Abstract
Heavy metals are known to cause deleterious effects on human health through food chain. Human health risks were evaluated from consumption of heavy metal contaminated fish from Buriganga River in Bangladesh. Whole body of five fish species (Puntius ticto, Puntius sophore, Puntius chola, Labeo rohita and Glossogobius giuris) were analyzed which contained various concentrations of Cd, As, Pb, Cr, Ni, Zn, Se, Cu, Mo, Mn, Sb, Ba, V and Ag. Concentrations of Mn, Zn, Se and Pb in all fish species were above the Food Safety Guideline (FSG) by WHO/FAO. Assessment of noncarcinogenic health hazard by target hazard quotient (THQ) indicated no concern from consumption of these fish except for Mn. However, all metals together may affect human health as revealed by hazard index (HI). The target cancer risk (TR) values suggested carcinogenic risk from Ni and As. Taken together it can be concluded that there is potential human health risk in consuming fish from river Buriganga.
Similar content being viewed by others
Background
Chemical contamination of food is considered of the most significant sources of human health risks. The most significant sources of foodborne diseases are microbiological and chemical hazards. Health risk due to consumption of food from aquatic ecosystems contaminated with hazardous chemicals including metals has increased globally especially in developing countries such as Bangladesh. The increasing usage of heavy metals in industry has led to increased release of harmful heavy metals into the aquatic environment (Agusa et al. 2005, 2007; Hajeb et al. 2009). The increasing demand of food safety research has accelerated regarding the risk associated with consumption of food contaminated by toxic metals (Mansour et al. 2009; Saha and Zaman 2013).
Metals and metalloids from natural and anthropogenic sources continuously enter the aquatic environment where they pose a serious threat to human and ecological health, owing to their toxicity, long persistence, bioaccumulation, and biomagnifications in the food chain (Rahman et al. 2013). Essential metals (Cu, Co, Zn, Fe, Ca, Mg, Se, Ni and Mn) are required in very trace quantities for the proper functioning of enzyme systems, hemoglobin formation and vitamin synthesis in human. However, excess of these essential metals results in metabolic disturbances (Hina et al. 2011). Pb, Ba, Cd, Hg, Cr, and As have no established role in biological system (Canli and Atli 2003) have been identified as toxic heavy metals and their maximum recommended residual levels have been set for humans (FAO 1983; European Commission 2006; FDA 2001). Heavy metals accumulate in vital organs in the human body such as the kidneys, bones, and liver which result in numerous serious health effects such as neurotoxic and carcinogenic effects (Duruibe et al. 2007; Sapkota et al. 2008). Among various heavy metals, Cr and Ni are known to cause various pulmonary disorders (Forti et al. 2011), while high intake of Cu can cause liver and kidney damage (Tuzen 2009; WHO 1995). Cd is toxic to the cardiovascular system, kidneys, and bones (Fang et al. 2014); excessive intake of Zn negative effects on immunological system (reduction in lymphocyte stimulation response) and cholesterol metabolism (Goldhaber 2003).
Fish assimilate metals by ingestion of particulate material suspended in water, ingestion of food, ion-exchange across lipophilic membranes (e.g., the gills), and adsorption on tissue and membrane surfaces. As a result, fish may accumulate large amounts of metals from the water (Mansour and Sidky 2002) and the accumulation rate is dependent both on uptake and elimination rates (Guven et al. 1999). So, there have been several studies and monitoring programs worldwide on metal accumulation in fish (Erdoĝrul and Ates 2006; Rashed 2001). Fish is known as integral component of a well-balanced diet worldwide because it provides energy, proteins, vitamins and different nutrients also (Pieniak et al. 2010). Studies have reported that dietary intake is the main route of exposure to heavy metals for most people (Zhuang et al. 2009). Fish have been reported in several studies as a source of heavy metals in human through consumption (Castro-González and Méndez-Armenta 2008). That’s why fish has been considered as one of the most indicative factors in freshwater systems, for the estimation of trace metals pollution and risk potential of human consumption (Alhashemi et al. 2012; Pan and Wang 2012; Yi et al. 2011; Vieira et al. 2011).
In Bangladesh, about 900 industries dispose untreated industrial wastes directly into rivers. The Buriganga River is the most polluted river in Bangladesh (Nouri et al. 2009) which is being increasingly polluted with huge volumes of toxic wastes from thousands of industrial units and sewerage lines (Islam et al. 2006). However, a wide variety of heavy metals originate from industrial waste discharge, batteries, lead based paint and gasoline discharge from cargos, launch and mechanized boat, traffic and improper domestic waste discharge etc. Although there has been several studies reporting enrichment of heavy metals in water, sediment and fish in various rivers including Buriganga (Begum et al. 2013; Ahmed et al. 2010b; Mohiuddin et al. 2011; Saha and Hossain 2011) studies reporting the risk assessment with special focus on human health are scant. But the risk assessment of these metals via daily dietary intake is a very important issue (Martí-Cid et al. 2008). Only Ahmed et al. (2015) and Islam et al. (2015) carried the carcinogenic and non-carcinogenic risk of several heavy metals in three fish and one prawn species collected from Buriganga. However, the accumulation and magnification vary in different fish species. In the context, it is important to monitor the concentration and potential human health risk associated with consumption of commonly consumed fish species. So, this study aims to determine various trace metals in fish tissue and to compare the estimated intakes with references toxicological and nutritional values. This study also evaluates the carcinogenic and non-carcinogenic health risk for humans through fish consumption.
Methods
Collection of samples
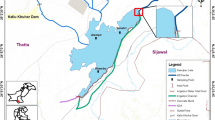
Fish samples were collected from different stations (Fig. 1) of the river Buriganga near Kamrangir Char in between the Bangladesh–China friendship bridge-01 (23°40′N 90°20′E) and Amin Bazzar Bridge (23°47′N 90°20′E) where fishing effort is high. Fishes were collected from the professional fishermen while they were fishing in the river during August to September, 2013. Individuals of the same species were of similar size and weight. The samples were immediately preserved in air sealed plastic bags and transported to the laboratory. Five fish species were identified namely: Puntius ticto, Puntius sophore, Puntius chola, Labeo rohita and Glossogobius giuris and their mean weight were 2.00 g, 2.40 g, 1.30 g, 47.18 g, and 5.44 g respectively.
Preparations of samples
Whole body of fish was used for analyses following Islam et al. (2015). A composite sample for each species was prepared and homogenized in a stainless steel blender cup and 50 g test portions were stored at −20 °C. The samples were then freeze dried and sealed in airtight plastic bags. The dried samples were transported to Yokohama National University, Japan for analytical methods.
Analytical methods
Sample digestion
0.2 g of dried fish sample were digested in DAP-Three step-digestion procedure with 5 ml HNO3 (Kanto Chemical Co, Japan) and 2 ml H2O2 (Wako Chemical Co, Japan) in microwave digestion system (Berghof Microwave MWS-2, Germany) as described by Islam et al. (2015). Three replicas were prepared for each sample was during digestion. Samples were then transferred into a Teflon beaker and total volume was made up to 50 ml with MilliQ water (Elix UV5 and MilliQ, Millipore, USA) and filtered with PTTF syringe filter (pore size = 0.45 mm) and stored in crew cap plastic tube.
Instrumental analysis and quality assurance
The samples were analyzed for cadmium (Cd), arsenic (As), lead (Pb), chromium (Cr), nickel (Ni), zinc (Zn), selenium (Se), copper (Cu), molybdenum (Mo), manganese (Mn), antimony (Sb), barium (Ba), vanadium (V), silver (Ag) using ICP-MS (Agilent 7700, USA). For each run of analysis blank, certified reference materials (CRM) as internal standard along with the samples were analyzed in duplicate to eliminate any batch-specific error. Multi-element standard solution was used to prepare standard curve. Five standards with standard linear regression and internal standardization were prepared at levels ranging from 0–50 µg/L. All test batches were evaluated using an internal quality approach and validated if they satisfied the defined internal quality controls (IQCs).
Non-carcinogenic Health hazard and carcinogenic risk estimation
The target hazard quotient (THQ) assessed the non-carcinogenic health hazards for each individual metal through fish consumption, while the hazard index (HI) was estimated as the sum of the THQs (USEPA 2011). The THQ assumes a level of exposure (i.e., RfD) below which it is unlikely for even sensitive populations to experience adverse health effects. On the other hand HI indicates the combined hazard of all metals.
For carcinogens, Target risks (TR) were estimated as the incremental probability of an individual to develop cancer over a lifetime, as a result of exposure to that potential carcinogen (i.e., incremental or excess individual lifetime cancer risk (USEPA 1989). The THQ, HI and TR values were estimated using Eqs. (1), (3), and (4) respectively. European Protection Agency (EPA) had declined RfD value for Pb (USEPA 2004) So, the THQ for Pb was calculated using the Eq. (2) (Liu et al. 1996):
or,
where, THQ is the target hazard quotient; EF is the exposure frequency (365 days/year); ED is the exposure duration (30 year for noncancer risk as used by USEPA 2011), FIR is the fish ingestion rate (49.41 g/person/day; (BBS 2014); Cf is the conversion factor (00.208) to convert fresh weight (FW) to dry weight (Dw.) considering 79 % of moisture content in fish; CM is the heavy metal concentration in fish (mg/kg d.w.); WAB is the average body weight (bw) (70 kg); ATn is the average exposure time for noncarcinogens (EF × ED) (365 days/year for 30 year (i.e., ATn = 10,950 days) as used in characterizing noncancer risk (USEPA 2011); RfD is the reference dose of the metal according to the Human health risk assessment. Regional screening level (RSL) summary table (USEPA 2015); MRL is maximum regulation limit for muscle meat for fish set by the Regulation (EC) No 1881/2006 (European Commission 2006). CPSo is the carcinogenic potency slope, oral (mg/kg bw-day-1); ATc is the averaging time, carcinogens (365 days/year for 70 year as used by (USEPA 2011). Since CPSo values were known for Ni, AS, Cd and Pb, so TR values were calculated for intake of these metals.
Results and discussion
Metal concentration in fish species
Fish muscle is the main part of human diet for most species throughout the world. Except L. rohita other fish species in this study were small indigenous species (SIS) which are generally consumed whole by Bangladeshi population. So, the whole body of fish was analyzed for different metals. All fish species contained V, Cr, Mn, Ni, Cu, Zn, As, Se, Mo, Ag, Cd, Sb, Ba, and Pb at different concentrations (Mean ± SD) (Table 1). Zn was found at the highest concentration in all fish species followed by Mn, Cu and Cr. The trend was Zn > Mn > Ba > Cu > Cr > Pb > V > Ni > Se > As > Cd in almost all fish species. Similar trend was also reported in L. rohita by Javed and Usman (2011) Earlier studies also reported Zn to be at highest concentration in fish collected from Buriganga river (Begum et al. 2013; Ahmed et al. 2010b, 2015; Khan et al. 2014 ). Concentration of various metals in fish usually follow Fe > Zn > Pb > Cu > Cd (Jezierska and Witeska 2006) which is the similar to the findings of the present study. However, the magnitude of bioaccumulation is a function of species and trophic transfer (Spry and Wiener 1991). So, species at different positions in the food chain accumulate different concentrations of metals. In the present study, there were considerable variations in the concentrations of heavy metals among different species. Among the five fish species L. rohita contained the highest concentrations of almost all metals (Table 1). This was due to the larger size individuals of the species because larger fish tend to accumulate more heavy metals (Farkas et al. 2003). This phenomenon might also be the due to extensive column feeding nature of L. rohita. In contrast, G. giuris was found to have the lowest metal accumulation due to the smaller body size which reduces the metal accumulation through surface action. The findings of Ahmed et al. (2010b) in G. giuris were very close to this study. Metal accumulation in P. sophore and L. rohita in the present study were different from the concentrations reported in these species from other rivers (Ahmed et al. 2010a; Hasan et al. 2011). This is probably due to the variation in the concentrations of metal in the ambient water along with the variation in the size and age of the fish under investigation. In addition, metal speciation in the aquatic system as well as the pH and temperature are also the factors of metal accumulation (Moiseenko and Kudryavtseva 2001; Dhanakumar et al. 2015). Although permissible limits of heavy metals in fish and food have been recommended by various international and regional bodies, but no such value were reported for V, Mo, Ag and Ba. However, permissible limits have not been recommended yet to all heavy metals. Concentrations of Mn, Zn, Se and Pb were higher than the Food safety guidelines (FSG) in fish recommended by various parties (Table 1) making these fish species from Buriganga unacceptable as food. No such standard for V, Mo, Ag and Ba were found.
V is an essential element for normal cell growth and essential component of some enzymes, particularly the vanadium-nitrogenase used by some nitrogen fixing microorganisms. Although V is toxic when present at higher concentrations, the V complexes can reduce growth of cancer cells and improve human diabetes mellitus. However, V has not been classified by the United State Environmental Protection Agency (USEPA) or World Health Organization (WHO) to have a permissible level. The estimated daily upper boundary range of vanadium is 0.2 mg/day (USEPA 2011). There is no maximum level established for dietary intake of V through fish consumption in Bangladesh.
The Cr concentrations in this study were higher than that of reported by Ahmed et al. (2010b) This increasing trend might have resulted from the effluent coming from the tannery industries near the Buriganga river. Metal accumulation in the body of organisms increase with time of exposure until it reaches the equilibrium with the environment based on the partitioning coefficient of the chemical. Several studies have shown that chromium (VI) compounds can increase the risk of lung cancer (Ishikawa et al. 1994). Fish health can also be affected by Cr exposure. The presence of Cr along with other metals was reported to increase the glycogen level in different organs indicating the stress due to the metal exposure (Javed and Usmani 2011). Equivalent concentration of Cr found in Buriganga river were reported to cause DNA damage and also induce micronucleus in air breathing catfish (Ahmed et al. 2013).
Mn occurs naturally and may be released into water bodies through runoff or leaching facilitated by agricultural activities while anthropogenic sources include agro chemicals. Mn concentrations in the present study were the highest among all metals. According to previous literature, the Mn concentrations in the samples ranged from 0.54 mg/kg to 79.08 mg/kg (Bashir et al. 2013); the muscles of fishes found in Indian fish markets ranged from 0.14 mg/kg to 3.36 mg/kg (Sivaperumal et al. 2007). Mn is an essential element in human and Mn deficiency causes skeletal and reproductive abnormalities (Sivaperumal et al. 2007). However, excess intake of Mn can result in psychological and neurologic disorder (Moreno et al. 2009).
Ni measured relatively low concentrations in the samples compared to Cr and Mn. The concentrations of Ni in the samples ranged between 0.73 ± 0.19 and 6.64 ± 0.24 mg/kg. Similar kinds of results were also observed in Cu, Zn, and Pb concentrations.
Zn is an essential micronutrient for all organisms. Zn is required at high level in organism for maintaining certain biological function as a constituent of various enzymes. Zn was found in very high concentrations in all fish species in the present study which exceeded the guideline values of FAO (1983). Both Zn and Mn were reported in Mastacembelus armatus at higher concentrations than FAO/WHO recommended levels in an effluent dominated rivulet in India (Javed and Usmani 2013).
As in natural surface water is less available settles down to sediment along with Fe and become unavailable to fish for uptake. A sublethal concentration of As can pose mutation or DNA damage to fish (Ahmed et al. 2011) Arsenic is present in our food in different chemical forms. The toxic inorganic arsenic becomes organic through methylation in aquatic environment which is less toxic. In the present study, the total arsenic concentrations were estimated. Although inorganic arsenic was estimated to be 10 % of total arsenic (USFDA 1993) but it is difficult to conclude about the contribution of different the forms of arsenic in total concentration. Chronic exposure to inorganic arsenic may cause several health effects, including to the gastrointestinal tract, respiratory tract, skin, liver, cardiovascular system, hematopoietic system and the nervous system (Mandal and Suzuki 2002).
Se is an essential micronutrient but at high concentrations it can be toxic (Coyle et al. 1993). About 1 mg/kg (ww) of Se in prey is the threshold for toxicity in some fish, of 2.6 mg/kg (ww) in muscle is associated with adverse in the fish themselves (Lemly 1993a, b).
Sb and Cd were present at a concentration lower than WHO/FAO standards (Table 1). This was due to the source of Sb and Cd is not significantly contributing the heavy metal load in Buriganga. Cd is a serious contaminant, a highly toxic element, which is transported in the air. Cd concentrations in all fish species were well below the maximum permissible limit in fish by FAO and European Commission (Table 1). Ahmed et al. (2010a) investigated the heavy metal concentration in fish and oyster from the Shitalakhya River, Bangladesh and found seasonal variation in Cd concentration ranged from 1.09 to 1.21 mg/kg. Industrial processes such as smelting or electroplating and the fertilizers can contribute to the environmental concentration of Cd. to Cd was reported to cause kidney failure and softening of bones following long-term or high dose exposure (Vannoort and Thomson 2006), and high levels of Cd have been reported to prostate cancer (Gray et al. 2005).
In the present study the highest concentration of Ba was 23.06 ± 1.30 (Table 1). Small amounts of water-soluble Ba may cause a person to experience breathing difficulties, increased blood pressures, heart rhythm changes, stomach irritation, muscle weakness, changes in nerve reflexes, swelling of brains and liver, kidney and heart damage. The maximum tolerable daily intake recommended by the Scientific Committee on Health and Environmental Risks as 12 mg/day (SCHER 2012). Pb is a ubiquitous pollutant which could find its way into the Buriganga River through discharge of industrial effluents from various industries such as printing, dyeing, oil refineries, textile around Dhaka City, and other sources.
Non-carcinogenic health hazard and carcinogenic risk
The health risk assessments are based on assumptions that for most chemicals with noncancer effects, exhibit a threshold response. The Target hazard quotient (THQ) estimated for individual heavy metals through consumption of different fish species are presented in Table 2. The acceptable guideline value for THQ is 1 (USEPA 2011). THQ values were less than 1 for all individual heavy metal except Mn in L. rohita (3.694) and P. ticto (1.027) indicating no potential non-carcinogenic health risk from ingestion of a single heavy metal through consumption of these fishes. In contrast, the combined impacts of all metals under consideration were higher than the acceptable limit of 1 for HI in all species. Consumption of L. rohita is of biggest concern with a HI value over 5 (Table 2). Humans are often exposed to more than one pollutant and suffer combined or interactive effects (Li et al. 2013). L. rohita was with the highest hazard potential, while there was no significant difference in HI among other fish species (Table 2). But the HI might overestimate the potential for noncancer health effects. This is because the toxicological effects associated with exposure to multiple chemicals, often through different exposure pathways, may not be additive. Moreover, the effect of one metal is supposed to be dependent on the others due to the competitive absorption of metal ions in specific tissues of concern. The risk associated with the carcinogenic effects of target metal is expressed as the excess probability of contracting cancer over a lifetime of 70 years. However, the THQ and HI are not direct measurement of risks because it does not define any dose–response relationship (USEPA 1989).
Epidemiological studies have shown that Ni and Cd correlate with increased incidences of cancer in humans (Nordberg 2010). Ni, As and Cd belong to group 1 of the International Agency for Research on Cancer classification system (IARC 2014) with sufficient evidence of carcinogenicity in human. The TR values were estimated for the metals reported with known carcinogenic effects. The TR values for Ni, As, Cd and Pb are presented in Table 3. In general, the TR values lower than E−6 are considered to be negligible for carcinogenic risk, cancer risks above E−04 are considered unacceptable (USEPA 1989, 2010) and risks lying between E−6 and E−4 are generally considered an acceptable range (Fryer et al. 2006). The present study found that all five fish species poses carcinogenic risk from Ni consumption, while only L. rohita had risk from As. Ahmed et al. (2015) reported similar result for some other fish species collected form river Buriganga.
Conclusions
The present study concludes that the commonly consumed fish species collected from river Buriganga contained various concentrations of heavy metals and the degree of accumulation vary among different species. Cr, Mn, Ni, Cu, Zn, Pb were present at higher concentrations than the Maximum allowable concentrations (MAC) in fish recommended by FAO/WHO. The metals do not pose non-carcinogenic health hazard individually but their combined effect is potentially hazardous to human health. The accumulation of Ni in all fish species suggests significant cancer risk through consumption of these fish species.
References
Agusa T, Kunito T, Yasunaga G, Iwata H, Subramanian A, Ismail A, Tanabe S (2005) Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar Pollut Bull 51(8):896–911
Agusa T, Kunito A, Sudaryanto T, Monirith SK, Klap A, Iwata H (2007) Exposure assessment for trace elements from consumption of marine fish in Southeast Asia. Environ Pollut 145:266–777. doi:10.1016/j.envpol.2006.04.034
Ahmed M, Bhowmik AC, Rahman S, Haque M (2010a) Heavy metal concentration in water, sediments, freshwater mussels and fishes of the river shitalakhya, Bangladesh. Asian J Water Env Pollut 7(1):77–90
Ahmed MK, Islam S, Rahman S, Haque MR, Islam MM (2010b) Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int J Environ Res 4:321–332
Ahmed MK, Habibullah-Al-Mamun M, Hossain MA, Arif M, Parvin E, Akter MS, Khan MS, Islam MM (2011) Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 4:143–149
Ahmed MK, Kundu GK, Al-Mamun MH, Sarkar SK, Akter MS, Khan MS (2013) Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol env saf 92:64–70
Ahmed MK, Baki MA, Islam MS, Kundu GK, Habibullah-Al-Mamun M, Sarkar SK, Hossain MM (2015) Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ Sci Pollut Res 22(20):15880–15890. doi:10.1007/s11356-015-4813-z
Alhashemi AH, Sekhavatjou MS, Kiabi BH, Karbassi AR (2012) Bioaccumulation of trace elements in water, sediment, and six fish species from a freshwater wetland Iran. Microchem J 104:1–6. doi:10.1016/j.microc.2012.03.002
Bashir FH, Othman MS, Mazlan AG, Rahim SM, Simon KD (2013) Heavy metal concentration in fishes from the coastal waters of Kapar and Mersing, Malaysia. Turk J Fish Aqua Sci 13:375–382
BBS (2014) Statistical Pocket book of Bangladesh 2013, Government of the People’s Republic of Bangladesh. Bangladesh Bureau of Statistics, Ministry of Planning, Dhaka, p 416
Begum A, Mustafa AI, Amin MN, Chowdhury TR, Quraishi SB, Banu N (2013) Levels of heavy metals in tissues of shingi fish (Heteropneustes fossilis) from Buriganga River Bangladesh. Environ Monit Assess 185(7):5461–5469. doi:10.1007/s10661-012-2959-4
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb & Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121:129–136
Castro-González MI, Méndez-Armenta M (2008) Heavy metals: implications associated to fish consumption. Environ Toxicol Pharmacol 26(3):263–271
Coyle JJ, Ingersoll DR, Fairchild CG, May TW (1993) Effects of dietary selenium on the reproductive success of bluegills (Lepomis macrochirus). Environ Toxicol Chem 12:551–565. doi:10.1002/etc.5620120315
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113:145–151
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118
Erdoĝrul Z, Ates DA (2006) Determination of cadmium and copper in fish samples from Sir and Menzelet dam lake Kahramanmaras, Turkey. Environ Monit Assess 117:281–290. doi:10.1007/s10661-006-0806-1
European Commission (2006) Commission Regulation (EC) No 1881/2006 of the European parliament and the council of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Communities, L364/18. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=EN. Accessed 12 June 2015
Fang Y, Sun X, Yang W, Ma N, Xin Z, Fu J, Liu X, Liu M, Mariga AM, Zhu X, Huuuu Q (2014) Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food chem. doi:10.1016/j.foodchem.2013.09.116
FAO (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish Circular 464:5–100. http://trove.nla.gov.au/version/22206109
FAO/WHO (FAO/World Health Organization) (2002) Codex alimentarius—general standards for contaminants and toxins in food. schedule 1 maximum and guideline levels for contaminants and toxins in food. Reference CX/FAC 02/16. Joint FAO/WHO Food Standards Programme, Codex Committee, Rotterdam, The Netherlands
Farkas A, Salánki J, Specziár A (2003) Age-and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res 37(5):959–964
FDA (2001) Fish and Fisheries Products Hazards and Controls Guidance, 3rd edn. Center for Food Safety and Applied Nutrition, US Food and Drug Administration, College Park
Forti E, Salovaara S, Cetin Y, Bulgheroni A, Tessadri R, Jennings P, Pfaller W, Prieto P (2011) In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicol In Vitro 25:454–461. doi:10.1016/j.tiv.2010.11.013
Fryer M, Collins CD, Ferrier H, Colvile RN, Nieuwenhuijsen MJ (2006) Human exposure modeling for chemical risk assessment: a review of current approaches and research and policy implications. Environ Sci Policy 9:261–274. doi:10.1016/j.envsci.2005.11.011
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharm 38:232–242. doi:10.1016/S0273-2300(02)00020-X
Gray MA, Harrins A, Centeno JA (2005) The role of cadmium, zinc, and selenium in prostate disease. In: Moore TA, Black A, Centeno JA, Harding JS, Trumm DA (eds) Metal contaminants in New Zealand: sources, treatments, and effects on ecology and human health. Resolutionz Press, Christchurch, pp 393–414
Guven K, Ozbay C, Unlu E, Satar A (1999) Acute lethal toxicity and accumulation of copper in Gammarus pulex (L.) (Amphipoda). Turk J Boil 23:513–521
Hajeb P, Jinap S, Ismail A, Fatimah AB, Jamilah J, Rahim MA (2009) Assessment of mercury level in commonly consumed marine fishes in Malaysia. Food Control 20:79–84. doi:10.1016/j.foodcont.2008.02.012
Hasan MN, Rashid H, Tanu MB, Parveen R, Sukhan ZP, Sattar MA, Mahmud Y (2011) Monitoring pollution level of the river Khiru at Mymensingh, Bangladesh. In: Proceedings of the international conference on environmental aspects of Bangladesh 2011. pp. 004
Hina B, Rizwani GH, Naseem S (2011) Determination of toxic metals in some herbal drugs through atomic absorption spectroscopy. Pak J Pharm Sci 24(3):353–358
IARC (International Agency for Research on Cancer) (2014) World Cancer Report 2014. http://www.iarc.fr/en/publications/books/wcr/wcr-order.php
Ishikawa Y, Nagakawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E (1994) Characteristics of chromate workers’cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br J Cancer 70:160
Islam MM, Akhtar MK, Masud MS (2006) Prediction of environmental flow to improve the water quality in the river Buriganga. Proceedings of the 17th IASTED International Conference on Modelling and Simulation, Montreal, QC, Canada, 2006
Islam MS, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Masunaga S (2015) Metal speciation in sediment and their bioaccumulation in fish species of three urban rivers in Bangladesh. Arch Environ Contam Toxicol 68(1):92–106
Javed M, Usmani N (2011) Accumulation of heavy metals in fishes: a human health concern. I J Env Sci 2(2):659
Javed M, Usmani N (2013) Assessment of heavy metal (Cu, Ni, Fe Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. SpringerPlus 2(1):1–13
Jezierska B, Witeska M (2006) The metal uptake and accumulation in fish living in polluted waters. Soil and water pollution monitoring, protection and remediation. Springer, Netherlands, pp 107–114
Khan F, Jolly YN, Islam GM, Akhter S, Kabir J (2014) Contamination status and health risk assessment of trace elements in foodstuffs collected from the Buriganga River embankments, Dhaka, Bangladesh. Int J Food Contam 1(1):1–8
Lemly AD (1993a) Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ Monit Assess 28:83–100. doi:10.1016/0166-445X(93)90051-2
Lemly AD (1993b) Metabolic stress during winter increases the toxicity of selenium to fish. Aquat Toxicol 27:133–158. doi:10.1016/0166-445X(93)90051-2
Li J, Huang ZY, Hu Y, Yang H (2013) Potential risk assessment of heavy metals by consuming shellfish collected from Xiamen, China. Environment Sci Pollut Res 20:2937–2947
Liu C, Huang HJ, Wang HC, Chung PY, Huang KH (1996) Liver metallothionein levels and metal content in fish of Chung-Kung stream, Taiwan–Tilapia and Liza macrolepsis. Chem Ecol 12:125–134
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201. doi:10.1016/S0039-9140(02)00268-0
Mansour SA, Sidky MM (2002) Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem 78(1):15–22. doi:10.1016/S0308-8146(01)00197-2
Mansour SA, Belal MH, Abou-Arab AA, Gad MF (2009) Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 75:601–609. doi:10.1016/j.chemosphere.2009.01.058
Martí-Cid R, Liobet JM, Castell V, Domingo JL (2008) Dietary intake of arsenic, cadmium, mercury, and lead by the population of Catalonia. Spain Biol Trace Elem Res 125(2):120–132. doi:10.1007/s12011-008-8162-3
MHSAC (2005) National Standard of the People’s Republic of China, Ministry of Hygienic and the Standardization Administration of China, http://www.apps.fas.usda.gov/gainfiles/200608/146208660.doc
Mohiuddin KM, Ogawa Y, Zakir HM, Otomo K, Shikazono N (2011) Heavy metals contamination in water and sediments of an urban river in a developing country. Int J Environ Sci Te 8:723–736. doi:10.1007/BF03326257
Moiseenko TI, Kudryavtseva LP (2001) Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola region Russia. Environ Pollut 114(2):285–297
Moreno JA, Yeimans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB (2009) Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol Sci 112(2):394–404
Nordberg GF (2010) Biomarkers of exposure, effects and susceptibility in humans and their application in studies of interactions among metals in China. Toxicol Lett 192(1):45–49
Nouri J, Khorasani N, Lorestani B, Karami M, Hassani AH, Yousefi N (2009) Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ Earth Sci 59(2):315–323. doi:10.1007/s12665-
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421:3–16. doi:10.1016/j.scitotenv.2011.03.013
Pieniak Z, Verbeke W, Olsen SO, Hansen KB, Brunso K (2010) Health-related attitudes as a basis for segmenting European fish consumers. Food Policy 35(5):448–455. doi:10.1016/j.foodpol.2010.05.002
Qiu YW, Lin D, Liu JQ, Zeng EY (2011) Bioaccumulation of trace metals in farmed fish from South China and potential risk assessment. Ecotoxicol Environ. Saf 74:284–293. doi:10.1016/j.ecoenv.2010.10.008
Rahman MM, Asaduzzaman M, Naidu R (2013) Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J Hazard Mater 262:1056–1063. doi:10.1016/j.jhazmat.2012.06.045
Rashed MN (2001) Monitoring of environmental heavy metals in fish from Nasser Lake. Env Int 27(1):27–33
Saha PK, Hossain MD (2011) Assessment of heavy metal contamination and sediment quality in the Buriganga River, Bangladesh. 2nd International Conference on Environmental Science and Technology, IPCBEE, Singapore, 2011
Saha N, Zaman MR (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City Bangladesh. Environ Monit Assess 185(5):3867–3878. doi:10.1007/s10661-012-2835-2
Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R (2008) Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int 34(8):1215–1226. doi:10.1016/j.envint.2008.04.009
SCHER (2012) Assessment of the tolerable daily intake of barium, scientific committee on health and environmental risks. European Union, Brussels
Sivaperumal P, Sankar TV, Nair PGV (2007) Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-à-vis international standards. Food Chem 102:612–620. doi:10.1016/j.foodchem.2006.05.041
Spry DJ, Wiener JG (1991) Metal bioavailability and toxicity to fish in low-alkalinity lakes—a critical review. Environ Pollut 71:243–244. doi:10.1016/0269-7491(91)90034-T
Tuzen M (2009) Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food Chem Toxicol 47:1785–1790. doi:10.1016/j.fct.2009.04.029
USEPA (1989) Risk assessment guidance for superfund, Vol. I. Human health evaluation. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=9&cad=rja&uact=8&ved=0ahUKEwiFjc3W7cbLAhUL1I4KHYtPBr8QFgg6MAg&url=https%3A%2F%2Frais.ornl.gov%2Fdocuments%2FHHEMB.pdf&usg=AFQjCNGKQY4Q-m5yQGZK-YogwChqBjJQA&sig2=3gbmDqk2D4D6Ut28pYSECQ
USEPA (US Environmental Protection Agency) (1989) Guidance manual for assessing human health risks from chemically contaminated, fish and shellfish. EPA-503/8-89- 002. USEPA, Washington DC
USEPA (2004) Integrated risk information system (IRIS) on lead and compounds (inorganic). National Center for Environmental Assessment. Washington, DC: Office of Research and Development. Last revised 2004. http://www.epa.gov/iris/subst/0277.htm#bib. Accessed on 4 Feb 2014
USEPA (2010) Risk-based concentration table. http://www.epa.gov/reg3hwmd/risk/human/index.htm Accessed April 15, 2014
USEPA (2011) USEPA regional screening level (RSL) summary table: November 2011. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables-november-2015
USEPA (2015) Human health risk assessment. Regional screening level (RSL)—summary table. http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/Generic_Tables/docs/master_sl_table_run_JAN2015.pdf. Accessed on 10 May 2015
USFDA (1993) Food and drug administration. Guidance document for nickel in shellfish. DHHS/PHS/FDA/CFSAN/Office of Seafood, Washington, DC. http://www.cfsan.fda.gov/%7Efrf/guid-as.html. Accessed March 17, 2016
Vannoort RW, Thomson BM (2006) 2003/2004 New Zealand total diet survey: agricultural compound residue, selected contaminants and nutrients. New Zealand Food Safety Authority 2006:144
Vieira C, Morais S, Ramos S, Delerue-Matos C, Oliveira MBPP (2011) Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food Chem Toxicol 49(4):923–932. doi:10.1016/j.fct.2010.12.016
WHO (1995) Environmental Health Criteria No 165: Inorganic Lead. Geneva (Switzerland): World Health Organization (WHO). http://www.inchem.org/documents/ehc/ehc/ehc165.htm
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585. doi:10.1016/j.envpol.2011.06.011
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407:1551–1561. doi:10.1016/j.scitotenv.2008.10.061
Authors’ contributions
MKA and MAB conceived and designed the experiments; MMH and MSI contributed reagents/materials/analysis tools and performed the experiments; GKK and MMI analyzed the data; GKK, MMI, MKA and MAB wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the authority of the Centre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh, and Yokohama National University, Japan, for providing laboratory facilities.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kawser Ahmed, M., Baki, M.A., Kundu, G.K. et al. Human health risks from heavy metals in fish of Buriganga river, Bangladesh. SpringerPlus 5, 1697 (2016). https://doi.org/10.1186/s40064-016-3357-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-3357-0