Abstract
As a hepatotropic virus, hepatitis B virus (HBV) can establish a persistent chronic infection in the liver, termed, chronic hepatitis B (CHB), which causes a series of liver-related complications, including fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). HCC with HBV infection has a significantly increased morbidity and mortality, whereas it could be preventable. The current goal of antiviral therapy for HBV infection is to decrease CHB-related morbidity and mortality, and achieve sustained suppression of virus replication, which is known as a functional or immunological cure. The natural history of chronic HBV infection includes four immune phases: the immune-tolerant phase, immune-active phase, inactive phase, and reactivation phase. However, many CHB patients do not fit into any of these defined phases and are regarded as indeterminate. A large proportion of indeterminate patients are only treated with dynamic monitoring rather than recommended antiviral therapy, mainly due to the lack of definite guidelines. However, many of these patients may gradually have significant liver histopathological changes during disease progression. Recent studies have focused on the prevalence, progression, and carcinogenicity of indeterminate CHB, and more attention has been given to the prevention, detection, and treatment for these patients. Herein, we discuss the latest understanding of the epidemiology, clinical characteristics, and therapeutic strategies of indeterminate CHB, to provide avenues for the management of these patients.
Similar content being viewed by others
Introduction
As a hepatotropic DNA virus, hepatitis B virus (HBV) can establish a persistent chronic infection, and cause a series of liver-related complications, including impaired liver function, fibrosis, cirrhosis, failure, and hepatocellular carcinoma (HCC) [1]. HBV is mainly transmitted by perinatal, percutaneous or sexual exposure, and by close person-to-person contact, among which perinatal transmission remains the most important cause of chronic infection [2]. The prevalence of HBV infection, as assessed by the presence of hepatitis B surface antigen (HBsAg), varies widely by regions [2]. Its morbidity and mortality have decreased owing to infant prophylaxis, early childhood vaccination, and medication to treat HBV infection; however, HBV has not yet been eradicated, mainly due to the lack of a virological cure for HBV infection [3,4,5]. Approximately 250 million people are living with HBV infection worldwide [3, 6], and a large proportion of liver fibrosis, cirrhosis, or HCC cases are associated with HBV infection [7]. Therefore, it is vital to understand the natural history of chronic hepatitis B (CHB) infection, and to investigate clinical preventive and therapeutic strategies to eliminate HBV.
According to the 2016 and 2018 Hepatitis B Guidelines proposed by the American Association for the Study of Liver Diseases (AASLD) [2, 4], CHB has been traditionally characterized into four immune phases, namely, the immune-tolerant phase, immune-active phase, inactive CHB phase, and immune reactivation phase, reflecting the dynamic correlations of HBV replication and evolution with host immune responses. Dynamic and serial monitoring of serum HBV antigens and antibodies, HBV DNA and alanine aminotransferase (ALT), as well as liver histopathology, helps to identify the immune phase of a chronic HBV infection [2, 4]. Patients with HBV infection can transition through different phases, and do not always evolve through these four phases in a subsequent manner [2, 4, 8, 9]. There is a high prevalence of HBV infection in China, and the Chinese Society of Hepatology, Chinese Medical Association [9, 10] has recommended that immune-active or reactivation CHB patients with elevated ALT levels should be treated with antiviral therapy; however, it may be controversial to treat CHB patients in immune-tolerant or inactive phase with HBsAg and HBV DNA positive and ALT beyond upper limits of normal (ULN). Furthermore, many CHB patients do not fit into any of these well-known phases, because their HBV DNA and/or ALT levels are outside of the defined ranges; these patients are considered “indeterminate patients” or in the “gray zone” [2, 4, 8]. The AASLD 2016 and 2018 HBV Guidelines [2, 4] suggest to dynamically monitor the serum HBV DNA and ALT levels of indeterminate patients rather than apply antiviral therapy. However, these patients may also be confronted with HBV-related complications [8, 11], and might benefit from antiviral therapy [12]. At present, there is no unified global definition of the indeterminate CHB phase. Although such a definition remains controversial, it is essential to reach a consensus on the management of indeterminate CHB patients, with accurate diagnostic and appropriate therapeutic strategies. This review discusses the epidemiology, clinical characteristics, and therapeutic strategies of indeterminate CHB to provide avenues for the management of these patients.
Epidemiology
Although the incidence rates of new HBV infections have gradually decreased worldwide owing to infant prophylaxis, early childhood vaccination, and medication to treat HBV infection, the World Health Organization (WHO) has reported that there were still approximately 250 million (3.5%) people with chronic HBV infection, and more than 1 million deaths were attributable to CHB-related complications [3,4,5,6]. Specifically, the implementation of birth three-dose coverage of HBV vaccination, considered as one of the largest strides, is limited in many developing countries, which seems to be the largest constrain on eliminating HBV infection [3]. Besides, patients with HBV infection are not always evaluated and treated adequately [13]. Thus, the effective strategies to prevent HBV infection and CHB progression have not been fully put into effect [3]. Moreover, the uneven geographic distribution of HBV prevalence has been emphasized, and the majority of HBV infections concentrates in the Western Pacific Region (116 million people) and the African Region (81 million people), which is inversely proportional to the income level [13, 14]. For instance, the prevalence rate of HBsAg positivity is 5–6% in the whole population in China, and approximately 70 million people have chronic HBV infection, including 20–30 million CHB cases [10, 15]. Furthermore, according to data from the 2019 Global Burden of Disease (GBD) Study (https://vizhub.healthdata.org/gbd-results/), estimated 331,000 deaths were from HBV-related chronic liver diseases in 2019, which did not change significantly compared to the previous decade [13, 16]; if the current situation remains, the annual mortalities from CHB-related complications are expected to increase by 39% from 2015 to 2030 [13]. Of note, the mortalities from CHB in different regions vary worldwide, with an uneven geographic distribution analogous to HBV prevalence mentioned above [13, 14, 17]. Although WHO has raised a goal of eliminating viral hepatitis as a public health problem by 2030 [15], the current data indicate that HBV infection remains a major global health problem, especially in developing countries, and more attention should be given to CHB patients.
The proportion of CHB patients with indeterminate phase
There have been considerably different epidemiological findings regarding indeterminate CHB patients [8, 18, 19]. One retrospective cohort study [8] recruited 3366 non-cirrhotic patients with untreated chronic HBV infection, and found that 38.7% of these patients were in indeterminate phase [19]; after a 10-year follow-up, 52.7% remained indeterminate, and 21.7% turned to the immune-active phase. Spradling PR et al. [11] demonstrated that more than half of 1598 CHB patients were indeterminate in a general US health care setting. Another retrospective study in China [18] that enrolled 4759 treatment-naïve CHB patients found that approximately 27.78% were defined as indeterminate (25.8%). Thus, the proportion of indeterminate patients has varied among studies based on distinct levels of upper/lower limits of normal (ULN/LLN) for HBsAg, HBV DNA, and ALT [2, 4, 10, 20]. These parameters are pivotal in the classification of immune phases for CHB [2, 4, 10, 20]. For instance, a study [21] of Asian Americans with CHB showed that 37% of these cases were indeterminate based on the conventional ULN for ALT (40 IU/L), whereas 33% were indeterminate when applying a modified ULN for ALT (30 IU/L for males and 19 IU/L for females). Although the epidemiological data of indeterminate patients may not be consistent in different studies due to different guidelines, the proportion of such CHB patients is not small and cannot be neglected, and more attention should be given to this population. A recent Chinese retrospective study [18] focused on untreated indeterminate CHB patients, and reported that indeterminate patients could be further divided into subgroups that were similar to the defined immune phases [2]. Specifically, 13.92% of indeterminate patients were similar to CHB patients with immune-tolerant phase but did not precisely fit into this phase; 7.79% were similar to CHB patients with immune-active phase, 24.73% were similar to patients with inactive phase, and 53.56% were similar to patients with reactivation phase [18]. Moreover, these subgroups of indeterminate patients showed differences regarding age and sex, suggesting that age and sex are crucial factors that may affect the distribution of indeterminate patients [18, 22].
Poor prognosis of CHB patients with indeterminate phase
The current evidence suggests that indeterminate CHB patients tend to have a poor prognosis [23]. Spradling et al. [11] found that 9% of indeterminate patients developed liver cirrhosis in 6.3 years, which was three times higher than CHB patients in immune inactive phase. Consistent with the above finding, Huang et al. [8] indicated that CHB patients who remained indeterminate had a higher 10-year cumulative HCC incidence than CHB patients who remained immune inactive. Moreover, hepatitis B e antigen (HBeAg)-negative indeterminate patients with serum HBV DNA levels ≥ 2 × 103 IU/mL and normal ALT levels (≤ ULN, 40 U/L) were found to have remarkably higher risks of HBV-related liver diseases, such as necroinflammation (≥ G2) and fibrosis (≥ F2), than patients in immune inactive phase (HBV DNA < 2 × 103 IU/mL) [24, 25]. Yao KF et al. [18] also reported that higher proportions of indeterminate CHB patients may experience liver fibrosis or even cirrhosis. Some factors, such as age, sex, a family history of HCC, virus load and genotype, may affect disease progression in indeterminate patients (Fig. 1) [4, 12, 18]. For instance, Huang DQ et al. [8] showed that age was independently related to HCC development and was nine times higher in indeterminate patients over 40 years, and 18.4 times higher for those older than 45, indicating that older age could be considered as an independent risk factor for other advanced liver diseases in indeterminate patients [8, 18]. Besides, the HBV genotype may also play an important role in the progression of HBV-related complications and in the therapeutic efficacy [2].
Therefore, more work is needed to clarify the global epidemiology of indeterminate CHB patients, and more attention should be given to these patients with regard to differences in age, sex, family history, race and geographic location.
Laboratory evaluations for chronic HBV infection
It is necessary to determine the status of a chronic HBV infection and any liver-related complications, which are crucial to guiding the therapeutic strategy [10]. Thus, novel biomarkers with good accuracy should be explored and thoroughly investigated to facilitate the screening, diagnosis, and prognosis of chronic HBV infection.
Laboratory evaluations of HBV
The covalently closed circular DNA (cccDNA) of HBV in hepatocytes is the main cause of persistent infection, and is an accurate indicator of HBV presence in the body [26]. HBV cccDNA is the only known template for pregenomic RNA transcription, which produces the template for reverse transcription and viral genome replication [5]. Thus, monitoring intrahepatic cccDNA is important for deciding an antiviral therapeutic strategy [27]; however, certain limitations exist. Specifically, HBV cccDNA is mainly located in the nucleus of infected hepatocytes; thus, its detection is complicated, because it requires invasive liver biopsy, and there can be interobserver variability as well [28, 29]. In addition, there is no standardized assay for cccDNA quantification, but quantitative PCR methods are being standardized [5].
Serum markers of cccDNA: HBV DNA
To address this problem, researchers have developed various non-invasive tests to assess HBV cccDNA levels and transcriptional activity, among which the most classic is serum HBV DNA quantitation [2, 10]. Serum HBV DNA is used to estimate viral replication, and guide decisions about antiviral therapeutic strategies [10, 30]. However, the detection results of HBV DNA levels are not consistent among the various available kits because of differences in the primers and reagents. Additionally, some CHB patients showed different levels after nucleos(t)ide analog (NA) treatment [31].
Serum markers of cccDNA: HBsAg
HBsAg is another potential biomarker, and is expressed by HBV cccDNA and viral DNA that has integrated into the host genome in infected hepatocytes [10, 27, 32,33,34,35]. HBsAg is correlated with HBV transcriptional activity, serum HBV DNA levels, and HBeAg status [35, 36]. The quantitative detection of serum HBsAg levels may help to distinguish patients among the different phases of chronic HBV infection [35,36,37,38,39]. Notably, HBsAg consists of three kinds of proteins, which are large (L), middle (M), and small (S) HBs. The ratio of LHBs and MHBs was considered as a better predictors of HBeAg-negative chronic HBV infection and HBeAg-negative CHB than the total HBsAg concentration [40].
Serum markers of cccDNA: hepatitis B core-related antigen (HBcrAg)
HBcrAg is also considered a useful and sensitive biomarker that can provide evidence for intrahepatic HBV cccDNA [41, 42]. Chen EQ et al. [42] identified a significant correlation between serum HBcrAg and intrahepatic HBV cccDNA, and this correlation was stronger than that of HBV cccDNA with serum HBsAg or HBV DNA. Further analysis showed an association between decreased HBV cccDNA and decreased serum HBsAg or HBcrAg. The serum HBcrAg level could reflect the presence and transcriptional activity of intrahepatic cccDNA in CHB patients, while it could not indicate the transcriptional activity of integrated HBV DNA in infected hepatocytes [34]. However, the correlations between HBcrAg and HBV RNA are unclear. Moreover, quantitative detection of serum HBcrAg levels may help determine the natural history of chronic HBV infection, and accurately predict spontaneous HBeAg seroconversion in CHB patients [42]. Specifically, serum HBcrAg levels are higher in immune-tolerant and immune-active phases, and lower in inactive and reactivation phases [43]. Furthermore, HBcrAg has been proven to be an effective indicator of the prognosis and antiviral therapeutic efficacy in CHB patients [44,45,46]. For instance, serum HBcrAg levels may be used to stratify HCC risk in CHB patients with indeterminate phase [47,48,49]. A study, enrolled two retrospective cohorts in Taiwan and Japan, reported that serum HBcrAg level of 10,000 U/mL could be an effective cut-off value for HCC risk stratification in HBeAg-negative CHB patients with indeterminate phase; and the 10-year HCC cumulative incidence was 5.33% in patients with high serum HBcrAg levels, which was significantly higher than that of 0.51% in patients with lower levels [49]. After that, Tseng TC et al. showed that HBcrAg-based score may be better than HBV DNA-based score to predict HCC risks in indeterminate CHB patients who are HBeAg-negative [50]. Another follow-up study also indicated that serum HBcrAg was better than HBV DNA and HBeAg in predicting HCC occurrence, and an HBcrAg level > 2.9 log U/mL was an independent predictor of HCC incidence [47]. In addition, Cheung et al. [48] found that pretreatment (NA) HBcrAg levels were significantly higher in an HCC group than in a non-HCC group, suggesting that pretreatment HBcrAg > 47.1 kU/mL independently predicted HCC development in CHB patients (OR 3.29, 95% CI 1.66–6.52). Of note, serum HBcrAg could also predict HCC recurrence after resection or radiofrequency ablation [46]. Therefore, serum HBcrAg may become a newly recognized biomarker for monitoring disease states, evaluating therapeutic efficacy and drug withdrawal, and predicting HCC development or recurrence [44,45,46,47,48, 51, 52]. Nevertheless, the optimal cut-off values of serum HBcrAg for the defined immune phases and indeterminate phase remain to be determined.
Serum markers of cccDNA: serum HBV RNA
In recent years, serum HBV RNA has been discovered and recognized as an HBV virological indicator [53,54,55]. Although serum HBV RNA levels vary during the natural phases of chronic HBV infection, the distribution pattern is similar to that of serum HBV DNA among the different immune phases, suggesting a predictive effect on the immune phases of chronic HBV infection [27]. Serum HBV RNA levels can also reflect the concentration and transcriptional activity of cccDNA in hepatocytes [56,57,58], and may have special significance in guiding NA administration [55, 59]. In contrast, some studies have indicated that serum HBV RNA does not have notable advantages in distinguishing the immune phases in CHB patients, compared with other traditional biomarkers [10]. Unfortunately, there is no standard or standardized method for the quantitative detection of HBV RNA; therefore, its standardization and traceability need to be clarified.
In summary, additional longitudinal studies with larger sample sizes are needed to further investigate the clinical utility of these biomarkers.
Laboratory evaluations of liver histopathology
For CHB patients, liver biopsy is regarded as the gold standard to assess the severity of liver inflammation, fibrosis, or cirrhosis [10]; however, patients are reluctant to have repeated biopsies to monitor disease progression [60] due to its invasiveness and risk of complications [61]. Non-invasive tests may contribute to evaluating liver histopathology (Fig. 2); these tests include serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, prothrombin time (PT), the AST-to-platelet ratio index (APRI), hyaluronidase (HA), laminin (LN), and N-terminal propeptide of collagen type I (PINP) [4, 62, 63]. ALT and AST are regarded as standard enzymes used to evaluate the degree of hepatocyte damage, and serum ALT detection is a sensitive indicator for the diagnosis of viral hepatitis [64]. The following is a brief description of these biomarkers of liver histopathology.
Liver histopathology and relevant serum markers. ALT: alanine aminotransferase; AST: aspartate aminotransferase; HBV: hepatitis B virus; HA: hyaluronidase; AFP: alpha-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein; PIVKA-II: prothrombin induced by vitamin K deficiency or antagonist-II
Serum markers of fibrosis and cirrhosis
As a high-molecular-weight glycosaminoglycan that is found in the extracellular matrix (ECM), HA could enter the circulation during the ECM turnover process, and elevated HA levels in circulation may indicate increased production of HA or reduced clearance of circulating HA, thus may correlate with liver inflammation and fibrosis [62]. Therefore, HA is considered as a sensitive and specific indicator of liver fibrosis among the various biochemical indicators, and is also an effective indicator of disease progression in CHB patients [62]. Transient elastography (FibroScan) [65] has also been used to the diagnose liver fibrosis with high accuracy, although it cannot provide information about intrahepatic inflammation. Additionally, thrombocytopenia has been suggested as a surrogate of liver cirrhosis and as an HCC predictor in patients with viral hepatitis [66].
Serum markers of HCC
Although tumor biomarkers, including alpha-fetoprotein (AFP), Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3) and prothrombin induced by vitamin K deficiency or antagonist-II (PIVKA-II), could not be used to directly diagnose HCC, they may predict tumor progression and even outcomes of HCC patients [67]. Since AFP was indicated in serum of HCC patients in 1964, it is considered as the primary biomarkers for HCC [68]. As an alpha globulin containing glycoprotein, the expression of AFP is increased during pregnancy by fetal liver and decreased to trace level after childbirth within less than 1 month [69]. High serum AFP level is usually associated with the presence and development of HCC [68, 70]. However, the elevation of serum AFP levels also exists in non-HCC diseases, such as hepatitis, cholangiocarcinoma, testicular germ cell tumor, and metastatic colon cancer [70]. With a sensitivity of 18–60% and a specificity of 85–90%, AFP alone is not recommended for HCC screening [70]. Based on its binding capacity to lectin lens culinaris agglutinin, AFP could be classified as three subtypes which are AFP-L1, AFP-L2, and AFP-L3 [69]. AFP-L1 increases in chronic hepatitis and liver cirrhosis, and AFP-L2 is increased in yolk sac tumors and may be detected in pregnant women [71]. With a higher specificity of 92.0–99.4%, AFP-L3 is regarded as a more specific biomarker for HCC [72, 73]; however, it has a low sensitivity ranging from 18.8 to 37% for HCC, and may be not relevant to HCC with a total AFP level lower than 20 ng/mL [72,73,74]. In a word, the concentrations of AFP and AFP-L3 should be detected together to facilitate early diagnosis of HCC [75]. Also known as Des-γ-carboxyprothrombin, PIVKA-II was considered as one of the tumor-related biomarkers [71, 76]. With a higher sensitivity of 72.7% and a specificity of 90% for HCC, the elevated serum PIVKA-II levels was not closely related to non-HCC liver diseases compared to serum AFP levels [70, 77]. It was reported that significant correlations existed between serum PIVKA-II levels and HCC clinicopathological characteristics, such as tumor size and TNM stage [76], and the performance of PIVKA-II plus AFP for HCC identification was superior to each of these biomarkers used alone [78]. Besides, PIVKA-II may improve the identification of patients with AFP-negative HCC [74]. Additionally, dickkopf-1 and circulating IgG are also regarded as novel promising biomarkers for HCC [79,80,81,82,83]. Altogether, combinations of these biomarkers might provide better prospects in clinical applications.
Laboratory evaluations of liver histopathology in indeterminate CHB patients
To more conveniently and accurately evaluate liver histopathology in indeterminate CHB patients, Pan AN et al. developed a new scoring system called the “Significant Histological Model (SHM) scoring system”, which could predict liver histopathological changes in indeterminate patients [84]. The SHM scoring system suggests that AST, platelet (PLT) counts, albumin, and HBV DNA (log10 IU/mL) are independent predictors of liver histological changes in indeterminate CHB patients. The model [84] showed good accuracy in identifying indeterminate patients with/without liver histological changes (logistic y = 3.339 + 0.06 × AST−0.06 × PLT−0.068 × albumin−0.246 × HBV DNA [log10 IU/mL]). Other non-invasive indicators, such as the APRI, Lok index, Forn index, FIB-4, and Zeng score, could not accurately predict the degree of liver fibrosis [63, 85,86,87,88]. The SHM scoring system has not been fully evaluated or directly compared with other non-invasive indicators, and its clinical application requires further investigation [84].
Although these indicators may have limited accuracies in identifying CHB patients with liver fibrosis, they may play crucial and guiding roles in decision-making within disease staging and therapeutic strategy selection [4]. However, the associations among these indicators should be thoroughly investigated to identify a more effective system that would facilitate the diagnosis and prognosis of indeterminate CHB patients.
Clinical characteristics of indeterminate CHB patients
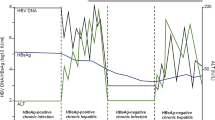
The interactions of HBV with the host and environmental factors are complicated [8, 12, 24, 89]. To our knowledge, HBV is not directly cytopathic, and host immune responses to HBV-infected hepatocytes are thought to mediate liver injury and the development of cirrhosis and HCC [2]. In addition, persistent or recurrent liver inflammation and incomplete HBV clearance might contribute to CHB development [10]. After primary HBV infection, the body initiates a nonspecific immune response, followed by a specific immune response [10]. Adult-acquired HBV is generally cleared by the host immune system, while chronic HBV infection is usually acquired from perinatal or horizontal infection [90]. Since chronic HBV infection is dynamic, it is necessary to regularly detect the levels of serum HBeAg, HBV DNA, and ALT [4, 10]. According to the liver disease severity, host immune response, and the natural history of chronic HBV infection, patients with chronic HBV infection are classified into four immune phases [2, 4, 10], but many CHB patients do not fit into any of these defined phases [2]. The guidelines [2, 4, 20] proposed by AASLD and the European Association for the Study of the Liver (EASL) have officially designated “indeterminate” gray zone (GZ), which indicate that the patient’s HBV DNA and ALT levels do not fall into the same phase; serial monitoring of the serum HBeAg, HBV DNA, and ALT levels is suggested in most instances, even after a complete assessment is conducted. A Chinese study [18] subdivided indeterminate CHB patients into four groups based on the following defined phases [2]: ① GZ-A: HBeAg positive, normal serum ALT and HBV DNA ≤ 106 IU/mL; ② GZ-B: HBeAg positive, elevated serum ALT and HBV DNA ≤ 2 × 104 IU/mL; ③ GZ-C: HBeAg negative, normal ALT and HBV DNA ≥ 2 × 103 IU/mL; and ④ GZ-D: HBeAg negative, elevated serum ALT and HBV DNA ≤ 2 × 103 IU/mL. To date, there is no unified definition of indeterminate CHB; therefore, this patient population needs to be further elucidated [2].
Immune-tolerant phase and indeterminate phase
Also known as HBeAg-positive chronic HBV infection [10, 20], the immune-tolerant phase is usually regarded as a benign disease course [91], and is characterized by high levels of serum HBsAg and HBV DNA, normal or minimally elevated ALT, and minor or no necroinflammation or fibrosis in the liver [2, 10, 92]. Different countries have distinct definitions of the immune-tolerant phase regarding the serum HBV DNA level. The diagnostic values of HBV DNA are > 2 × 107 IU/mL in China [10], > 1 × 107 IU/mL in European countries [20], > 1 × 106 IU/mL in the United States [2], and > 2 × 104 IU/mL according to the Asia–Pacific Guidelines [93]. Therefore, the proportions of indeterminate CHB patients with immune tolerance are quite different according to these distinct guidelines. Specifically, indeterminate CHB patients are characterized by HBeAg and HBsAg positivity, HBV DNA ≤ 2 × 104 IU/mL, and normal serum ALT levels (≤ 35 U/L for men and 25 U/L for women) in accordance with the AASLD Guidelines [2].
The underlying mechanisms of immune tolerance are mainly described as follows: ① Persistence of cccDNA. Upon infection, HBV nucleic acid enters the hepatocellular nucleus, and forms a primitive template for viral replication, namely, cccDNA. It remains in the nucleus “wrapped” by histones, which act as the virus template being copied and transcribed in the nucleus, and then generates new HBV particles causing persistent infection [28]. Although the current antiviral medications are designed to inhibit HBV DNA replication, it is difficult to target intrahepatic cccDNA. In addition, HBV cccDNA has high stability and a long half-life, which could also explain the difficulty of HBV clearance [26]. ② High variability of the HBV gene. The HBsAg antigenicity or serum concentrations could be changed after mutations of the pre-S/S region [94, 95], which may help the virus to escape neutralization by the corresponding antibodies and induce T cells to develop immune tolerance to the target antigen. ③ Presence of cellular immunity. Cellular immunity and the production of cytokines related to this process may play important roles in mediating host immune tolerance [93, 96, 97]. ④ Polymorphism of host genes. Some studies [98, 99] have shown that host gene polymorphisms are associated with immune tolerance. For instance, HLA-DPA1 and HLA-DPB are protective against chronic HBV infection in Asian populations [98], while rs7453920-G(HLA-DQ) and rs2856718-A(HLA-DQ) are associated with chronic HBV infection [99]. During the tolerant phase, HBV remains quiescent for several weeks during which the host immune system does not respond to the HBV infection [100]. Some researchers have suggested changing the “immune-tolerant phase” to the “high replication low inflammation period (HRLI)” due to the absence of immunological evidence [92]. However, there is no sufficient evidence to rename this phase [100, 101], but the EASL 2017 Guidelines [20] use the term “HBeAg-positive chronic HBV infection” based on the serum HBeAg status. Nevertheless, no specific definition has been recognized worldwide to replace the term “immune-tolerant phase”.
Immune-active phase and indeterminate phase
Not all CHB patients go through the four phases in order, for instance, most patients who were infected with HBV in adolescence or adulthood may directly enter the immune clearance phase rather than the immune tolerance phase, and the perinatally or early childhood-acquired chronic HBV may have a long immune-tolerant phase [9, 102]. During the immune-active phase, CHB patients are considered to have HBeAg-positive chronic hepatitis B [20], and are characterized by the presence of serum HBeAg, elevated serum HBV DNA, and intermittently or persistently elevated serum ALT, in conjunction with chronic moderate-to-severe necroinflammation or fibrosis [2, 4]. In this phase, host immune tolerance to HBV is lost, and the immune system attacks HBV-infected hepatocytes, resulting in decreased HBV DNA levels and elevated ALT levels. However, some CHB patients with similar characteristics fall outside the above ranges, and are considered indeterminate. For instance, indeterminate CHB patients are usually characterized by HBeAg and HBsAg positivity, HBV DNA positivity (between 2 × 103 IU/mL and 2 × 104 IU/mL), and continuously or repeatedly abnormal ALT levels (≤ 2 ULN) [2].
Immune inactive phase and indeterminate phase
The immune inactive phase, previously the inactive carrier phase [4, 103], is known as HBeAg-negative chronic HBV infection [20], and is characterized by the presence of HBeAg seroconversion, low or undetectable HBV DNA levels, persistently normal ALT levels, and minimal necroinflammation and variable fibrosis in the liver [2, 4, 10]. Similarly, some HBeAg-negative CHB patients are considered indeterminate, with serum HBV DNA levels ≥ 2 × 103 IU/mL and normal ALT levels, or HBV DNA levels < 2 × 103 IU/mL and elevated serum ALT levels [2].
Immune reactivation phase and indeterminate phase
Spontaneously or subsequent to antiviral therapy [104], some CHB patients enter the reactivation phase, which is also called HBeAg-negative chronic hepatitis B [2, 10, 20]. The immune reactivation phase is characterized by antibody to hepatitis B e antigen (anti-HBe) positivity, elevated HBV DNA levels, fluctuating or persistently elevated ALT, and moderate-to-severe necroinflammation or fibrosis (≥ G2/S2) [2, 4, 10, 20]. The EASL 2012 and 2017 Clinical Practice Guidelines [20, 105] state that CHB patients in reactivation phase usually have low rates of spontaneous disease remission. Some patients do not meet the diagnostic criteria of the other three defined phases, but do not fit into the reactivation phase due to one of the above indicators being out of range. These indeterminate CHB patients are usually characterized by low levels of HBV DNA (< 2 × 103 IU/mL) and abnormal ALT levels (≥ 2 ULN), or HBV DNA ≥ 2 × 103 IU/mL and slightly elevated ALT levels (< 2 ULN) [2].
Therapeutic strategies for indeterminate CHB patients
The EASL 2017 Guidelines on the management of HBV infection and the AASLD 2018 Hepatitis B Guidelines have proposed the optimal goal of CHB treatment, which is to suppress HBV replication in a sustained manner and even eliminate the virus from infected hepatocytes to prevent disease progression, resulting in longer survival and improved quality of life [2, 20]. Afterwards, the Chinese Medical Association has proposed a comprehensive and dynamic assessment for chronic HBV infection [10], including serum HBV DNA and ALT levels, liver function and disease severity, age, family history of HCC, and concomitant diseases, to guide clinical decisions and therapeutic strategies [4, 106].
Clinical treatment of HBsAg-positive and HBeAg-positive CHB patients in immune-tolerant, immune-active, or indeterminate phase
The immune-tolerant phase is characterized by a higher HBV load without obvious liver histopathological changes, indicating a "peaceful coexistence" of HBV and the host [107, 108]. Most HBV-related guidelines recommend to not treat these patients with antiviral therapy [2, 4, 10, 100, 109, 110]. Different guidelines have distinct diagnostic values of serum indicators for staging CHB patients. For example, the AASLD 2018 Guidelines [2] state that immune-tolerant and indeterminate CHB patients [2] with HBV DNA levels > 2 × 104 IU/mL and ALT ≤ ULN should not be treated with antiviral therapy; in addition, it is necessary to monitor the HBV DNA and ALT levels every 3–6 months and HBeAg every 6–12 months. Notably, serum ALT levels have no direct correlation with the liver viral load; however, serum ALT levels within the normal range could not indicate no or slight inflammatory activity in the liver either [111, 112]. A multinational systematic analysis [112] of 830 CHB patients with normal serum ALT levels found that 20.7% were in the severe liver fibrosis stage (≥ F2), suggesting that serum ALT levels did not accurately reflect the liver histopathology. A meta-analysis and systematic review [113] indicated that CHB patients with normal or slightly elevated serum ALT levels may also have HBV-related complications, and could benefit from antiviral therapy. Additionally, a study [114] conducted in South Korea found that the cumulative incidence of HCC in untreated immune-tolerant patients (ALT < 30 U/L for males, ALT < 19 U/L for females) was 12.7%, which was significantly higher than that in treated patients in immune reactivation phase (6.1%, ALT > 80 U/L). Obviously, these so-called immune-tolerant patients should be treated with antiviral medication. The AASLD HBV Guidelines [2, 4] suggest antiviral therapy for indeterminate CHB patients over 40 years old with normal ALT, elevated HBV DNA and liver biopsy showing moderate-to-severe necroinflammation or fibrosis. Some indeterminate CHB patients are characterized by mildly elevated serum ALT levels (< 2 ULN) and HBV DNA levels (> 2 × 104 IU/mL) [2]. For these patients, other causes of ALT elevation should be excluded, and an assessment of disease severity should be performed with non-invasive tests and/or liver biopsy [2]. If liver histopathological changes indicate ≥ F2 or ≥ A3 and slightly elevated ALT (< 2 ULN) persists, these indeterminate patients should be treated with antiviral therapy, especially if they are over 40 years old [2]. For indeterminate patients with HBV DNA levels between 2 × 103 IU/mL and 2 × 104 IU/mL, regular monitoring should be performed every 1–3 months; if HBV DNA persists for > 6 months, antiviral therapy should be considered, regardless of the serum ALT level [2].
For CHB patients in either immune-tolerant or indeterminate phase, comprehensive and dynamic assessments should be performed to evaluate whether they have real immune tolerance and have any intra- or extra-liver complications. Other factors, such as age, sex, and family history of HCC, should be considered in this process [106].
The immune-active phase is believed to be the best time for antiviral therapy to achieve satisfactory therapeutic efficacy with decreased risk of liver-related complications [2, 4, 10]. In this phase, HBV is actively replicating, and the host’s immune system is also activated; hence, HBV can be recognized and attacked by the immune system, but the patient may experience recurrent abnormal liver function [10]. Therefore, it is recommended to treat immune-active patients with antiviral therapy [10]. For certain indeterminate patients with HBV DNA levels < 2 × 104 IU/mL and elevated ALT (≥ 2 ULN), regular monitoring of HBeAg, HBV DNA, and ALT should be performed, as well as non-invasive tests and/or biopsy for liver histopathological changes [2]. These indeterminate patients (with elevated ALT and HBV DNA levels between 2 × 103 IU/mL and 2 × 104 IU/mL) should be treated with antiviral therapy, especially if they are older than 40 years, or have liver cirrhosis, a family history of HCC, previous treatment history, extrahepatic manifestations, or a long duration of HBV infection [2, 4].
Clinical treatment of HBsAg-positive and HBeAg-negative CHB patients in immune inactive, reactivation phases, or indeterminate phase
Theoretically, immune inactive patients usually have low serum HBsAg levels, undetectable or low levels of HBV DNA (< 2 × 103 IU/mL), and persistently normal ALT levels [2, 10]; however, some patients with immune inactivation have higher HBV DNA levels or slightly elevated ATL levels (< 2 ULN), and are considered indeterminate [2]. The AASLD Hepatitis B Guidance [2, 4] suggests that antiviral therapy is generally not recommended for immune inactive patients, and regular monitoring of the HBV DNA and ALT levels is recommended every 3 ~ 6 months, as well as HBsAg annually. Some researchers believe that immune inactive patients should not be treated with antiviral therapy for the following reasons: ① There is a lower risk of disease progression. For example, a study [108] of 361 immune inactive CHB patients showed that only 2.8% experienced disease progression within a 4-year follow-up. A similar study by Tong MJ et al. [115] recruited 146 HBeAg-negative CHB patients with normal ALT levels and HBV DNA ≤ 10 × 103 IU/mL and followed them for 8 ± 6.3 years; none of the patients progressed to liver cirrhosis, and only 2 developed HCC. Bonacci M et al. [12] found that the proportion of HBeAg-negative patients among indeterminate CHB patients was only 6.3%, and none developed liver fibrosis or cirrhosis during an 8.2-year follow-up. The prognosis of immune inactive patients is favorable, and transiently elevated ALT and HBV DNA levels may have minimal clinical significance [115]. ② Economic burden. Once started, antiviral therapy usually lasts for 5–10 years, and can even last a lifetime, which can result in a large economic burden on these patients and their families [116]. And Zhang H et al. [117] have already revealed the economic burden on CHB patients in China. ③ Adverse effects of antiviral therapy. Kwon JH et al. [118] and Buti M et al. [119] found that some patients develop renal dysfunction with different severities after 7 years of antiviral therapy with tenofovir, suggesting cautious consideration of antiviral strategies for patients with chronic HBV infection. However, whether immune inactive patients should be treated with antiviral therapy remains controversial. Duan MH et al. [24] found that ALT > 20 U/L was a good independent predictive factor for evaluating liver histopathology for immune inactive CHB patients, and liver biopsy or non-invasive methods should be performed to evaluate the liver histopathological changes to make decisions about antiviral therapy [2]. Older (> 40 years) male CHB patients with delayed HBeAg seroconversion and a family history of liver cirrhosis or HCC may have significantly higher risks of clinical events [120, 121]. Thereafter, the EASL 2017 Guidelines [20] and the China 2019 Guidelines [10] recommend antiviral therapy for immune inactive patients with a family history of liver cirrhosis or cancer, regardless of liver histopathology.
Different clinical studies have reported different risks of disease progression in HBeAg-negative indeterminate patients. For example, Spradling PR et al. [11] conducted a large cohort study of CHB patients from 2006 to 2013, and found that 9% of indeterminate patients progressed to liver cirrhosis, and indeterminate patients with similar immune inactive characteristics may have a higher risk of disease progression than defined immune inactive patients. Moreover, the HBV DNA load was found to be an independent risk factor for related clinical events in HBeAg-negative CHB patients [122]. In addition, the proportion of patients with liver necrotizing inflammation and fibrosis was significantly higher in HBeAg-negative indeterminate patients with normal ALT levels (≤ ULN, 40 U/L) and HBV DNA levels ≥ 2 × 103 IU/mL than in immune inactive patients with normal ALT and HBV DNA levels < 2 × 103 [24, 25]. A large clinical and community study from the United States and Taiwan, China found that antiviral therapy for patients with HBV DNA ≥ 2 × 103 IU/mL reduced the risk of HCC by 77%, regardless of HBeAg, ALT, sex, age, and liver cirrhosis status or treatment medication [123]. However, a few studies have focused on antiviral therapy for immune inactive patients and the related indeterminate phase, and more studies are needed to provide evidence-based data to facilitate the clinical application of antiviral therapy. According to the AASLD guidelines [2], for indeterminate patients with HBV DNA levels ≥ 2 × 103 IU/mL and normal ALT levels, regular monitoring should be performed every 3 months for 1 year and every 6 months thereafter. For indeterminate patients with slightly elevated ALT levels (≤ 2 ULN), regardless of HBV DNA levels, other causes of ALT elevation should be excluded, and an assessment of disease severity should be performed with non-invasive tests and/or biopsy for liver histopathological changes [2]. If liver histopathological changes indicate ≥ F2 or ≥ A3, antiviral therapy should be performed; if slightly elevated ALT levels (> ULN) with HBV DNA ≥ 2 × 103 IU/mL persist, these indeterminate patients should be treated with antiviral therapy, especially if they are over 40 years old [2].
Antiviral therapy is also recommended for patients in immune reactivation phase [2, 4, 10]. According to the AASLD guidelines [2], indeterminate patients with HBV DNA levels < 2 × 103 IU/mL and elevated ALT (≥ 2 ULN) should undergo regular monitoring of HBeAg, HBV DNA and ALT, as well as non-invasive tests and/or biopsy for liver histopathological changes.
Future potential of therapeutic expansion for indeterminate CHB
It is worth noting that there is still no international consensus to guide the management of indeterminate CHB patients, and some differences exist among different guidelines. The AASLD 2016 and 2018 Hepatitis B Guidelines [2, 4] suggest to dynamically monitor the serum HBV DNA and ALT levels in indeterminate patients rather than apply antiviral therapy. While, the Chinese Expert Opinion on expanding anti-HBV treatment for CHB recommends to start antiviral therapy for untreated indeterminate patients with uncertain HBV DNA and ALT patterns after 1-year follow-up [124]. Certain studies indicate that indeterminate CHB patients may be confronted with HBV-related complications, and propose antiviral therapy to prevent disease progression and reduce HCC risks [8, 11, 12, 23,24,25, 125]. For instance, a randomized, double-blind, placebo-controlled study conducted in Taiwan, China showed that tenofovir disoproxil fumarate could reduce the risk of liver fibrosis in patients with non-cirrhotic CHB and minimally raised ALT [126]. Moreover, another multi-center study conducted in 14 centers in U.S., Europe and Asia reported that HCC risk in CHB is higher in indeterminate phase compared to the immune inactive phase, and antiviral therapy could reduce HCC risk by 70% among indeterminate CHB patients without advanced fibrosis [127]. In a word, expanding antiviral therapy may benefit indeterminate CHB patients from preventing disease progression, which is of great significance.
Conclusion
Chronic HBV infection is a major health problem worldwide. One of the main issues for this infection is to decide which patients will benefit from antiviral therapy. The precise classification of the immune phases of CHB patients could help in evaluating disease prognosis and developing therapeutic strategies [10, 128]. However, certain CHB patients do not fit into any of the defined phases, and are considered to be in an “indeterminate” gray zone [4]. Although the distributions of indeterminate CHB patients vary due to distinct epidemiological data and classification strategies, approximately 30% ~ 40% of CHB patients are in indeterminate phase [2], and the proportions of indeterminate patients with distinct characteristics can vary [18]. Current evidence has shown that the prognosis of indeterminate patients is not optimistic, and they also have a higher risk of disease progression to liver cirrhosis or HCC. Factors, such as age, male sex, HBeAg positivity, higher HBV DNA or ALT levels, lower albumin levels or PLT counts, and a family history of HCC, have been associated with disease progression [8, 18, 122]. Accurate diagnosis is crucial to establish the immune phase and choose the optimal therapeutic strategy. Novel efficient assays should be investigated and verified. Furthermore, the therapeutic strategy for indeterminate CHB patients remains controversial. Clinicians should make a comprehensive assessment of these patients, based on the virology and immunology indicators, imaging examination, age, sex, and a family history of HCC. Regular monitoring of these patients should be performed, and antiviral therapy should be given to patients who are at high risk of disease progression. Although many advances have been made in providing therapeutic strategies for indeterminate CHB patients, there is still a long way to go to improve the prediction, detection, therapeutic timing, scheme, course, efficacy, and prognosis in this patient population. Multi-center and multi-country cohort clinical trials with large numbers of indeterminate CHB patients should be launched.
Availability of data and materials
Not applicable.
References
Shimona S, Laura M. Hepatitis B virus infection. Nat Rev Dis Prime. 2018;4:18036.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99.
Collaborators TPO. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403.
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–83.
Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18(11):827–44.
Organization WH. Global hepatitis report, 2017. Geneva: World Health Organization; 2017.
Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, et al. Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017. https://doi.org/10.1016/j.jhep.2017.07.033.
Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clini Gastroenterol Hepatol. 2022;20(8):1803-1812.e1805.
Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). Chin J Hepatol. 2022;30(12):1309–31.
Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Chin J Hepatol. 2019;27(12):938–61.
Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Teshale ET, et al. Distribution of disease phase, treatment prescription and severe liver disease among 1598 patients with chronic hepatitis B in the Chronic Hepatitis Cohort Study, 2006–2013. Aliment Pharmacol Ther. 2016;44(10):1080–9.
Londoño MC, Bonacci M, Lens S, Rodríguez-Tajes S, García-López M, Forns X, et al. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. Aliment Pharmacol Ther. 2018;47(10):1397–408.
Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20(8):524–37.
World Health Organization. Hepatitis B. 2024. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed 9 Apr 2024
Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–8.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7(9):796–829.
Yao K, Liu J, Wang J, Yan X, Xia J, Yang Y, et al. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28(7):1025–33.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50.
European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98.
Hsu YN, Pan CQ, Abbasi A, Xia V, Bansal R, Hu KQ. Clinical presentation and disease phases of chronic hepatitis B using conventional versus modified ALT criteria in Asian Americans. Dig Dis Sci. 2014;59(4):865–71.
Shan S, You H, Niu J, Shang J, Xie W, Zhang Y, et al. Baseline characteristics and treatment patterns of the patients recruited to the China registry of hepatitis B. J Clin Transl Hepatol. 2019;7(4):322–8.
Liu J, Wang J, Yan X, Xue R, Zhan J, Jiang S, et al. Presence of liver inflammation in asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis. Hepatol Commun. 2022;6(4):855–66.
Duan M, Chi X, Xiao H, Liu X, Zhuang H. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B. Hepatol Int. 2021;15(2):318–27.
Koc ÖM, Robaeys G, Topal H, Bielen R, Busschots D, Fevery J, et al. Outcome in Caucasian patients with hepatitis B e antigen negative chronic infection: a long-term observational cohort study. J Med Virol. 2020;92(12):3373–80.
Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64(12):1972–84.
Liu Y, Jiang M, Xue J, Yan H, Liang X. Serum HBV RNA quantification: useful for monitoring natural history of chronic hepatitis B infection. BMC Gastroenterol. 2019;19(1):53.
Allweiss L, Dandri M. The role of cccDNA in HBV maintenance. Viruses. 2017;9(6):156.
Kerwin SC. Hepatic aspiration and biopsy techniques. Vet Clin N Am Small Anim Pract. 1995;25(2):275–91.
Lin CL, Kao JH. New perspectives of biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2016;22(4):423–31.
Liu Y, Liu H, Hu Z, Ding Y, Pan XB, Zou J, et al. Hepatitis B virus virions produced under nucleos(t)ide analogue treatment are mainly not infectious because of irreversible DNA chain termination. Hepatology. 2020;71(2):463–76.
Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011—a core group report. J Hepatol. 2011;55(5):1121–31.
Su TH, Hsu CS, Chen CL, Liu CH, Huang YW, Tseng TC, et al. Serum hepatitis B surface antigen concentration correlates with HBV DNA level in patients with chronic hepatitis B. Antivir Ther. 2010;15(8):1133–9.
Chen EQ, Wang ML, Tao YC, Wu DB, Liao J, He M, et al. Serum HBcrAg is better than HBV RNA and HBsAg in reflecting intrahepatic covalently closed circular DNA. J Viral Hepat. 2019;26(5):586–95.
Chan HL, Wong VW, Wong GL, Tse CH, Chan HY, Sung JJ. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010;52(4):1232–41.
Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52(4):508–13.
Kim YJ, Cho HC, Choi MS, Lee JH, Koh KC, Yoo BC, et al. The change of the quantitative HBsAg level during the natural course of chronic hepatitis B. Liver Int. 2011;31(6):817–23.
Jang JW, Yoo SH, Kwon JH, You CR, Lee S, Lee JH, et al. Serum hepatitis B surface antigen levels in the natural history of chronic hepatitis B infection. Aliment Pharmacol Ther. 2011;34(11–12):1337–46.
Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52(4):514–22.
Pfefferkorn M, Böhm S, Schott T, Deichsel D, Bremer CM, Schröder K, et al. Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers. Gut. 2018;67(11):2045–53.
Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70(4):615–25.
Chen EQ, Feng S, Wang ML, Liang LB, Zhou LY, Du LY, et al. Serum hepatitis B core-related antigen is a satisfactory surrogate marker of intrahepatic covalently closed circular DNA in chronic hepatitis B. Sci Rep. 2017;7(1):173.
Song G, Yang R, Rao H, Feng B, Ma H, Jin Q, et al. Serum HBV core-related antigen is a good predictor for spontaneous HBeAg seroconversion in chronic hepatitis B patients. J Med Virol. 2017;89(3):463–8.
Inoue T, Kusumoto S, Iio E, Ogawa S, Suzuki T, Yagi S, et al. Clinical efficacy of a novel, high-sensitivity HBcrAg assay in the management of chronic hepatitis B and HBV reactivation. J Hepatol. 2021;75(2):302–10.
Hsu YC, Nguyen MH, Mo LR, Wu MS, Yang TH, Chen CC, et al. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment Pharmacol Ther. 2019;49(1):107–15.
Hosaka T, Suzuki F, Kobayashi M, Hirakawa M, Kawamura Y, Yatsuji H, et al. HBcrAg is a predictor of post-treatment recurrence of hepatocellular carcinoma during antiviral therapy. Liver Int. 2010;30(10):1461–70.
Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, et al. HBcrAg predicts hepatocellular carcinoma development: an analysis using time-dependent receiver operating characteristics. J Hepatol. 2016;65(1):48–56.
Cheung KS, Seto WK, Wong DK, Lai CL, Yuen MF. Relationship between HBsAg, HBcrAg and hepatocellular carcinoma in patients with undetectable HBV DNA under nucleos(t)ide therapy. J Viral Hepat. 2017;24(8):654–61.
Tseng TC, Hosaka T, Liu CJ, Suzuki F, Hong CM, Kumada H, et al. Hepatitis B core-related antigen stratifies the risk of liver cancer in HBeAg-negative patients with indeterminate phase. Am J Gastroenterol. 2022;117(5):748–57.
Tseng TC, Hosaka T, Liu CJ, Suzuki F, Chiang C, Hong CM, et al. HBcrAg-based risk score performs better than the HBV DNA-based scores for HCC prediction in grey zone patients who are HBeAg-negative. JHEP Rep. 2024;6(1):100956.
Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44(Suppl S1):1–58.
Hosaka T, Suzuki F, Kobayashi M, Fujiyama S, Kawamura Y, Sezaki H, et al. Impact of hepatitis B core-related antigen on the incidence of hepatocellular carcinoma in patients treated with nucleos(t)ide analogues. Aliment Pharmacol Ther. 2019;49(4):457–71.
van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, et al. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61(1):66–76.
Giersch K, Allweiss L, Volz T, Dandri M, Lütgehetmann M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol. 2017;66(2):460–2.
Hong X, Hu J. Serum HBV RNA composition dynamics as a marker for intrahepatic HBV cccDNA turnover. J Med Virol. 2020;92(8):935–7.
Fan R, Zhou B, Xu M, Tan D, Niu J, Wang H, et al. Association between negative results from tests for HBV DNA and RNA and durability of response after discontinuation of nucles(t)ide analogue therapy. Clin Gastroenterol Hepatol. 2020;18(3):719-727.e717.
Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65(4):700–10.
Cornberg M, Lok AS, Terrault NA, Zoulim F, EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B—report from the 2019 EASL-AASLD HBV treatment endpoints conference. Hepatology. 2020;71(3):1070–92.
Liu Y, Xue J, Liao W, Yan H, Liang X. Serum HBV RNA dynamic and drug withdrawal predictor value in patients with chronic HBV infection on long-term nucleos(t)ide analogue (NA) therapy. J Clin Gastroenterol. 2020;54(8):e73–82.
Castera L. Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol. 2011;25(2):291–303.
Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12(23):3682–94.
Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21(41):11567–83.
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26.
Akkaya O, Kiyici M, Yilmaz Y, Ulukaya E, Yerci O. Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World J Gastroenterol. 2007;13(41):5481–5.
Wilder J, Patel K. The clinical utility of FibroScan(®) as a noninvasive diagnostic test for liver disease. Med Devices. 2014;7:107–14.
Lu SN, Wang JH, Liu SL, Hung CH, Chen CH, Tung HD, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006;107(9):2212–22.
Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 2015;4(2):126–36.
IuS T. Detection of embryo-specific alpha-globulin in the blood serum of a patient with primary liver cancer. Vopr Med Khim. 1964;10:90–1.
Force M, Park G, Chalikonda D, Roth C, Cohen M, Halegoua-DeMarzio D, et al. Alpha-fetoprotein (AFP) and AFP-L3 is most useful in detection of recurrence of hepatocellular carcinoma in patients after tumor ablation and with low AFP level. Viruses. 2022;14(4):775.
Ayoub WS, Steggerda J, Yang JD, Kuo A, Sundaram V, Lu SC. Current status of hepatocellular carcinoma detection: screening strategies and novel biomarkers. Ther Adv Med Oncol. 2019;11:1758835919869120.
Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10573–83.
Kudo M. Alpha-fetoprotein-L3: useful or useless for hepatocellular carcinoma? Liver Cancer. 2013;2(3–4):151–2.
Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, et al. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7(1):104–13.
Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020;26(3):261–79.
Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69(5):1983–94.
Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21(1):401.
Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848–54.
Caviglia GP, Ribaldone DG, Abate ML, Ciancio A, Pellicano R, Smedile A, et al. Performance of protein induced by vitamin K absence or antagonist-II assessed by chemiluminescence enzyme immunoassay for hepatocellular carcinoma detection: a meta-analysis. Scand J Gastroenterol. 2018;53(6):734–40.
Erdal H, Gül Utku Ö, Karatay E, Çelik B, Elbeg Ş, Doğan İ. Combination of DKK1 and AFP improves diagnostic accuracy of hepatocellular carcinoma compared with either marker alone. Turk J Gastroenterol. 2016;27(4):375–81.
Ge T, Shen Q, Wang N, Zhang Y, Ge Z, Chu W, et al. Diagnostic values of alpha-fetoprotein, dickkopf-1, and osteopontin for hepatocellular carcinoma. Med Oncol. 2015;32(3):59.
Xu Y, Gu L, Wang J, Wang Z, Zhang P, Zhang X. Detection of circulating antibodies to p16 protein-derived peptides in hepatocellular carcinoma. Lab Med. 2020;51(6):574–8.
Wang J, Xu Y, Zhao H, Zhang X. Change of circulating antibodies against CD25-derived peptide antigen in hepatocellular carcinoma. J Cancer Res Ther. 2017;13(5):813–6.
Wang J, Xu Y, Wang Y, Zhang X, Zhang G. Further study of circulating antibodies to P16, CD25 and FOXP3 in hepatocellular carcinoma. OncoTargets Ther. 2019;12:10487–93.
Pan AN, Xu WW, Luo YL, Yu HH, Hu YB, Sun QF, et al. A novel system for predicting liver histopathology in patients with chronic hepatitis B. Medicine. 2017;96(14): e6465.
Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42(2):282–92.
Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36(4 Pt 1):986–92.
Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection: comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6.
Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, et al. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42(6):1437–45.
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73.
Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci USA. 1990;87(17):6599–603.
Lee HW, Chan HL. Unresolved issues of immune tolerance in chronic hepatitis B. J Gastroenterol. 2020;55(4):383–9.
Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol. 2015;12(3):258–63.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Kim MH, Kang SY, Lee WI. Occult HBV among anti-HBc alone: mutation analysis of an HBV surface gene and Pre-S gene. Yonsei Med J. 2017;58(3):557–63.
Yin Y, Zhang P, Tan Z, Zhou J, Wu L, Hou H. The association of Pre-S/S gene mutations and hepatitis B virus vertical transmission. Hepat Mon. 2016;16(3): e32160.
Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143(4):963-973.e969.
Shao X, Ma J, Jia S, Yang L, Wang W, Jin Z. Interleukin-35 suppresses antiviral immune response in chronic hepatitis B virus infection. Front Cell Infect Microbiol. 2017;7:472.
Nishida N, Sawai H, Kashiwase K, Minami M, Sugiyama M, Seto WK, et al. New susceptibility and resistance HLA-DP alleles to HBV-related diseases identified by a trans-ethnic association study in Asia. PLoS ONE. 2014;9(2): e86449.
Xu T, Sun M, Wang H. Relationship between HLA-DQ gene polymorphism and hepatitis B virus infection. Biomed Res Int. 2017;2017:9679843.
Milich DR. The concept of immune tolerance in chronic hepatitis B virus infection is alive and well. Gastroenterology. 2016;151(5):801–4.
Liaw YF, Chu CM. Immune tolerance phase of chronic hepatitis B. Gastroenterology. 2017;152(5):1245–6.
Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(Suppl 1):100–7.
Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053–63.
Chen YC. Hepatitis B surface antigen (HBsAg) levels in the prediction of spontaneous HBsAg seroclearance. Hepatology. 2013;57(4):1675.
European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85.
Andreani T, Serfaty L, Mohand D, Dernaika S, Wendum D, Chazouillères O, et al. Chronic hepatitis B virus carriers in the immunotolerant phase of infection: histologic findings and outcome. Clin Gastroenterol Hepatol. 2007;5(5):636–41.
Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57(1):196–202.
Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol. 2013;28(12):1842–8.
Sherman M. Does hepatitis B treatment reduce the incidence of hepatocellular carcinoma? Hepatology. 2013;58(1):18–20.
Attar BM. CON: all patients with immune-tolerated hepatitis B virus do not need to be treated. Clin Liver Dis. 2020;15(1):25–30.
Liao B, Wang Z, Lin S, Xu Y, Yi J, Xu M, et al. Significant fibrosis is not rare in Chinese chronic hepatitis B patients with persistent normal ALT. PLoS ONE. 2013;8(10): e78672.
Chao DT, Lim JK, Ayoub WS, Nguyen LH, Nguyen MH. Systematic review with meta-analysis: the proportion of chronic hepatitis B patients with normal alanine transaminase ≤ 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther. 2014;39(4):349–58.
Chen X, Zheng X, Wu H, Zhang B, Peng L, Xie C. Virological changes of chronic hepatitis b patients with minimally elevated levels of alanine aminotransferase: a meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2022;2022:7499492.
Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67(5):945–52.
Tong MJ, Trieu J. Hepatitis B inactive carriers: clinical course and outcomes. J Dig Dis. 2013;14(6):311–7.
Zhang S, Ma Q, Liang S, Xiao H, Zhuang G, Zou Y, et al. Annual economic burden of hepatitis B virus-related diseases among hospitalized patients in twelve cities in China. J Viral Hepat. 2016;23(3):202–10.
Zhang H, Chao J, Zhu L, Song L, Li X, Liu P. Direct economic burden and influencing factors in patients with hepatitis B virus related diseases in Jiangsu, China. Asia-Pac J Public Health. 2015;27(2 Suppl):41s–8s.
Kwon JH, Song MJ, Jang JW, Bae SH, Choi JY, Yoon SK, et al. Efficacy and safety of tenofovir disoproxil fumarate in treatment-naïve patients with chronic hepatitis B in Korea. Dig Dis Sci. 2019;64(7):2039–48.
Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60(5):1457–64.
Ruggieri A, Gagliardi MC, Anticoli S. Sex-Dependent Outcome of Hepatitis B and C Viruses Infections: Synergy of Sex Hormones and Immune responses? Front Immunol. 2018;9:2302.
Liu X, Baecker A, Wu M, Zhou JY, Yang J, Han RQ, et al. Family history of liver cancer may modify the association between HBV infection and liver cancer in a Chinese population. Liver Int. 2019;39(8):1490–503.
Choi GH, Kim GA, Choi J, Han S, Lim YS. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment Pharmacol Ther. 2019;50(2):215–26.
Lin D, Yang HI, Nguyen N, Hoang J, Kim Y, Vu V, et al. Reduction of chronic hepatitis B-related hepatocellular carcinoma with anti-viral therapy, including low risk patients. Aliment Pharmacol Ther. 2016;44(8):846–55.
Chinese Society of Hepatology, Chinese Medical Association. Expert opinion on expanding anti-HBV treatment for chronic hepatitis B. Chin J Hepatol. 2022;30(2):131–6.
Teng W, Chang TT, Yang HI, Peng CY, Su CW, Su TH, et al. Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines. Hepatol Int. 2021;15(6):1421–30.
Hsu YC, Chen CY, Chang IW, Chang CY, Wu CY, Lee TY, et al. Once-daily tenofovir disoproxil fumarate in treatment-naive Taiwanese patients with chronic hepatitis B and minimally raised alanine aminotransferase (TORCH-B): a multicentre, double-blind, placebo-controlled, parallel-group, randomised trial. Lancet Infect Dis. 2021;21(6):823–33.
Huang DQ, Tran A, Yeh ML, Yasuda S, Tsai PC, Huang CF, et al. Antiviral therapy substantially reduces HCC risk in patients with chronic hepatitis B infection in the indeterminate phase. Hepatology. 2023;78(5):1558–68.
Hadziyannis SJ, Vassilopoulos D, Hadziyannis E. The natural course of chronic hepatitis B virus infection and its management. Adv Pharmacol. 2013;67:247–91.
Acknowledgements
Not applicable.
Funding
This review was supported by the Youth Program of Xi’an Municipal Health Commission of China, No. 2022qn07; the General Program of Xi’an Municipal Health Commission of China, No. 2023ms11 and No. 2020ms14; the Shaanxi Natural Science Foundation of China, No. 2019JQ-978.
Author information
Authors and Affiliations
Contributions
Liu JY and Lv J searched and reviewed published articles, and wrote the manuscript; Liu JY, Yu Y, Zhao HP, Guo L, Yang WJ, Yan YZ, and Lv J critically reviewed and revised the manuscript; Liu JY and Lv J made substantial contributions to the conception and design of this study; all authors approved the final version of the article to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agree to the publication of this work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Yu, Y., Zhao, H. et al. Latest insights into the epidemiology, characteristics, and therapeutic strategies of chronic hepatitis B patients in indeterminate phase. Eur J Med Res 29, 343 (2024). https://doi.org/10.1186/s40001-024-01942-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01942-0