Abstract
Background
Renally adjusted lamivudine dosages are effective. However, some of the kidney failure patients managed with lamivudine-containing regimens are failing to suppress HIV in peritoneal dialysis (CAPD) effluent. The steady-state lamivudine pharmacokinetics among these patients was evaluated.
Methods
This overnight open-label pharmacokinetic study enrolled participants living with HIV and managed with CAPD. Lamivudine levels in blood serum and CAPD effluent samples were quantified using liquid chromatography coupled with a mass spectrometer. Pharmacokinetic measures were obtained through non-compartmental analysis.
Results
Twenty-eight participants were recruited with a median antiretroviral (ARV) drug duration of 8 (IQR,4.5–10.5) years and a CAPD duration of 13.3 (IQR,3.3–31.9) months. 14.3% (4/28) had detectable unsuppressed HIV-1 viral load in CAPD effluents. The majority (78,6%,22/28) of participants received a 50 mg dose, while 10.7% (3/28), and another 10.7% (3/28) received 75 mg and 300 mg dosages, respectively. Among those treated with 75 and 300 mg, 66.7% (2/3) and 33.3% (1/3) had detectable HIV-VL in CAPD, respectively. The peritoneal membrane characteristics and CAPD system strengths were variable across the entire study population. Lamivudine exposure was increased in blood serum (50 mg-AUC0-24 h, 651.3 ng/mL; 75 mg-AUC0-24 h, 677.84 ng/mL; 300 mg-AUC0-24 h, 3135.89 ng/mL) compared to CAPD effluents (50 mg-AUC0-24 h, 384.91 ng/mL; 75 mg-AUC0-24 h, 383.24 ng/mL; 300 mg-AUC0-24 h, 2001.60 ng/mL) among the entire study population. The Cmax (50 mg, 41.5 ng/mL; 75 mg, 53.2 ng/mL; 300 mg, 199.1 ng/mL) and Cmin (50 mg, 17.8 ng/mL; 75 mg, 16.4 ng/mL; 300 mg, 76.4 ng/mL) measured in serum were within the therapeutic levels.
Conclusions
Steady-state lamivudine pharmacokinetic measures were variable among the entire study population. However, the total lamivudine exposure was within the therapeutic levels.
Key Learning Points
-
What was known: steady-state lamivudine pharmacokinetics in persons living with HIV (PLHIV) and managed with peritoneal dialysis (PD).
-
This study adds: HIV-1 shedding into CAPD effluents is not necessarily contributed by reduced lamivudine dosages pharmacokinetics.
-
Potential impact: Renally adjusted lamivudine dosages are effective even in patients shedding HIV-1 into CAPD effluents.
Similar content being viewed by others
Introduction
Lamivudine (3TC) is a nuclease reverse transcriptase inhibitor (NRTI) agent used in combination with other antiretroviral drugs [1]. It is recommended by the World Health Organisation as the first-and second-line antiretroviral therapy (ART) regimen in the management of HIV and Hepatitis B infection [2]. Lamivudine should always be used with other antiretroviral drugs to achieve more effective viral suppression and to prevent the development of resistance [1]. Because lamivudine is excreted in urine, dose adjustment is required in patients with renal impairment [3].
The altered lamivudine dosages have shown to be effective even in people having kidney failure (KF) and managed with continuous ambulatory peritoneal dialysis (CAPD) [4, 5]. In comparison to subjects with normal renal function, those with impaired renal function had higher peak concentrations in the serum (Cmax), longer terminal elimination half-lives (T1/2), and areas under the serum concentration–time curves (AUCs) [6, 7]; based on the results from these studies, lamivudine dose adjustment is required in KF patients. Dose reduction for renal insufficiency can be accomplished by altering either dose size or dosing interval [8]. The current local HIV treatment guidelines in persons managed with CAPD recommend daily dosing of an oral tablet or solution at 50 mg or 75 mg depending on the creatinine clearance, glomerular filtration rate, and tolerance [4].
After oral administration lamivudine has a rapid dissolution rate and a bioavailability of approximately 82% in adults; it takes roughly 0.5 to 1.5 h to reach maximum serum concentrations (Cmax) in the non-KF population [1]. However, in the KF population, the time of maximum concentration (Tmax) is delayed in patients receiving drugs such as phosphate binders that interact with its active metabolite, 5′-triphosphate, and may lower the bioavailability [9]. Approximately 70% of an oral dose of lamivudine is excreted unchanged by the kidneys in patients with normal renal function [1] and 16% is removed by CAPD [6] because of its low molecular weight and decreased serum protein binding capacity; furthermore, CAPD dwell duration, peritoneal membrane type, blood supply to the peritoneal cavity as well as the strength of dialysate solution may be possible inter-patient pharmacokinetic variability factors likely to contribute to variable lamivudine clinical effects and outcomes [9].
Lamivudine dosages of 10 mg/mL solution and 150 mg tablet in the KF population managed with dialysis were shown to have similar AUC0-24, Cmax, and longer T1/2 compared to populations with normal kidney function [5, 6]. However, shedding of HIV-1 into CAPD effluents has been suggested in patients who are on steady-state lamivudine concentration [10,11,12,13]. Thus, the aim of this study was to evaluate the steady-state lamivudine pharmacokinetics in KF cohorts of participants managed with CAPD and in relation to HIV-1 shedding in CAPD effluents.
Materials and methods
Ethics statement
The study protocol was approved by the University of the Free State-Health Sciences Research Ethics Committee (UFS-HSREC) (UFS-HSD2020/0318/2710 & UFS-HSD2021/0267/2505 & UFS-HSD2021/0267/2505-0006), and permission for the study was granted by the Free State Department of Health. All participants provided written informed consent before enrolment, and study procedures were done in accordance with the Helsinki Declaration of Clinical Research on Human subjects [14].
Study population
This is a sub-study of the main prospective cross-sectional study [12]. It recruited people living with HIV and kidney failure and managed with an ART-lamivudine-containing regimen and CAPD at Universitas Academic Hospital. The eligibility criteria included participants who were 18 years and older at the time informed consent was signed and living with HIV on ART for more than 3 months. Participants were excluded if they had active peritonitis at the time of enrolment, had an ART treatment duration of less than 3 months, showed any significant hematologic, hepatic, or pancreatic dysfunction, any documented ART non-adherence and history of substance abuse.
Study design
This was an open-label non-randomised pharmacokinetics study performed at one of the clinical research organisations (FARMOVs) at the University of the Free State main campus. Participants were invited for a three-day admission after their routine clinic visit day. On the 1st day of admission participants’ ART and CAPD prescriptions were assessed and recorded by the research nurse. Vital signs and anthropometric measurements were recorded, and laboratory data were derived from the electronic database of Universitas Academic Hospital. Y-sets, twin-bag systems, and conventional peritoneal dialysis (PD) solutions (Adcock Ingram and Fresenius, South Africa) were used in all CAPD patients. The ART dosages were according to the patient's local clinic prescription.
On the 2nd day of admission, a pre-dose sample of blood and CAPD effluent were taken 30 min before the morning or night exchange; CAPD exchange was completed to ensure the participant does not ‘dry-up’ and a regular ART prescription comprising of lamivudine dosage either one of 50, 75 or 300 mg was taken at 07:00 or 19:00 depending on the previous time of the participants’ daily dosage intake. On the 3rd day of admission, the ‘24-h’ samples were collected from each participant. During the entire admission period, participants were fed on a standardised’ renal-friendly’ diet.
6 mL serum samples were obtained at pre-dose, 0.5 h, and post-dose 1, 2, 4, 6, 8, 12, 16, and 24 h using a red-cap serum separation vacutainer (Lasec, South Africa). During this period, participants continued their usual CAPD schedule, four times post-dose as per the participants’ clinic CAPD prescription. Before each exchange, a CAPD effluent sample was collected using a sterile 10 mL syringe, directly from a functioning Tenckhoff catheter into a sterile specimen bottle. The first 10 mL of CAPD effluent was aspirated and discarded. All samples were transported on ice to the research laboratory, where whole blood was centrifuged at 2500 rpm for 20 min, and serum was separated in hooded benchtops. Both separated serum and aliquoted CAPD effluent samples were freeze-stored in 2 mL Eppendorf tubes at -70 °C throughout the study period.
Quantification of lamivudine levels by LC–MS/MS
The lamivudine concentrations were measured using a validated LC–MS/MS method. Lamivudine and internal standard (abacavir-d4) in both serum and CAPD effluents were extracted, desalted, and concentrated onto methanol-conditioned C18 1 mL solid phase extraction cartridges (Oasis Prime HLB 1 cc/30 mg, Waters) at a flow rate of 1 mL/min. The eluents were vacuum dried, reconstituted in a 500 µL H2O with 0.1% formic acid and separated on a C18 (50 mm × 2.0 mm, Aqua, Phenomenex) column and detected by positive-ion MRM (multiple reaction monitoring) using two transitions per analyte.
The nominal concentrations ranged from 0.005 to 1.25 ppm for the lamivudine calibration curve using 500 µL sample volume. The intraassay precision and accuracy of the method (coefficient of variation) were 9.1% and 10.6%, respectively, while at the lowest limit of quantification, it was 15.5% and 17.2%, respectively.
Safety
The participants’ vital signs were checked during admission and monitored by research nurses during the course of this study; thereafter, by a nephrologist.
Steady-state lamivudine pharmacokinetic analysis
The non-compartmental pharmacokinetic analysis was performed to estimate the standard pharmacokinetic parameters for lamivudine. The mean area under the time-versus-concentration curve from 0 till 24-h period (AUC0-24 h), half-life (T1/2), elimination constant rate (ke), as well as AUC from 0 till infinity (AUC0-∞) were extrapolated from the concentration–time curve using Stata version 15 (StataCorp LP, College Station, TX, USA). The AUC0-∞ was estimated using three different models, estimated with a linear fit, estimated with an exponential fit, and estimated with a linear fit of the natural log concentrations. Furthermore, the maximum concentration (Cmax) in serum and CAPD samples and the time of maximum concentration (Tmax) as well as trough levels before the next dosage (Cmin) were estimated from the concentration–time curve.
Statistical analysis
Continuous variables were summarized as medians and interquartile ranges (IQRs) and compared using Kruskal‒Wallis’s equality-of-populations rank test. Categorical and ordinal variables were summarized using proportions and percentages and were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate. All analyses were performed using Stata version 15 (StataCorp LP, College Station, TX, USA). The level of significance was set at p < 0.05.
Results
Patient characteristics
We enrolled 28 participants with KF receiving lamivudine-based regimens and managed with CAPD. Participants had a median age of 42.6 (IQR, 38.9–48.1) years at the time of enrolment with a median body mass index (BMI) of 22.5 (IQR, 20.8–24.1) Kg/m2. More females were enrolled [71.4% (20/28)]. Most participants received lamivudine 50 mg (78.6%, 22/28). The median duration of ARV treatment was 8 (IQR, 4.5–10.5) years, and CAPD treatment was 13.3 (IQR, 3.3–31.9) months. The median T-cell CD4 count was 356 (IQR, 234–500) cells/mm3. The frequency of HIV-1 detectable in CAPD effluents was 14.3% (4/28) with median viral load in serum and CAPD effluents 43,400 (IQR, < 20–101000) and 388 (< 20–675) copies/mL, respectively. Furthermore, the majority (85.7%, 24/28) of participants had suppressed serum and CAPD viral loads. Lastly, the peritoneal membrane type and pharmacokinetic measures were variable among study participants who underwent PET, Table 1.
Safety
Lamivudine and CAPD procedures were well tolerated among the study population. No adverse reactions were observed during the three-day admission.
Steady-state serum lamivudine pharmacokinetic analysis
50 mg oral solution lamivudine pharmacokinetic measures
The observed geometric mean of lamivudine AUC (AUC0-24) was higher (666.04 ± 434.8 vs 341.76 ± 0 ng/mL) among the undetectable CAPD HIV-1 viral load cohort when compared to a cohort with detectable HIV-1 in CAPD. T1/2 was decreased (10.47 ± 0 vs 18.70 ± 9.0 h per hour) in the detectable cohort of HIV-1 in CAPD than in a cohort with undetectable HIV-1 in CAPD effluents. The Ke was increased (0.07 vs 0.04) among the HIV-1 detectable cohort compared to the undetectable cohort. The Cmax (42.03 ± 23.4 vs 30.2 ± 0 ng/mL) and Cmin (18.3 ± 13.7 vs 6.7 ± 0 ng/mL) were increased in a cohort undetectable of HIV-1 in CAPD than a detectable cohort. However, Tmax among the two groups was comparable (4.05 ± 2.2 vs 4 ± 0 h); although it was lower compared to Tmax in the peritoneal compartment (11.81 ± 4.5 vs 12 ± 0 h), Table 2.
75 mg oral tablet lamivudine pharmacokinetic measures
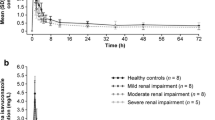
Lamivudine exposure in serum was higher, AUC0-24 (964.93 ± 628.0 vs 103.67 ± 0 ng/mL), and Cmax (76.80 ± 33.9 vs 6.04 ± 0 ng/mL) in a cohort of HIV detectable in CAPD than an undetectable cohort. T1/2 was decreased (10.03 ± 4.3 vs 28.40 ± 0 h) in a cohort with detectable HIV in CAPD compared to the undetectable cohort. Alternatively, the rate of elimination was increased (0.08 vs 0.05) in the detectable than undetectable cohort, Table 2. The total blood serum exposure of lamivudine between participants who received 50 mg and 75 mg was comparable, Fig. 1.
300 mg oral FDC lamivudine pharmacokinetics measures
The mean serum lamivudine exposure was increased in a cohort undetectable of HIV-1 in CAPD, AUC0-24 (3814.33 ± 836.8 vs 1779.02 ± 0 ng/mL) than in a detectable cohort. The Ke (0.05 vs 0.04) and T1/2 (15.41 ± 2.3 vs 16.86 ± 0) were almost comparable among the two cohorts. Although the Tmax (3 ± 1.7 vs 4 h ± 0) was comparable, the Cmax (245.20 ± 15.7 vs 107.00 ± 0 ng/mL) and Cmin (92.2 ± 17.5 vs 44.8 ± 0 ng/mL) was variable and increased among a cohort of undetectable HIV-1 in CAPD than the detectable cohort, Table 2. All participants were over-exposed to lamivudine at 300 mg compared to 50 and 75 mg dosages, Fig. 1.
Steady-state lamivudine pharmacokinetics in the peritoneal compartment
50 mg lamivudine oral solution pharmacokinetic measures
The lamivudine exposure in the peritoneal cavity was increased in a cohort undetectable of HIV-1 in CAPD effluents, AUC0-24 (398.14 ± 264.3 vs 107.12 ± 0 ng/mL), and Cmax (22.72 ± 14.4 vs 7.38 ± 0 ng/mL) compared to a cohort detectable of HIV in CAPD. T1/2 was decreased (7.57 ± 0 vs 176.02 ± 614.7 h) in a cohort detectable of HIV-1 in CAPD than undetectable cohort. Alternatively, ke was increased (0.09 vs 0.05) in a cohort detectable of HIV-1 in CAPD than undetectable cohort, Table 2.
75 mg lamivudine oral tablet pharmacokinetic measures
The exposure of lamivudine in the peritoneal cavity was decreased in a cohort undetectable of HIV-1 in CAPD effluents, AUC0-24 (72.38 ± 0 vs 538.67 ± 378.9 ng/mL), and Cmax (3.78 ± 0 vs 39.20 ± 31.7 ng/mL) compared to a cohort detectable of HIV-1 in CAPD effluents. T1/2 was lower (14.2 ± 7.3 vs 26.96 ± 0) in a cohort detectable of HIV-1 in CAPD. However, ke was higher (0.05 vs 0.03) in the CAPD HIV-1 detectable cohort than the undetectable cohort, Table 2.
300 mg lamivudine FDC oral table pharmacokinetic measures
The mean lamivudine exposure was lower in a cohort detectable of HIV-1 in CAPD effluents, AUC0-24 (1278.20 ± 0 vs 2363.30 ± 415.4 ng/mL) and Cmax (65.60 ± 0 vs 132.50 ± 17.4 ng/mL) with a decreased Tmax (4.00 ± 0 vs 12.00 ± 8.5 h) compared to the undetectable cohort. Although the apparent T1/2 was decreased (13.76 ± 0 vs 22.86 ± 6.8 h) in a cohort detectable of HIV-1 in CAPD, the ke was increased (0.05 vs 0.03) compared to a cohort undetectable of HIV-1 in CAPD, Table 4.
Discussion
This study evaluated the steady-state lamivudine pharmacokinetics in KF patients with suppressed and unsuppressed CAPD viral load. The blood serum and CAPD pharmacokinetic measures were variable among cohorts with detectable and undetectable HIV-1 in CAPD effluents. The total exposure of lamivudine was higher in blood compared to CAPD effluents for all lamivudine dosages. Serum and CAPD exposure was decreased in two participants with detectable CAPD viral load and treated with 50 and 300 mg, respectively; however, it was within the therapeutic ranges in serum for all participants.
Lamivudine serum exposure (AUC0-24) was variable among participants (4/28) shedding HIV-1 in CAPD effluents, possibly due to inter-patient pharmacokinetic variability. Furthermore, lamivudine concentrations, including Cmin, in these participants, were above the reported lamivudine 90% inhibitory concentration (IC90) range against HIV-1 in various cell lines (0.0087 to 0.464 µg/ml) [15]; thus, suggesting adequate therapeutic exposure. Studies on steady-state lamivudine pharmacokinetics by Bohjanen et al. [6] reported the serum AUC0-24 (49,800 ng/h/mL), Cmax (3770 ng/mL), and Cmin (1410 ng/mL) in HIV-suppressed CAPD participants treated with a daily dosage of 150 mg; furthermore, Yuen et al. [15] reported AUC0-24 (9210 ng/h/mL) and Cmax (1190 ng/mL) and Cmin (90 ng/mL) in steady-state non-KF patients who received 150 mg bi-daily dosage. A study by Heald et al. [7] looked at non-steady-state KF patients receiving 300 mg lamivudine dosage and reported a Cmax of 5684 ng/mL. These mean concentration values were also above the reported IC90 and IC50 [16,17,18] and within a comparable range to our findings. The concentrations shown in Bohjanen study [6] were effective and tolerated and suggested a reduced daily dosage of 25 mg in dialysis patients which is suggested to provide the same exposure to 150 mg bi-daily dosage in non-KF patients. This may suggest 50 mg, 75 mg and 300 mg daily dosages to be effective in our study population. However, safety studies may be necessary to evaluate the tolerability of these dosages.
The Tmax in participants shedding HIV-1 in CAPD effluents was almost comparable to participants with suppressed HIV-1 in CAPD. This was an expected finding, since impaired renal function does not affect lamivudine absorption in the gastrointestinal tract. Furthermore, this may suggest an almost comparable absorption rate of lamivudine into the systemic circulation and penetrance into CAPD effluents among these participants and the majority with a suppressed HIV-1 viral load.
The rate of elimination in participants shedding HIV-1 in CAPD effluents was slightly increased in CAPD effluents (5–9% per hour per mL) compared to serum (4–8% per hour per mL) samples among all lamivudine dosages. The elimination rate in CAPD effluents was almost half-fold lower compared to 16% reported in a study by Bohjanen et al. [6]. Notably, a patient who received 50 mg and shedding HIV-1 in CAPD effluents had an increased elimination rate in the PD compartment (9%) with a decreased half-life (7.6 h) compared to the majority of patients who were HIV-suppressed. Although the hypertonic dialysate solution and peritoneal membrane type are hypothesised to influence drug elimination in CAPD effluents [19, 20], this was not the case in this patient because the elimination rate could not reduce the AUC0-24 and Cmax and Cmin (6.7 ng/mL) below the therapeutic levels, and was not below Cmin reported as 1410 ng/mL in [6]. Thus, it may not affect the bioavailable lamivudine pro-drug effective concentrations in the serum.
The steady-state lamivudine exposure in serum and CAPD samples does not suggest inadequate lamivudine dosing across all study participants (Table 2). Lamivudine is maintained even in 'renal-friendly' ART or second-line regimens due to its ability to enhance the susceptibility/pharmacodynamics of NRTI, Zidovudine [21], and Tenofovir [22] against HIV drug resistance mutations. However, viral suppression is unlikely in the presence of M184V mutation. HIV-1 shedding among participants who received a higher lamivudine dosage did not alter pharmacokinetics parameters. Thus, suggesting HIV drug resistance mutation may be possible influencers of HIV-1 shedding in CAPD effluents as demonstrated in a cross-sectional study by Mooko et al. [12].
This study had several limitations, first, this sub-study was not powered for pharmacokinetic analysis of 75 mg and 300 mg lamivudine doses. Second, our study did not quantify the active lamivudine metabolite, 5′-triphosphate in study samples as it is only generated from a small (30%) fraction of lamivudine pro-drug, which undergoes a minor route excretion.
Conclusions
The lamivudine dosages evaluated in this study do not suggest inadequate dosing in KF patients managed with CAPD even in those shedding HIV-1 in CAPD effluents. Thus, highlighting a need for improved ARV treatment stratagems to mitigate challenges associated with adherence and intensify health education in this patient population.
Data availability
Not applicable.
References
Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66.
World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021.
Bouazza N, Tréluyer J, Ghosn J, Hirt D, Benaboud S, Foissac F, et al. Evaluation of effect of impaired renal function on lamivudine pharmacokinetics. Br J Clin Pharmacol. 2014;78(4):847–54.
National Department of Health South Africa. 2023 ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates. https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-07/National%20ART%20Clinical%20Guideline%20AR%204.5%2020230713%20Version%204%20WEB.pdf. Accessed 22 Aug 2023.
Asari A, Iles-Smith H, Chen Y, Naderer OJ, Johnson MA, Yuen GJ, et al. Pharmacokinetics of lamivudine in subjects receiving peritoneal dialysis in end-stage renal failure. Br J Clin Pharmacol. 2007;64(6):738–44.
Bohjanen PR, Johnson MD, Szczech LA, Wray DW, Petros WP, Miller CR, et al. Steady-state pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with end-stage renal disease receiving chronic dialysis. Antimicrob Agents Chemother. 2002;46(8):2387–92.
Heald AE, Hsyu PH, Yuen GJ, Robinson P, Mydlow P, Bartlett JA. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob Agents Chemother. 1996;40(6):1514–9.
Gupta SK, Eustace JA, Winston JA, Boydstun II, Ahuja TS, Rodriguez RA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2005;40(11):1559–85.
Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65:757–73.
Mooko T, Bisiwe B, Chikobvu P, Morobadi D, Nyaga M, Bester P, et al. # 4355 the influence of antiretroviral drugs and hiv resistance mutations on the shedding of hiv-1 into peritoneal dialysis effluent. Nephrol Dial Transpl. 2023;38(Supplement_1):gfad063c_4355.
Mooko T, Bisiwe B, Mondleki E, Morobadi D, Chikobvu P, Nyaga M, et al. # 3017 Renal-friendly lamivudine dosages are unlikely to contribute to HIV-1 shedding into PD effluents—an open label non-randomized pharmacokinetics study. Nephrol Dial Transpl. 2024;39(Supplement_1):gfae069-1654.
Mooko T, Bisiwe FB, Chikobvu P, Morobadi MD, Mofokeng TRP, Nyaga MM, et al. The prevalence of HIV resistance mutations and their influence on the shedding of HIV-1 into peritoneal dialysis effluent. J Med Virol. 2024;96(6): e29734.
Ndlovu KC, Sibanda W, Assounga A. Detection of human immunodeficiency virus-1 ribonucleic acid in the peritoneal effluent of renal failure patients on highly active antiretroviral therapy. Nephrol Dial Transpl. 2017;32(4):714–21.
General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14–8.
Yuen GJ, Lou Y, Bumgarner NF, Bishop JP, Smith GA, Otto VR, et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48(1):176–82.
Coates J, Cammack N, Jenkinson H, Mutton I, Pearson B, Storer R, et al. The separated enantiomers of 2’-deoxy-3’-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992;36(1):202–5.
Soudeyns H, Yao X, Gao Q, Belleau B, Kraus JL, Nguyen-Ba N, et al. Anti-human immunodeficiency virus type 1 activity and in vitro toxicity of 2’-deoxy-3’-thiacytidine (BCH-189), a novel heterocyclic nucleoside analog. Antimicrob Agents Chemother. 1991;35(7):1386–90.
Quercia R, Perno CF, Koteff J, Moore K, McCoig C, Clair MS, et al. Twenty-five years of lamivudine: current and future use for the treatment of HIV-1 infection. J Acquir Immune Defic Syndr 1999. 2018;78(2):125.
Matzke GR, Millikin SP. Influence of renal function and dialysis on drug disposition. Appl Pharmacokinet Princ Ther Drug Monit 3rd Ed Appl Ther Inc Vanc Wash. 1992;1–49.
Rowland M, Tozer TN. Clinical pharmacokinetics: concepts and applications. Clinical pharmacokinetics: concepts and applications. 1980.
Larder BA, Kemp SD, Harrigan PR. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995;269(5224):696–9.
Wolf K, Walter H, Beerenwinkel N, Keulen W, Kaiser R, Hoffmann D, et al. Tenofovir resistance and resensitization. Antimicrob Agents Chemother. 2003;47(11):3478–84.
Acknowledgements
The authors wish to thank all study participants for their voluntary participation in this study and research nurses, Mrs. AL Ngcezula, Mrs. TC Walaza, and Ms. TR Mahamotse who assisted with study samples collection and monitoring of participants during the three-day admission. We would also like to thank FARMOVs for renting out their facilities as well as medical equipment which were used to collect data during this study. Parts of this manuscript were presented during the 61st European Renal Association Congress 2024, Stockholm, Sweden, May 23-26, 2024
Funding
The study was made possible by funding from Thuthuka National Research Foundation (NRF) grant TTK190417431220 awarded to Prof. KC Ndlovu and BMGF grant INV-046917 awarded to Prof. Nyaga MM. A PhD scholarship and travel grants were granted to Dr. Teboho Mooko by the CSIR-DSI Inter-bursary Support Programme (IBS).
Author information
Authors and Affiliations
Contributions
Mooko Teboho organized the project, collected and analysed the data, and contributed majorly to manuscript writing. Ndlovu Kwazi and all other co-authors supervised Mooko Teboho and supported the study through their resources.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the University of the Free State-Health Sciences Research Ethics Committee (UFS-HSREC) [UFS-HSD2020/0318/2710 (date: 09/10/2022) & UFS-HSD2021/0267/2505 (date: 15/02/2021) & UFS-HSD2021/0267/2505-0006 (date: 19 May 2022)], and permission for the study was granted by the Free State Department of Health. All participants provided written consent before enrolment.
Competing interests
No conflict of interest was declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mooko, T., Bisiwe, F.B., Mondleki, E. et al. Steady-state pharmacokinetics of lamivudine in end-stage kidney failure persons with detectable and undetectable HIV-1 RNA in peritoneal dialysis effluent. Eur J Med Res 29, 374 (2024). https://doi.org/10.1186/s40001-024-01972-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01972-8