Abstract
An incubation study was conducted to test the effects of phosphorus (P) addition on nitrous oxide (N2O) emissions from the soils taken from two tropical rain forests established on different parent materials [meta-sedimentary (MS) and ultrabasic (UB) rock] on Mt. Kinabalu, Borneo. Earlier studies suggest that the forest on UB soils is more strongly limited by P than that on MS soils is. In MS soils, P addition significantly reduced N2O emissions. Since neither ammonium (NH4 +) nor nitrate (NO3 −) contents were reduced by P addition, we assumed that the decrease in N2O emissions were not due to the previously-reported mechanism: P addition stimulated microbial nitrogen (N) immobilization and collateral inorganic N consumption, reducing resources for producing N2O. Since P addition enhanced the ratios of microbial biomass to CO2 and N2O emissions (indicators of nitrifying and/or denitrifying respiratory efficiency), it was suggested that the N required for the respiration of nitrifying and/or denitrifying bacteria was reduced, leading to reduced N2O emissions. On the other hand, P addition had no effects on N2O emissions in UB soils. The respiratory efficiency did not change significantly by P addition, possibly because the microbial community in the highly-P-depleted UB soils shifted by P addition, with which the enhancement of respiration efficiency did not co-vary. We concluded that (1) P addition may control N2O emissions through increasing respiratory efficiency, and (2) the effects may be different depending on the differences in P availability.
Similar content being viewed by others
Background
Nitrous oxide (N2O) is the third most important global warming gas (IPCC 2007) and also the most important ozone-depleting gas (Ravishankara et al. 2009). Soils are one of the major sources for N2O, which is a by-product or an intermediate of microbial nitrification and denitrification, respectively (Firestone and Davidson 1989; Wrage et al. 2001; Ishizuka et al. 2002; Keller et al. 2005). Various factors are suggested to control N2O emission including direct factors such as the availability of soil inorganic nitrogen (N) (Firestone and Davidson 1989; Davidson and Verchot 2000; Arai et al. 2008; Liu and Greaver 2009) and organic carbon (C) (Nobre et al. 2001), soil temperature (Cavelier et al. 2000; Dobbie and Smith 2001; Schindlbacher and Zechmeister-Boltenstern 2004), moisture (Klemedtsson et al. 1988; Firestone and Davidson 1989; Davidson and Verchot 2000; Erickson et al. 2001; Konda et al. 2010), bulk density (Sitaula et al. 2000), and pH (Kesik et al. 2006; Baggs et al. 2010), and indirect factors such as land use (Ishizuka et al. 2002), land use history (Van Lent et al. 2015), vegetation (Erickson et al. 2001; Konda et al. 2008), and soil parent material (Hall et al. 2004).
In tropical forest ecosystems, which account for 14–23 % of the current N2O budget (IPCC 2007), phosphorus (P) availability may be another important factor controlling N2O emissions. Phosphorus is generally believed to be the main limiting factor in tropical forest ecosystems on Ultisoils and Oxisols due to the low P supply from highly weathered soil and relatively high N input (Walker and Syers 1976; Elser et al. 2007; Vitousek et al. 2010). Microbial activity including nitrification or denitrification is also suggested to be limited by P availability (Minami and Fukushi 1983; Kitayama et al. 1997, 1998; Cleveland et al. 2002; Ilstedt et al. 2003; Kitayama et al. 2004; Ilstedt and Singh 2005; Cleveland and Townsend 2006; Mori et al. 2010b, 2013a; He and Dijkstra 2015).
Recently several studies reported that P application reduced N2O emissions. They suggested that added-P stimulated plant N uptake and reduced N resources for N2O production (Mori et al. 2013b; Baral et al. 2014; Zhang et al. 2014; Chen et al. 2015). This idea was experimentally confirmed by Mori et al., demonstrating that P addition reduced N2O emissions from Acacia mangium plantation sites with roots, while conversely stimulated the emissions from root-excluded sites (Mori et al. 2014).
Contrasting with the observations in the field with vegetation, results regarding how P controls the microbial activity (without the interference of vegetation) and accompanying N2O emissions are not consistent. Hall and Matson (1999) observed that N addition to P-limited forest soils generated 10–100 times higher N2O fluxes than to N-limited forest soils. They also demonstrated that the 15N-labeled inorganic N added to the N-limited soils readily became a microbial form, while that added to the P-limited soils largely remained as inorganic form. From these results, they suggested that P shortage in tropical soils restricts microbial N immobilization, which supplies more N sources for nitrification and/or denitrification, stimulating N2O emissions (Hall and Matson 1999). Sundareshwar et al. (2003) demonstrated that N2O emissions from sediments from a coastal salt marsh in South Carolina decreased by P addition because of an increase in N immobilization and a subsequent decrease in denitrification (Sundareshwar et al. 2003). On the other hand, Mori et al. reported that P addition stimulated N2O emissions both from nitrification and denitrification (Mori et al. 2010a, 2013c). They attributed the results to the following mechanisms: (1) P addition directly stimulated nitrifying and/or denitrifying activities; and (2) P addition stimulated heterotrophic CO2 consumption and promoted a more reductive condition, which produces more N2O emissions.
Thus, so far, it is not clear how P controls soil microbial activities and accompanying N2O emissions in P-limited tropical forest soils. Especially, the reason why N2O emissions respond differently to P addition (or P shortage) among studies is unknown. In the present paper we hypothesized that P addition affects N2O emissions differently depending on the strength of P shortage. Long term ecological study sites in Mt. Kinabalu (Kitayama and Aiba 2002) is an ideal sites for testing this hypothesis, because the sites consist of two types of study sites on two different soils with different P availability (Kitayama et al. 2004; Kitayama 2013). We conducted an incubation experiment using soils taken from two primary tropical rain forests established on different parent materials [meta-sedimentary (MS) and ultrabasic (UB) rock] on a lower eastern slope of Mt. Kinabalu, Sabah, Malaysia. Earlier studies suggest that the forest on UB soils is more strongly limited by P than that on MS soils is (Kitayama and Aiba 2002).
Methods
Field location and soil sampling
The study field is located on the lower eastern slope of Mt. Kinabalu (4095 m, 6°05′N, 160°33′E) within Kinabalu Park, Sabah, Malaysia. We selected a pair of lowland dipterocarp forests established at the same altitude (700 m) with the same rock age (Tertiary) but with contrasting parent materials of MS and UB rocks (Table 1). Both sites are intact primary rain forests with no prior land use history and have similar basal areas and stem densities (Table 1). The climate is aseasonal in monthly temperature with a mean annual temperature of approximately 23.8 °C and precipitation ranging from 2300 to 2500 mm year−1 (Aiba and Kitayama 1999). The studied site is a subset of the long-term ecological study described in Kitayama and Aiba (2002). Selected site characteristics are shown in Table 1.
At each site, we laid five transects (40 m) and took 20 soil cores (0–15 cm) at 2 m intervals from each transect using a stainless soil corer (3.4 cm diameter and 60 cm long). The cored soils were immediately taken back to the laboratory and kept under 4 °C after composited across the 20 soil cores by each transect (yielding a total of five composites) and put through a 2 mm sieve. After sieving, soil bulk density became lower (0.51 and 0.46 in UB soil and MS soil, respectively) than field condition (see Table 1), which may have influenced the microbial activities and gas emissions to some extent. Bray-1 P content in each composited sample was determined (data shown in Table 1) by extracting P after shaking 1 g air-dry soil and 7 ml Bray-1 solution for 1 min (Kuo 1996).
Incubation
Twenty g fresh soil was placed in a 223 ml wide mouth jar for a gas emission analysis, 5 g in a 50 ml plastic bottle for analyzing inorganic N, dissolved organic C (DOC) and dissolved N (DN), and 5 g in a 50 ml glass bottle for a soil microbial biomass analysis. We prepared two subsamples for each analysis, one for P addition and the other for control. We added P as KH2PO4 solution (100 μg P g soil−1, dissolved in distilled water) to each soil so that soil water condition became equivalent to 80 % water holding capacity (WHC). Controls were prepared without P addition in the same manner. The samples were incubated at 25 °C in the dark for 48 h. In the present study, our purpose was not measuring the precise fluxes of the gases, but comparing gas emissions between P-added soil and non-added control, not missing the emission peaks. Thus we chose to measure gas emissions by closing lids for 48 h. Although gas concentration may have not increased linearly and the differences among treatment may have been underestimated, we considered it more important not to miss the emission peaks. Previous incubation studies showed that N2O emissions declined to low level at 1–3 days (Mori et al. 2010a, 2013c). Wide mouth jars were closed with butyl rubber stoppers equipped with sampling ports, and gas samples were taken 0 and 48 h after the closure of the stoppers. N2O, NO and CO2 emissions were measured by calculating the changes of the gas concentrations during the incubation period. The N2O concentration in the gas sample was analyzed using a gas chromatograph (GC-14B, SHIMADZU, Kyoto, Japan) equipped with an electron capture detector. The column, injector, and detector temperatures were kept at 60, 80 and 330 °C, respectively. Argon containing 5 % CH4 was used as a carrier gas. The NO concentration was analyzed with a NO–NO2–NOx Analyzer (Model 42i, Nippon Thermo Co. Ltd., Kyoto, Japan). The CO2 concentration was analyzed with a gas chromatograph (GC-14B, SHIMADZU, Kyoto, Japan) equipped with a thermal conductivity detector, using He as a carrier gas. The column, injector and detector temperatures were kept at 60, 60 and 100 °C, respectively.
Inorganic N (NH4 + and NO3 −), DOC and DN were extracted at the end of the incubation by shaking 5 g soil with 50 ml 0.5 M K2SO4 extractant for 30 min. The supernatants were filtered and refrigerated until the analysis. Ammonium was determined by indophenol blue absorptiometry and NO3 − by naphthyl ethylenediamine method using a flow injection analyzer (AQLA-700-NO, AQUA LAB, Japan). DOC and DN were analyzed by a total organic carbon analyzer with a total organic nitrogen measurement unit (TOC-VE/TNM-1, SHIMADZU, Kyoto, Japan). Soil microbial biomass C (MBC) and N (MBN) were determined by a chloroform fumigation extraction method (Vance and Jenkinson 1987). Five g fresh soils were exposed to CHCl3 vapor for 24 h in a vacuum desiccator at 25 °C after 48-h incubation. After residual CHCl3 was removed, fumigated soils were shaken with 50 ml of 0.5 M K2SO4 extractant for 30 min and soluble C and N were extracted. Equivalent portions of unfumigated soils were also extracted. Soluble C and N were analyzed on a total organic carbon analyzer with a total organic nitrogen measurement unit (TOC-VE/TNM-1, SHIMADZU, Kyoto, Japan). Soil microbial biomass element contents were calculated from the differences between the fumigated and unfumigated samples using a conversion factor of 0.45 (Jenkinson et al. 2004). Since measuring the real C use efficiency or respiratory efficiency was technically difficult (Sinsabaugh et al. 2013), we used the ratio of MBC to CO2 or N2O (an inverse of respiratory quotient) as indicators of microbial respiratory efficiency. A number of studies have used respiratory quotient as an indicator of microbial C use efficiency (Giller et al. 1998; Priess and Fölster 2001; Schipper and Lee 2004).
Statistical analysis
Statistical analyses were performed by Excel 2010 with Statcel 3 (OMS Ltd.) (ex. Ohyagi-Hara et al. 2013; Shigenobu et al. 2014; Mori et al. 2015) or Excel 2013 with statistical add-in software (Social Survey Research Information Co., Ltd.) (ex. Mori et al. 2016). The level of significance was examined by a paired t test after confirming the normality of data. Some data sets not normally distributing were log-transformed prior to statistical analysis. For some data sets not following the normal or log-normal distribution, we adopted non-parametric Mann–Whitney’s U test. Correlation coefficient was obtained sing simple regression analysis.
Results
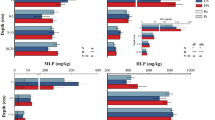
In MS soils, P addition significantly reduced N2O emissions (Fig. 1), while it did not change NO or CO2 emissions. On the other hand, P addition stimulated CO2 emissions in UB soils, but had no significant effects on N2O and NO emissions (Fig. 1). N2O emissions from UB soils showed higher values than MS soils did, with high variability. We could not observe NO emissions (under detectable level) in UB soils. A higher bulk density (0.51 and 0.46 in UB soil and MS soil, respectively) and a higher clay content (Table 1) in UB soils than MS soils probably provided a more reductive condition in the UB soils, causing a higher N2O/NO ratio (Fig. 1). P addition did not change NH4 +, NO3 −, DOC or DN contents at the end of the incubation period (Table 2). Soil microbial biomass (MBC and MBN) tended to increase by P addition in MS soils (P = 0.09 and 0.07 in MBC and MBN, respectively), but not in UB soils (Table 3). P addition increased respiratory efficiency (i.e. the ratio of MBC to CO2 or N2O) significantly in MS soils, but not in UB soils (Table 3). The inconsistence was due to the differences in the relationship between MBC/CO2 with and without P addition (Fig. 2a). In MS soils, MBC/CO2 ratio was consistently higher in P-added soils than controls in MS. On the other hand, in UB soils, P addition stimulated MBC/CO2 ratio when the intact soil (control) was low in MBC/CO2 ratio (lower than 30), and in contrast reduced MBC/CO2 ratio when the intact soil (control) was high in MBC/CO2 ratio (greater than 30). In the UB soils, higher initial respiratory efficiency (MBC/CO2 ratio) was associated with lower soil P availability (Bray-1 P content) with significant correlations (P = 0.01), but the trend was not significant in MS soils (Fig. 2b). The differences of the MBC/CO2 ratio between control and P added soils (ΔMBC/CO2) were correlated well with Bray-1 P contents, especially in UB soils, with larger ΔMBC/CO2 in soils with higher P availability (Fig. 2c).
a Effects of P addition on the ratio of soil microbial biomass C (MBC) to CO2 emissions. b Relationship between soil Bray-1 P content and the MBC/CO2 ratio in the control soils. c Relationship between soil Bray-1 P contents and the differences of the MBC/CO2 ratios between control and P added soils (ΔMBC/CO2). MS meta-sediment rock soil, UB ultrabasic rock soil. Because of the experimental failure about CO2 measurement analysis in P-added soil, we report only 4 of 5 replications in MS in a, c
Discussion
In MS soils, P addition significantly reduced N2O emissions (Fig. 1), which is in contradiction to the accounts by Mori et al. (2010a, 2013c). They reported that P addition stimulated N2O emissions both from nitrification and denitrification, possibly because of the following two mechanisms: (1) P addition directly activated nitrifying and/or denitrifying bacteria; (2) P addition stimulated O2 consumption by heterotrophic activities and created a more reduced condition, which is suitable for denitrifying bacteria and stimulates denitrification. In our study, however, we could observe neither the rise in inorganic N contents as a result of activated nitrification (Table 2) nor microbial respiration as a result of stimulated O2 consumption (Fig. 1c) in MS soils.
Decrease in N2O emissions by P addition in MS soils could have been due to the stimulated microbial N immobilization and collateral inorganic N consumption, which reduced the resources for producing N2O. Hall and Matson (1999, 2003) demonstrated that N addition in a P-limited forest resulted in a 10–100 times greater amount of N2O than that from an N-limited forest did. They suggested that P shortage restricted microbial N immobilization and that the surplus N was used by nitrifying and/or denitrifying bacteria, boosting N2O emission. According to their hypothesis, P addition will reduce N2O emissions because P addition will alleviate the limitation of microbial N immobilization process and subsequently reduce both inorganic N pool and the activities of nitrification and/or denitrification. Sundareshwar et al. (2003) experimentally demonstrated that P addition reduced N2O emissions from coastal marsh soils in California via increasing microbial N immobilization and reducing denitrifying activity. In fact our data showed that P addition tended to increase MBN in MS soils (P = 0.07). However, we assume that the decrease in N2O emissions by P addition was not caused by the increase in MBN, because the same amount of N resources were available for nitrification and/or denitrification in the P added soils and the control without P addition [see that no significant reductions in NH4 + and NO3 − contents were observed in P added soils (Table 2)], although substantial amount of increase in MBN may reduce N2O emissions in a longer period.
Instead, we attributed the decrease in N2O emissions by P addition to the improvement of respiratory efficiency (both nitrifying and denitrifying respiration). It is well known that nutrient shortage drives microbes to require more energy to maintain their activities and causes a lower efficiency of respiration (Schimel 2003; López-Urrutia and Morán 2007; Sinsabaugh et al. 2013); nutrient supply could increase respiratory efficiency conversely. Since N2O is a by-product and an intermediate of nitrifying and denitrifying respiration, respectively (Davidson and Verchot 2000), P addition may increase microbial biomass per N2O emission as well as that per CO2 emission through improving the respiratory efficiency (López-Urrutia and Morán 2007). In fact, both MBC/CO2 ratio and MBC/N2O ratio significantly increased by P addition (Table 3) in MS soil, suggesting that the increase in nitrifying and/or denitrifying respiratory efficiency may be a reason for the suppressed N2O emissions by P addition in MS soils. We assumed that P addition mainly improved the efficiency of denitrifying respiration, because water condition was adjusted to a relatively high value of WHC 80 % in the present study, where the contribution of nitrification to N2O emissions must have been lower compared with that of denitrification (Davidson and Verchot 2000).
As we hypothesized, effects of P addition on N2O emissions differed in UB soils from in MS soils. In UB soils, we could not observe any differences in N2O emissions between control and P-added soils. Neither MBC/CO2 nor MBC/N2O changed significantly by P addition. We could not explain about this phenomena from our data. But one assumption is as follows. In UB soils, where the ecosystem processes were more-severely limited by P availability than in MS soils (Kitayama and Aiba 2002), P addition might have changed the microbial community (Li et al. 2010; Liu et al. 2012) from a “high P use efficiency but low growth rate (highly adapted to low P condition) community” to a “lower P use efficiency but higher growth rate (less adapted to low P condition) community” (here we need to admit we did not analyze the microbial community indicators). A higher respiration rate and a higher turnover of the “less adapted to low P condition community” might have resulted in a lowered respiratory efficiency, which offset the promoting effects of P addition on respiratory efficiency (the mechanisms observed in MS soils). Although the assumption is highly speculative, the fact that the initial respiratory efficiency was higher in more-severely P-limited condition in UB soils (Fig. 2b), and the more-severely P-limited condition caused fewer increase in respiratory efficiency (even negative) (Fig. 2c) may support this idea. Based on this idea, Fig. 1c also suggests that the shift in microbial community (highly adapted to low P condition community to less adapted to low P condition community) have also occurred in MS soils, because the decrease in ΔMBC/CO2 with decreasing P availability was also observed in MS soils. The increase in the respiratory efficiency may have been also partly offset by the shift in soil microbial community in MS soils. According to this idea, the magnitude of offset was probably smaller in MS soils than that in UB soils, which may have caused a clear decrease in N2O emissions in P added soils in MS soils. This idea is not based on the data, and needs to be tested in the future. However, at least, we demonstrated that the effects of P addition (or P shortage) on N2O emissions may be different depending on the degree of P shortage. Our suggestion may partly explain the inconsistency about the effects of P addition on N2O emissions among previous studies (Hall and Matson 1999; Sundareshwar et al. 2003, Mori et al. 2010a, b, 2013c).
Our study also provided a new hypothesis about P shortage in tropical soils (Vitousek and Sanford 1986; Elser et al. 2007) and N2O emissions; P shortage in tropical soils (but with ample N) causes a lower nitrifying and/or denitrifying respiratory efficiency, which in turn causes higher N2O losses through respiration processes. More data are needed from various types of soils from broader areas to verify or falsify our hypothesis. Changes in microbial community composition by P addition should also be clarified.
Conclusion
We suggested that P application to the P-limited tropical forest soils enhanced the respiratory efficiency and reduced the gases emitted from respiration (both CO2 and N2O). We also suggested that the effects of P addition on N2O emissions may be different depending on the degree of P shortage. This is the first study that tried to elucidate the factors causing contradictory effects of P addition on N2O emission in laboratory condition (without vegetation interaction). Further observations with microbial community analysis using more variety of soils are necessary to fully understand the effects of P addition on N2O emissions.
References
Aiba SI, Kitayama K (1999) Structure, composition and species diversity in an altitude-substrate matrix of rain forest tree communities on Mount Kinabalu, Borneo. Plant Ecol 140:139–157
Arai S, Ishizuka S, Ohta S et al (2008) Potential N2O emissions from leguminous tree plantation soils in the humid tropics. Global Biogeochem Cycles. doi:10.1029/2007GB002965
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol Fertil Soils 46:793–805. doi:10.1007/s00374-010-0484-6
Baral BR, Kuyper TW, Van Groenigen JW (2014) Liebig’s law of the minimum applied to a greenhouse gas: alleviation of P-limitation reduces soil N2O emission. Plant Soil 374:539–548. doi:10.1007/s11104-013-1913-8
Cavelier J, Tanner E, Santamaría J (2000) Effect of water, temperature and fertilizers on soil nitrogen net transformations and tree growth in an elfin cloud forest of Colombia. J Trop Ecol 16:83–99. doi:10.1017/S0266467400001280
Chen H, Gurmesa GA, Zhang W et al (2015) Nitrogen saturation in humid tropical forests after 6 years of nitrogen and phosphorus addition: hypothesis testing. Funct Ecol. doi:10.1111/1365-2435.12475
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci USA 103:10316–10321. doi:10.1073/pnas.0600989103
Cleveland C, Townsend A, Schmidt S (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:680–691. doi:10.1007/s10021-002-0202-9
Davidson EA, Verchot LV (2000) Testing the hole-in-the-pipe model of nitric and nitrous oxide emissions from soils using the TRAGNET database. Global Biogeochem Cycles 14:1035–1043. doi:10.1029/1999GB001223
Dobbie KE, Smith A (2001) The effects of temperature, water filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur J Soil Sci 52:667–673
Elser JJ, Bracken MES, Cleland EE et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. doi:10.1111/j.1461-0248.2007.01113.x
Erickson H, Keller M, Davidson EA (2001) Nitrogen oxide fluxes and nitrogen cycling during postagricultural succession and forest fertilization in the humid tropics. Ecosystems 4:67–84. doi:10.1007/s100210000060
Firestone MK, Davidson E (1989) Microbiological basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, New York, pp 7–21
Giller KE, Witter E, Mcgrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414. doi:10.1016/S0038-0717(97)00270-8
Hall SJ, Matson PA (1999) Nitrogen oxide emissions after nitrogen additions in tropical forests. Nature 400:152. doi:10.1038/22094
Hall SJ, Matson PA (2003) Nutrient status of tropical rain forests influences soil N dynamics after N additions. Ecol Monogr 73:107–129
Hall SJ, Asner GP, Kitayama K (2004) Substrate, climate, and land use controls over soil N dynamics and N-oxide emissions in Borneo. Biogeochemistry 70:27–58. doi:10.1023/B:BIOG.0000049335.68897.87
He M, Dijkstra FA (2015) Phosphorus addition enhances loss of nitrogen in a phosphorus-poor soil. Soil Biol Biochem 82:99–106. doi:10.1016/j.soilbio.2014.12.015
Ilstedt U, Singh S (2005) Nitrogen and phosphorus limitations of microbial respiration in a tropical phosphorus-fixing acrisol (ultisol) compared with organic compost. Soil Biol Biochem 37:1407–1410. doi:10.1016/j.soilbio.2005.01.002
Ilstedt U, Giesler R, Nordgren A, Malmer A (2003) Changes in soil chemical and microbial properties after a wildfire in a tropical rainforest in Sabah, Malaysia. Soil Biol Biochem 35:1071–1078. doi:10.1016/S0038-0717(03)00152-4
IPCC (2007) Climate Change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change
Ishizuka S, Tsuruta H, Murdiyarso D (2002) An intensive field study on CO2, CH4, and N2O emissions from soils at four land-use types in Sumatra, Indonesia. Glob Biogeochem Cycles 16:22-1–22-11. doi:10.1029/2001GB001614
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7. doi:10.1016/j.soilbio.2003.10.002
Keller M, Varner R, Dias JD et al (2005) Soil–atmosphere exchange of nitrous oxide, nitric oxide, methane, and carbon dioxide in logged and undisturbed forest in the Tapajós National Forest, Brazil. Earth Interact 9:1–28
Kesik M, Blagodatsky S, Papen H, Butterbach-Bahl K (2006) Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p. J Appl Microbiol 101:655–667. doi:10.1111/j.1365-2672.2006.02927.x
Kitayama K (2013) The activities of soil and root acid phosphatase in the nine tropical rain forests that differ in phosphorus availability on Mount Kinabalu, Borneo. Plant Soil 367:215–224. doi:10.1007/s11104-013-1624-1
Kitayama K, Aiba SI (2002) Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu, Borneo. J Ecol 90:37–51. doi:10.1046/j.0022-0477.2001.00634.x
Kitayama K, Schurr EAG, Drake DR, Mueller-Dombois D (1997) Fate of a wet montane forest during soil aging in Hawaii. J Ecol 85:669–679
Kitayama K, Aiba SI, Majalap-Lee N, Ohsawa M (1998) Soil nitrogen mineralization rates of rainforests in a matrix of elevations and geological substrates on Mount Kinabalu, Borneo. Ecol Res 13:301–312. doi:10.1046/j.1440-1703.1998.00264.x
Kitayama K, Majalap-Lee N, Aiba SI (2000) Soil phosphorus fractionation and phosphorus-use efficiencies of tropical rain forests along altitudinal gradients of Mount Kinabalu, Borneo. Oecologia 123:342–349
Kitayama K, Aiba SI, Takyu M et al (2004) Soil phosphorus fractionation and phosphorus-use efficiency of a Bornean tropical montane rain forest during soil aging with podozolization. Ecosystems 7:259–274. doi:10.1007/s10021-003-0229-6
Klemedtsson L, Svensson BH, Rosswall T (1988) Relationships between soil moisture content and nitrous oxide production during nitrification and denitrification. Biol Fertil Soils 6:106–111. doi:10.1007/BF00257658
Konda R, Ohta S, Ishizuka S et al (2008) Soil biology & biochemistry spatial structures of N2O, CO2, and CH4 fluxes from Acacia mangium plantation soils during a relatively dry season in Indonesia. Soil Biol Biochem 40:3021–3030. doi:10.1016/j.soilbio.2008.08.022
Konda R, Ohta S, Ishizuka S et al (2010) Seasonal changes in the spatial structures of N2O, CO2, and CH4 fluxes from Acacia mangium plantation soils in Indonesia. Soil Biol Biochem 42:1512–1522. doi:10.1016/j.soilbio.2010.05.022
Kuo S (1996) Phosphorus. In: Bgham JM (ed) Methods of analysis. Part 3. Chemical methods. Soil Science Society of America, Madison, pp 869–919
Li X, Upadhyaya G, Yuen W et al (2010) Changes in the structure and function of microbial communities in drinking water treatment bioreactors upon addition of phosphorus. Appl Environ Microbiol 76:7473–7481. doi:10.1128/AEM.01232-10
Liu L, Greaver TL (2009) A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol Lett 12:1103–1117. doi:10.1111/j.1461-0248.2009.01351.x
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38. doi:10.1016/j.soilbio.2011.08.017
López-Urrutia Á, Morán XAG (2007) Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88:817–822. doi:10.1890/06-1641
Minami K, Fukushi S (1983) Effects of phosphate and calcium carbonate application on emission of N2O from soils under aerobic conditions. Soil Sci Plant Nutr 29:517–524. doi:10.1080/00380768.1983.10434654
Mori T, Ohta S, Ishizuka S et al (2010a) Effects of phosphorus addition on N2O and NO emissions from soils of an Acacia mangium plantation. Soil Sci Plant Nutr 56:782–788. doi:10.1111/j.1747-0765.2010.00501.x
Mori T, Ohta S, Konda R et al (2010b) Phosphorus limitation on CO2, N2O, and NO emissions from a tropical humid forest soil of South Sumatra, Indonesia. In: 2010 international conference on environmental engineering and applications (ICEEA), pp 18–21. doi:10.1109/ICEEA.2010.5596085
Mori T, Ohta S, Ishizuka S et al (2013a) Effects of phosphorus and nitrogen addition on heterotrophic respiration in an Acacia mangium plantation soil in South Sumatra, Indonesia. Tropics 22:83–87. doi:10.1111/j.1747-0765.2010.00501.x
Mori T, Ohta S, Ishizuka S et al (2013b) Soil greenhouse gas fluxes and C stocks as affected by phosphorus addition in a newly established Acacia mangium plantation in Indonesia. For Ecol Manag 310:643–651. doi:10.1016/j.foreCO2013.08.010
Mori T, Ohta S, Ishizuka S et al (2013c) Effects of phosphorus addition with and without ammonium, nitrate, or glucose on N2O and NO emissions from soil sampled under Acacia mangium plantation and incubated at 100% of the water-filled pore space. Biol Fertil Soils 49:13–21. doi:10.1007/s00374-012-0690-5
Mori T, Ohta S, Ishizuka S et al (2014) Phosphorus application reduces N2O emissions from tropical leguminous plantation soil when phosphorus uptake is occurring. Biol Fertil Soils 50:45–51. doi:10.1007/s00374-013-0824-4
Mori T, Ishizuka S, Konda R et al (2015) Phosphorus addition reduced microbial respiration during the decomposi- tion of Acacia mangium litter in South Sumatra, Indonesia. Tropics 24:113–118
Mori T, Wachrinrat C, Staporn D et al (2016) Seasonal changes in soil respiration and microbial biomass in five tropical tree plantations in Thailand. Topics 25:85–89
Nobre AD, Keller M, Crill PM, Harriss RC (2001) Short-term nitrous oxide profile dynamics and emissions response to water, nitrogen and carbon additions in two tropical soils. Biol Fertil Soils 34:363–373. doi:10.1007/s003740100396
Ohyagi-Hara C, Sawada K, Kamiura S et al (2013) MiR-92a inhibits peritoneal dissemination of ovarian cancer cells by inhibiting integrin α5 expression. Am J Pathol 182:1876–1889. doi:10.1016/j.ajpath.2013.01.039
Priess JA, Fölster H (2001) Microbial properties and soil respiration in submontane forests of Venezuelian Guyana: characteristics and response to fertilizer treatments. Soil Biol Biochem 33:503–509. doi:10.1016/S0038-0717(00)00191-7
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. doi:10.1126/science.1176985
Schimel J (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563. doi:10.1016/S0038-0717(03)00015-4
Schindlbacher A, Zechmeister-Boltenstern S (2004) Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J Geophys Res 109:1–12. doi:10.1029/2004JD004590
Schipper LA, Lee WG (2004) Microbial biomass, respiration and diversity in ultramafic soils of West Dome, New Zealand. Plant Soil 262:151–158. doi:10.1023/B:PLSO.0000037027.56794.08
Shigenobu Y, Fujimoto K, Ambe D et al (2014) Radiocesium contamination of greenlings (Hexagrammos otakii) off the coast of Fukushima. Sci Rep 4:6851. doi:10.1038/srep06851
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16:930–939. doi:10.1111/ele.12113
Sitaula BK, Hansen S, Sitaula JIB, Bakken LR (2000) Effects of soil compaction on N2O emission in agricultural soil. Chemosph Glob Chang Sci 2:367–371. doi:10.1016/S1465-9972(00)00040-4
Sundareshwar PV, Morris JT, Koepfler EK, Fornwalt B (2003) Phosphorus limitation of coastal ecosystem processes. Science 299:563–565. doi:10.1126/science.1079100
Van Lent J, Hergoualc’h HK, Verchot LV (2015) Reviews and syntheses: soil N2O and NO emissions from land use and land-use change in the tropics and subtropics: a meta-analysis. Biogeosciences 12:7299–7313. doi:10.5194/bg-12-7299-2015
Vance ED, Jenkinson DS (1987) Microbial biomass measurement in forest soils: the use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol Biochem 19:697–702
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167. doi:10.1146/annurev.es.17.110186.001033
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Wagai R, Mayer LM, Kitayama K, Knicker H (2008) Climate and parent material controls on organic matter storage in surface soils: a three-pool, density-separation approach. Geoderma 147:23–33
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19. doi:10.1016/0016-7061(76)90066-5
Wrage N, Velthof GL, Van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732. doi:10.1016/S0038-0717(01)00096-7
Zhang W, Zhu X, Luo Y et al (2014) Responses of nitrous oxide emissions to nitrogen and phosphorus additions in two tropical plantations with N-fixing vs. non-N-fixing tree species. Biogeosci Discuss 11:1413–1442. doi:10.5194/bgd-11-1413-2014
Authors’ contributions
TM analyzed samples and prepared Figs. 1, 2 and Tables 1, 2, 3. All authors contributed to the plot design and sampling. All authors discussed about the results. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Jamili Nais and all staff members of Sabah Parks for general support of this study. This study was financially supported by a grant from Grant-in-Aid for JSPS Fellowes (Number 25·2647) to TM, and by the MEXT Grant-in-Aid 22255002 to KK.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mori, T., Yokoyama, D. & Kitayama, K. Contrasting effects of exogenous phosphorus application on N2O emissions from two tropical forest soils with contrasting phosphorus availability. SpringerPlus 5, 1237 (2016). https://doi.org/10.1186/s40064-016-2587-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2587-5