidester S, James SR, Karpf AR, Cairns BR, et al. Dnmt3 and G9a cooperate for tissue-specific development in zebrafish. J Biol Chem. 2010;285:4110–21.
Bikond Nkoma G, Leduc F, Jaouad L, Boissonneault G. Electron microscopy analysis of histone acetylation and DNA strand breaks in mouse elongating spermatids using a dual labelling approach. Andrologia. 2010;42:322–5.
Wang F, Kou Z, Zhang Y, Gao S. Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol Reprod. 2007;77:1007–16.
Keniry A, Gearing LJ, Jansz N, Liu J, Holik AZ, Hickey PF, et al. Setdb1-mediated H3K9 methylation is enriched on the inactive X and plays a role in its epigenetic silencing. Epigenetics Chromatin. 2016;9:16.
Ryu HY, Rhie BH, Ahn SH. Loss of the Set2 histone methyltransferase increases cellular lifespan in yeast cells. Biochem Biophys Res Commun. 2014;446:113–8.
Li S, Liu L, Li S, Gao L, Zhao Y, Kim YJ, et al. SUVH1, a Su(var)3-9 family member, promotes the expression of genes targeted by DNA methylation. Nucleic Acids Res. 2016;44:608–20.
Yu X, Zhang L, Wen G, Zhao H, Luong LA, Chen Q, et al. Upregulated sirtuin 1 by miRNA-34a is required for smooth muscle cell differentiation from pluripotent stem cells. Cell Death Differ. 2015;22:1170–80.
Zhao H, Yang L, Cui H, et al. Biochem Biophys Res Commun. 2015;464:1163–70.
Chen IY, Lypowy J, Pain J, Sayed D, Grinberg S, Alcendor RR, et al. Histone H2A.z is essential for cardiac myocyte hypertrophy but opposed by silent information regulator 2alpha. J Biol Chem. 2006;281:19369–77.
Han L, Zhao G, Wang H, Tong T, Chen J. Calorie restriction upregulated sirtuin 1 by attenuating its ubiquitin degradation in cancer cells. Clin Exp Pharmacol Physiol. 2014;41:165–8.
Roxburgh P, Hock AK, Dickens MP, Mezna M, Fischer PM, Vousden KH. Small molecules that bind the Mdm2 RING stabilize and activate p53. Carcinogenesis. 2012;33:791–8.
Nihira NT, Oqura K, Shimizu K, North BJ, Zhang J, Gao D, et al. Acetylation-dependent regulation of MDM2 E3 ligase activity dictates its oncogenic function. Sci Signal. 2017;10:eaai8026.
Woo SR, Byun JG, Kim YH, Park ER, Joo HY, Yun M, et al. SIRT1 suppresses cellular accumulation of β-TrCP E3 ligase via protein degradation. Biochem Biophys Res Commun. 2013;441:831–7.
Inoue K, Oikawa M, Kamimura S, Ogonuki N, Nakamura T, Nakano T, et al. Trichostatin A specifically improves the aberrant expression of transcription factor genes in embryos produced by somatic cell nuclear transfer. Sci Rep. 2015;5:10127.
Jee BC, Jo JW, Lee JR, Suh CS, Kim SH, Moon SY. Effect of trichostatin A on fertilization and embryo development during extended culture of mouse oocyte. Zygote. 2012;20:27–32.
Jeseta M, Petr J, Krejcová T, Chmelíková E, Jílek F. In vitro ageing of pig oocytes: effects of the histone deacetylase inhibitor trichostatin A. Zygote. 2008;16:145–52.
Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 2015;112:13699–704.
Mao J, Zhao MT, Whitworth KM, Spate LD, Walters EM, O'Gorman C, et al. Oxamflatin treatment enhances cloned porcine embryo development and nuclear reprogramming. Cell Reprogram. 2015;17:28–40.
Whitworth KM, Mao J, Lee K, Spollen WG, Samuel MS, Walters EM, et al. Transcriptome analysis of pig in vivo, in vitro-fertilized, and nuclear transfer blastocyst-stage embryos treated with histone deacetylase inhibitors postfusion and activation reveals changes in the lysosomal pathway. Cell Reprogram. 2015;17:243–58.
Acknowledgements
We would like to thank Ms. Hee-Jung Lee for help with experiments and Ms. Kathryn Craighead for manuscript editing.
Funding
This work was supported by Agriculture and Food Research Initiative Competitive (Grant no. 2015–67,015-23,231) from the USDA National Institute of Food and Agriculture and by seed funding from the Food for the twenty-first Century program of the University of Missouri to P.S. Work in Y-J.Y. laboratory was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2013R1A6A3A04063769), M.K. and J.N. was supported by Charles University (PROGRES Q-39) and the National Sustainability Program I (NPU I) Nr. LO1503 provided by the Ministry of Education, Youth and Sports of the Czech Republic, K.A., T.Z., K.H., J.P. and J.N. were supported by the National Agency of Agriculture Sciences (NAZV QJ1510138), the Czech Ministry of Agriculture (MZeRO 0714).
Avaliability of data and materials
Please contact author for data requests.
Author information
Authors and Affiliations
Contributions
KZ, PJ and JN carried out the experiments and analysis of zygotes, TZ and KH participated in zygote production, YJY performed blastocyst production and following analysis, JM did proteomic analysis, MS participated the introduction of zygote analysis, KZ, YJY and JN did manuscript writing, JP, JN and PS designed experiments and edited the manuscript. MK, JP and PS provided wherewithal for all experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional file
Additional file 1:
Detection of SIRT1 (A) and MDM2 (B) in matured MII oocytes and their cumulus cells. (DOCX 107 kb)
Additional file 2:
Results of IVF after 22 h of IVC with SIRT1 activators and inhibitors. (DOCX 19 kb)
Additional file 3:
Embryonic development and blastocyst formation after treatment with SIRT1 activators and inhibitors. (DOCX 163 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Adamkova, K., Yi, YJ., Petr, J. et al. SIRT1-dependent modulation of methylation and acetylation of histone H3 on lysine 9 (H3K9) in the zygotic pronuclei improves porcine embryo development. J Animal Sci Biotechnol 8, 83 (2017). https://doi.org/10.1186/s40104-017-0214-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-017-0214-0