Abstract
Background and purpose
Peginterferon Lambda was being developed as an alternative to alfa interferon for the treatment of chronic hepatitis C virus (HCV) infection. We compared peginterferon Lambda-1a plus ribavirin (Lambda/RBV) and Lambda/RBV plus daclatasvir (DCV; pangenotypic NS5A inhibitor) with peginterferon alfa-2a plus RBV (alfa/RBV) in treatment-naive patients with HCV genotype 2 or 3 infection.
Methods
In this multicenter, double-blind, phase 3 randomized controlled trial, patients were assigned 2:2:1 to receive 24 weeks of Lambda/RBV, 12 weeks of Lambda/RBV + DCV, or 24 weeks of alfa/RBV. The primary outcome measure was sustained virologic response at post-treatment Week 12 (SVR12).
Results
Overall, 874 patients were treated: Lambda/RBV, n = 353; Lambda/RBV + DCV, n = 349; alfa/RBV, n = 172. Patients were 65 % white and 33 % Asian, 57 % male, with a mean age of 47 years; 52 % were infected with genotype 2 (6 % cirrhotic) and 48 % with genotype 3 (9 % cirrhotic). In the Lambda/RBV + DCV group, 83 % (95 % confidence interval [CI] 78.5, 86.5) achieved SVR12 (90 % genotype 2, 75 % genotype 3) whereas SVR12 was achieved by 68 % (95 % CI 63.1, 72.9) with Lambda/RBV (72 % genotype 2, 64 % genotype 3) and 73 % (95 % CI 66.6, 79.9) with peginterferon alfa/RBV (74 % genotype 2, 73 % genotype 3). Lambda/RBV + DCV was associated with lower incidences of flu-like symptoms, hematological abnormalities, and discontinuations due to adverse events compared with alfa/RBV.
Conclusion
The 12-week regimen of Lambda/RBV + DCV was superior to peginterferon alfa/RBV in the combined population of treatment-naive patients with genotype 2 or 3 infection, with an improved tolerability and safety profile compared with alfa/RBV.
Similar content being viewed by others
Background
Chronic hepatitis C virus (HCV) infection affects up to 170 million people worldwide based on serologic data (Lavanchy 2009), resulting in approximately 500,000 deaths each year (World Health Organization 2012). HCV comprises 7 major genotypes and 67 subtypes; genotypes 1, 2, and 3 are the most widely distributed and the most studied therapeutically (Smith et al. 2014). Genotype 2 is prevalent in South America and Asia, whereas genotype 3 is common in Europe (European Association for the Study of the Liver 2014), the United States, Australia, and southern Asia (Ansaldi et al. 2014).
Previously, HCV genotype 2 or 3 infection was treated primarily with 24 weeks of peginterferon alfa-2a plus ribavirin (alfa/RBV). Alfa/RBV therapy is subject to a number of limitations; among these are frequent, sometimes treatment-limiting adverse events (AEs) including hemolytic anemia and other cytopenias (Sulkowski et al. 2011). Genotype 3 infection has been associated with poorer outcomes than genotype 2, including a higher incidence of steatosis (Matos et al. 2006), accelerated fibrosis (Bochud et al. 2009; Probst et al. 2011), increased risk of hepatocellular cancer, and lower sustained virologic response (SVR) rates following treatment with alfa/RBV or oral direct-acting antiviral (DAA)-based regimens (Zeuzem et al. 2004; Andriulli et al. 2008).
Oral DAA regimens are now replacing interferon-based treatment for chronic HCV infection; however, DAA options for genotypes 2 and 3 are more restricted than those for genotype 1. Currently approved regimens for genotype 2-infected patients include sofosbuvir (SOF) plus RBV and peginterferon plus RBV in the US and EU, and SOF plus velpatasvir (VEL) in the US. Approved regimens for genotype 3-infected patients include combinations of SOF plus RBV, SOF plus daclatasvir (DCV) with or without RBV, SOF plus peginterferon and RBV, and peginterferon plus RBV; in addition, SOF plus VEL was recently approved in the US (Bristol Myers Squbb Pharmaceuticals Ltd 2014; Bristol-Myers Squibb 2016). DCV + SOF with or without RBV is a preferred option for treating HCV genotype 3-infected patients according to guidelines issued by the American Association for the Study of Liver Disease and the European Association for the Study of the Liver (European Association for the Study of the Liver 2014; AASLD/IDSA HCV Guidance Panel 2015).
Peginterferon lambda-1a (Lambda) is a pegylated Type III interferon with HCV antiviral activity similar to that of alfa interferons; however, Lambda utilizes a unique receptor with more restricted tissue distribution than the alfa receptor (Andersen et al. 2013). In the phase 2 EMERGE study, similar SVR rates were achieved with Lambda/RBV and alfa/RBV in previously untreated genotype 1–4 infection. However, Lambda/RBV exhibited improved tolerability characterized by fewer musculoskeletal and influenza-like events (Muir et al. 2014) and a better hematologic profile, including amelioration of RBV-associated anemia through compensatory erythropoiesis (Everson et al. 2011).
Daclatasvir (DCV) is an NS5A replication complex inhibitor DAA with in vitro activity and clinical data against HCV genotypes 1–6 (Hézode et al. 2016; Welzel et al. 2016; Sulkowski et al. 2014; Gao 2013; Poordad et al. 2016; Nelson et al. 2015; Wyles et al. 2015). DCV has been approved in the US, Europe, Japan, and multiple countries across the Americas, Middle East, and Asia Pacific region. We report the results of a phase 3 study of Lambda/RBV, with and without DCV, versus alfa/RBV in previously untreated patients with genotype 2 or 3 infection.
Methods
Study design
This randomized, double-blind, multinational phase 3 study (ClinicalTrials.gov identifier NCT01616524) enrolled treatment-naive patients with genotype 2 or 3 infection from 124 clinical centers in 18 countries in Europe (Belgium, Finland, France, Greece, Italy, Netherlands, United Kingdom), Asia (Japan, Korea, Singapore, Hong Kong, Taiwan), and South America (Argentina, Mexico), plus the USA, Russia, Australia, and New Zealand between 20 July 2012 and 15 August 2013. Eligible patients were randomly assigned 2:2:1 to treatment with either (a) 24 weeks of Lambda/RBV (first 12 weeks with a DCV placebo); (b) 12 weeks of Lambda/RBV plus DCV 60 mg once daily, or (c) 24 weeks of alfa/RBV (first 12 weeks with a DCV placebo). Patients receiving 24 or 12 weeks of therapy were followed up for 24 or 48 weeks post-treatment, respectively. Both peginterferons were self-administered at a dose of 180 μg once weekly by subcutaneous injection. RBV 400 mg was taken orally twice daily with food. DCV 60 mg was taken orally once daily, with or without food.
Randomization was via an interactive voice response system designated by the study sponsor. Randomization was stratified by baseline HCV RNA (<800,000 IU/mL or ≥800,000 IU/mL), cirrhosis status, region (Japan vs the rest of the world), and HCV genotype. Study enrolment was capped by genotype (neither genotype 2 nor 3 could comprise more than 60 % of total enrollment), cirrhosis status (no more than 20 % of patients with compensated cirrhosis in any treatment arm), and region (Japanese patients limited to approximately 70 overall).
Ethics, consent and permissions
This study was conducted in accordance with Good Clinical Practice guidelines and in accordance with the ethical principles originating in the Declaration of Helsinki. All patients provided written informed consent. The study protocol and all relevant documents were approved by the appropriate independent ethics committee or institutional review board for each participating site prior to initiation.
Patients
Eligible patients were men and women aged ≥18 years with a body mass index between 18 and 35 kg/m2 at screening. HCV RNA levels were required to be ≥100,000 IU/mL, based on reports of lower SVR rates with alfa/RBV therapy in patients with high viral load, particularly those with genotype 3 infection, suggesting a greater medical need for improved therapies for this patient population (Andriulli et al. 2008; Mecenate et al. 2010). Cirrhosis status was determined by liver biopsy or FibroScan. For liver biopsies, absence of cirrhosis (Ishak fibrosis score ≤4 or Metavir score ≤3) was documented within 3 years prior to study enrollment, while biopsies demonstrating cirrhosis (Ishak score ≥5 or Metavir score = 4) could be from any time prior to enrollment. FibroScan results (cirrhosis defined as ≥14.6 kPa) were required within 1 year of enrollment. Women of child-bearing potential and men with female partners of child-bearing potential were required to use highly effective contraception throughout treatment. Major exclusion criteria included coinfection with hepatitis B virus or human immunodeficiency virus, or a history of hepatocellular carcinoma, decompensated liver disease, or any chronic liver disease other than HCV. Laboratory exclusion criteria included hemoglobin ≤12 g/dL for women and ≤13 g/dL for men, platelets <90 × 106/L, absolute neutrophil count ≤1.5 × 109/L, albumin ≤35 g/L, creatinine clearance ≤50 mL/min, total bilirubin ≥2 mg/dL or >1.8 × the upper limit of normal [ULN for cirrhotics (unless Gilbert’s disease was present)], alanine aminotransferase (ALT) ≥5 × ULN, and electrocardiographic abnormality (QTcF >500 ms).
Endpoints and assessments
The primary endpoint was sustained virologic response at post-treatment Week 12 (SVR12) in the combined genotype 2 and 3 patient population, defined as a plasma HCV RNA measurement below the assay lower limit of quantitation (LLOQ; 25 IU/mL) target detected or not detected. Secondary efficacy endpoints included the proportion of patients with undetectable HCV RNA at Week 4 (rapid virologic response; RVR), Weeks 4 and 12 (extended rapid virologic response on treatment; eRVR), Week 12 (complete early virologic response; cEVR), end-of-treatment response (EOTR), and the proportion of genotype 3-infected patients with SVR12.
Virologic failure was defined as either on-treatment virologic breakthrough or post-treatment relapse. Breakthrough was defined as a confirmed increase in HCV RNA >1 log10 IU/mL above nadir or a confirmed HCV RNA level ≥LLOQ after being <LLOQ. Relapse was defined as HCV RNA ≥LLOQ post-treatment following an EOTR. Virologic futility criteria for early discontinuation of study drug were failure to achieve ≥2 log10 IU/mL reduction from baseline at treatment Week 12 with either peginterferon without DCV, and virologic breakthrough in patients receiving Lambda/RBV with DCV.
Safety endpoints included proportions of patients with AEs, serious AEs, dose reductions and discontinuations for AEs, proportions of patients with treatment-emergent cytopenic abnormalities (hemoglobin <10 g/dL, absolute neutrophils <0.75 × 109/L, platelets <50 × 109/L), proportions of patients with on-treatment interferon-related flu-like symptoms (pyrexia, chills, pain), musculoskeletal symptoms (myalgia, arthralgia, back pain), or constitutional symptoms (fatigue, asthenia), and treatment-emergent laboratory abnormalities.
HCV genotype was assessed using the VERSANT HCV genotype 2.0 assay (LiPA; Siemens, Washington, DC, USA) and HCV RNA using the COBAS® AmpliPrep/COBAS® TaqMan® HCV Test version 2.0 (Roche, Pleasanton, CA, USA). HCV RNA measurements were taken at screening, baseline, on-treatment Weeks 1, 2, 4, 8, 12, 16, 20, and 24 (as applicable), and post-treatment Weeks 4, 12, 24, 36, and 48 (as applicable). Population-based sequencing of the HCV NS5A region derived from plasma samples from all patients at baseline, and from patients experiencing virologic failure was performed when HCV RNA was ≥1000 IU/mL.
Statistical analysis
Efficacy analyses were based on a modified intention-to-treat approach (mITT) including all patients who received at least one dose of study medication. Observed values based on all patients with HCV RNA data at the relevant time point were also derived. Treatment comparisons were conducted for each of the two Lambda-containing arms versus the alfa/RBV arm, each at a significance level of 0.025. For the primary endpoint of SVR12 in the combined genotype 2 and genotype 3 patient population, treatment comparisons of both the Lambda arms versus the alfa arm were conducted as two-stage evaluations where superiority was tested only if non-inferiority was first established. The treatment difference in SVR12 and the associated two-sided 97.5 % confidence interval (CI) were estimated using a Mantel–Haenszel approach stratified by the randomization strata. Non-inferiority was inferred if the lower bound of the 97.5 % CI for the treatment difference exceeded −10 %. Superiority was inferred subsequently if the comparison showed non-inferiority and the lower bound of the 97.5 % CI exceeded 0 %. Because superiority was not evaluated unless non-inferiority was established, no adjustment to the significance level was made.
Target enrollment was 875 patients, randomly assigned in a 2:2:1 distribution of 350 in each Lambda arm and 175 in the alfa/RBV comparator arm. Assuming a −10 % non-inferiority margin and response rates of 80 % for alfa/RBV and 82 % for both Lambda/RBV and Lambda/RBV + DCV, these sample sizes were predicted to give 90 % power to demonstrate the non-inferiority of Lambda to alfa for each comparison (SVR12). Assuming an 80 % response on alfa/RBV and a 92 % response in each Lambda arm, and also assuming a two-sided type I error of 0.025, the above sample sizes were also predicted to give 90 % power to demonstrate superiority of Lambda to alfa for each comparison. A multivariate logistic regression analysis was performed to evaluate the effects of baseline factors on SVR12 rates in patients treated with the DCV-containing regimen. In the logistic regression, the dependent variable was SVR12 status (yes or no) and the independent variables (covariates) were the baseline factors. The probability of achieving SVR12 based on those covariates was estimated using a logistic function, which is the cumulative logistic distribution.
Results
Patient disposition and baseline characteristics
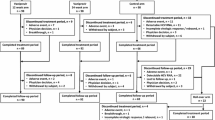
A total of 1243 patients were screened; 880 were randomized and 874 treated (Fig. 1). Most of the patients screened but not randomized (324/363; 89 %) did not meet study inclusion criteria. Six patients were randomized but not treated; one withdrew consent, one was lost to follow-up, one was a screening failure randomized in error, and three were found not to meet study criteria for drug/alcohol use or other significant protocol deviations.
Cirrhotic patients comprised 7 % (65/874) of the total population (Table 1), with 77 % of diagnoses (50/65) made using FibroScan. Baseline characteristics were generally balanced between treatment arms and between HCV genotype groups, although there was a slightly higher incidence of cirrhosis among patients with genotype 3 (9 %) than genotype 2 (6 %), and a smaller proportion of patients with genotype 3 (46 %) than genotype 2 (60 %) had an IL28B CC genotype (rs12979860).
Efficacy
SVR12 was achieved by 83 % (95 % CI 78.5, 86.5) in the Lambda/RBV + DCV group, 68 % (95 % CI 63.1, 72.9) in the Lambda/RBV group, and 73 % (95 % CI 66.6, 79.9) in the alfa/RBV group. In the primary analysis, Lambda/RBV + DCV demonstrated superiority to alfa/RBV, with a treatment difference of 9 % and a 97.5 % CI (0.3, 17.6). In contrast, SVR12 for Lambda/RBV versus alfa/RBV did not meet prespecified non-inferiority criteria, with a treatment difference of −6 % and a 97.5 % CI (−14.9, 3.4) whose lower limit was not above −10 % (Table 2). SVR12 rates were lower in patients with cirrhosis than in those without cirrhosis in all three treatment arms. SVR12 rates in patients with cirrhosis versus those without cirrhosis were 48 % versus 69 % for Lambda/RBV, 65 % versus 84 % for Lambda/RBV + DCV, and 57 % versus 74 % for alfa/RBV.
In subgroup analyses, there were no notable effects on Lambda/RBV versus alfa/RBV treatment differences with respect to gender, race (white or Asian), Hispanic/Latino ethnicity, HCV genotype, IL28B genotype, geographic region, or cirrhosis status (Fig. 2a). The treatment difference slightly favored alfa/RBV for patients with a body mass index ≥30 kg/m2 (97.5 % CI did not cross zero), although patient numbers were relatively small (Lambda/RBV, n = 54; alfa/RBV, n = 21). Treatment differences favored Lambda/RBV + DCV over alfa/RBV (97.5 % CI did not cross zero) in patients >65 years old and in Hispanic patients, although patient numbers were low for both comparisons (Fig. 2b). Lambda/RBV + DCV was also favored in patients with HCV genotype 2, patients with IL28B non-CC genotypes, non-cirrhotic patients, and patients in Asia (Fig. 2).
Treatment differences and 97.5 % confidence intervals by subgroup. a Subgroup analysis of the Lambda/RBV versus alfa/RBV treatment difference and 97.5 % confidence intervals. b Subgroup analysis of the Lambda/RBV + DCV versus alfa/RBV treatment difference and 97.5 % confidence intervals. alfa peginterferon alfa-2a, BMI body mass index, DCV daclatasvir, GT genotype, Lambda peginterferon Lambda-1a, RBV ribavirin
Genotype 2
Both Lambda arms tended to have higher SVR12 rates among patients infected with genotype 2 compared with genotype 3 (Table 2). Among genotype 2-infected patients, SVR12 was achieved by 90 % in the Lambda/RBV + DCV group, 72 % in the Lambda/RBV group, and 74 % in the alfa/RBV group. One additional patient receiving Lambda/RBV + DCV had HCV RNA <LLOQ TD at week 12 and was therefore defined as a treatment failure per the study protocol; however, HCV RNA was <LLOQ TND at all post-treatment visits. The 12-week regimen of Lambda/RBV + DCV achieved non-inferiority to the 24-week regimen of alfa/RBV for SVR12 in patients infected with genotype 2, with a treatment difference of 16 % and 97.5 % CI (4.2, 27.2), whose lower limit was above −10 %. In patients with genotype 2 infection, SVR12 rates were similar in patients with or without cirrhosis: 73 % versus 71 % (Lambda/RBV), 92 % versus 89 % (Lambda/RBV + DCV), and 67 % versus 74 % (alfa/RBV). Further assessment of patients treated with Lambda/RBV + DCV, based on multivariate logistic regression analysis, found no significant effect of patient baseline factors on SVR12 rates in patients with HCV genotype 2.
Genotype 3
Among genotype 3-infected patients, SVR12 was achieved by 75 % in the Lambda/RBV + DCV group, 64 % in the Lambda/RBV group, and 73 % in the alfa/RBV group. The 12-week regimen of Lambda/RBV + DCV did not achieve non-inferiority to the 24-week regimen of alfa/RBV for SVR12 in patients infected with genotype 3. The failure of Lambda/RBV + DCV to achieve non-inferiority for SVR12 in genotype 3 infection was driven by a higher rate of post-treatment relapse in genotype 3 (19 %) than genotype 2 (6 %). As noted earlier, SVR12 rates were higher among patients without cirrhosis in all treatment arms; this difference was driven primarily by SVR12 rates in patients with genotype 3 infection, among whom SVR12 rates for those with or without cirrhosis were 29 % versus 67 % (Lambda/RBV), 43 % versus 78 % (Lambda/RBV + DCV), and 50 % versus 75 % (alfa/RBV). In addition, there were more patients with cirrhosis among the genotype 3-infected group. Multivariate logistic regression analysis found both cirrhosis and the baseline NS5A-Y93H polymorphism to be negative predictors of SVR12 among genotype 3-infected patients when treated for 12 weeks with Lambda/RBV + DCV.
Resistance
Genotype 2
Baseline NS5A sequences were available for 173/184 genotype 2-infected patients who were treated with Lambda/RBV + DCV (Additional file 1: Table S1a). Among these 173 patients, 17 were reported as not achieving SVR12. Three of these 17 non-SVR12 patients received ≤4 weeks of treatment due to death, withdrawn consent, or loss to follow-up while one patient actually achieved SVR (described above). All 17 non-SVR12 patients had baseline NS5A polymorphisms at positions F28 and/or L31. However, most patients with these common polymorphisms achieved SVR12. SVR12 was achieved by 89 % (57/64) and 91 % (99/109) of patients with or without baseline F28 polymorphisms, respectively, and by 88 % (103/117) and 95 % (53/56) of patients with or without baseline L31 polymorphisms, respectively. Thirteen genotype 2-infected patients with virologic failure had NS5A sequence data at both baseline and failure (Additional file 1: Table S2a). At failure, NS5A resistance-associated variants (RAVs) were detected in these 13 patients at positions F28 (1/13), L31 (5/13), or F28 and L31 (7/13). Treatment-emergent NS5A RAVs were present in 46 % (6/13) of patients at F28 (n = 4) or L31 (n = 2).
Genotype 3
Baseline NS5A sequences were available for all 165 patients with genotype 3 infection who were treated with Lambda/RBV + DCV (Additional file 1: Table S1b). SVR12 was achieved by 63 % (24/38) of patients with NS5A polymorphisms at M28, A30, L31, and/or Y93, and by 78 % (99/127) of patients without polymorphisms at any of these positions. SVR12 rates in patients with or without baseline NS5A polymorphisms at M28 or A30 were comparable. SVR12 was achieved by 50 % (2/4) and 75 % (121/161) of patients with or without M28 polymorphisms, respectively, and by 72 % (18/25) or 75 % (105/140) of patients with or without A30 polymorphisms, respectively. SVR12 rates in patients without baseline NS5A-Y93H (77 %, 119/154) were higher than in patients with this polymorphism (36 %, 4/11). Two patients had L31 polymorphisms at baseline; both achieved SVR12. NS5A sequencing data were available at both baseline and failure for 38/42 genotype 3-infected patients with virologic failure (Additional file 1: Table S2b). NS5A-Y93H was the most frequent RAV detected in this group, including 26/38 patients (68 %) with treatment-emergent Y93H and 7/38 (18 %) with Y93H at both baseline and failure. Emergent M28A, A30K, and L31F were present in 1/38, 1/38, and 1/38 patients, respectively.
Safety
There were no unexpected AE signals; observed events were consistent with known alfa/RBV toxicities and with previous clinical study data for Lambda/RBV (Table 3). The incidence of all-grade AEs ranged from 86 % to 97 % in the three treatment arms. Dose reductions of both interferon and RBV were less common in the two Lambda arms than in the alfa/RBV arm. Almost all dose reductions in alfa/RBV recipients were due to AEs, whereas the majority of reductions in Lambda recipients were due to elevated liver function tests.
Grade 3–4 laboratory abnormalities were more common in the alfa/RBV arm, due primarily to cytopenias. Patients receiving Lambda/RBV had numerically fewer cytopenic abnormalities, flu-like and musculoskeletal events than those receiving alfa/RBV. Patients receiving Lambda/RBV + DCV had significantly fewer cytopenic abnormalities than patients receiving alfa/RBV (3 % vs 36 %; p < 0.0001), and numerically fewer flu-like and musculoskeletal events. RBV dose reduction was most common in the alfa/RBV arm (19 %) whereas the Lambda/RBV + DCV arm had the lowest rate of RBV dose reduction (3 %). Grade 3–4 anemia was observed in 5 % of the alfa/RBV group versus <1 % in the Lambda/RBV + DCV group. Grade 3–4 bilirubin elevations were observed in 8 % of the Lambda/RBV group and in 3 % of the Lambda/RBV + DCV group with no grade 3–4 elevations in the alfa/RBV group. Grade 3–4 ALT elevations were observed in 6 % of the Lambda/RBV group, 4 % of the Lambda/RBV/DCV group, and 3 % of the alfa/RBV group. Overall, the 12-week Lambda/RBV + DCV regimen appeared better tolerated than either of the 24-week arms (Table 3) with the lowest proportion of grade 3–4 AEs, discontinuations for AEs, and RBV dose reductions.
Three hepatic decompensation events were reported. A 63-year-old female with cirrhosis and a history of esophageal varices experienced hepatic decompensation during treatment with Lambda/RBV + DCV, and was discontinued from study drug on study Day 35. This patient later died of renal failure and septic shock at post-treatment Week 4. A 58-year-old male with cirrhosis experienced hepatic failure leading to discontinuation of Lambda/RBV on Day 91; the event was considered resolved on Day 115. A 56-year-old male experienced decompensated cirrhosis with elevated total bilirubin, encephalopathy, and ascites leading to discontinuation of Lambda/RBV + DCV therapy on Day 64. The bilirubin elevation improved progressively post-treatment and was considered resolved on day 162. In addition to these patients, a 40-year-old male with a history of fatty liver and Gilbert’s syndrome experienced ALT and aspartate aminotransferase (AST) increases leading to interruption of Lambda/RBV treatment on Day 56; treatment at a reduced dose resumed on Day 78. The patient completed blinded therapy but subsequent extended therapy with Lambda/RBV was discontinued on Day 146 after the events worsened to grade 4. ALT and AST levels were normal on Day 189.
Discussion
This phase 3, randomized, double-blind study compared the efficacy and safety of Lambda/RBV, with and without DCV, with that of alfa/RBV in treatment-naive patients with genotype 2 or 3 infection. In a direct comparison of interferon/RBV treatment over 24 weeks, the endpoint of non-inferiority of Lambda/RBV versus alfa/RBV was not met for the primary endpoint of SVR12. This finding was unexpected, since phase 2 clinical data for genotypes 2 and 3 from the dose-ranging EMERGE study (Muir et al. 2014) showed a numerically higher SVR12 rate with Lambda (180 μg) plus RBV than with alfa/RBV after 24 weeks of treatment (76 vs 57 %). However, whereas the proportions of genotype 3 to genotype 2 in the Lambda and alfa arms of the EMERGE study (41–50 %) were similar to this study, patient numbers (29–30 patients per arm) were much lower.
In contrast, 12 weeks of triple therapy with Lambda/RBV and DCV was superior to 24 weeks of alfa/RBV for the primary endpoint, and also superior for early on-treatment (RVR) response. High SVR12 rates were achieved in patients with genotype 2 infection, with no significant impact of baseline demographic or disease characteristics, including cirrhosis status. However, the 12-week regimen was found to be less efficacious among patients with genotype 3, primarily due to a higher rate of relapse. This finding is similar to that noted with 12 weeks of sofosbuvir with RBV; extension of that regimen to 24 weeks resulted in a significantly higher SVR rate (Zeuzem et al. 2014). Genotype 3-infected patients with cirrhosis had substantially lower responses than those without cirrhosis; this difference was significant in multivariate analysis. The difference was larger in the Lambda arms than in the alfa/RBV arm. Treatment differences related to cirrhosis status in genotype 3 infection, together with the shorter duration of treatment with Lambda/RBV + DCV, may have contributed to the higher relapse rate for Lambda/RBV + DCV in genotype 3. Prolonging therapy for 24 weeks may potentially increase SVR12 rates in genotype 3-infected patients and reduce the impact of baseline factors; however, this has not been studied.
Baseline NS5A polymorphisms associated with DCV resistance in patients treated with Lambda/RBV + DCV had a greater impact in genotype 3 infection than in genotype 2. Most patients with genotype 2 infection had baseline polymorphisms at F28 and/or L31; however, differences in SVR12 rates in patients with or without these polymorphisms at baseline were modest. Baseline NS5A polymorphisms associated with DCV resistance were less common in patients with genotype 3 infection. However, 36 % of patients with Y93H at baseline achieved SVR12, compared with 77 % of patients without this baseline polymorphism. Moreover, Y93H was present post-failure in 86 % of genotype 3-infected patients who experienced virologic failure. Multivariate logistic regression analysis identified the presence of Y93H as having a significant adverse impact on SVR12 among genotype 3-infected patients. In follow-up studies, persistence of NS5A resistance variants was variable in patients who failed therapy with DCV-based regimens (Reddy et al. 2014). Partial or complete replacement of NS5A variants was observed in some patients after 1–2 years of post-treatment follow-up; however, the potential relevance of these findings to retreatment strategies has not yet been established.
The safety profiles of Lambda/RBV and Lambda/RBV + DCV in this study were consistent with previous studies of Lambda/RBV (Muir et al. 2014; Vierling et al. 2013). The lower incidences of hematologic, musculoskeletal, and flu-like symptoms and lower rates of dose adjustment for Lambda-based regimens versus alfa-2a that were observed are consistent with the more restricted extrahepatic distribution of the Type III receptor and its virtual absence from bone marrow progenitors and peripheral blood cells (Andersen et al. 2013). There were no unexpected safety findings. The proportions of grade 3–4 aminotransferase and bilirubin elevations over 24 weeks on Lambda/RBV in this study were similar to those seen over 24 weeks in the EMERGE genotype 2 and 3 population among patients receiving 120 or 180 μg of Lambda. Similarly, the proportions of grade 3–4 aminotransferase and bilirubin elevations over 12 weeks of Lambda/RBV + DCV (≈3.5 and ≈3 %, respectively) were comparable to those observed with the same regimen over 24 weeks in the phase 2 D-LITE study (5 and 3 %, respectively; Muir et al. 2014; Vierling et al. 2013). Hepatic decompensation occurred in 3 patients, all with prior evidence of portal hypertension. Similar events have been reported in previous studies using alfa interferons (Hezode et al. 2013).
In conclusion, the results of this large multinational phase 3 study in treatment-naive HCV genotype 2 and 3 infection demonstrated that 12 weeks of treatment with Lambda/RBV and DCV gave a superior SVR12 rate compared with 24 weeks of alfa/RBV in the combined population of patients with genotype 2 or 3 infection. However, the regimen was less effective among genotype 3-infected patients with cirrhosis. The shorter, Lambda-containing regimen also demonstrated better tolerability than alfa/RBV and a lower incidence of interferon and/or RBV dose adjustments, consistent with phase 2 clinical data for Lambda/RBV with and without DCV. Despite the encouraging efficacy and safety outcomes of the Lambda/RBV + DCV regimen in this study, further development of this regimen was discontinued due to the emergence of highly efficacious and well-tolerated all-oral DAA combination regimens. However, data from the current study remain relevant in the context of understanding the clinical profile of DCV, which has been widely approved as a component of all-oral HCV regimens.
Abbreviations
- HCV:
-
hepatitis C virus
- Alfa:
-
peginterferon alfa-2a
- RBV:
-
ribavirin
- AE:
-
adverse event
- SVR:
-
sustained virologic response
- DAA:
-
direct-acting antiviral
- Lambda:
-
peginterferon lambda-1a
- DCV:
-
daclatasvir
- ULN:
-
upper limit of normal
- ALT:
-
alanine aminotransferase
- SVR12:
-
sustained virologic response at post-treatment Week 12
- LLOQ:
-
lower limit of quantitation
- RVR:
-
rapid virologic response
- eRVR:
-
extended rapid virologic response
- cEVR:
-
complete early virologic response
- EOTR:
-
end-of-treatment response
- mITT:
-
modified intention-to-treat
- CI:
-
confidence interval
- RAV:
-
resistance-associated variant
- AST:
-
aspartate aminotransferase
References
AASLD/IDSA HCV Guidance Panel (2015) Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62(3):932–954
Andersen H, Meyer J, Freeman J, Doyle SE, Klucher K, Miller DM et al (2013) Peginterferon lambda-1a, a new therapeutic for hepatitis C infection, from bench to clinic. J Clin Transl Hepatol 1(2):116–124. doi:10.14218/JCTH.2013.00014
Andriulli A, Mangia A, Iacobellis A, Ippolito A, Leandro G, Zeuzem S (2008) Meta-analysis: the outcome of anti-viral therapy in HCV genotype 2 and genotype 3 infected patients with chronic hepatitis. Aliment Pharmacol Ther 28(4):397–404
Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G (2014) Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol 20(29):9633–9652
Bochud PY, Cai T, Overbeck K, Bochud M, Dufour JF, Mullhaupt B et al (2009) Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 51(4):655–666
Bristol Myers Squbb Pharmaceuticals Ltd (2014) Daklinza (daclatasvir) summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/003768/WC500172848.pdf. Accessed 27 June 2016
Bristol-Myers Squibb (2016) Daklinza (daclatasvir) prescribing information. http://packageinserts.bms.com/pi/pi_daklinza.pdf. Accessed 27 June 2016
European Association for the Study of the Liver (2014) EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 60(2):392–420
Everson G, Gray T, Hillson JL, Horga A, Xu D, Fontana D et al (2011) Pegylated interferon lambda ameliorates ribavirin (RBV)-induced anemia in HCV patients by maintaining compensatory erythropoiesis: analysis of EMERGE phase 2b results through week 12 [Abstract]. Hepatology 54(4 suppl):993A–994A
Gao M (2013) Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol 3(5):514–520
Hezode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V et al (2013) Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC): NCT01514890. J Hepatol 59(3):434–441
Hézode C, Abergel A, Chas J, Conti F, Cotte L, Tateo M, et al. (2016) Sustained virologic response to daclatasvir and sofosbuvir, with or without ribavirin, among patients in the French daclatasvir ATU programme infected with HCV genotypes 4, 5, and 6 [abstract]. The International Liver Congress, April 13–17, Barcelona, Spain
Lavanchy D (2009) The global burden of hepatitis C. Liver Int 29(Suppl 1):74–81
Matos CA, Perez RM, Pacheco MS, Figueiredo-Mendes CG, Lopes-Neto E, Oliveira EB Jr et al (2006) Steatosis in chronic hepatitis C: relationship to the virus and host risk factors. J Gastroenterol Hepatol 21(8):1236–1239
Mecenate F, Pellicelli AM, Barbaro G, Romano M, Barlattani A, Mazzoni E et al (2010) Short versus standard treatment with pegylated interferon alfa-2A plus ribavirin in patients with hepatitis C virus genotype 2 or 3: the cleo trial. BMC Gastroenterol 10(1):1
Muir AJ, Arora S, Everson G, Flisiak R, George J, Ghalib R et al (2014) A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J Hepatol 23(61):1238–1246
Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N et al (2015) All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61(4):1127–1135
Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R et al (2016) Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplant recurrence. Hepatology 63(5):1493–1505
Probst A, Dang T, Bochud M, Egger M, Negro F, Bochud PY (2011) Role of hepatitis C virus genotype 3 in liver fibrosis progression: a systematic review and meta-analysis. J Viral Hepat 18(11):745–759
Reddy KR, Pol S, Thuluvath PJ, Dumada H, Toyota J, Chayama K et al (2014) Long-term follow-up of patients treated with daclatasvir-based regimens in phase 2 and 3 studies [abstract]. Hepatology 60:1154A–1155A
Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT et al (2014) Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment Web resource. Hepatology 59(1):318–327
Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M et al (2011) Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol 8(4):212–223
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I et al (2014) Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 370(3):211–221
Vierling JM, Lataillade M, Gane E, Lueth S, Serfaty L, Taliani G et al (2013) Sustained Virologic Response (SVR12) in HCV Genotype 1 Patients Receiving Peginterferon Lambda in Combination With Ribavirin and Either Daclatasvir or Asunaprevir: interim Results From the D-LITE Study [Abstract 2607]. Hepatol Int 7(suppl 1):S438–S439
Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Cornberg M, et al (2016) Daclatasvir plus sofosbuvir with or without ribavirin for treatment of chronic HCV infection in patients wtih advanced liver disease: results of a European compassionate use programme. The International Liver Congress. April 13–17, Barcelona, Spain
World Health Organization (2012) Prevention and control of viral hepatitis infection: framework for global action. http://www.who/int/csr/disease/hepatitis/GHP_framework.pdf. Accessed 27 June 2016
Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR et al (2015) Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 373(8):714–725
Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J et al (2004) Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol 40(6):993–999
Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH et al (2014) Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 370(21):1993–2001
Authors’ contributions
GRF, KC, WLC, HF, MF, AG, GBG, CH, YI, JH, HK, SNL, PM, CM, SR, SS, AT, JT, SWP, JMV, and ALZ participated in study design, data acquisition, analysis and interpretation, and manuscript preparation and critical review. SS, HM, and SP participated in study concept and design, administration, technical support, and manuscript preparation and critical review. DC participated in study design, conducted statistical analyses, and reviewed the manuscript. FM participated in technical support, analysis of resistance data, and manuscript critical review. MH and MWR participated in study data acquisition and manuscript critical review. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Hiroki Ishikawa for supporting the study execution. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Competing interests
GRF served in advisory committees or review panels for Bristol-Myers Squibb, AbbVie, GlaxoSmithKline, Novartis, Boehringer Ingelheim, Tibotec, Chughai, Gilead, Janssen, Idenix, Roche, Gilead, Merck, and Janssen; received Grant/Research Support from Chughai, Roche; and served as a speaker for Bristol-Myers Squibb, AbbVie, Roche, Gilead, Tibotec, Merck, Boehringer Ingelheim, and Janssen. KC served as a consultant for AbbVie; received Grant/Research Support from Bristol-Myers Squibb, Dainippon Sumitomo, Chugai, Mitsubishi Tanabe, Daiichi Sankyo, Toray, and MSD; and served as a speaker for Bristol-Myers Squibb, Chugai, Mitsubishi Tanabe, Daiichi Sankyo, KYORIN, Nihon Medi-Physics, Dainippon Sumitomo, MSD, ASKA, Astellas, AstraZeneca, Eisai, Olympus, GlaxoSmithKline, ZERIA, Bayer, Minophagen, Janssen, Jimro, Tsumura, Otsuka, Taiho, Nippon Kayaku, Nippon, Shinyaku, Takeda, Ajinomoto, Meiji Seika, and Toray. WLC served in advisory committees or review panels for Gilead, Roche, and Novartis; and served as a speaker for Bristol-Myers Squibb. HF served as a speaker for Bristol-Myers Squibb. AG served in advisory committees or review panels of Bristol-Myers Squibb; served as a speaker for Bristol-Myers Squibb; and has participated in Clinical Trials. GBG served in the advisory committees or review panels of Janssen, Merck, AbbVie, and Roche; and served as a speaker for Bristol-Myers Squibb and Gilead. SKR served on the advisory boards of Bristol-Myers Squibb, Janssen, Roche, Gilead, and AbbVie; and served as a speaker for Roche, BMS, and Janssen. SIS served in advisory committees or review panels of Bristol-Myers Squibb, Janssen, AbbVie, Roche Products Australia, MSD, Gilead, Norgine, and Bayer Healthcare; and served as a speaker for Bristol-Myers Squibb, Bayer Healthcare, MSD, Roche Products Australia, Gilead, and Janssen. AJT is supported by an NHMRC Fellowship; served as a consultant for Bristol-Myers Squibb, Merck, Roche, AbbVie, Gilead Sciences, Janssen, and Spring Bank Pharmaceuticals; received Grant/Research Support from Bristol-Myers Squibb, Merck, Gilead Sciences, and AbbVie; and served as a speaker for Bristol-Myers Squibb, Gilead, AbbVie, and Merck. CH served as a speaker for Bristol-Myers Squibb, Roche, MSD, Janssen, AbbVie, and Gilead. JH served in advisory committees for Bristol-Myers Squibb, AbbVie, Gilead, and Janssen; received Grant/Research Support from Roche; and served as a speaker for Bristol-Myers Squibb. HK served as a speaker for Bristol-Myers Squibb and Pharma International. SNL served in advisory committees for Bristol-Myers Squibb, and Abbvie; and served as a speaker for Bayer. PM served as a consultant for Bristol-Myers Squibb, Roche, Gilead, Vertex, Novartis, Janssen-Tibotec, MSD, Boehringer, Pfizer, Abbott, and Alios BioPharma; received Grant/Research Support from Bristol-Myers Squibb, Roche, Gilead, Novartis, Janssen-Tibotec, MSD, and Alios BioPharma; and served as a speaker for Bristol-Myers Squibb, Roche, Gilead, Vertex, Novartis, Janssen-Tibotec, MSD, and Abbott. CM served as a consultant for Bristol-Myers Squibb, AbbVie, Gilead, and MSD; received Grant/Research Support from Roche, Janssen, Novartis, Astellas, MSD, and Gilead; and served as a speaker for Bristol-Myers Squibb, MSD, and Janssen, Abbvie, Novartis, Promethera and Gilead. JMV served in advisory committees or review panels of Bristol-Myers Squibb, AbbVie, Gilead, Hyperion, Intercept, Janssen, Novartis, Merck, Sundise, HepQuant, and Salix; received Grant/Research Support from Bristol-Myers Squibb, AbbVie, Eisai, Gilead, Hyperion, Intercept, Janssen, Novartis, Merck, Sundise, Ocera, and Mochida; and served as a speaker for GALA, Chronic Liver Disease Foundation, and ViralEd. JT served as a speaker for Bristol-Myers Squibb, MSD. SWP received Grant/Research Support from Bristol-Myers Squibb, AbbVie, Merck, Gilead, Janssen, Roche, Boehringer Ingelheim and Norvatis. DC and SP were employees of Bristol-Myers Squibb during the study. MWR, FM, SS, MH, and HM are employees of Bristol-Myers Squibb. YI, MF, and ALZ declare that they have no competing interests.
Funding
This study was funded by Bristol-Myers Squibb.
Writing assistance
Writing assistance was provided by Satarupa Sen, Ph.D., of Articulate Science, and was funded by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Additional file
40064_2016_2920_MOESM1_ESM.pdf
Additional file 1. Table S1. Baseline NS5A polymorphisms at 28, 30, 31, and/or 93 in HCV genotype 2-infected (Table 1a) and genotype 3-infected (Table 1b) patients who received DCV-containing regimen. Table S2. Newly emergent NS5A substitutions in virologic failures with HCV genotype 2 (Table 2a) or genotype 3 (Table 2b).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Foster, G.R., Chayama, K., Chuang, WL. et al. A randomized, controlled study of peginterferon lambda-1a/ribavirin ± daclatasvir for hepatitis C virus genotype 2 or 3. SpringerPlus 5, 1365 (2016). https://doi.org/10.1186/s40064-016-2920-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2920-z