Abstract
Background
Multidrug resistant Pseudomonas aeruginosa and Acinetobacter baumannii are common causes of health care associated infections worldwide. Carbapenems are effective against infections caused by multidrug resistant Gram-negative bacteria including Pseudomonas and Acinetobacter species. However, their use is threatened by the emergence of carbapenemase-producing strains. The aim of this study was to determine the prevalence of carbapenem-resistant P. aeruginosa and A. baumannii at Mulago Hospital in Kampala Uganda, and to establish whether the hospital environment harbors carbapenem-resistant Gram-negative rods.
Results
Between February 2007 and September 2009, a total of 869 clinical specimens were processed for culture and sensitivity testing yielding 42 (5 %) P. aeruginosa and 29 (3 %) A. baumannii isolates, of which 24 % (10/42) P. aeruginosa and 31 % (9/29) A. baumannii were carbapenem-resistant. Additionally, 80 samples from the hospital environment were randomly collected and similarly processed yielding 58 % (46/80) P. aeruginosa and 14 % (11/80) A. baumannii, of which 33 % (15/46) P. aeruginosa and 55 % (6/11) A. baumannii were carbapenem-resistant. The total number of isolates studied was 128. Carbapenemase genes detected were bla IMP-like (36 %, 9/25), bla VIM-like (32 %, 8/25), bla SPM-like (16 %, 4/25); bla NDM-1-like (4 %, 1/25) in carbapenem-resistant P. aeruginosa, and bla OXA-23-like (60 %, 9/15), bla OXA-24-like (7 %, 1/15), bla OXA-58-like (13 %, 2/15), and bla VIM-like (13 %, 2/15) in carbapenem-resistant A. baumannii. Furthermore, class 1 integrons were detected in 38 % (48/128) of P. aeruginosa and Acinetobacter, 37 % (26/71) of which were in clinical isolates and 39 % (22/57) in environment isolates. Gene cassettes were found in 25 % (12/48) of integron-positive isolates. These were aminoglycoside adenylyltransferase ant(4′)-IIb (3 isolates); trimethoprim-resistant dihydrofolate reductase dfrA (2 isolates); adenyltransferase aadAB (3 isolates); QacE delta1 multidrug exporter (2 isolates); quinolone resistance pentapeptide repeat protein qnr (1 isolate); and metallo-β-lactamase genes bla VIM-4-like, bla IMP-19-like, and bla IMP-26-like (1 isolate each). Gene cassettes were missing in 75 % (36/48) of the integron-positive isolates.
Conclusions
The prevalence of carbapenem-resistant P. aeruginosa and Acinetobacter among hospitalized patients at Mulago Hospital is low compared to rates from South-East Asia. However, it is high among isolates and in the environment, which is of concern given that the hospital environment is a potential source of infection for hospitalized patients and health care workers.
Similar content being viewed by others
Background
Multidrug resistant Pseudomonas aeruginosa and Acinetobacter baumannii are common causes of nosocomial infections worldwide (Falagas et al. 2005; Giamarellos-Bourboulis et al. 2003; Gootz and Marra 2008; Tam et al. 2010; Turton et al. 2005). Carbapenems are the most effective drugs against infections caused by multidrug resistant Gram-negative bacteria including Pseudomonas and Acinetobacter species (Papp-Wallace et al. 2011; Manenzhe et al. 2015). However, their use in the management of these infections is threatened by the emergence of carbapenemase producing strains. Carbapenemases are β-lactamase enzymes with capacity to hydrolyze carbapenems, penicillins, and cephalosporins; they were first described in the 1990s and have continued to be reported every year with increasing rates (Papp-Wallace et al. 2011; Tenover 2006; Queenan and Bush 2007).
Carbapenemases are assigned to three of four classes of β-lactamases; Ambler classes A, B, and D that are differentiated based on the hydrolytic mechanisms at their active sites (Manenzhe et al. 2015; Queenan and Bush 2007). Class A and D carbapenemases are referred to as Serine carbapenemases because they have Serine (amino acid) at the active site (Serine-dependent), whereas class B carbapenemases have zinc (zinc-dependent) and are referred to as metallo-β-lactamases. Ambler class A carbapenemases can be plasmid encoded or chromosomal and are inhibited by clavulanic acid, a β-lactamase inhibitor; SME, IMI, NMC, GES, and KPC families are the most frequently identified class A carbapenemases mostly in Klebsiella pneumoniae. Class B metallo-β-lactamases are plasmid-encoded (in some cases chromosomal) and the most common enzymes include the VIM, IMP, SPM, GIM, SIM and NDM families. Metallo-β-lactamases have been detected primarily in P. aeruginosa but they are also increasingly being detected in Acinetobacter species (Tsakris et al. 2006) and the Enterobacteriaceae (Turton et al. 2005, 2006; Okoche et al. 2015); NDM producing Enterobacteriaceae are currently a major concern due to their rapid spread worldwide (Manenzhe et al. 2015). Furthermore, class B enzymes are able to hydrolyze β-lactams except aztreonam (a monobactam) and their hydrolytic activity is inhibited by EDTA (ethylenediammine tetra acetic acid), but not clavulanic acid. On the other hand, class D enzymes, also referred to as the OXA-type carbapenemases, are subdivided into five families; OXA-23, OXA-24/40, OXA-48 and OXA-58 families that are mainly plasmid-encoded (Queenan and Bush 2007), and OXA-51 that is chromosomally encoded and intrinsic (naturally found) in A. baumannii (Manenzhe et al. 2015; Turton et al. 2006). Though, OXA-51 confers resistance or reduced susceptibility to carbapenems only when its expression is up-regulated by genetic reorganization (SMI P 8 2014). Class D enzymes are not inhibited by clavulanic acid or EDTA.
Whereas carbapenemase-producing bacteria are well characterized in high-income countries, little is known about them in Uganda and Africa at large (Manenzhe et al. 2015). Metallo-β-lactamase producing bacteria from a tertiary-care center in Nairobi, Kenya, was characterized in 2008, in a first report of VIM-2-producing P. aeruginosa in East Africa (Pitout et al. 2008). Furthermore, in a systematic review of 83 surveillance studies on carbapenemases in Africa (Manenzhe et al. 2015), it was revealed that most studies were from 15 of the 54 countries in Africa, mainly in Northern and Southern Africa with no report of negative results in studies that screened for carbapenemase-producing bacteria in humans in hospitals (Manenzhe et al. 2015). Indeed, prior to 2010 there were only seven reports of carbapenemase-producing bacteria in Africa with OXA-58, OXA-48, OXA-23, VIM-2 and VIM-4 documented as prevalent in carbapenemase-producing bacteria in outbreaks (Manenzhe et al. 2015). Carbapenemase-producing bacteria elaborating OXA-23, OXA-24, OXA-58, VIM-2 and IMP-1 were also isolated from hospital environments (Manenzhe et al. 2015). Recently in Uganda, we characterized carbapenem-resistant Enterobacteriaceae isolated from patients admitted at Mulago National Referral Hospital in Kampala (Okoche et al. 2015).
The aim of this study was to determine the prevalence of carbapenem resistant P. aeruginosa and A. baumannii at Mulago National Referral Hospital, and to establish whether the Mulago Hospital environment harbors carbapenem resistant Gram-negative rods. Herein we describe the susceptibility patterns and carbapenemase genes harbored by the isolates, as it might help in the global comparisons of resistance mechanisms of multidrug resistant Gram-negative bacteria.
Methods
Study setting and design
This study was conducted at Mulago National Referral Hospital in Kampala, Uganda. Mulago is a 1500-bed tertiary hospital belonging to the Ministry of Health, Uganda. With its free medical care, the hospital is highly attractive for the peri-urban low-income population around the capital Kampala where the infectious disease burden is high. The laboratory procedures were performed in Clinical Microbiology and Molecular Biology Laboratories of the Department of Medical Microbiology, College of Health Sciences, Makerere University.
Between February 2007 and September 2009, carbapenem resistant P. aeruginosa and A. baumannii were isolated from hospitalized patients at Mulago Hospital, in a laboratory surveillance study that aimed to identify carbapenem resistant Gram-negative rods at Mulago Hospital. Within this period, 869 clinical specimens from hospitalized patients were processed by the Clinical Microbiology Laboratory for culture and sensitivity testing (one specimen per patient). Specimens processed were blood (51), cerebral spinal fluid (49), tracheal aspirates (163), ear swabs (197), sputum (204), urine catheters (98), and pus (107). Following detection of P. aeruginosa and Acinetobacter species in the specimens, 80 samples were randomly collected from the hospital environment (surgical/medical wards including the intensive care units, ICUs); they included water (13), disinfectants like chlorhexidine gluconate (15), cleaning materials like mops and squeezers (15), sink swabs (22), and floor swabs (15). Water and disinfectants were sampled using sterile syringes with needles while swabs were used to sample sinks and wet floors.
Identification of P. aeruginosa and A. baumannii
All clinical and environment samples were processed within 2 h of collection for identification of Gram-negative bacteria. Isolates were recovered on blood agar after incubating at 35–37 °C for 24–48 h. Then, single colonies were sub-cultured on MacConkey agar and incubated at 35–37 °C for 24–48 h. Isolates were presumptively identified based on colony morphology, Gram-staining properties and biochemical characteristics [oxidase, triple sugar iron (TSI), sulphur indole and motility (SIM), citrate, and urease tests]. Colonial morphological features (i.e. colonies with characteristic spreading pattern and serrated edges, fruity sweet-grape smell, and bright green color) were used to identify Pseudomonas isolates. Positive catalase and oxidase tests, negative TSI and glucose fermentation tests and growth at 42 °C were used to distinguish P. aeruginosa from other lactose non-fermenting Gram-negative rods. Acinetobacter was presumptively identified based on negative motility and catalase tests, negative oxidase and glucose fermentation tests, and inability to grow under anaerobic conditions.
Identification of isolates to species level and drug susceptibility testing
To confirm A. baumannii to species level, PCR-amplification followed by DNA sequencing of the species-specific region of the bla OXA-51-like gene intrinsic to A. baumannii (Manenzhe et al. 2015; Turton et al. 2006) was performed, using chromosomal DNA extracted from presumptuously identified isolates as template. To confirm P. aeruginosa to species level, and to determine the antimicrobial susceptibility profiles of both P. aeruginosa and A. baumannii, minimum inhibitory concentrations (MICs) were performed using the ‘Phoenix Automated Microbiology System’ from Becton and Dickson, Franklin Lakes, NJ, USA. This system has combination testing panels that include (a) an identification (ID) side with dried substrates for bacterial identification; the ID portion of the Phoenix panels utilizes a series of conventional, chromogenic, and fluorogenic biochemical tests to identify the organism, (b) an antimicrobial susceptibility testing (AST) side with varying concentrations of antimicrobial agents, and (c) growth and fluorescent controls at appropriate well locations. Specimen processing and Gram staining procedure was performed according to the manufacturer’s guidelines.
Phoenix panels were inoculated with standardized inoculum according to the manufacturer’s guidelines; occasionally, minor modifications were performed as described elsewhere (Carroll et al. 2006). Briefly, after determining the Gram staining properties of the isolates, nonselective medium (blood agar) was used to prepare fresh pure cultures for isolate identification (ID) and antimicrobial susceptibility testing (AST). Isolates were inoculated into appropriate ID/AST combination panels for Gram-negative isolates that were loaded into the instrument and incubated at 35 °C. The ID broth was inoculated with bacterial colonies adjusted to a 0.5 McFarland standard, and the suspension poured into the ID side of the Phoenix panel after a 30 µl aliquot was removed and saved for AST. For AST, the Phoenix AST Indicator Solution was added to the AST broth tubes and mixed by inversion. The AST side of the combination panel contains 84 wells with dried antimicrobial panels and one growth control well. One free-falling drop of the AST indicator was added to the AST broth tube, and 30 µl of the standardized ID broth suspension transferred to the AST broth and incubated for 16 h at 35 °C. Samples were read automatically at the instrument’s set parameters.
Phoenix default MIC breakpoints were used; amikacin >32 µg/ml, gentamicin >8 µg/ml, imipenem >8 µg/ml, meropenem >8 µg/ml, ceftazidime >16 µg/ml, cefepime >16 µg/ml, aztreonam >16 µg/ml, piperacine-tazobactam 16/4 µg/ml, and ciprofloxacin >2 µg/ml for P. aeruginosa; amikacin >32 µg/ml, gentamicin >8 µg/ml, imipenem ≤1 µg/ml, meropenem ≤1 µg/ml, ceftazidime >4 µg/ml, cefepime >4 µg/ml, aztreonam >8 µg/ml, piperacine-tazobactam 4/4 µg/ml, and ciprofloxacin >2 µg/ml for A. baumannii. Quality control and maintenance were performed according to the manufacturer’s recommendations. Reference strains Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were included in the ID and AST Panels.
Following isolate identification and AST, carbapenem-susceptible isolates were retested with the disc diffusion susceptibility method (10 µg imipenem or 10 µg meropenem, BiolabZrt, Budapest, Hungary) to detect isolates with inhibition zone diameters of ≤25 mm as Clinical and Laboratory Standards Institute (CLSI) recommends screening them for carbapenemase production (Wikler 2006).
Carbapenemase assays
To detect carbapenemase activity in carbapenem-resistant isolates, carbapenemase assays were performed with the modified Hodge test (MHT) and the imipenem-EDTA test using K. pneumoniae ATCC 700603 and E. coli ATCC 25922 as indicator strains (Okoche et al. 2015; Miriagou et al. 2010; Asthana et al. 2014). In the MHT assay, a 1:10 dilution of the indicator strains was made by diluting 0.5 ml of culture (at 0.5 McFarland) to 5 ml with sterile saline, which was streaked all over the Mueller–Hinton Agar (MHA) plate using a sterile swab. Then, 10 µg meropenem disk (BiolabZrt, Budapest, Hungary) was placed at the center of the MHA plate. Each test isolate was streaked in a straight line from the disk to the edge of the plate. K. pneumoniae ATCC BAA-1705 and K. pneumoniae ATCC BAA-1706 served as positive and negative controls, respectively. Strain BAA-1705 possesses a K. pneumoniae carbapenemase KPC-2 that is highly active against cephamycins, carbapenems, and to several extended spectrum beta-lactamases (ESBLs) (Broberg et al. 2013). Positive or negative results were interpreted according to the guidelines of CLSI and the UK Standards for Microbiology Investigations (SMI P 8 2014; Wikler 2006; Asthana et al. 2014).
To detect metallo-β-lactamase activity, the imipenem-EDTA double-disk synergy test was performed using an overnight liquid culture of the test isolate adjusted to a turbidity of 0.5 McFarland standard, and spread on the surface of MHA plates. Then, two discs with 10 µg imipenem each were placed on the agar 15 mm apart (center-to-center); 10 µl of 0.5 M EDTA was added to one of the imipenem disc to achieve a disc content of 1.5 mg. After incubating at 37 °C overnight, an increase in inhibition zone diameter of ≥5 mm in the EDTA-supplemented disc was interpreted as positive for metallo-β-lactamase production (SMI P 8 2014; Asthana et al. 2014).
Furthermore, carbapenem-susceptible isolates with disc inhibition zone diameters of ≤25 mm were also tested for carbapenemase activity (Wikler 2006); isolates with positive results were screened by PCR for carbapenemase genes.
Detection of carbapenemase genes and integrons
As the patterns of carbepenemase-encoding genes differ significantly between countries (Woodford et al. 2010), PCR was performed to identify the profiles of these genes at Mulago Hospital. All carbapenem-resistant isolates were screened for metallo-β-lactamase genes (bla IMP, bla VIM, bla SPM, bla NDM) using PCR primers and conditions described elsewhere (Queenan and Bush 2007; Ma et al. 2015; Pitout et al. 2005; Fallah et al. 2013, 2014); Acinetobacter were screened for OXA-carbapenemase genes (bla OXA-23, bla OXA-24, bla OXA-58) using PCR primers and conditions described Ma et al. (2015). Class 1 integrons were detected with previously described primers 5′-CS (GGCATCCAAGCAGCAAG) and 3′-CS (AAGCAGACTTGACCTGA) that are specific to the 5′ and 3′ conserved segments (CS) of class 1 integrons (Levesque et al. 1995).
Chromosomal DNA used as templates in PCRs was extracted by the cetyltrimethyl ammonium bromide (CTAB) method (Andreou 2013; William and Feil 2012) and dissolved in 100 µl of sterile Tris-EDTA (TE) buffer. PCRs were performed in 10 µl volumes with 100 ng DNA template, custom Master-mix (1×), 0.5 µM each of forward and reverse primer, and 1.25 U Taq DNA polymerase, in a Techne TC-412 thermal cycler (Techne, UK). 5 µl of the amplified PCR product was analyzed by electrophoresis on 1 % agarose gels at 120 constant voltage for 1 h. PCR products were cleaned with the QIAquick PCR-purification kit (Qiagen, Hilden, Germany) and shipped to the United States for sequencing (ACGT Inc., Wheeling IL). Although our focus on PCR was screening mainly carbapenem-resistant isolates, carbapenem-susceptible isolates with disc inhibition zone diameters of ≤25 mm were also screened; for class 1 integron gene cassettes, all isolates were screened by PCR.
Quality control
Negative controls for the PCR-amplified carbapenemase genes included reactions with only water (no DNA), and DNA template extracted from carbapenemase-negative strains K. pneumoniae DSMZ 9377, E. coli ATCC 25922 and P. aeruginosa ATCC 27853. Positive control reactions included template DNA extracted from carbapenemase-producing strains (K. pneumoniae Nr.8 for bla NDM-1, K. pneumoniae 714 for bla OXA-48) and a previously characterized P. aeruginosa clinical strain from Giessen for bla IMP that was obtained from the Institute of Microbiology, Giessen, Germany [see Mushi et al. (2014)]. For bla VIM, the positive control strain was obtained from the RESET research collaboration, Germany [see Fischer et al. (2012)]. Additionally, targeted DNA sequencing of PCR-products and confirmation of sequenced amplicons through BLAST-searching at National Center for Biotechnology Information (NCBI) was performed. Isolates with sequenced amplicons that did not match sequences for the genes being studied were excluded.
Results
Patients and isolates
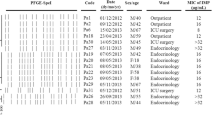
A total of 869 clinical specimens processed yielded 42 (5 %) P. aeruginosa isolates and 29 (3 %) A. baumannii isolates (one per specimen/patient). P. aeruginosa was isolated from tracheal aspirates (15 isolates), ear swabs (12 isolates), pus (7), sputum (5), blood (2) and urine catheter (1), while A. baumannii was isolated from ear swabs (8), tracheal aspirates (9), pus (4), sputum (2), blood (4), and cerebral spinal fluid (2), Additional file 1: Table S1. Mixed populations of P. aeruginosa and Acinetobacter species in the same sample were recovered from a total of nine specimens (1 %). The median age of participants with samples yielding P. aeruginosa was 18.5 years of whom 22 (52 %) were females while 20 (48 %) were males. On the other hand, the median age of participants with samples yielding Acinetobacter was 24 years of whom 13 (45 %) were females while 16 (55 %) were males. Furthermore, a total of 80 samples from the hospital environment that were processed yielded 46 (58 %) P. aeruginosa isolates and 11 (14 %) A. baumannii isolates. Overall, 88 P. aeruginosa and 40 A. baumannii were isolated (128 isolates in total), Table 1 and Additional file 1: Table S1.
Carbapenem resistance
P. aeruginosa
Of the 42 P. aeruginosa isolates from patients, 10 were carbapenem resistant giving a prevalence of 1 % (10/869) in patients or 24 % (10/42) in isolates. Carbapenem resistant P. aeruginosa was isolated from pus (three patients with wounds), sputum (three patients with pneumonia), tracheal aspirates (three patients with hospital acquired pneumonia), and an ear swab (from a patient with otitis media), Additional file 1: Table S1. On the other hand, 15 (33 %, 15/46) P. aeruginosa from the environment were carbapenem resistant. Overall, 25 P. aeruginosa isolates were carbapenem-resistant, Table 1 and Additional file 1: Table S1. Furthermore, most isolates were multidrug resistant (resistance to three or more classes of antimicrobials); 73 % (52/71) and 54 % (31/57) for isolates from hospitalized patients and the environment, respectively (Table 1), with high resistance to ciprofloxacin (64 %, 27/42), ceftazidime (69 %, 29/42), gentamicin (69 %, 29/42), aztreonam (40 %, 17/42), and cefepime (55 %, 23/42) among isolates from hospitalized patients, Additional file 1: Table S1. However, compared to isolates from patients, the environment isolates were more susceptible to ciprofloxacin (43 %, 20/46), ceftazidime (41 %, 19/46) and gentamicin (37 %, 17/46), but more resistant to piperacillin-tazobactam (63 %, 29/46), Additional file 1: Table S1.
A. baumannii
Of the 29 Acinetobacter isolates from hospitalized patients, nine were carbapenem resistant giving a prevalence of 1 % (9/869) or 31 % (9/29) in isolates. Carbapenem resistant Acinetobacter was isolated from tracheal aspirates (four patients with hospital acquired pneumonia), ear swabs (three patients with otitis media), sputum (patient with pneumonia), and cerebral spinal fluid (patient with meningitis). For the environment, six isolates were carbapenem resistant (55 %, 6/11), Table 1 and Additional file 1: Table S1. Overall, 15 A. baumannii isolates were carbapenem resistant, Table 1 and Additional file 1: Table S1, and similar to P. aeruginosa, most Acinetobacter were also multidrug resistant; 62 % (18/29) in isolates from patients and 55 % (6/11) in isolates from environment, with high resistance to ciprofloxacin, gentamicin, piperacillin-tazobactam, ceftazidime and aztreonam, Table 1 and Additional file 1: Table S1.
Carbapenemase activity
Carbapenemase activity in carbapenem-resistant P. aeruginosa was 40 % (4/10) and 27 % (4/15) with MHT in isolates from patients and the environment, respectively; 60 % (6/10) and 67 % (10/15) with imipenem/EDTA test in isolates from patients and the environment, respectively. Carbapenemase activity in carbapenem-resistant Acinetobacter was 33 % (4/9) and 50 % (3/6) with MHT in isolates from patients and the environment, respectively; 22 % (2/9) and 17 % (1/6) with imipenem/EDTA test in isolates from patients and the environment, respectively. Hence, not all carbapenem resistant isolates studied were positive both with MHT and imipenem/EDTA test; however, the latter was more sensitive in detecting metallo-β-lactamase activity. Overall, 28 % (11/40) of carbapenem resistant isolates tested negative both with MHT and imipenem/EDTA test, Additional file 1: Table S1.
Carbapenemase genes
Metallo-β-lactamase genes detected in carbapenem-resistant P. aeruginosa were bla IMP1-like (36 %, 9/25), bla IMP2-like (4 %, 1/25), bla VIM1-like (32 %, 8/25), bla SPM-like (20 %, 5/25), and bla NDM1-like (4 %, 1/25), Table 2 and Additional file 1: Table S1. Most isolates with metallo-β-lactamase genes were positive with the imipenem-EDTA test, and bla VIM-1-like was the only metallo-β-lactamase that was detected in carbapenem-resistant A. baumannii at 13 % (2/15), Table 3 and Additional file 1: Table S1. As expected, OXA-carbapenemase genes were detected mainly in A. baumannii; bla OXA-23-like (60 %, 9/15), bla OXA-24-like (7 %, 1/15), and bla OXA-58-like (13 %, 2/15), Additional file 1: Table S1.
Furthermore, two (8 %, 2/25) carbapenem resistant P. aeruginosa isolates lacked carbapenamase activity and they were also negative for the carbapenemase genes studied, Table 2 and Additional file 1: Table S1. Additionally, six (40 %, 6/15) carbapeneme-resistant Acinetobacter lacked carbapenemase activity, two of which (A081-7 and J093) were negative for carbapenemase genes (except bla OXA51-like), Table 3 and Additional file 1: Table S1. Two carbapenem-susceptible P. aeruginosa from the environment and seven carbapenem-susceptible Acinetobacter from patients carried carbapenemase genes, Additional file 1: Table S1. The carbapenem-susceptible P. aeruginosa isolates with genes were positive with the imipenem/EDTA test (implying metallo-β-lactamase activity) and had disc inhibition zone diameters of ≤25 mm for carbapenems.
Integrons
Class 1 integron amplicons ranging from 600 to 1500 bp were found in 38 % (48/128) of P. aeruginosa and Acinetobacter, of which 37 % (26/71) were found in clinical isolates while 39 % (22/57) in environment isolates. Further, gene cassettes were found only in 25 % (12/48) of the integron-positive isolates. These were aminoglycoside adenylyltransferase ant(4′)-IIb that encodes amikacin and tobramycin resistance (Sabtcheva et al. 2003) (3 isolates); trimethoprim-resistant dihydrofolate reductase dfrA that encodes resistance to trimethoprims (Huovinen 2001; Levings et al. 2006) (2 isolates); adenyltransferase aadAB (3 isolates); QacE delta1 multidrug exporter associated with multidrug resistance to antiseptics and disinfectants (Paulsen et al. 1993) (2 isolates); quinolone resistance pentapeptide repeat protein qnr (1 isolate); and metallo-β-lactamase genes bla VIM-4-like, bla IMP-19-like, and bla IMP-26-like that are associated with resistance to carbapenems (Kim et al. 2013; Libisch et al. 2004; Yamamoto et al. 2011) (1 isolate each). Gene cassettes were not found in 75 % (36/48) of the integron-positive isolates. Two isolates (PA1107 and S20) with the ant(4′)-IIb gene cassette were also resistant to amikacin while another isolate (AC1107) with a similar cassette exhibited intermediate resistance to amikacin, Additional file 1: Table S1. Except for isolates S20 (A. baumannii) and J851 (P. aeruginosa), all the detected gene cassettes occurred in carbapenem-susceptible isolates, Additional file 1: Table S1.
Other genes detected in integrons were the putative glucose dehydrogenase precursor and hypothetical genes common to A. baumannii class 1 integrons (3 isolates) and non-ribosomal peptide synthetase (pyoverdine sidechain peptide synthetase) (1 isolate) in P. aeruginosa, Additional file 1: Table S1.
Discussion
In this study, we have described carbapenem-resistant P. aeruginosa and A. baumannii isolated from hospitalized patients and the environment at Mulago Hospital in Kampala, Uganda. As species identification is highly desirable to allow proper interpretation of the results (SMI P 8 2014), the isolates were successfully identified to species level using a rigorous methodology. While the isolates from hospitalized patients were from clinically relevant specimens referred to the diagnostic laboratory for culture and sensitivity testing, we could not rule-out colonization implying that some of the isolates might have been not clinically relevant, given the high rate of colonization on various body parts particularly by Acinetobacter. The purpose of this study was to detect carbapenem resistance and while important, confirmation of the isolates as infectious was beyond the scope of this study.
The prevalence of carbapenem resistant strains was relatively low among hospitalized patients; however, it was comparatively high in isolates particularly in the environment. This is of concern as the hospital environment is known to be a potential source of infection for hospitalized patients and health care workers. Furthermore, most isolates in this study were multidrug resistant with rates (i.e. 65 % overall; 73 % hospitalized patients, 54 % environment) comparable to those reported by Pitout et al. in Kenya (Pitout et al. 2008), Lee et al. in Korea (Lee et al. 2007) and Gu et al. in China (Gu et al. 2007), but lower than rates reported from Latin America (Labarca et al. 2016) and India (Uma Karthika et al. 2009). This could be due to differences in antibiotic usage between Uganda and the countries where these studies were done (Manikal et al. 2000).
It is important to note that carbapenemases are not the only mechanisms of acquired resistance to carbapenems; other resistance mechanisms in P. aeruginosa include upregulated efflux pumps and loss of the outer-membrane protein encoding gene oprD (SMI P 8 2014). As such, when screening for carbapenemases, two confounders have to be ruled-out (SMI P 8 2014); (a) not all carbapenem-resistant isolates produce a carbapenemase, (b) not all carbapenemase producers are resistant to carbapenems. In this study we did not screen for carbapenemases in carbapenem-susceptible isolates with disc inhibition zone diameters of >25 mm. Furthermore, while the automated systems efficiently detect carbapenem resistance, the inbuilt software in these systems is not always accurate at correctly inferring the presence of carbapenemases. The Phoenix BD expert system that we used is noted for its high sensitivity (ability to detect carbapenem resistance) but on the other hand, low specificity (ability to distinguish true carbapenemase producers) (SMI P 8 2014; Woodford et al. 2010). Hence, carbapenem-resistant isolates in this study were screened for carbapenemase production with the MHT and imipenem/EDTA test that have been effectively used to validate carbapenemase producers. These tests also can distinguish carbepenem-resistance mediated by carbapenemases from the one mediated by other mechanisms (SMI P 8 2014; Asthana et al. 2014). Our data showed that carbapeneme-resistance in this study particularly in P. aeruginosa, was mainly mediated by metallo-β-lactamases. Nevertheless, the reported low rates of carbapenemase activity (particularly with MHT) among carbapenem-resistant isolates could be due to the fact that the detection of carbapenemase activity in clinical isolates is challenging (Queenan and Bush 2007); the MHT assay suffers from low sensitivity, and interpretation of its results can be subjective (i.e. the identification of the clover-leaf indentation).
Molecular tests have been reliably used to confirm the presence of carbapenemase genes (SMI P 8 2014; Asthana et al. 2014). In this study, carbapenem resistance correlated well with carbapenemase gene detection; that is, 72 % (18/25) and 13 % (2/15) of carbapenem-resistant P. aeruginosa and Acinetobacter respectively, possessed metallo-β-lacatamase genes while all (15/15) carbapenem-resistant Acinetobacter carried OXA-carbapenemases. However, three carbapenem-resistant Acinetobacter isolates (A081-7, J093 and J044-2) carried only bla OXA-51–like gene intrinsic to A. baumannii and it likely does not confer carbapenem resistance unless it is up-regulated (SMI P 8 2014). Overall, organisms producing OXA-23, OXA-24, IMP, and VIM, the most prevalent carbapenemases in many settings (Manenzhe et al. 2015; Pitout et al. 2008; Amudhan et al. 2011), also appear to be the circulating A. baumannii and P. aeruginosa strains at Mulago Hospital. These enzymes also were the most prevalent among Enterobacteriaceae isolated from patients at Mulago (Okoche et al. 2015). Although only one isolate carried the bla NDM-1-like gene, its detection is of concern as NDM-1 producing bacteria are rapidly spreading worldwide (Manenzhe et al. 2015).
Furthermore, carbapenem resistant isolates (both P. aeruginosa and A. baumannii with carbapenemase genes), which lacked carbapenemase activity were also detected. As outlined above, this might reflect the difficulty in detecting carbapenemase production particularly in Acinetobacter (SMI P 8 2014); OXA-carbapenemases often have poor enzymatic activity leading to sub-optimal activity in some strains. Furthermore, carbapenem resistant isolates without carbapenemase genes and carbapenemase activity detected in this study alludes to the occurrence of additional non-carbapenemase resistance mechanisms in these isolates (outlined earlier) particularly in P. aeruginosa [e.g. upregulated efflux pumps, oprD loss (SMI P 8 2014)]. Non-carbapenemase mediated mechanisms need further study as we did not extensively characterize them in this study.
Integrons carry novel metallo-β-lactamase genes (Liakopoulos et al. 2013); in this study three isolates carried gene cassettes encoding VIM-4, IMP-19 and IMP-26 metallo-β-lactamases. Also, the other cassettes and genes identified in this study were previously confirmed to be associated with class 1 integrons (Levesque et al. 1995; Sabtcheva et al. 2003; Huovinen 2001; Levings et al. 2006).
Conclusions
The prevalence of carbapenem resistant P. aeruginosa and A. baumannii is relatively low among hospitalized patients at Mulago Hospital. In the 2 year study period we detected only 40 carbapenem-resistant isolates combined. However, carbapenem-resistance prevalence is comparatively high in isolates and in the environment. The detection of carbapenem-resistant organisms in the environment is of concern, as the hospital environment is a potential source of infection for patients and health care workers. VIM-1, IMP-1, IMP-2, SPM and NDM-1 producing P. aeruginosa and OXA-23, OXA-58 and VIM-1 producing A. baumannii are the carbapenemase-producing strains circulating at Mulago Hospital.
Abbreviations
- ant(4′)-IIb :
-
aminoglycoside adenylyltransferase gene
- AST:
-
antimicrobial Susceptibility Testing
- ATCC:
-
American type culture collection
- BLAST:
-
basic local alignment search tool
- Carbapenemases (Queenan and Bush 2007):
-
SME (for “Serratia marcescens enzyme”); IMI (for “imipenem-hydrolyzing β-lactamase”); NMC (for “not metalloenzyme carbapenemase”); GES (for “guiana extended spectrum”); KPC (for “Klebsiella pneumoniae carbapenemase”); VIM (for “verona integron-encoded metallo-β-lactamase”); IMP (for “active on imipenem”); SPM (for “Sao Paulo metallo-β-lactamase”); GIM (for “German imipenemase”); SIM (for “Seoul imipenemase”); and NDM (for “New Delhi metallo-β-lactamase”); OXA (for “oxacillin-hydrolyzing”)
- CLSI:
-
Clinical and Laboratory Standards Institute
- CTAB:
-
cetyltrimethyl ammonium bromide
- dfrA :
-
trimethoprim-resistant dihydrofolate reductase gene
- EDTA:
-
ethylenediammine tetra acetic acid
- ESBLs:
-
extended spectrum beta-lactamases
- ID:
-
identification
- ICU:
-
intensive care unit
- MHA:
-
Mueller–Hinton Agar
- MHT:
-
modified Hodge test
- NCBI:
-
National Center for Biotechnology Information
- PCR:
-
polymerase chain reaction
- SIM:
-
sulphur indole and motility
- TE:
-
Tris–EDTA buffer
- TSI:
-
triple sugar iron
References
Amudhan SM, Sekar U, Arunagiri K, Sekar B (2011) OXA beta-lactamase-mediated carbapenem resistance in Acinetobacter baumannii. Indian J Med Microbiol 29(3):269–274
Andreou LV (2013) Preparation of genomic DNA from bacteria. Methods Enzymol 529:143–151
Asthana S, Mathur P, Tak V (2014) Detection of carbapenemase production in Gram-negative bacteria. J Lab Physicians 6(2):69–75
Broberg CA, Palacios M, Miller VL (2013) Whole-genome draft sequences of three multidrug-resistant Klebsiella pneumoniae strains available from the American type culture collection. Genome Announc 1(3). doi:10.1128/genomeA.00312-13
Carroll KC, Glanz BD, Borek AP, Burger C, Bhally HS, Henciak S, Flayhart D (2006) Evaluation of the BD Phoenix automated microbiology system for identification and antimicrobial susceptibility testing of Enterobacteriaceae. J Clin Microbiol 44(10):3506–3509
Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A (2005) Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis 5:24
Fallah F, Borhan RS, Hashemi A (2013) Detection of bla(IMP) and bla(VIM) metallo-beta-lactamases genes among Pseudomonas aeruginosa strains. Int J Burns Trauma 3(2):122–124
Fallah F, Noori M, Hashemi A, Goudarzi H, Karimi A, Erfanimanesh S, Alimehr S (2014) Prevalence of bla NDM, bla PER, bla VEB, bla IMP, and bla VIM genes among Acinetobacter baumannii isolated from two hospitals of Tehran, Iran. Scientifica 2014:245162
Fischer J, Rodriguez I, Schmoger S, Friese A, Roesler U, Helmuth R, Guerra B (2012) Escherichia coli producing VIM-1 carbapenemase isolated on a pig farm. J Antimicrob Chemother 67(7):1793–1795
Giamarellos-Bourboulis EJ, Sambatakou H, Galani I, Giamarellou H (2003) In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa. J Chemother 15(3):235–238
Gootz TD, Marra A (2008) Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev Anti Infect Ther 6(3):309–325
Gu B, Tong M, Zhao W, Liu G, Ning M, Pan S (2007) Prevalence and characterization of class I integrons among Pseudomonas aeruginosa and Acinetobacter baumannii isolates from patients in Nanjing, China. J Clin Microbiol 45(1):241–243
Huovinen P (2001) Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis 32(11):1608–1614
Kim MJ, Bae IK, Jeong SH, Kim SH, Song JH, Choi JY, Yoon SS, Thamlikitkul V, Hsueh P-R, Yasin RM et al (2013) Dissemination of metallo-β-lactamase-producing Pseudomonas aeruginosa of sequence type 235 in Asian countries. J Antimicrob Chemother 68(12):2820–2824
Labarca JA, Salles MJ, Seas C, Guzman-Blanco M (2016) Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbiol 42(2):276–292
Lee YC, Ahn BJ, Jin JS, Kim JU, Lee SH, Song DY, Lee WK, Lee JC (2007) Molecular characterization of Pseudomonas aeruginosa isolates resistant to all antimicrobial agents, but susceptible to colistin, in Daegu, Korea. J Microbiol 45(4):358–363
Levesque C, Piche L, Larose C, Roy PH (1995) PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother 39(1):185–191
Levings RS, Lightfoot D, Elbourne LD, Djordjevic SP, Hall RM (2006) New integron-associated gene cassette encoding a trimethoprim-resistant DfrB-type dihydrofolate reductase. Antimicrob Agents Chemother 50(8):2863–2865
Liakopoulos A, Mavroidi A, Katsifas EA, Theodosiou A, Karagouni AD, Miriagou V, Petinaki E (2013) Carbapenemase-producing Pseudomonas aeruginosa from central Greece: molecular epidemiology and genetic analysis of class I integrons. BMC Infect Dis 13:505
Libisch B, Gacs M, Csiszár K, Muzslay M, Rókusz L, Füzi M (2004) Isolation of an integron-borne bla(VIM-4) type metallo-β-lactamase gene from a carbapenem-resistant Pseudomonas aeruginosa clinical isolate in Hungary. Antimicrob Agents Chemother 48(9):3576–3578
Ma Z, Zhou L, Wang H, Luo L (2015) Investigation on the genomic diversity of OXA from isolated Acinetobacter baumannii. Int J Clin Exp Med 8(3):4429–4432
Manenzhe RI, Zar HJ, Nicol MP, Kaba M (2015) The spread of carbapenemase-producing bacteria in Africa: a systematic review. J Antimicrob Chemother 70(1):23–40
Manikal VM, Landman D, Saurina G, Oydna E, Lal H, Quale J (2000) Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis 31(1):101–106
Miriagou V, Cornaglia G, Edelstein M, Galani I, Giske CG, Gniadkowski M, Malamou-Lada E, Martinez-Martinez L, Navarro F, Nordmann P et al (2010) Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin Microbiol Infect 16(2):112–122
Mushi MF, Mshana SE, Imirzalioglu C, Bwanga F (2014) Carbapenemase genes among multidrug resistant gram negative clinical isolates from a tertiary hospital in Mwanza, Tanzania. BioMed Res Int 2014:303104
Okoche D, Asiimwe BB, Katabazi FA, Kato L, Najjuka CF (2015) Prevalence and characterization of carbapenem-resistant Enterobacteriaceae isolated from Mulago National Referral Hospital, Uganda. PLoS ONE 10(8):e0135745
Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA (2011) Carbapenems: past, present, and future. Antimicrob Agents Chemother 55(11):4943–4960
Paulsen IT, Littlejohn TG, Rådström P, Sundström L, Sköld O, Swedberg G, Skurray RA (1993) The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother 37(4):761–768
Pitout JD, Gregson DB, Poirel L, McClure JA, Le P, Church DL (2005) Detection of Pseudomonas aeruginosa producing metallo-beta-lactamases in a large centralized laboratory. J Clin Microbiol 43(7):3129–3135
Pitout JD, Revathi G, Chow BL, Kabera B, Kariuki S, Nordmann P, Poirel L (2008) Metallo-beta-lactamase-producing Pseudomonas aeruginosa isolated from a large tertiary centre in Kenya. Clin Microbiol Infect 14(8):755–759
Queenan AM, Bush K (2007) Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20(3):440–458 (table of contents)
Sabtcheva S, Galimand M, Gerbaud G, Courvalin P, Lambert T (2003) Aminoglycoside resistance gene ant(4′)-IIb of Pseudomonas aeruginosa BM4492, a clinical isolate from Bulgaria. Antimicrob Agents Chemother 47(5):1584–1588
Seni J, Najjuka CF, Kateete DP, Makobore P, Joloba ML, Kajumbula H, Kapesa A, Bwanga F (2013) Antimicrobial resistance in hospitalized surgical patients: a silently emerging public health concern in Uganda. BMC Res Notes 6:298
SMI P 8: Laboratory detection and reporting of bacteria with carbapenem-hydrolysing beta-lactamases (carbapenemases) (2014). https://www.gov.uk/government/publications/smi-p-8-laboratory-detection-and-reporting-of-bacteria-with-carbapenem-hydrolysing-beta-lactamases-carbapenemases
Tam VH, Chang KT, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, Weston JS, Caeiro JP, Garey KW (2010) Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 54(3):1160–1164
Tenover FC (2006) Mechanisms of antimicrobial resistance in bacteria. Am J Med 119(6 Suppl 1):S3–S10 (discussion S62–S70)
Tsakris A, Ikonomidis A, Pournaras S, Tzouvelekis LS, Sofianou D, Legakis NJ, Maniatis AN (2006) VIM-1 metallo-beta-lactamase in Acinetobacter baumannii. Emerg Infect Dis 12(6):981–983
Turton JF, Kaufmann ME, Glover J, Coelho JM, Warner M, Pike R, Pitt TL (2005) Detection and typing of integrons in epidemic strains of Acinetobacter baumannii found in the United Kingdom. J Clin Microbiol 43(7):3074–3082
Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL (2006) Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol 44(8):2974–2976
Uma Karthika R, Srinivasa Rao R, Sahoo S, Shashikala P, Kanungo R, Jayachandran S, Prashanth K (2009) Phenotypic and genotypic assays for detecting the prevalence of metallo-beta-lactamases in clinical isolates of Acinetobacter baumannii from a South Indian tertiary care hospital. J Med Microbiol 58(Pt 4):430–435
Wikler MA (2006) Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement, vol 26. Clinical and Laboratory Standards Institute, Wayne, PA
William S, Feil H (2012) Bacterial genomic DNA isolation using CTAB. Sigma 50:6876
Woodford N, Eastaway AT, Ford M, Leanord A, Keane C, Quayle RM, Steer JA, Zhang J, Livermore DM (2010) Comparison of BD Phoenix, Vitek 2, and MicroScan automated systems for detection and inference of mechanisms responsible for carbapenem resistance in Enterobacteriaceae. J Clin Microbiol 48(8):2999–3002
Yamamoto M, Nagao M, Matsumura Y, Matsushima A, Ito Y, Takakura S, Ichiyama S (2011) Interspecies dissemination of a novel class 1 integron carrying blaIMP-19 among Acinetobacter species in Japan. J Antimicrob Chemother 66(11):2480–2483
Authors’ contributions
DPK and CFN conceived and designed the study; they also supervised the molecular assays, performed data analysis and drafted the manuscript. Sequence analysis was performed by DPK. RN performed the microbiology and molecular assays in partial fulfilment of the requirements for the award of the Degree of Master of Science in Molecular Biology of Makerere University (under supervision of CFN and JE). JN participated in culture and drug sensitivity testing. MLJ provided the laboratory supplies. All authors read and approved the final manuscript.
Acknowledgements
We thank Ms. Martha F. Mushi and Professor Stephen E. Mshana of the Department of Microbiology, Catholic University of Health and Allied Sciences, Mwanza, Tanzania, for providing the control strains for carbapenemase PCRs. We also thank Mr. Moses Okee and Mr. Alfred Okeng for assistance in performing the molecular assays.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article (and its additional file).
Ethics approval and consent to participate
This study was nested within previous studies at Mulago Hospital (Seni et al. 2013) that were approved by the Ethics Committees of the School of Medicine, Makerere University, and the Mulago Hospital Research Ethics Committee. The informed consent procedure (or assent for minors) was approved by the same committee, including the future use of the samples or isolates.
Funding
There was no specific funding for this study. The Phoenix 100 ID/AST BDexpert system was purchased with funds from previous research support from the Swedish International Development Cooperation (Sida) and the Directorate of Research and Graduate Training, Makerere University. The Molecular Biology and Clinical Microbiology laboratories of the Department of Medical Microbiology where the research was performed were previously supported by the National Institutes of Health (Grant #s RO3 AI062849 and RO1 AI075637). DNA sequencing costs were met by DPK.
Author information
Authors and Affiliations
Corresponding author
Additional file
40064_2016_2986_MOESM1_ESM.xlsx
Additional file 1: Table S1. Isolates, drug susceptibility profiles, carbapenemase genes, class 1 integrons and gene cassettes found.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kateete, D.P., Nakanjako, R., Namugenyi, J. et al. Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago Hospital in Kampala, Uganda (2007–2009). SpringerPlus 5, 1308 (2016). https://doi.org/10.1186/s40064-016-2986-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2986-7