Abstract
Background
Hepatitis B virus (HBV) genotypes have distinct geographical distributions and are associated with different clinical courses. HBV genotype G (HBV/G) is extremely rare among Human immunodeficiency virus (HIV) infected populations in Japan. Genetic analysis and clinical course of recombinant forms with HBV/G infection are seldom reported in the literature.
Case presentation
A 36-year old homosexual man with HIV infection was referred to a general hospital for assessment of chronic HBV infection. We cloned full-length HBV isolates and determined the complete genome sequences of 2 obtained clones, although mixture of multiple variant with different length is detected by HBV-DNA genotyping. The Bootscaning analysis using a full-length HBV genome revealed the clones represented as the HBV/A2 and the HBV/G/A2 recombinant strain. The HBV-DNA decreased from >9.1 to 2.5 log copies/mL after 24 months of antiretroviral therapy.
Conclusions
This patient was co-infected with HBV/A2 and HBV/G/A2 recombinant strain. This recombinant strain was not identical to HBV/G/A2 strains previously reported from Japan. Recombination with other genotypes could alter the clinical manifestations of chronic hepatitis B in people living with HIV.
Similar content being viewed by others
Background
Hepatitis B virus (HBV) infection is a major public health issues among human immunodeficiency virus (HIV) -infected people. HIV-HBV co-infection can accelerate the progression of chronic hepatitis resulting in fibrosis and hepatocelullar carcinoma (Thio et al. 2002). HIV-HBV co-infection rate is high due to the similar transmission route and approximately 6.3 % of the HIV-infected patients in Japan are coinfected with HBV (Koike et al. 2008).
HBV is classified into ten genotypes on the basis of 8 % divergence in the entire genomic sequence. HBV genotypes have distinct geographical distributions and are associated with different clinical courses. In HIV-HBV coinfected patients in Japan, HBV genotype A2 (HBV/A2) is the most frequently detected, followed far behind by genotype C and genotype B which are common in the entire chronic hepatitis B population (Yanagimoto et al. 2012). HBV genotype G (HBV/G) was first reported in 2000 from France and characterized by a unique 36 nt insertion in the core region and the possession of two stop codons in the precore region that prevents the expression of hepatitis B envelope antigen (HBeAg) (Stuyver et al. 2000). HBV/G infections were found to occur predominantly in males (92 %) and were primarily associated with male homosexual sex (67 %) in Canada (Osiowy et al. 2008). French studies found a high prevalence of patients infected with HBV/G among HIV-HBV co-infected patients (12 and 25 %) (Lacombe et al. 2006; Desire et al. 2010). In contrast, HBV/G is extremely rare in Japan even in HIV-infected patients (Yanagimoto et al. 2012; Kato et al. 2004). One of its unique characteristics is frequent co-infection with the other genotypes. Recombinant forms between HBV/G and the other genotypes have been observed. However, these recombinant forms have been detected normally as a minority species within the HBV quasispecies population and specific prevalent strains have not been identified (Kato et al. 2002a, b). Some epidemiological data exhibited patients infected with HIV-HBV/G were at increased risk of liver fibrosis compared to those infected with other HBV genotypes (Lacombe et al. 2006; Dao et al. 2011) but clinical data on HBV/G is limited due to the low prevalence throughout the world. We report here a case of chronic hepatitis caused by HIV-HBV/G/A2 recombinant strain co-infection. These epidemiological and clinical findings have warranted the need for HBV genotyping in the management of HIV-HBV co-infected patients.
Case presentation
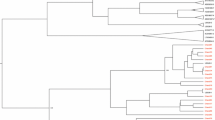
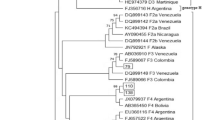
In 2013, a 36 year-old Japanese man was diagnosed with chronic hepatitis B based on the elevated levels of liver enzyme and the positive hepatitis B surface antigen (HBsAg) at a local clinic during follow-up for flu-like symptoms. He was referred to the hepatology division, in a general hospital for further assessment of chronic hepatitis B. The liver biopsy showed septal fibrosis and infiltrating lymphocyte in his liver tissue associated with degeneration of limiting plate, and histologic activity index—METAVIR score (Thio et al. 2002) was A2-F3. He had been living in Japan all his life and had no family histories of viral hepatitis. He denied intravenous drug use and dissolute sexual behavior in Japan, but admitted occasional sexual intercourse with men in Thailand since he was 20 years old. The screening for HIV antigen/antibody test was positive and HIV infection was confirmed by the positive Western-blot HIV antibody test. The Serological HBV genotyping test by enzyme immunoassay (EIA) in the clinical laboratory testing industry (SRL Inc. Japan) showed genotype D. However, his social history was not compatible with the risk of chronically HBV gonotype D (HBV/D) infection because HBV/D is rare throughout Japan except for some areas and not prevalent among men who have sex with men (MSM). Then, HBV-DNA genotyping was performed using obtained isolates from this patient. We cloned full-length HBV isolates and determined the complete genome sequences of 2 obtained clones as previously reported (Sugauchi et al. 2001). Briefly, the full length of HBV genome was amplified as two overlapping fragments by using the primer set to yield a 3200 bp amplicon for fragment A and another primer set to yield a 462 bp amplicon for fragment B. The fragment A or B was transferred to pGEM-T easy vector by TA cloning. The cloned plasmids of fragment A or B were sequenced and analyzed by phylogenetic analysis using MEGA6 (Tamura et al. 2013). The Bootscaning analysis using RDP4 software (Martin et al. 2010) revealed the clones represented as the HBV/A2 and the HBV/G/A2 recombinant strain (Fig. 1a, b). These findings indicated co-infection of HBV/A2 and HBV/G/A2. 36 nt insertion in the core region and two stop codons in the precore region were not detected in the recombinant strain because two estimated recombination junction of HBV/G were from approximately nt.192 to nt.1795 (Fig. 1b). The HBV/A2 and the HBV/G/A2 recombinant strain were compared with other registered strains including G/A recombinants by MEGA6. Figure 2 shows the phylogenetic tree of these strains. Our HBV/G/A2 were located near the clusters of HBV/G and G/A recombinants. Our HBV/A2 belonged to the HBV/A2 cluster.
The HIV-RNA and CD4 T cell count were 4300 copies/mL and 226 cells/μL, respectively. The HIV subtype was CRF-01 AE and no primary mutations associated with drug resistance mutations were detected. Laboratory findings were as follows: platelet count, 10.6 × 104/dL; asparate aminotransferase, 84 IU/L; alanine aminotransferase, 122 IU/L; alkaline phosphatase, 272 IU/L; γ-glutamyltranspeptidase, 34 IU/L; hepatitis B surface antigen (HBsAg), 93,297 IU/mL; antibodies to the HBsAg (anti-HBsAg), negative; total hepatitis B core antibodies, 11.9 S/CO; HBeAg, 42.9 S/CO; antibodies to the HBeAg (anti-HBeAg), negative; HBV-DNA, >9.1 log copies/mL; hyaluronic acid, 124 ng/mL; Type IV Collagen 7 s, 7.3 ng/mL. HBV drug resistance mutations related to lamivudine, adefovir, entecavir or tenofovir were not detected. He received antiretroviral therapy (ART) consisting of tenofovir/emtricitabine (TDF/FTC) and raltegravir to treat HIV-HBV/G/A2 recombinant infection. Three months after the initiation of ART, while the HBV-DNA decreased steadily, the levels of liver enzyme increased progressively with the rapid decline of HBeAg and HBsAg. The CD4 T-cell count increased to 487 cells/μL. This presentation was consistent with HIV-HBV immune reconstitution inflammatory syndrome (IRIS). The levels of liver enzymes improved without specific treatments. The HBV-DNA decreased to 2.5 log copies/mL after 24 months of ART.
Discussion
HIV infection accelerates chronic hepatitis B related liver diseases, and precise assessment of HBV infection is imperative in the management of HIV-HBV co-infected patients. Different HBV genotypes induce different clinical features in HBV-infected patients. Recently, acute HBV infection in metropolitan areas of Japan has been increasingly attributed to the HBV/A2, that is transmitted through sexual contacts (Tamada et al. 2012). Another study in Japan found HBV/A2 is the most frequent in HIV infected patients and is detected almost exclusively in homosexual patients (Yanagimoto et al. 2012). Several HBV/G/A2 recombinant strains have been reported among MSM patients in Japan (Tsuzuki et al. 2015; Kojima et al. 2015) and all of these strains have a 36-nt insertion of the core gene. However, the insertion was not detected in the HBV/G/A2 recombinant from our patient, suggesting a possibility that he was infected with the strain outside Japan. The co-infection with HBV/A2 and HBV/G/A2 recombinant was a rare case in HIV-infected patients. The HIV subtype of this patient is CRF_01 AE, which is a prevalent strain in South East Asia (Hemelaar et al. 2006). Hence, we speculated he had been infected with HIV-HBV in Thailand. However, epidemiological data regarding the prevalence of HBV/G or recombinant forms among HIV-infected patients are scarce in Asia (Araujo 2015).
There was a discrepancy between results of the HBV-DNA genotyping and the serological genotyping test by EIA in this case. The reason was unclear, but recombination in the preS2-region might affect the results of the serological test by EIA that used monoclonal antibodies of a combination of epitopes on preS2-region products. HBV/G may be overlooked if a tool designed to detect recombinant forms with other genotypes is not used. It is assumed that detection technique could affect the epidemiologic study of HBV genotype. Full-genome sequence was occasionally difficult to be determined because of the presence of mixture of multiple variant with different length.
Two studies suggest that HBV/G may accelerate liver fibrosis progression and associated with HBeAg positivity and high HBV-DNA levels (Lacombe et al. 2006; Dao et al. 2011). The clinical findings in this case showed the advanced liver fibrosis. Recombination with HIV-HBV/A2 co-infection, which was frequently associated with high HBV-DNA levels, would explain his extremely high HBV-DNA level. Some studies revealed co-infection with HBV/A2 enhanced HBV/G replication (Dao et al. 2011; Kato et al. 2002a, b; Tanaka et al. 2008) and a in vivo study demonstrated the introduction of the HBV/A2 core promoter or core protein or both genomic regions into the HBV/G genome increased HBV/G replication efficiently (Tsuzuki et al. 2015; Sakamoto et al. 2013). Meanwhile, his CD4+ cell count was maintained at 226/μL, nevertheless HIV-HBV-IRIS occurred after the initiation of ART and HBV-specific CD8+ cell responses could be impaired. Thereafter, the impaired cellular immunity caused by HIV infection may affect the progress of liver fibrosis. In contrast, a recent study reports HBV/G does not have a harmful effect on fibrosis progression in efficiently treated HIV–HBV co-infected patients (Calin et al. 2013). Prognosis of chronic hepatitis caused by HBV/G or recombinant forms remain controversial. Long-term precise observation of clinical course is needed to evaluate the prognosis of this patient.
Clinical data regarding treatment for HBV/G is limited. As a rule, HIV-HBV co-infected patients regardless of HBV genotype receive TDF/FTC-containing ART. A study reported three of six patients who experienced HBV-DNA rebound with correct TDF serum concentration were infected with HIV-HBV/G or HIV-HBV/G/A2 mixture (Karine Lacombe et al. 2009). Another French report showed 15 % (7/45) of HIV-HBV co-infected patients who added TDF to lamivudine therapy experienced delayed decrease in plasma HBV-DNA levels, of whom four of them were infected with HBV/G (Lada et al. 2012). A recent report from Japan showed HBV/G/A2 recombinant did not always cause rapid progression of disease (Kojima et al. 2015). In this present case detectable HBV-DNA levels have lasted for 2 years after the initiation of antiviral therapy. Clinical and virological factors to affect TDF/FTC response to HBV/G remained to be determined. Additionally, a case of occult infection with HBV/G/A2 recombinant following acute hepatitis B caused by HBV/A2 is reported (de Barros et al. 2015). Co-infection with HBV/G/A2 recombinant could be a risk of chronic infection caused by HBV/A2 and persistent viremia.
Conclusions
This patient was infected with HBV/A2 and HBV/G/A2 recombinant which is extremely rare in Japan. Although these HBV strains had no mutations related to anti-HBV drugs, detectable HBV-DNA levels have lasted for 2 years under TDF/FTC-containing ART. Clinicians should keep the HBV genotyping in mind using tools designed to detect recombinant forms in the management of HIV-HBV co-infected patients.
Abbreviations
- HBV:
-
hepatitis B virus
- HIV:
-
human immunodeficiency virus
- ART:
-
antiretroviral therapy
- TDF/FTC:
-
tenofovir/emtricitabine
- IRIS:
-
immune reconstitution inflammatory syndrome
References
Araujo NM (2015) Hepatitis B virus intergenotypic recombinants worldwide: an overview. Infect Genet Evol 36:500–510
Calin R, Guiguet M, Desire N, Imbert-Bismut F, Munteanu M, Poynard T, Valantin MA, Stitou H, Katlama C, Thibault V (2013) Role of genotype G hepatitis B virus mixed infection on the progression of hepatic fibrosis in HIV positive patients over 5 years of follow-up. J Clin Virol 58:408–414
Dao DY, Balko J, Attar N, Neak E, Yuan HJ, Lee WM, Jain MK (2011) Hepatitis B virus genotype G prevalence and impact in patients co-infected with human immunodeficiency virus. J Med Virol 83:1551–1558
de Barros JJ, Peres LR, de Sousa PS, do Amaral Mello FC, de Araujo NM, de Andrade Gomes S, Niel C, Lewis-Ximenez LL (2015) Occult infection with HBV intergenotypic A2/G recombinant following acute hepatitis B caused by an HBV/A2 isolate. J Clin Virol 67:31–35
Desire N, Sanchis T, Ben Moussa F, Stitou H, Katlama C, Thibault V (2010) Development and validation of a specific method for relative HBV-genotype G (G-HBV) quantification in the context of co-infection with other genotypes. Pathol Biol 59:e13–e19
Hemelaar J, Gouws E, Ghys PD, Osmanov S (2006) Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20:W13–W23
Kato H, Orito E, Gish RG, Bzowej N, Newsom M, Sugauchi F, Suzuki S, Ueda R, Miyakawa Y, Mizokami M (2002a) Hepatitis B e antigen in sera from individuals infected with hepatitis B virus of genotype G. Hepatology 35:922–929
Kato H, Orito E, Gish RG, Sugauchi F, Suzuki S, Ueda R, Miyakawa Y, Mizokami M (2002b) Characteristics of hepatitis B virus isolates of genotype G and their phylogenetic differences from the other six genotypes (A through F). J Virol 76:6131–6137
Kato H, Sugauchi F, Ozasa A, Kato T, Tanaka Y, Sakugawa H, Sata M, Hino K, Onji M, Okanoue T, Tanaka E, Kawata S, Suzuki K, Hige S, Ohno T, Orito E, Ueda R, Mizokami M (2004) Hepatitis B virus genotype G is an extremely rare genotype in Japan. Hepatol Res 30:199–203
Koike K, Kikuchi Y, Kato M, Takamatsu J, Shintani Y, Tsutsumi T, Fujie H, Miyoshi H, Moriya K, Yotsuyanagi H (2008) Prevalence of hepatitis B virus infection in Japanese patients with HIV. Hepatol Res 38:310–314
Kojima Y, Kawahata T, Mori H, Furubayashi K, Taniguchi Y, Itoda I, Komano J (2015) Identification of novel recombinant forms of hepatitis B virus generated from genotypes Ae and G in HIV-1-positive Japanese men who have sex with men. AIDS Res Hum Retroviruses 31:760–767
Lacombe K, Massari V, Girard PM, Serfaty L, Gozlan J, Mialhes PG, Molina JM, Lascoux-Combe C, Wendum D, Carrat F, Zoulim F (2006) Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS 20:419–427
Lacombe K, Boyd A, Bonnard P, Molina J-M, Miailhes P, Lascoux-Combe C, Zoulim F, Pacanowski J, Girard P-M (2009) HBV blippers and rebounders under treatment with tenofovir in HIV/HBV co-infection. Conference on retroviruses and opportunistic infections, Montreal, Canada. 009
Lada O, Gervais A, Branger M, Peytavin G, Roquebert B, Collin G, Fraqueiro G, Moucari R, Hamet G, Martinot-Peignoux M, Matheron S, Marcellin P (2012) Long-term outcome of primary non-responders to tenofovir therapy in HIV/HBV-co-infected patients: impact of HBV genotype G. Liver Int 32:93–101
Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26:2462–2463
Osiowy C, Gordon D, Borlang J, Giles E, Villeneuve JP (2008) Hepatitis B virus genotype G epidemiology and co-infection with genotype A in Canada. J Gen Virol 89:3009–3015
Sakamoto T, Tanaka Y, Watanabe T, Iijima S, Kani S, Sugiyama M, Murakami S, Matsuura K, Kusakabe A, Shinkai N, Sugauchi F, Mizokami M (2013) Mechanism of the dependence of hepatitis B virus genotype G on co-infection with other genotypes for viral replication. J Viral Hepat 20:e27–e36
Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R (2000) A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol 81:67–74
Sugauchi F, Mizokami M, Orito E, Ohno T, Kato H, Suzuki S, Kimura Y, Ueda R, Butterworth LA, Cooksley WG (2001) A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: complete genome sequence and phylogenetic relatedness. J Gen Virol 82:883–892
Tamada Y, Yatsuhashi H, Masaki N, Nakamuta M, Mita E, Komatsu T, Watanabe Y, Muro T, Shimada M, Hijioka T, Satoh T, Mano Y, Komeda T, Takahashi M, Kohno H, Ota H, Hayashi S, Miyakawa Y, Abiru S, Ishibashi H (2012) Hepatitis B virus strains of subgenotype A2 with an identical sequence spreading rapidly from the capital region to all over Japan in patients with acute hepatitis B. Gut 61:765–773
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka Y, Sanchez LV, Sugiyama M, Sakamoto T, Kurbanov F, Tatematsu K, Roman S, Takahashi S, Shirai T, Panduro A, Mizokami M (2008) Characteristics of hepatitis B virus genotype G coinfected with genotype H in chimeric mice carrying human hepatocytes. Virology 376:408–415
Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Munoz A, Thomas DL (2002) HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 360:1921–1926
Tsuzuki Y, Watanabe T, Iio E, Fujisaki S, Ibe S, Kani S, Hamada-Tsutsumi S, Yokomaku Y, Iwatani Y, Sugiura W, Okuse C, Okumura A, Sato Y, Tanaka Y (2015) Virological characteristics of hepatitis B genotype G/A2 recombination virus in Japan. Hepatol Res. doi:10.1111/hepr.12612
Yanagimoto S, Yotsuyanagi H, Kikuchi Y, Tsukada K, Kato M, Takamatsu J, Hige S, Chayama K, Moriya K, Koike K (2012) Chronic hepatitis B in patients coinfected with human immunodeficiency virus in Japan: a retrospective multicenter analysis. J Infect Chemother 18:883–890
Authors’ contributions
EA and MS equally contributed to this work. EA involved in the clinical management of the patient.and drafted the manuscript. MS carried out the genotyping analysis and identification of inter-genotypic recombination. SS and KK involved in the clinical management of the patient. EA, KT, SS, MS and MM designed the project. TK and MK supported the clinical management. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Shunsuke Aoi, MD, for his comments on drafts of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Informed consent
Informed consent was obtained for publication of this case report.
Nucleotide sequence accession numbers
Nucleotide sequence data reported are submitted in the DDBJ/EMBL/GenBank databases under the accession numbers LC150335 and LC150336.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Adachi, E., Sugiyama, M., Shimizu, S. et al. Human immunodeficiency virus and hepatitis B genotype G/A2 recombinant co-infection: a case study. SpringerPlus 5, 1502 (2016). https://doi.org/10.1186/s40064-016-3169-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-3169-2