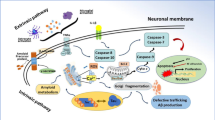
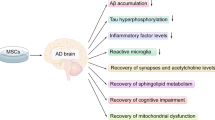
Abstract
Background
Alzheimer’s disease is a neurodegenerative disorder. Therapeutically, a transplantation of bone marrow mesenchymal stem cells (BMMSCs) can play a beneficial role in animal models of Alzheimer’s disease. However, the relevant mechanism remains to be fully elucidated.
Main body
Subsequent to the transplantation of BMMSCs, memory loss and cognitive impairment were significantly improved in animal models with Alzheimer’s disease (AD). Potential mechanisms involved neurogenesis, apoptosis, angiogenesis, inflammation, immunomodulation, etc. The above mechanisms might play different roles at certain stages. It was revealed that the transplantation of BMMSCs could alter some gene levels. Moreover, the differential expression of representative genes was responsible for neuropathological phenotypes in Alzheimer’s disease, which could be used to construct gene-specific patterns.
Conclusions
Multiple signal pathways involve therapeutic mechanisms by which the transplantation of BMMSCs improves cognitive and behavioral deficits in AD models. Gene expression profile can be utilized to establish statistical regression model for the evaluation of therapeutic effect. The transplantation of autologous BMMSCs maybe a prospective therapy for patients with Alzheimer’s disease.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is a chronic disorder of central nervous system. Its clinical manifestations are characterized by memory loss, cognitive dysfunction, abnormal behavior, etc. With the deterioration of AD, patients fall into stupor state and usually die of exhaustion within 5—10 years [1].
In pathology, AD is manifested by the decreased number of neurons and synapses in cerebral regions, resulted in different degrees of memory loss and cognitive impairment. Amyloidal plaques, mostly insoluble deposits of amyloid β peptide (Aβ), are observed around neurons [1]. Neurofibrillary tangles, hyperphosphorylated aggregates of the microtubule-associated protein tau, are accumulated inside the neuron [2]. As compared with the general aging brain, many plaques and tangles in patients with AD are discovered in specific brain regions such as temporal lobe and hippocampus [3, 4].
Currently, there is no cure for the Alzheimer’s disease. Therapeutic strategy in the treatment of AD is to alleviate symptoms through pharmacological intervention, such as an enhancement of neurotransmitter acetylcholine [5, 6]. A few medicines can slow down the exacerbation and improve behavioral deficits in some patients. Two types of medication are presently used to treat cognitive symptoms, including (i) cholinesterase inhibitors (AChE inhibitors) such as donepezil, galantamine and rivastigmine [6]. These drugs boost levels of acetylcholine that is decreased in the brain of Alzheimer’s disease, which may improve neuropsychiatric agitation or depression; (ii) memantine, an uncompetitive NMDA antagonist, is used to improve memory and awareness in moderate to severe patients with AD. It works in cell communication network and delays the exacerbation of symptoms due to AD. Sometimes, the memantine is utilized in combination with AChE inhibitors. Antidepressants may be prescribed to control the behavioral symptoms associated with Alzheimer’s disease. The therapeutic effect of above drugs is limited in advanced patients with poor condition. Recent nanotechnological advancements provide effective options of drug carriers [7, 8]. For instance, when the rivastigmine was assisted with biocompatible nanoparticles (NPs), the NPs-based drug delivery could effectively cross the blood-brain barrier and improved its bioavailability [7]. The biocompatible NPs also showed significant effect on the kinetics of Aβ-fibrinogen [9, 10]. In addition, non-pharmacological approaches such as diet, regular exercise or other healthy lifestyle choice are supplemented for the improvement of patients’ life quality.
Transplantation of mesenchymal stem cells (MSCs) as a therapeutic technique has been well developed in the recent decades. It has also been explored in the treatment of animal models with nervous disease. The accumulative evidence demonstrated that the transplanted MSCs could be differentiated into cell lineage such as neurons and reconnected synaptic network, which played a critical role in the functional improvement of nervous system [11, 12]. A comparison had been carried out among stem cells derived from different resources such as brain, fat, bone marrow, umbilical blood or fetal tissues [13,14,15]. Owing to ethical issue and alloimmunogenicity, stem cells from embryos and allogenic umbilical cord may be not suitable for the treatment of AD. Autologous neurons from brain biopsy are confronted with unacceptable attitude and challenge. Still, it is long way to go for the preparation of stem cells through iPSc method. Therefore, autologous stem cells from bone marrow or fat were additional choices. Interestingly, the stem cells from bone marrow had a better therapeutic effect as compared with that from the fatty tissue based on previous studies [16, 17]. The MSCs from autologous bone marrow could be delivered into AD subjects via different approaches such as intracerebral, peripheral vein and intracerebroventricular injection. The therapeutic effect of bone marrow mesenchymal stem cells (BMMSCs) was verified in several animal models. The results indicated that the BMMSCs could alleviate the memory loss, behavioral deficits and neuropathology. Technical advantages in autologous BMMSCs have provided a prospective therapy for patients with Alzheimer’s disease.
The early studies demonstrated that the therapeutic effect of exogenous stem cells could improve pathological manifestations in animal models with Alzheimer’s disease. Furthermore, there were seldom adverse responses following a transplantation of bone marrow mesenchymal stem cells [17, 18]. Advantages of bone marrow mesenchymal stem cells were reflected by its efficiency and safety. At present, the transplantation of bone marrow mesenchymal stem cell has been optimized through appropriate facilities as well as experimental conditions. A series of research data proved a dramatic improvement in cognitive deterioration and neuropathological symptoms among AD-like animal models. Autologous bone marrow-derived mesenchymal stem cells may be used in the clinical treatment of Alzheimer’s disease in near future. The present study aims to explore the potential mechanisms by which the transplantation of BMMSCs improves cognitive and behavioral deficits in animal models of Alzheimer’s disease, which can lay a foundation for the clinical application of autologous BMMSCs in AD patients.
Methods
Systematical search of published literature
Database PubMed, Medline, and Embase were systematically screened, and the time point was set at the end of February 2019. Keywords “Alzheimer’s disease” and “stem cell transplantation” were used to identify literature. Total 414 references were acquired, which were not restricted by the type of publication. The published work was further scrutinized according to the integrity of data and article types.
Study selection
Studies eligible for inclusion were based on quality of resultant data, included randomized controlled trials and cohort-controlled trials. We excluded studies using therapeutic stem cells from umbilicus cord, fat and brain. Also, the exclusion covered studies that provided incomplete data relevant to the pre-specified outcome variables. The inclusion studies were restricted to the transplantation of BMMSCs. Data extraction was accomplished by two investigators independently.
Data collection and outcome measures
The extracted data were based on general characteristics of all included studies, such as source of reference, study design, animal species, surgery procedure, delivery route of stem cells, outcome measures, etc. A primary comparison was performed among primary data derived from BMMSCs and control groups. The data analysis involved cognitive function, behavioral change, neurogenesis, angiogenesis, apoptosis, inflammatory response, immunomodulation, reactive gliosis, microglial activation, level of Aβ peptide, tau hyperphosphorylation and so forth. Morbidity of adverse response was calculated by the number of animals with at least one complication after stem cell transplantation and mortality was computed by death number during or after operation due to any causes. Meta-analysis based on outcome variables was further carried out, including Y-maze test, escape latency, histone H3-positive cells, expression of VEGF, TNF-α, IL-1β, Aβ level, activation of Aβ-degrading enzyme ECE, percentage of Iba-1 positive cells, percentage of AT8 positive cells, etc.
Statistical analysis
Review Manager (RevMan version: 5.3.5; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to pool data and meta-analysis. For categorical variable, treatment effect was expressed as odds ratio (OR) with corresponding 95% confidence intervals (CI). Results were compared through a random-effects model. For continuous variable, treatment effect was expressed as weighted mean difference (WMD) with corresponding 95% CI. Chi-square (Chi2 or χ2) and I2 statistics estimate the appropriateness of pooling individual study. Heterogeneity was evaluated by χ2-test with significance set at P value 0.10. The heterogeneity was measured by I2 more than 50% as statistical significance. Forest plots were constructed, P values of < 0.05 as significant difference. Gene data on microarray and high-throughput DNA sequencing were retrieved out of Geo DataSets (https://www.ncbi.nlm.nih.gov/pubmed/). The linear relationship between the two variables was measured with Pearson’s correlation coefficient. Principal component analysis (PCA) of gene expression data was performed based on the correlation matrix. The number of principal components would satisfy more than 80% variability of differential gene expression. The clusters were combined based on similar expression profiles and enriched gene ontology (GO) categories. The cluster analysis was performed using correlation for hierarchical clustering and Euclidean distance for K-means clustering. Difference was considered significant at p values < 0.05. Data were analyzed with software SPSS 19.0 (IBM Corp., Armonk, NY, USA), JMP 13.0 software (SAS Institute Inc., Cary, North Carolina, USA), and R 3.5.3 for Windows.
Main text
Quality assessment of the included studies
Systematic review on therapeutic effect of mesenchymal stem cells for Alzheimer’s disease was summarized according to animal species, sources of mesenchymal stem cells, cognitive improvement, route of delivery, position of delivery, mechanisms, and so on (Supplementary table). Original studies with complete data were kept in the present meta-analytic review (Fig. 1, Table 1). General characteristics of the included studies in the meta-analysis were reflected by source of transplanted stem cells, amount of transplanted stem cells, species of recipient animals, gender ratio of recipients, age or body weight of recipients, route of delivery, position of delivery, and sustainability of transplanted stem cells (Table 2). Study quality was assessed via bias in primary studies. Potential bias in the identified studies were also evaluated (Fig. 2). The interpretation of results was weighed in terms of existed bias and sources of heterogeneity. The methodology of included studies was evaluated through random sequence generation, blinding of outcome assessors, incomplete outcome data, and selective reporting, etc. Priori criteria of high-quality study include (i) randomized trial; (ii) controlled study; (iii) adequately reported methodology of measurement.
Improvement of cognitive and behavioral deficits
The present review summarized therapeutic role of bone marrow mesenchymal stem cells in animal models of Alzheimer’s disease. The therapeutic effect of the bone marrow mesenchymal stem cells was demonstrated via behavioral changes in experimental subjects. After transplantation of bone marrow mesenchymal stem cells into Alzheimer-like animal models, symptom and sign were significantly alleviated as exhibited in APP mice, DAL mice or scopolamine-induced rats [12, 19, 20]. Benefits of the transplanted stem cells in the behavioral changes were confirmed through diverse tests such as Morris water maze test, Y-maze alternation test (Y-maze), plus-maze discriminative avoidance task, social recognition test and open-field evaluation (Fig. 3a, b). There was an improvement in learning ability and spatial memory performance subsequent to a transplantation of BMMSCs. The functional improvement of model brains was evidenced by preventive treatment against spatial learning and memory impairment. Of note, behavioral measurement was not performed in all experiments, because some animals were too young to conduct behavioral tests in certain studies [21].
Transplantation of bone marrow mesenchymal stem cells could improve behavioral deficits in animal models of Alzheimer’s disease, which was generally characterized by abnormal manifestation or relationship. The beneficial change might be a temporary or permanent effect when compared to previous behavior. a. Behavioral changes as demonstrated through Y-maze test; b. Behavioral changes by Morris water maze test
Importantly, the BMMSCs treatment was beneficial in both young and aged Alzheimer-like animals. This therapeutic approach could reverse cognitive impairments induced by cerebral amyloidosis as observed in mouse AD models [3, 18, 21]. The treatment of transplanted BMMSCs could ameliorate spatial learning and memory impairment. Also, the BMMSCs treatment might improve impaired spatial memory in APP/PS1 mice as detected via Morris water maze test [3]. The APP/PS1 mice treated with BMMSCs had shorter escape latency than that of PBS-treated controls. These results indicated that the transplantation of BMMSCs was able to reduce the cognitive impairment of spatial memory [3]. Moreover, 3xTg-AD mice lost their working memory, but this impairment was improved in the transgenic mice after having received transplanted MSCs. The BMMSCs could dramatically alleviate working memory in the 3xTg-AD mice [22]. The transgenic DAL mice express a dominant-negative mutant form of mitochondrial aldehyde dehydrogenase 2 and exhibit AD-like phenotypes. By having employed a spontaneous Y-maze alternation test, an alternation rate of BMMSC-treated DAL mice was significantly higher than that of vehicle-treated mice in 3 months after transplantation. Even a single transplantation of stem cells was enough to have an effective result [12]. The cognitive decline could be ameliorated and even reversed via the beneficial role of BMMSCs in the AD animals [18].
Above-mentioned improvement was associated with input concentration of stem cells, cell viability, passage number, and delivery methods. The delivery routes of stem cells included (i) intravenous delivery. Animal models might receive either a single injection or a weekly injection more than 10 weeks through the tail vein [21]; (ii) intranasal administration of active factors secreted by stem cells. The animal was restrained by hand without anesthesia. An appropriate amount of soluble MSC factors was placed at nares of the animal via a pipette until the liquid drop disappeared into the nares [21]. A repeated intranasal delivery of soluble factors from cultured MSCs was enough to improve behavioral deficits in the mice; (iii) intracerebral or intracerebroventricular injection of stem cells. Intracerebral transplantation of grafted cells circumvents the prohibitive blood brain barrier and the cells can reach the discreet brain site. Benefits of mesenchymal stem cells on memory improvement in AD models had been detected [23]. However, the intracerebral delivery, compared to peripheral route, is an invasive procedure to implant stem cells into particular brain area [15]. Thus, it is a major hurdle for clinical applications. In contrast, intravenous delivery of transplanted stem cells is fast and easy route, and complications are rarely observed. To date, some preclinical studies have evaluated the impact of intravenous MSC injections on cerebral amyloidosis [21, 24].
Neuropathological changes
Removal of Aβ plaques
Amyloid β peptide deposits in brain tissue and forms plaques. Moreover, the Aβ plaques are accumulated in special areas of AD brain. Nowadays cumulative level of Aβ plaques is a hallmark of AD. It is still a long way to demonstrate the actual role of Aβ plaques in the pathogenesis of Alzheimer’s disease, but the number of Aβ plaques is increased along with the deterioration of AD stage. The deposition of amyloid plaques in the form of spots and streaks could induce neuronal cell death via oxidative stress in the hippocampus [20, 25]. The transplantation of stem cells was able significantly to decrease the number of hippocampal Aβ plaques, which was demonstrated in APP/PS1 model mice as early as 1 week after intravenous delivery (Fig. 4a). Further investigation indicated that the impact of stem cells could activate several Aβ-degrading enzymes such as neprilysin-degrading enzyme, insulin degrading enzyme (I), endothelin-converting enzyme, etc. Those enzymes may play a critical role during degradation of amyloid β plaques. In the aspect of feasibility, the therapeutic application of stem cells via intravenous delivery is convenient and sufficient to diminish cerebral amyloidosis [21, 25].
Meta-analysis on potential mechanisms. The transplantation of BMMSCs could alleviate neuropathology through diverse mechanisms, such as to decrease the number of hippocampal Aβ plaques as demonstrated in AD animal models (a). The Fig. 4a was plotted by relative ratio. The value in experimental group was assigned as 1 and the same as the following figures; to stimulate neurogenesis, neuronal differentiation, and neuronal integration (b); to promote angiogenesis in brain tissue as reflected by VEGF marker (c, d); to attenuate Aβ-induced apoptotic cell death in both primary hippocampal neurons and Aβ-injected animal models (e, f); immunomodulation and neuroprotection (g); to inhibit neuroinflammation in AD animal models (h)
Neurogenesis, differentiation and integration
The intravenous transplantation of stem cells was readily detected in brain parenchyma, i.e. hippocampus as revealed in 1 h after administration [21]. The expression of sry gene in the brain tissue of female AD model treated with male BMMSCs confirmed the migratory ability of the intravenously infused foreign stem cells to the site of brain injury [25]. The BMMSCs could differentiate into neuron-like cells and partially express ChAT [26]. Neural cells express nestin that can be as a marker of neural precursors. Brain nestin expression was up-regulated subsequent to the treatment of BMMSCs [27]. Bone marrow cells migrate throughout the brain and differentiate into neurons and glial cells [11]. In the hippocampus, there were different neurogenic phases such as proliferation, differentiation, migration, targeting, and integration respectively [28]. The transplanted stem cell may play a beneficial part in different phases of cell growth, although exact mechanism remains to be determined. The MSCs produce various trophic factors, including BDNF, NGF, and IGF-1 [29,30,31]. The MSCs could upregulate the trophic factors like NGF, FGF-2 and BDNF. This result could be attributed to the positive expression of growth factor, chemokine and extracellular matrix receptors on the surface of MSCs [25]. All these factors contribute to recover neurobehavioral function and stimulate endogenous regeneration. The BMMSCs could significantly increase the intensity of ChAT spots as well as the number of positive cells for ChAT expression in AD group. Cholinergic change is potential mechanism for the neurogenesis subsequent to a transplantation of BMMSCs. After BMMSCs treatment, the improvement in these biomarkers might be attributed to the powerful neurogenesis, neuronal differentiation and integration [11, 32] (Fig. 4b).
Angiogenesis
Angiogenesis is a pathophysiological process that is involved in regeneration and tissue reconstruction. Transplantation of the BMMSCs can promote angiogenesis in brain tissue as proved by (a) the fold change of expression marker such as VEGF; (b) interaction between VEGF and Aβ protein in experimental animal study; and (c) therapeutic effects of the VEGF in the murine model of Alzheimer’s disease [33,34,35]. The role of MSC in the cerebrovasculature had been correlated with angiogenesis and revascularization, mainly through secretion of various angiogenic factors (Fig. 4c, d). An administration of MSCs stimulated revascularization at the site of injury via secreting VEGF, FGF-2, Ang-1 and EGF [36]. The injection of hMSC into rats would increase angiogenesis by enhancing endogenous VEGF and VEGFR2 levels in the ischemic zone [37, 38]. Moreover, transplanted stem cells were able to differentiate into mural cells that accelerated the formation of peripheral vascular layers [39]. In the context of neurodegenerative disorders, these mesenchymal stem cells might contribute to neuroprotection by secreting trophic factors such as EGF, VEGF, FGF-2, NT-3, HGF, and BNDF [40]. Further study on potential mechanisms in AD models will be required to understand the contribution of above factors to the disruption of amyloid plaques following intravenous implementation of stem cells [21]. In the brains of AD patients, the soluble VEGF concentration is decreased because Aβ binds to VEGF forming aggregate that leads to the loss of angiogenic and neuroprotective activities [41]. Therefore, provide additional VEGF would have high therapeutic effect. An overexpressing VEGF in mesenchymal stem cells could promote neovascularization in the hippocampus and recovered the memory deficit in the 2xTg-AD animals. More interestingly, only intraperitoneal injection of VEGF could improve cognitive function through the hippocampal angiogenesis and decreased Aβ deposition in the brain [35, 42].
Inhibition of apoptosis
The Aβ peptide in AD animal models could induce neuronal apoptosis via caspase pathway [13, 43, 44]. The neuronal apoptosis was responsible for the memory impairment in AD brain. The transplantation of BMMSCs attenuated Aβ-induced apoptotic cell death in primary hippocampal neurons as well as intrahippocampally Aβ-injected AD animal models (Fig. 4e, f). The neuroprotective mechanisms of BMMSCs may be through (a) to reduce Aβ deposition. The Aβ peptide induced the stress-activated protein kinases p38 and c-jun N-terminal kinase, and upregulated p53 expression, which were closely associated with apoptosis [1]. Furthermore, the MSCs expressed seladin-1, which inhibits the activation of caspase-3 and is a neuroprotective factor. The transplantation of BMMSCs could significantly increase seladin-1 gene expression in AD groups [45]; (b) activation of the cell survival signal pathway. The BMMSCs treatment upregulated the survivin expression as showed by the increased number of survivin-positive cells in AD models [46]. The MSCs could inhibit P53 activation [47]. Also, the MSCs produce VEGF, BDNF, NGF, and FGF2, which were supposed to exert an anti-apoptotic effect [48]. The BMMSCs could significantly down-regulate caspase-3 expression, thus protecting seladin-1 from cleavage [49]; (c) to decrease oxidative stress-induced neurotoxicity in the hippocampus [18]. ER-oxidative stress and mitochondrial failure involve the pathogenesis of Alzheimer’s disease. The transplantation of stem cells led to a significant improvement of memory deficits in AD mouse models via the suppression of apoptosis and the maintenance of functional synaptic contacts [4, 13, 50]. The MSCs could up-regulate the cellular antioxidant defense through their capability to secrete trophic factors like NGF, FGF2 and BDNF. MSCs could also attenuate oxidative damage by reducing ROS and increasing expression of endogenous antioxidants in neurons [47]. The apoptotic mechanism not only took part in neuronal cell death, but also involved survival of transplanted mesenchymal stem cells in brain tissue. Actually, the later also hampered the clinical application of stem cell therapy for Alzheimer disease [51].
Immunomodulation
Histopathological examination disclosed that immunomodulatory property of the BMMSCs play an important role in therapeutic role against AD as well [25]. The intracerebral transplantation of BMMSCs was applied to acute AD model induced through Aβ peptide injection into the dentate gyrus of hippocampus of C57BL/6 mice. The activation of microglia promoted the diminution of Aβ deposits due to microglial phagocytosis. The BMMSCs could accelerate the activation of microglia and the removal of Aβ deposition in AD brain [52]. In vitro study demonstrated the bone marrow-derived mesenchymal stem cells could decrease expressional levels of pro-inflammatory genes (IL-1β, TNF-α, IL-6) in astrocytes [53]. The MSCs regulated a series of gene expression, including intermediate filaments (GFAP, vimentin), pro-inflammatory enzymes (iNOS, COX-2) and receptors (TLR4, CD14, mGluR3, mGluR5). Immunomodulatory influence of MSCs may be through diverse cell types to participate in the neuroinflammation (Fig. 4g). The observation of decreased neuroinflammation in hMSC-treated APP/PS1 mice further suggests that hMSC delivery does not elicit a major immune response from the host. In addition, preclinical study demonstrated that a repeated intravenous hMSC treatment could safely reduce cerebral Aβ pathology in a typical mouse model of AD.
Inhibition of inflammation
The neuroinflammation was reduced in APP/PS1 mice following hMSC treatment [21] (Fig. 4h). There was a dramatic decline on the panel of cerebral cytokines such as IFNγ, diverse interleukins (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, and IL-12p70), KC/GRO, and TNF-α, suggesting an anti-inflammatory impact of hMSCs. The hMSC treatment significantly down-regulated cerebral IBA-1. Among multiple cell types of brain tissue, the IBA-1 gene is specifically expressed in microglia. Upon activation of microglia due to inflammation, expression of the IBA-1 is up-regulated, which allows the discrimination between surveilling and activated microglia. Microglial coverage was examined to evaluate neuroinflammation changes in transgenic brains following repeated hMSC treatment [21]. There was an overall decrease of the microglia coverage in brains of APP/PS1 transgenic mice of both young and aged groups. A qualitative observation was confirmed by quantitative image analysis of IBA-1 immunoreactivity. TNFα and IL-12p70 were reduced following a single hMSC intravenous injection. Interestingly, TNFα has been implicated in chronic inflammation, cancer, and other inflammatory diseases. Notably, levels of the cytokine IL-10 were decreased following stem cell treatment, which might be therapeutically relevant for AD although this cytokine was reported to be anti-inflammatory. Accordingly, AD patients showed abnormally high IL-10 signaling, which highlighted that blocking the IL-10 anti-inflammatory response could be therapeutically relevant for AD [54]. The repeated intravenous hMSC injections or even single administration reduced cerebral neuroinflammation. The anti-inflammatory role of BMMSCs was also verified in a rat model of spinal cord injury [55]. Obviously, the stem cell therapy significantly inhibited the inflammatory response.
Gene-specific patterns of Alzheimer’s disease
In pathology, the pathogenesis of Alzheimer’s disease can be classified into different stages, which involves various mechanisms such as proliferation, apoptosis, angiogenesis, immunomodulation, inflammation, etc. These mechanisms are reflected by differential gene levels as compared with normal control (Fig. 5a). In recent decades, gene analysis based on microarray assay and high-throughput DNA sequencing has provided abundant information on gene expression profile of Alzheimer’s disease. It is reasonable to hypothesize that the Alzheimer’s disease has gene-specific patterns by which its progression and severity are mediated.
Construction of gene-specific regression model. a. Differential gene expression was compared between control and samples of patients with Alzheimer’s disease. b. Hierarchical cluster analysis based on the comparison between control and gene data from samples of patients with Alzheimer’s disease. c. Heatmap of gene data from brain samples of patients with Alzheimer’s disease. d. Sigmoid curve of gene pattern. e. Logistic regression equation for prediction of gene-specific patterns of Alzheimer’s disease
Gene data from microarray assay and high-throughput DNA sequencing were collected and analyzed through comprehensive comparison. In gene ontology and signal pathway analyses, principal components of differential genes were identified [56]. The guideline for the construction of gene-specific patterns was summarized as follows:
-
1)
Comparison of differential gene expression in brain samples of patients with AD (Fig. 5b).
-
Quantification of hippocampal key genes, such as BDNF, NGF, VEGF, etc.
-
Estimation of inflammatory cytokines, such as TNF-α, IL-1β, IL-10, etc.
-
Determination of oxidative damage, e.g., hippocampal Nrf2 level.
-
-
2)
Cluster analysis of all relevant gene data (Fig. 5c).
-
3)
To screen principal variables via PCA analysis.
-
4)
Statistical regression model. After correlation and regression analysis, a multinomial logistic equation was obtained (Fig. 5d, e).
-
Based on big data analysis, a predictive model was composed of representative gene variables in the pathogenesis of Alzheimer’s disease.
-
Logistic regression equation can classify gene variables into gene-specific patterns.
-
-
5)
Pathophysiological significance of the gene-specific patterns.
-
To diagnose patient based on differential gene levels. Logistic regression model can distinguish AD patient from normal control.
-
To predict progression of AD, severity, and patient’s life expectancy.
-
In the context of neurodegenerative AD, the transplantation of BMMSCs could improve cognitive deficits and alleviated neuropathology at various degrees. The grafted MSCs contributed to neuroprotection through secretion of neurotrophic factors such as BDNF, EGF, VEGF, FGF-2, NT-3, HGF and so forth [40]. Differential gene expression involved a series of functional results of paracrine secretion of neurotrophic factors and cytokines. The aforementioned changes might be weighed by differential levels of responsible genes. In fact, therapeutic effect of the BMMSCs was determined by comprehensive role of representative genes. As presented in this study, there are gene-specific patterns in the pathogenesis of Alzheimer’s disease. The gene patterns would be an appropriate method to assess the therapeutic effect subsequent to stem cell transplantation in AD models. Accordingly, relative levels of representative genes can be used to evaluate the progress and prognosis of the disease. Next, it is necessary to expand the sample size of representative gene data and further to confirm real contribution of these key genes to the pathogenesis of Alzheimer’s disease.
Discussion
Therapeutic effect of the transplanted BMMSCs was demonstrated with the improvement of memory loss and behavioral deficits in animal models with Alzheimer’s disease [18, 57, 58]. Positive results have been acquired not only through the repeated transplantation of BMMSCs, but also via a single injection or even soluble MSC factors over nasal mucosa. In future, it is possible to use BMMSCs for the clinical treatment of Alzheimer’s disease. Potential mechanisms are associated with a broad coverage of neurogenesis, differentiation, apoptosis, angiogenesis, inflammation, immunomodulation and so on [17, 18, 20, 22]. However, the exact mechanism remains to be determined. Based on data analysis, a gene-specific pattern was revealed in brain tissue of patients with Alzheimer’s disease. The above gene patterns were altered with the severity of neuropathology, which maybe a useful tool for the molecular diagnosis and therapeutic evaluation of Alzheimer’s disease.
It is a long way to clarify the pathogenesis of Alzheimer’s disease. However, an investigation on its potential mechanisms is still an essential work, since any progress in clinical treatment depends on a comprehensive understanding of the relevant mechanisms. Neuropathological mechanism is associated with differential panel of gene expression [21, 54, 56]. Gene change in brain tissue can be clustered into diverse patterns based on expressional levels and functional features. Therefore, a novel concept of the gene pattern is proposed. The gene pattern may be utilized as a surveillance marker for the dynamic assessment of neuropathology. Its significance will be reflected in the molecular diagnosis and therapeutic evaluation of Alzheimer’s disease.
Beneficial results of BMMSCs transplantation had been observed in different animal models that were induced using genetic modification, Aβ protein injection, or administration of chemicals. The transplantation of stem cells from autologous BMMSCs did not cause any immune response. Enormous experiment data showed therapeutic effects of the BMMSCs, which included the improvement in cognitive deficits and pathological changes [18, 20]. It is quite possible for the BMMSCs to be utilized in clinical treatment of AD patients in future, because (a) stem cells are easily obtained through bone marrow aspiration; (b) peripheral vein delivery; (c) autologous stem cells without immunogenicity.
A combination of transplanted BMMSCs with drug therapy may be a future direction. In clinical, cholinesterase inhibitors and NMDA antagonist have been now used to improve memory loss and behavioral symptom of patients with Alzheimer’s disease [5, 6]. Therapeutic effect had been observed in certain patients, but not all patient community. If above-mentioned medications are combined with a transplantation of BMMSCs, what will happen? So far, it is only a rational hypothesis. In addition, the soluble factors from stem cells could also produce positive result, which encourages further investigation using the combination of neurotransmitter drugs with cytokines [21]. Their joint application may trigger a synergistic effect.
It seems that the stem cells from autologous bone marrow have some advantages as compared with those from allogeneic embryos and umbilical cord. However, there is still weakness in the transplantation of BMMSCs. There are some side-effects from bone marrow aspiration. Another drawback is from the preparation of stem cells. Moreover, there are diverse subtypes of stem cells according to CD markers on the cell membrane [59]. They can be also classified into disparate subgroups. Different cell subtypes may play distinct roles during neurogenesis and functional reconstruction. Unfortunately, it is remains to be identified for specific subtypes to give rise to precise roles and neuroprotective mechanisms.
Conclusion
In summary, the beneficial effect was confirmed in animal models with Alzheimer’s disease subsequent to the transplantation of bone marrow mesenchymal stem cells. The therapeutic efficacy and safety were verified through the improvement of behavioral deficits and the alleviation of neuropathology. Multiple signal pathways involved therapeutic mechanisms, including neurogenesis, apoptosis, angiogenesis, immunomodulation, inflammation and so on. Gene expression profiles might reflect relative importance of above mechanisms in different stages. The transplantation of BMMSCs could alter gene expression levels. Differential expression of representative genes could be used to establish statistical regression model for the evaluation of therapeutic effect and the prediction of prognosis. There is a great possibility for the clinical application of autologous BMMSCs in patients with Alzheimer’s disease.
Availability of data and materials
All generated or analyzed data are included in this published article.
Abbreviations
- AD:
-
Alzheimer’s disease
- BMMSCs:
-
Bone marrow mesenchymal stem cells
- Aβ:
-
Amyloid β peptide
- AChE:
-
Cholinesterase
- NMDA:
-
N-methyl-D-aspartic acid
- iPSc:
-
Induced pluripotent stem cell
- MSCs:
-
Mesenchymal stem cells
- VEGF:
-
Vascular endothelial growth factor
- TNF-α:
-
Tumor necrosis factor alpha
- IL-1β,:
-
Interleukin 1 beta
- ECE:
-
Endothelin converting enzyme
- Iba-1:
-
Induction of brown adipocytes 1
- AT8:
-
ATPase subunit 8
- APP:
-
Amyloid beta precursor protein
- Y-maze:
-
Y-maze alternation test
- APP/PS1:
-
Amyloid beta precursor protein/ presenilin 1
- 3xTg-AD:
-
APP/PS1/Tau transgenic AD
- ChAT:
-
Choline acetyltransferase
- BDNF:
-
Brain-derived neurotrophic factors
- NGF:
-
Nerve growth factor
- IGF-1:
-
Insulin-like growth factor-1
- FGF-2:
-
Fibroblast growth factor 2
- Ang-1:
-
Angiopoietin 1
- EGF:
-
Epidermal growth factor
- hMSC:
-
Human mesenchymal stem cells
- VEGFR2,:
-
Vascular endothelial growth factor receptor 2
- NT-3:
-
Neurotrophin-3
- HGF:
-
Hepatocyte growth factor
- 2xTg-AD,:
-
APP/PS1 transgenic AD
- p38:
-
P38 kinase
- p53:
-
Tumor protein p53
- ER:
-
Endoplasmic reticulum
- ROS:
-
Reactive oxygen species
- IL-6:
-
Interleukin 6
- GFAP:
-
Glial fibrillary acidic protein
- iNOS:
-
Inducible nitric oxide synthase
- COX-2:
-
Prostaglandin-endoperoxide synthase 2
- TLR4:
-
Toll like receptor 4
- CD14:
-
CD14 molecule
- mGluR3:
-
Metabotropic glutamate receptor 3
- mGluR5:
-
Metabotropic glutamate receptor 5
- IFNγ:
-
Interferon gamma
- IL-2:
-
Interleukin 2
- IL-4:
-
Interleukin 4
- IL-5:
-
Interleukin 5
- IL-10:
-
Interleukin 10
- KC/GRO:
-
Cxcl1 chemokine (C-X-C motif) ligand 1
- ChIP:
-
Chromatin immunoprecipitation
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PCA:
-
Principle component analysis
References
Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–44.
Matchynski-Franks JJ, Pappas C, Rossignol J, Reinke T, Fink K, Crane A, et al. Mesenchymal stem cells as treatment for behavioral deficits and neuropathology in the 5xFAD mouse model of Alzheimer's disease. Cell Transplant. 2016;25(4):687–703.
Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–43.
Blurton-Jones M, Spencer B, Michael S, Castello NA, Agazaryan AA, Davis JL, et al. Neural stem cells genetically-modified to express neprilysin reduce pathology in Alzheimer transgenic models. Stem Cell Res Ther. 2014;5(2):46.
Verma S, Kumar A, Tripathi T, Kumar A. Muscarinic and nicotinic acetylcholine receptor agonists: current scenario in Alzheimer's disease therapy. J Pharm Pharmacol. 2018;70(8):985–93.
Connelly PJ, Adams F, Tayar ZI, Khan F. Peripheral vascular responses to acetylcholine as a predictive tool for response to cholinesterase inhibitors in Alzheimer's disease. BMC Neurol. 2019;19(1):88.
Leszek J, Md Ashraf G, Tse WH, Zhang J, Gasiorowski K, Avila-Rodriguez MF, et al. Nanotechnology for Alzheimer disease. Curr Alzheimer Res. 2017;14(11):1182–9.
Sadegh Malvajerd S, Izadi Z, Azadi A, Kurd M, Derakhshankhah H, Sharifzadeh M, et al. Neuroprotective potential of Curcumin-loaded nanostructured lipid carrier in an animal model of Alzheimer's disease: behavioral and biochemical evidence. J Alzheimers Dis. 2019;69(3):671–86.
Derakhshankhah H, Hajipour MJ, Barzegari E, Lotfabadi A, Ferdousi M, Saboury AA, et al. Zeolite nanoparticles inhibit Aβ-fibrinogen interaction and formation of a consequent abnormal structural clot. ACS Appl Mater Interfaces. 2016;8(45):30768–79.
Lotfabadi A, Hajipour MJ, Derakhshankhah H, Peirovi A, Saffar S, Shams E, et al. Biomolecular Corona dictates Aβ fibrillation process. ACS Chem Neurosci. 2018;9(7):1725–34.
Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290(5497):1779–82.
Kanamaru T, Kamimura N, Yokota T, Nishimaki K, Iuchi K, Lee H, et al. Intravenous transplantation of bone marrow-derived mononuclear cells prevents memory impairment in transgenic mouse models of Alzheimer's disease. Brain Res. 1605;2015:49–58.
Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer's disease. Brain Res. 1691;2018:87–93.
Ehrhart J, Darlington D, Kuzmin-Nichols N, Sanberg CD, Sawmiller DR, Sanberg PR, et al. Biodistribution of infused human umbilical cord blood cells in Alzheimer's disease-like murine model. Cell Transplant. 2016;25(1):195–9.
Reyes S, Tajiri N, Borlongan CV. Developments in intracerebral stem cell grafts. Expert Rev Neurother. 2015;15(4):381–93.
Shen Z, Li X, Bao X, Wang R. Microglia-targeted stem cell therapies for Alzheimer disease: a preclinical data review. J Neurosci Res. 2017;95(12):2420–9.
Naaldijk Y, Jager C, Fabian C, Leovsky C, Bluher A, Rudolph L, et al. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43(4):299–314.
Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res. 2010;7(6):540–8.
Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci. 2008;28(24):6239–49.
Safar MM, Arab HH, Rizk SM, El-Maraghy SA. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced Alzheimer-like pathological aberrations. Mol Neurobiol. 2016;53(3):1403–18.
Harach T, Jammes F, Muller C, Duthilleul N, Cheatham V, Zufferey V, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer's disease. Neurobiol Aging. 2017;51:83–96.
Ruzicka J, Kulijewicz-Nawrot M, Rodrigez-Arellano JJ, Jendelova P, Sykova E. Mesenchymal Stem Cells Preserve Working Memory in the 3xTg-AD Mouse Model of Alzheimer's Disease. Int J Mol Sci. 2016;17(2).
Yun HM, Kim HS, Park KR, Shin JM, Kang AR, il Lee K, et al. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Abeta1–42-infused mouse model of Alzheimer's disease. Cell Death Dis. 2013;4:e958.
Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, et al. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer's disease model. Neurobiol Aging. 2013;34(10):2408–20.
Salem AM, Ahmed HH, Atta HM, Ghazy MA, Aglan HA. Potential of bone marrow mesenchymal stem cells in management of Alzheimer's disease in female rats. Cell Biol Int. 2014;38(12):1367–83.
Li CQ, Liu D, Wu XQ. Differentiation of rat bone marrow stromal cells into neuron like cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29(1):18–20.
Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–56.
Perry EK, Johnson M, Ekonomou A, Perry RH, Ballard C, Attems J. Neurogenic abnormalities in Alzheimer's disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol Dis. 2012;47(2):155–62.
Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3(1):63–70.
Cho YI, Choi JS, Jeong SY, Yoo HS. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010;6(12):4725–33.
Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88(5):1017–25.
Mezey E, Chandross KJ. Bone marrow: a possible alternative source of cells in the adult nervous system. Eur J Pharmacol. 2000;405(1–3):297–302.
Li L, Chu L, Ren C, Wang J, Sun S, Li T, et al. Enhanced migration of bone marrow-derived Mesenchymal stem cells with Tetramethylpyrazine and its synergistic effect on angiogenesis and neurogenesis after cerebral ischemia in rats. Stem Cells Dev. 2019;28(13):871–81.
Mitkari B, Nitzsche F, Kerkela E, Kuptsova K, Huttunen J, Nystedt J, et al. Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav Brain Res. 2014;259:50–9.
Garcia KO, Ornellas FL, Martin PK, Patti CL, Mello LE, Frussa-Filho R, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer's disease. Front Aging Neurosci. 2014;6:30.
Gallina C, Turinetto V, Giachino C. A new paradigm in cardiac regeneration: the Mesenchymal stem cell Secretome. Stem Cells Int. 2015;2015:765846.
Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92(6):692–9.
Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287(6):H2670–6.
Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93(5):429–37.
Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52.
Yang SP, Bae DG, Kang HJ, Gwag BJ, Gho YS, Chae CB. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer's disease. Neurobiol Aging. 2004;25(3):283–90.
Wang P, Xie ZH, Guo YJ, Zhao CP, Jiang H, Song Y, et al. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2011;411(3):620–6.
Borghi R, Pellegrini L, Lacana E, Diaspro A, Pronzato MA, Vitali A, et al. Neuronal apoptosis is accompanied by amyloid beta-protein accumulation in the endoplasmic reticulum. J Alzheimers Dis. 2002;4(1):31–7.
Demeester N, Baier G, Enzinger C, Goethals M, Vandekerckhove J, Rosseneu M, et al. Apoptosis induced in neuronal cells by C-terminal amyloid beta-fragments is correlated with their aggregation properties in phospholipid membranes. Mol Membr Biol. 2000;17(4):219–28.
Benvenuti S, Saccardi R, Luciani P, Urbani S, Deledda C, Cellai I, et al. Neuronal differentiation of human mesenchymal stem cells: changes in the expression of the Alzheimer's disease-related gene seladin-1. Exp Cell Res. 2006;312(13):2592–604.
Okazaki T, Magaki T, Takeda M, Kajiwara Y, Hanaya R, Sugiyama K, et al. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci Lett. 2008;430(2):109–14.
Liu L, Cao JX, Sun B, Li HL, Xia Y, Wu Z, et al. Mesenchymal stem cells inhibition of chronic ethanol-induced oxidative damage via upregulation of phosphatidylinositol-3-kinase/Akt and modulation of extracellular signal-regulated kinase 1/2 activation in PC12 cells and neurons. Neuroscience. 2010;167(4):1115–24.
Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, et al. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005(1–2):21–8.
Mo SJ, Zhong Q, Zhou YF, Deng DB, Zhang XQ. Bone marrow-derived mesenchymal stem cells prevent the apoptosis of neuron-like PC12 cells via erythropoietin expression. Neurosci Lett. 2012;522(2):92–7.
Marei HE, Farag A, Althani A, Afifi N, Abd-Elmaksoud A, Lashen S, et al. Human olfactory bulb neural stem cells expressing hNGF restore cognitive deficit in Alzheimer's disease rat model. J Cell Physiol. 2015;230(1):116–30.
Han L, Zhou Y, Zhang R, Wu K, Lu Y, Li Y, et al. MicroRNA let-7f-5p promotes bone marrow Mesenchymal stem cells survival by targeting Caspase-3 in Alzheimer disease model. Front Neurosci. 2018;12:333.
Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 2009;450(2):136–41.
Schafer S, Calas AG, Vergouts M, Hermans E. Immunomodulatory influence of bone marrow-derived mesenchymal stem cells on neuroinflammation in astrocyte cultures. J Neuroimmunol. 2012;249(1–2):40–8.
Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J Jr, Leung BP, Rezai-Zadeh K, et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85(3):534–48.
Kim M, Kim KH, Song SU, Yi TG, Yoon SH, Park SR, et al. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J Tissue Eng Regen Med. 2018;12(2):e1034–e45.
Song M, Lee JH, Bae J, Bu Y, Kim EC. Human dental pulp stem cells are more effective than human bone marrow-derived Mesenchymal stem cells in cerebral ischemic injury. Cell Transplant. 2017;26(6):1001–16.
Wu W, Yang JQ, He ZY. Effect of ginsenoside Rg1 on the spatial learning-memory ability in dementia rats after transplanted with bone marrow mesenchymal stem cells. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi. 2011;31(6):799–802.
Li WY, Jin RL, Hu XY. Migration of PKH26-labeled mesenchymal stem cells in rats with Alzheimer's disease. Zhejiang da xue xue bao Yi xue ban. 2012;41(6):659–64.
Kastrinaki MC, Andreakou I, Charbord P, Papadaki HA. Isolation of human bone marrow mesenchymal stem cells using different membrane markers: comparison of colony/cloning efficiency, differentiation potential, and molecular profile. Tissue Eng Part C Methods. 2008;14(4):333–9.
Acknowledgments
Not applicable
Funding
This work was supported by grants Beijing Natural Science Foundation (No. 517100), National Key Research and Development Project (No. 2017YFA0105200) and CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-2-006).
Author information
Authors and Affiliations
Contributions
CQ conceived and designed the manuscript. YL and KW were responsible for data collection and statistical analysis. Additional data were provided by LB. YL, KW, LB, GS, YH and YL supported data analysis and interpretation. KW wrote the first draft that was revised by CQ and YL. All authors approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have nothing to disclose.
Supplementary information
Additional file 1: Table 1.
Stem cell transplantation for the treatment of Alzheimer’ disease. Present study utilized keywords “Alzheimer’s disease” and “stem cell transplantation” to identify literature. The supplementary table further scrutinized relevant information of stem cell transplantation in different animal models.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qin, C., Lu, Y., Wang, K. et al. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: a meta-analytic review on potential mechanisms. Transl Neurodegener 9, 20 (2020). https://doi.org/10.1186/s40035-020-00199-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40035-020-00199-x