Abstract
Background
To determine the effect of colchicine on cancer risk in patients with the immune-mediated inflammatory diseases (IMIDs)-related to colchicine use.
Methods
This is a time-dependent propensity-matched general population study based on the National Health Insurance Research Database (NHIRD) of Taiwan. We identified the IMIDs patients (n = 111,644) newly diagnosed between 2000 and 2012 based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)—274,712, 135, 136.1, 279.49, 518.3, 287.0, 696.0, 696.1, 696.8, 420, 429.4, 710.0, 710.1, 710.3, 710.4, 714.0, 720, 55.0, 55.1, 55.9, 556. Inclusion criteria: aged ≧ 20 years, if a patient had at least these disease diagnosis requirements within 1 year of follow-up, and, these patients had at least two outpatient visits or an inpatient visit. After propensity-matched according to age, sex, comorbidities, medications and index date, the IMIDs patients enter into colchicine users (N = 16,026) and colchicine nonusers (N = 16,026). Furthermore, time-dependent Cox models were used to analyze cancer risk in propensity-matched colchicine users compared with the nonusers. The cumulative cancer incidence was analyzed using Cox proportional regression analysis. We calculated adjusted hazard ratios (aHRs) and their 95% confidence intervals (95% CIs) for cancer after adjusting for sex, age, comorbidities, and use of medicine including acetylcysteine, medication for smoking cessation such as nicotine replacement medicines (the nicotine patch) and pill medicines (varenicline), anti-inflammatory drugs and immunosuppressant drugs.
Results
Comparing the colchicine nonusers, all cancer risk were mildly attenuated, the (aHR (95% CI)) of all cancer is (0.84 (0.55, 0.99)). Meanwhile, the colchicine users were associated with the lower incidence of the colorectal cancer, the (aHRs (95% CI)) is (0.22 (0.19, 0.89)). Those aged < 65 years and male/female having the colchicine users were associated with lower risk the colorectal cancer also. Moreover, the colchicine > 20 days use with the lower aHR for colorectal cancer.
Conclusion
Colchicine was associated with the lower aHR of the all cancer and colorectal cancer formation in patients with the IMIDs.
Similar content being viewed by others
Introduction
The pyrin domain-containing protein 3 [or NOD-like receptor protein 3 (NLRP3)] inflammasome and NLRP3 inflammasome have been associated with colorectal cancer formation [1]. Notably, these markers would increase, especially in patients with the gout, diabetes, obesity and cancer [1]. The role of the NLRP3 inflammasome varies according to sex, age, and tumor type. For example, estrogen enhances the expression of NLRP3 inflammasome, whereas testosterone inhibits its expression. The estrogen acts through estrogen receptor β (ERβ) to enhance the activation of NLPR3 inflammasome and promote the progression of endometrial cancer [2]. In contrast, NLRP3 inflammasome protects against the bowel inflammation associated-tumor formation in chronic stages but promotes colon cancer in the early stage of the chronic colitis-ulcerative colitis, Crohn’s disease [1, 3, 4]. Thus, the NLRP3 inflammasome may be critically involved in the maintenance of intestinal homeostasis and protection against colitis [4]. Hence, NLRP3 inflammasome exhibits contrasting roles in cancer development [1, 5].
Colchicine is an anti-inflammatory drug, it have been used in the gout and immune-mediated inflammatory diseases (IMIDs) such as arthritis-crystal arthropathies, systemic inflammatory diseases such as sarcoidosis, Behcet’s syndrome, autoimmune disease, chronic idiopathic or spontaneous urticarial skin diseases, allergic purpura, psoriasis, collagen vascular diseases, and pericarditis [3, 6,7,8,9]. In addition, colchicine exerts its unique effects by the retardation of new tubulin formation, dissociation of tubulin, thus leading to cancer cell death and it could inhibit angiogenesis and cancer cell migration and metastasis, limit ATP influx into mitochondria, and release caspases and cytochrome-c, thus leading to apoptosis [1, 10]. In recent study, the colchicine could reduce the NLRP3 inflammasome activity or its downstream mediators in the cancer related to chronic inflammation diseases such as the hyperlipidemia with atherosclerosis, tobacco use with chronic obstructive pulmonary disease (COPD) and chronic colitis [1, 5, 11,12,13]. The nano-drug delivery of colchicine by means of mesoporous silica nanoparticles could enhance the anticancer effect for attenuating the risk of colon cancer cell formation support these speculations [14].
The important evidence that links inflammation and cancer is that non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, reduce the risk and mortality from many cancers [15]. Kuo et al. showed that male gout patients using colchicine had less colorectal cancer than other gout patients and colchicine use was associated with a decreased risk of incident all-cause cancers [16]. The IMIDs such as gout, chronic colitis are associated with the risk of cancer [9, 17,18,19]. However, to best our knowledge in English literature, no research has examined the role of colchicine in the evolution of cancer among patients with IMIDs. Thus, in this study, we investigated the relationship between the effects of colchicine and the incident cancer in patients with IMIDs by using a National Health Insurance Research Database (NHIRD).
Methods
Data source
On March 1, 1995, Taiwan launched a single-payer mandatory enrollment National Health Insurance (NHI) Program. Taiwan’s National Health Research Institutes (NHRI) established from 2002. The NHRI continue to maintain NHIRD for the purpose of public research. Up to 99.99% of Taiwan’s population are enrolled under this well-established program. Thus, the NHIRD, derived from claims data of NHI beneficiaries, could illuminate the disease burden and health care process of the entire Taiwanese population and the population included in this database representative of the population of Taiwan.
We analyzed the Longitudinal Health Insurance Database, a subset of the NHIRD that contains demographic information, inpatient and outpatient records, medications, and treatments of 2 million insured individuals.
Study population
Between the 2000-01-01 and the 2012-12-31, the patients with the ICD-9CM (274, 712, 135, 136.1, 279.49, 518.3, 287.0, 696.0, 696.1, 696.8, 420, 429.4, 710.0, 710.1, 710.3, 710.4, 714.0, 720, 55.0, 55.1, 55.9, 556)-IDIMs enter into study. The inclusion criteria (N = 111,644) as below: aged ≧ 20 years, if a patient had at least these disease diagnosis requirements within 1 year of follow-up. And, these patients had at least two outpatient visits or an inpatient visit.
The patients who used colchicine between 2000 and 2012 were defined as colchicine users among patients with IMIDs (N = 23,612) [6,7,8, 20, 21]. The colchicine users need to fulfill criteria as below: in Lin et al.’s study, the nude mice experiment showed that colchicine-treated mice after 14 days of treatment had lower increased tumor volume ratios and tumor growth rates than the control. In accordance with this previous study, we set the duration of the colchicine use > 15 days [22]. Nonusers defined as those who never used colchicine or the date of the first prescription of colchicine, the duration of the colchicine use ≤ 14 days (N = 88,032) [15, 23] (Additional file 1: Figure S1).
Excluding criteria (N = 1148) in colchicine users as below: not Taiwanese citizens, or aged < 20 years, had no IMIDs-related prescription requirements or related management or procedure 1 year after the first IMIDs diagnosis; had a disease history of IMIDs or received colchicine before the first IMIDs diagnosis or previous history of cancer (ICD-9-CM codes 140–239) [7, 21, 24]. The colchicine may be considered as an immunosuppressant agent, and the colchicine may interact with other immunosuppressant. And these immunosuppressants could interact with each other and perhaps affecting the cancer formation [25, 26]. For those who had more than one prescription of immunosuppressant, a minimum of < 7 days between prescriptions were excluded. Incident use of drugs increases in the months prior to a cancer diagnosis. To avoid reverse causation, 6 months lag time would be sufficient for most drug–cancer associations. In this study, to avoid this reverse causation, the lag time would be set as ≧ 12 months from the first prescription of colchicine to any diagnosis of cancer [23, 27]. Therefore, prescriptions within 12 months of diagnosis of cancer were not considered as exposed, again to avoid reverse causation [25]. And the same exclusion criteria was used in the nonusers.
Before propensity matching, 22,464 cases without cancer at baseline in colchicine users and 76,224 controls were selected (n = 98,688). The 1:1 propensity score matching was used in cohort studies And, we excluded unmatched 66,636 patients. Finally, total 16,026 colchicine used cases and 16,026 control subjects were identified in the study cohort (Additional file 1: Figure S1).
Since this a time-dependent study, the index date for the colchicine users was the date of the first prescription of colchicine, and a date between 2000 and 2012 was the index date for the nonusers. Colchicine users and nonusers were then matched by propensity scores according to age, sex, comorbidities (alcohol-related disease, coronary artery disease, diabetes, hypertension, hyperlipidemia, chronic obstructive pulmonary disease stroke, tobacco use, depression, chronic kidney disease), medications (acetylcysteine, smoking cessation-related drugs, anti-inflammatory drugs and immunosuppressants) and index date. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes considered for patients with the MD (Full names, Additional file 2: Table S1).
Licensing Committee (ethics statement)
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. All methods were carried out in accordance with relevant guidelines and regulations. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University & Hospital Research Ethics Center (CMUH104-REC2-115[CR-7]). The IRB also specifically waived the consent requirement.
Data availability statement
The dataset used in this study is held by the Taiwan Ministry of Health and Welfare (MOHW). The Ministry of Health and Welfare must approve our application to access this data. Any researcher interested in accessing this dataset can submit an application form to the Ministry of Health and Welfare requesting access. Please contact the staff of MOHW (Email: stcarolwu@mohw.gov.tw) for further assistance. Taiwan Ministry of Health and Welfare Address: No.488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.). Phone: +886-2-8590-6848. All relevant data are within the paper.
Main outcome and covariates
The primary endpoint of this study was the development of cancer (ICD-9-CM codes 140–239), as evidenced by a major illness or injury certificate of cancer. The study endpoint was defined as the occurrence of cancer, withdrawal from the insurance program if they are missing for 6 months or more or an individual is missing because of a natural disaster, coverage can be withdrawn from the day the disaster occurred or they lose Taiwan citizenship, move overseas, or have an expired Alien Resident Certificate, or December 31, 2013.
In addition to age and sex, other potential confounders included comorbidities (Full names, Additional file 2: Table S1). The use of acetylcysteine, smoking cessation-related medication, anti-inflammatory drugs (aspirin, NSAID) and immunosuppressant drugs was evaluated as a confounder also. The severity and duration of IMIDs have impact on the risk of cancer [28,29,30]. Owing to the comorbidities and medications were close relation to the IMIDs severity and duration [28]. Thus, these comorbidities, medications and Charlson comorbidity index enter into analysis in this study. Meanwhile, we used alcohol-related diseases as a proxy for drinking, chronic obstructive pulmonary disease (COPD) and tobacco use for smoking. The Anatomical Therapeutic Chemical code (ATC) of medications were defined as a prescription with one of the following National Health Insurance (NHI) codes: Additional file 1: Figure S1 displays the flowchart for selection of the patients.
Statistical analysis
Categorical variables were expressed as number and percentage, and differences between the two cohorts were examined using the Chi-square test. Continuous variables were presented as median and interquartile range, and differences between the two cohorts were assessed using the Mann–Whitney U test. The incidence rate was expressed as per 1000 person-years. The disease duration might impact on the risk of cancer and should be taken into account. Thus, the Cox proportional hazards (PH) model with time-dependent exposure covariates was used as the main model in the study to analyze the effect of colchicine on cancer. The association between the duration of colchicine use and the development of cancer was analyzed using the general Cox PH model. The Kaplan–Meier method was used to obtain the cumulative curves, and the results were then examined using the log-rank test. SAS statistical software (version 9.1, SAS Institute, Cary, NC) was used to perform the analysis. A p-value less than 0.05 was set as the significant level.
Results
A total of 22,464 patients were defined as colchicine users in this study (Table 1). The median age of the colchicine users was higher than that of the colchicine nonusers: 54.3 years versus 53.7 years, respectively. A male preponderance was observed in the colchicine users compared with the colchicine nonusers. As this is a time-dependent study, more patients had developed comorbidities such as alcohol-related diseases, diabetes, hypertension and hyperlipidemia in the colchicine users than in the colchicine nonusers. In addition, the colchicine users included more patients who used acetylcysteine, smoking cessation-related medication, anti-inflammatory drugs such as oral steroids (OSs), NSAIDs, acetylsalicylic acid, and immunosuppressant drugs such as azathioprine, sulfasalazine, cyclophosphamide, methotrexate, hydroxychloroquine, and cyclosporine. After propensity score matching, both cohorts included 16,026 patients each. Both cohorts had a similar distribution of age, comorbidities, and medications except the sex and alcohol-related disease, hypertension and hyperlipidemia.
However, Charlson comorbidity index (CCI) have been found to be strongly predictive of survival among colon cancer patients [29]. The CCI index ≧ 1 of the both cohorts was without significant differences [31]. Furthermore, we use the multiple variants logic regression with time-dependent analysis for minimize these baseline imbalance [32].
The higher frequency of the gout in colchicine users was in parallel with the higher frequency of alcohol-related disease, hypertension and hyperlipidemia. In our study, the 75% colchicine users (n = 16,026 × 0.75 = 12,019) having the gout (Additional file 2: Table S1). The number of the colchicine users with gout is 12,019 which is similar to the Kuo et al. study (a total of 13,679 patients with gout having received colchicine).
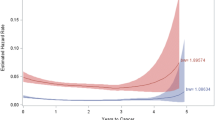
Table 2 illustrates the association of colchicine use with different types of cancer in the colchicine users. For example, comparing with colchicine nonusers, the colorectal cancer and brain tumor in the colchicine users were associated with the different adjusted HRs [aHRs], the aHRs (95% CI) were 0.22 (0.19, 0.89), and 0.68 (0.42, 2.01), respectively. The colchicine users were associated with the lower incidence of the colorectal cancer (Fig. 1). Additional file 3: Table S2 displays the lower aHR for all cancer revealed in the colchicine users.
Table 3 illustrates the colchicine use with > 20 days having the lower aHR for the chronic colitis related to colorectal cancer.
Investigating the impact of sex and age on the IMIDs and subcohort of IMIDs-chronic colitis-related to colorectal cancers formation
The elderly with colchicine use having the null effect for the aHR of colorectal cancer. Regarding the young adults (aged < 65 years and male/female), the aHRs for colorectal cancer were associated with the lower aHRs for the colchicine users in the IMIDs (Table 4).
The subcohort of the IDIMs-chronic colitis is well representative of these speculations. We display these points in Additional file 4: Table S3
Additional file 4: Table S3 displays the lower aHR of colorectal cancer for the colchicine users among the chronic colitis-(ulcerative colitis, Crohn’s disease) in young adult [9, 18, 33].
Covariates as proxies to the NLRP3 expression for the incident cancer
The NLRP3 expression were unavailable in the NHIRD. However, the NLRP3 expression was in accordance with the cell type, duration of use, age, sex. Meanwhile, these variables were independent and critical factors of the colchicine on the cancer promotion [22, 34,35,36,37]. Moreover, those covariates such as comorbidities (e.g., alcohol-related disease, hypertension, diabetes, depression, tobacco use) were proxies to the NLRP3 expression for the incident cancer [35, 38]. Therein, these variants were entered into the analysis [1, 39].
Additional file 5: Table S4a and Additional file 6: Table S4b display the aHR for the individual comorbidity and medication. The comorbidities display the higher aHR for all cancer except the hypertension and coronary artery disease. The smoking cessation-related medications and anti-inflammatory medications revealed the lower aHR for all cancer.
Sensitivity analysis-propensity score analysis for time-dependent exposure in different cancer subtype and comorbidities
The colchicine users have the null effect for the aHR of most of the individual cancer such as the brain tumor except the lower aHR of colorectal cancer. Combination of the NLRP3 dual effect for cancer, colchicine modulates NLRP3 effect for cancer, duration of drug effect and detection bias could play a role for contributing to these results. We did sensitivity analysis for clarifying these points for colorectal cancer. We displayed these speculations in Table 5: (1) the combination effect of the colchicine, NLRP3, comorbidities and detection bias on the risk of cancer in colorectal cancer with chronic colitis; (2) sex and age for modulating the effect of colchicine on the risk of colorectal cancer [40, 41].
Discussion
The main finding of our study was that colchicine use was associated with a lower risk of the colorectal cancer in patients with the IMIDs. The Kaplan–Meier was used only one variable (event of interest-colchicine use) for estimating the survival curves. Log-rank test compares two or more survival curves and does not consider additional independent variables. Similar to multiple regression model, Cox PH model considers additional independent variables (covariates-age, sex, comorbidities and medications) for estimating the differences between the survival curves. To the best of our knowledge, this study is the first study to report an association between colchicine use and incidental cancers in patients with the IMIDs, who were associated with the lower risk of colorectal cancer based on the Cox PH model with time-dependent exposure. Similar to that, the colchicine users were associated with the lower cumulative incidence of the colorectal cancer in analysis of the Kaplan–Meier method. Meanwhile, the lower aHR for all cancer was revealed in the colchicine users also.
The methods of this study are different from the Kuo et al. study. The Kuo et al.’s study focusses on the gout cohort, ever user (colchicine usage within 3 years after gout) and they excluded the DM and cancer within 1 year after gout. In our study, the study cohort is the IMIDs cohort, the patients with colchicine use are all new colchicine users and the colchicine users excluding the lag time within 1 year for incident cancer formation. However, some results are similar to Kuo et al. study. For example, Kuo et al. found that colchicine ever-users had significantly lower incidence of all-cause cancers after adjustment for age, compared with colchicine never-users (aHR = 0.85, 95% CI (0.77, 0.94)). In our study, the lower risk of the all cancer (aHR = 0.84, 95% CI (0.55, 0.99)) was found in the colchicine users excluding the lag time within 1 year for incident cancer. In Kuo et al., the comorbidity such as the hyperlipidemia has higher risk of cancer, on comparing the colchicine ever-users with the colchicine never-users. Similar to that, in our study, the colchicine users with comorbidities such as the alcohol-related disease, diabetes, hyperlipidemia, COPD, depression, tobacco use and chronic kidney disease had higher aHR for cancer.
An important confounding factor of this study is the detection bias. Detection bias is hypothesized, in part, because of observed differences between the colchicine users and colchicine nonusers. Detection bias occurs when screening and diagnostic patterns vary in association with potential risk factors. Detection bias could exaggerate or attenuate estimated cancer–colchicine associations and affecting the aHR for cancer. For example, those aged > 65 received the higher frequency of neurodegenerative diseases-related procedure and brain CT, these procedures let us to early detection of brain lesion. In similar indication, brain CT is a popular examination for resolving the critical neurological patients at emergency department in Taiwan. These feasible brain CT examinations in practice, increase the early detection of the brain tumor [42].
Moreover, the colchicine users have the higher frequency of the alcohol-related diseases, hypertension and hyperlipidemia. The alcohol-related diseases, diabetes, hyperlipidemia have the higher risk of the cancer in our study. Bao et al. report that high glucose promotes human glioblastoma cell growth by increasing the expression and function of chemoattractant and growth factor receptors, leading to the growth of human glioblastoma cells [43]. Rogers et al. report that hyperlipidemia and diabetes are associated with the higher risk of brain tumor such as glioblastoma [44]. These previous reports support these findings. These comorbidities combined with the overactivity of NLRP3 inflammasome attenuate the auxiliary anti-tumor effect of colchicine in patients with IMIDs [37, 45]. Therefore, these combination factors (age, comorbidities and detection bias) lead to null effect for aHR of brain tumor in the IMIDs with colchicine user [46].
Another important finding in this report is that the young adult colchicine users were associated with the lower aHR of colorectal cancer among the IMIDs [21, 47].
As mention before, the NLRP3 inflammasome could play a positive role for protecting the colorectal cancer (negative effect on the aHR) in the late stage of chronic colitis. In the young male with high level of the NLRP3 inflammasome activity especially the IMIDs-chronic colitis patients, testosterone could modulate the NLRP3 inflammasome activity and promoting the NLRP3 inflammasome-related tumor genesis activity [37]. However, colchicine could impair testosterone synthesis, attenuating the effect the testosterone on the NLRP3 inflammasome-related tumor genesis activity, leading to colchicine may play a positive role for protecting the cancer formation (negative effect on the aHR) [48]. Taking these together, the colchicine users were associated with the optimal counterbalance effect on the NLRP3 inflammasome, thus the young male patients in the colchicine users were associated with the lower risk of the colorectal cancer (negative effect on the aHR) in a later course of the chronic colitis even with the higher frequency of the comorbidities (Tables 4, 5) [48, 49]. Similar to that, the colchicine could modulate the NLRP3 inflammasome among the female, leading to colchicine users being associated with the lower aHR for colorectal cancer [35].
The chronic colitis is a key factor of the colorectal cancer. In previous study, the colchicine may play an auxiliary for management of the chronic colitis such as Crohn’s disease with amyloidosis in young adult, perhaps leading to the lower risk of the colorectal cancer in accordance with our findings [3, 50, 51].
In summary, age, sex, comorbidities and detection bias are confounding factors for the effect of colchicine on cancer. However, the colchicine users having the lower risk of the all cancer. Furthermore, especially in young adults and long-term use (> 100 days) having the lowest the aHR for colorectal cancer among the IMIDs.
Finally, subcohort such as the IMIDs-chronic colitis in young adult, those with colchicine use having the lower risk of the colorectal cancer in accordance with initial results.
Altogether, colchicine users are more likely to engage in health-seeking behaviors than colchicine nonusers. Meanwhile, those colchicine users who often use other medications such as anti-hypertension, statins and oral sugar or insulin sometimes forego preventive services such as cancer screening. Differential screening rates by colchicine use could then inflate the aHR for the tumor such as the brain tumor. It is to be noted that when we interpret these findings, we need to take these confounding factors into account [42, 52]. Further research is warranted to confirm these observations.
Clinical implication in practice
The microtubule-destabilizing agents bind to the colchicine-binding site (CBS) of tubulin, termed colchicine-binding site inhibitors (CBSIs). While colchicine itself could not be used in the treatment of cancer due to systemic toxicity, several CBSIs show promise in the future treatment of different subtypes cancers such as gastro-entero-pancreatic neuroendocrine cancers, including those with acquired multiple drug resistance characteristics [53].
Recently, Hamid et al. report that out of the 69,260,780 patients in the database, they identified 209, 020 patients with ulcerative colitis (0.30%) of whom 9130 had gout (4.3%). Additionally, 249,480 had Crohn’s disease (0.36%) of whom 14,000 had gout (5.61%) [33], such that, the number of the gout with IMIDs-chronic colitis is 9130 + 14,000 = 23,130. We assumed the 75% gout patients with colchicine use, the number of colchicine user is about (23,130) × 0.75 = 17,348. This number of colchicine users is similar to our study cohort (16,026). Therefore, our results indicated IMIDs, especially gout with chronic colitis having the colchicine use are associated with the lower risk of colorectal cancer. These speculations could infer to future research for relationship between the colchicine use in chronic colitis with gout among the IMIDs cohort.
Strengths
This is the first general population study to investigate the effect of colchicine on the development of cancer in patients with the IMIDs-chronic colitis. The time-dependent and propensity score matching for avoiding the bias. Furthermore, in Taiwan, diagnoses of the cancer and the IMIDs follow strict guidelines. The role of colchicine in NLRP3 inflammasome activity has been examined in a Taiwanese’s study [54]. The diagnosis, treatment, or follow-up of these groups of patients drawn from the NHIRD were based on previous experiments. The strict policy contributing to our result would be representative of the real world in Taiwan. However, the result in our study warrants future research into the relationship between cancer, and the IMIDs-chronic colitis.
Limitations
This study had a few limitations. First, the NHIRD provides no detailed information on patients regarding factors such as their lifestyle, body mass index (or obesity), habits (e.g., smoking, alcoholic drinking), physical activity, socioeconomic status, or family history; all of which are possible confounding factors in this study. We used alcohol-related diseases as a proxy for drinking, tobacco use and COPD for smoking, gout, hypertension and hyperlipidemia for exercise and life style [55, 56]. Yet, there are gaps between the proxy and real world. Second, the diagnosis of IMIDs in Taiwan is based on clinical history, imaging, laboratory data and pathology. The coding of the IMIDs in the NHIRD was established according to these strict standard [55, 56]. Notably, the registries in the NHI claims are primarily used for administrative billing and are not verified for scientific purposes. Third, recording of cancer diagnoses and survival estimates based on these diagnosis codes in the NHI database are generally consistent with the National Cancer Registry (NCR) [57]. However, lack of individual laboratory data, imaging finding, and pathologic result (such as cancer staging) in the NHIRD may be the other study limitation. Fourth, the retrospective study is usually lower evidence than the randomized controlled trials because a retrospective study is subject to have many unknown or uncontrolled confounding factors. Fifth, the incidence of some specific cancers was relatively low, thus limiting the power of our study. Sixth, as mention before, detection bias is present when the exposure (e.g., newly diagnosed patients with the IMIDs leads to higher detection of the outcome of interest (cancer) due to the increased frequency of clinic visits, which also results in an overestimation of risk-cancer. Thus, including a lag period, such as starting follow-up after 1 year of the initiation of a drug in this study, simultaneously considers a latency period while also minimizing protopathic and detection bias. However, colchicine use may just indicate developments around an imminent cancer diagnosis; these two bias were another limitation in this study.
Conclusion
This study implied colchicine is associated with lower risk of all cancer and colorectal cancer formation in patients with IMIDs. However, age, sex comorbidities and detection bias may modulate the aHRs for brain and colorectal cancer in the colchicine users among the IMIDs.
Availability of data and materials
The dataset used in this study is held by the Taiwan Ministry of Health and Welfare (MOHW). The Ministry of Health and Welfare must approve our application to access this data. Any researcher interested in accessing this dataset can submit an application form to the Ministry of Health and Welfare requesting access. Please contact the staff of MOHW (Email: stcarolwu@mohw.gov.tw) for further assistance. Taiwan Ministry of Health and Welfare Address: No.488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.). Phone: + 886-2-8590-6848. All relevant data are within the paper.
Abbreviations
- aHRs:
-
Adjusted hazard ratios
- CIs:
-
Confidence intervals
- NLRP3:
-
NOD-like receptor protein 3
- ICD-9-CM:
-
International Classification of Diseases, Ninth revision, Clinical Modification
- NHIRD:
-
National Health Insurance Research Database
- CAD:
-
Coronary artery disease
- COPD:
-
Chronic obstructive pulmonary disease
- ATC:
-
Anatomical Therapeutic Chemical
- PH:
-
Proportional hazards
- OSs:
-
Oral steroids
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- IMIDs:
-
Immune-mediated inflammatory diseases
References
Missiroli S, Perrone M, Boncompagni C, et al. Targeting the NLRP3 inflammasome as a new therapeutic option for overcoming cancer. Cancers. 2021;13:2297.
Liu SG, Wu XX, Hua T, et al. NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. Onco Targets Ther. 2019;12:6927–36.
Shi Y, Li J, Yang P, et al. Colchicine increases intestinal permeability, suppresses inflammatory responses, and alters gut microbiota in mice. Toxicol Lett. 2020;334:66–77.
Zaki MH, Boyd KL, Vogel P, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–91.
Wagatsuma K, Nakase H. Contradictory effects of NLRP3 inflammasome regulatory mechanisms in colitis. Int J Mol Sci. 2020;21:8145.
Sardana K, Sinha S, Sachdeva S. Colchicine in dermatology: rediscovering an old drug with novel uses. Indian Dermatol Online J. 2020;11:693–700.
Surma S, Basiak M, Romańczyk M, et al. Colchicine—from rheumatology to the new kid on the block: coronary syndromes and COVID-19. Cardiol J. 2023;30:297–311.
Shelley-Fraser G, Borley NR, Warren BF, et al. The connective tissue changes of Crohn’s disease. Histopathology. 2012;60:1034–44.
Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol. 2019;17:2704-2712.e3.
Lee HE, Lee JY, Yang G, et al. Inhibition of NLRP3 inflammasome in tumor microenvironment leads to suppression of metastatic potential of cancer cells. Sci Rep. 2019;9:12277.
Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262–71.
Yao J, Sterling K, Wang Z, et al. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct Target Ther. 2024;9:10.
Otani K, Watanabe T, Shimada S, et al. Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome. Sci Rep. 2016;6:32587.
AbouAitah K, Hassan HA, Swiderska-Sroda A, et al. Targeted nano-drug delivery of colchicine against colon cancer cells by means of mesoporous silica nanoparticles. Cancers (Basel). 2020;12:144.
Bastiaannet E, Sampieri K, Dekkers OM, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106:1564–70.
Kuo MC, Chang SJ, Hsieh MC. Colchicine significantly reduces incident cancer in gout male patients: a 12-year cohort study. Medicine. 2015;94: e1570.
Wang W, Xu D, Wang B, et al. Increased risk of cancer in relation to gout: a review of three prospective cohort studies with 50,358 subjects. Mediators Inflamm. 2015;2015: 680853.
Wu X, Wang J, Ye Z, et al. Risk of colorectal cancer in patients with irritable bowel syndrome: a meta-analysis of population-based observational studies. Front Med (Lausanne). 2022;9: 819122.
Yu KH, Kuo CF, Huang LH, et al. Cancer risk in patients with inflammatory systemic autoimmune rheumatic diseases: a nationwide population-based dynamic cohort study in Taiwan. Medicine (Baltimore). 2016;95: e3540.
Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken). 2010;62:460–4.
Albayrak B, Sebin E. A novel inflammatory marker for extensive ulcerative colitis. Endocan BMC Gastroenterol. 2023;23:118.
Lin ZY, Kuo CH, Wu DC, et al. Anticancer effects of clinically acceptable colchicine concentrations on human gastric cancer cell lines. Kaohsiung J Med Sci. 2016;32:68–73.
Hicks B, Kaye JA, Azoulay L, et al. The application of lag times in cancer pharmacoepidemiology: a narrative review. Ann Epidemiol. 2023;84:25–32.
Leung YY, Yao Hui LL, Kraus VB. Colchicine-update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–50.
Tsai TL, Wei JCC, Wu YT, et al. The association between usage of colchicine and pneumonia: a nationwide, population-based cohort study. Front Pharmacol. 2019;10:908.
Bakouny Z, Labaki C, Grover P, et al. Interplay of immunosuppression and immunotherapy among patients with cancer and COVID-19. JAMA Oncol. 2023;9:128–34.
Tope P, Farah E, Ali R, et al. The impact of lag time to cancer diagnosis and treatment on clinical outcomes prior to the COVID-19 pandemic: a scoping review of systematic reviews and meta-analyses. Elife. 2023;12: e81354.
Yagyu H, Murohashi K, Hara Y, et al. Clinical utility of a composite scoring system including Charlson Comorbidity Index score in patients with interstitial lung disease. J Thorac Dis. 2020;12:5774–82.
Hall WH, Ramachandran R, Narayan S, et al. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94.
Kwon MJ, Han KM, Kim JH, et al. Gout and colorectal cancer likelihood: insights from a nested case-control study of the korean population utilizing the Korean national health insurance service-national sample cohort. Cancers (Basel). 2023;15:5602.
Jørgensen TL, Hallas J, Friis S, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353–60.
Wilkinson JD, Mamas MA, Kontopantelis E. Logistic regression frequently outperformed propensity score methods, especially for large datasets: a simulation study. J Clin Epidemiol. 2022;152:176–84.
Hamid O, Alsabbagh Alchirazi K, Eltelbany A, et al. Increased prevalence of gout in patients with inflammatory bowel disease: a population-based study. JGH Open. 2023;7:640–4.
Cho JH, Joo YH, Shin EY, et al. Anticancer effects of colchicine on hypopharyngeal cancer. Anticancer Res. 2017;37:6269–80.
Chuang JP, Lee JC, Leu TH, et al. Association of gout and colorectal cancer in Taiwan: a nationwide population-based cohort study. BMJ Open. 2019;9: e028892.
Kim SR, Kim EK, Cho J, et al. Effect of anti-inflammatory drugs on clinical outcomes in patients with malignant pericardial effusion. J Am Coll Cardiol. 2020;76:1551–61.
Zhang H, Tang Y, Tao J. Sex-related overactivation of NLRP3 inflammasome increases lethality of the male COVID-19 patients. Front Mol Biosci. 2021;8: 671363.
Robinson PC, Terkeltaub R, Pillinger MH, et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. 2022;135:32–8.
Wei Q, Guo P, Mu K, et al. Estrogen suppresses hepatocellular carcinoma cells through ERβ-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95:804–16.
Ghisa M, Marinelli C, Savarino V, et al. Idiopathic pulmonary fibrosis and GERD: links and risks. Ther Clin Risk Manag. 2019;15:1081–93.
Gu JP, Tsai CL, Wysham NG, et al. Chronic hypersensitivity pneumonitis in the southeastern United States: an assessment of how clinicians reached the diagnosis. BMC Pulm Med. 2020;20:32.
Russell B, Garmo H, Beckmann K, et al. A case-control study of lower urinary-tract infections, associated antibiotics and the risk of developing prostate cancer using PCBaSe 3.0. PLoS ONE. 2018;13:e0195690.
Bao Z, Chen K, Krepel S, et al. High glucose promotes human glioblastoma cell growth by increasing the expression and function of chemoattractant and growth factor receptors. Transl Oncol. 2019;12:1155–63.
Rogers LR, Ostrom QT, Schroer J, et al. Association of metabolic syndrome with glioblastoma: a retrospective cohort study and review. Neurooncol Pract. 2020;7:541–8.
Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–9.
Hu SY, Hsieh MS, Lin MY, et al. Trends of CT utilisation in an emergency department in Taiwan: a 5-year retrospective study. BMJ Open. 2016;6: e010973.
Tavassoli S, Shahabinasab I, Norouzi A, et al. From bowel inflammation to the bone and joints: musculoskeletal examination in inflammatory bowel disease (IBD). BMC Musculoskelet Disord. 2021;22:1019.
Ding J, Shang X, Zhang Z, et al. FDA-approved medications that impair human spermatogenesis. Oncotarget. 2017;8:10714–25.
Haimov-Kochman R, Ben-Chetrit E. The effect of colchicine treatment on sperm production and function: a review. Hum Reprod. 1998;13:360–2.
You G, Ferges W, Das K. Infliximab and colchicine therapy in the treatment of systemic secondary amyloidosis in Crohn’s disease: 1502. Am J Gastroenterol. 2014;109:S443.
Barkhodari A, Lee KE, Shen M, et al. Inflammatory bowel disease: focus on enteropathic arthritis and therapy. Rheumatol Immunol Res. 2022;3:69–76.
Peng YC, Lin CL, Hsu WY, et al. The risk of colorectal cancer is related to frequent hospitalization of IBD in an Asian population: results from a nationwide study. QJM. 2015;108:457–63.
McLoughlin EC, O’Boyle NM. Colchicine-binding site inhibitors from chemistry to clinic: a review. Pharmaceuticals (Basel). 2020;13:8.
Lin TY, Tsai MC, Tu W, et al. Role of the NLRP3 inflammasome: insights into cancer hallmarks. Front Immunol. 2021;11: 610492.
Yeh JJ, Lai JN, Lin CL, et al. Time-dependent propensity-matched general population study of the effects of statin use on cancer risk in an interstitial lung disease and pulmonary fibrosis cohort. BMJ Open. 2021;11: e047039.
Yeh JJ, Lin CL, Hsu NH, et al. Effects of statins and steroids on coronary artery disease and stroke in patients with interstitial lung disease and pulmonary fibrosis: a general population study. PLoS ONE. 2021;16: e0259153.
Kao WH, Hong JH, See LC, et al. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol Drug Saf. 2018;27:1060–6.
Acknowledgements
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW111-TDU-B-212-124004), China Medical University Hospital (DMR-113-048, DMR-113-060, DMR-113-061, DMR-111-105); Ministry of Science and Technology (MOST 110-2321-B-039-003). We are grateful to the Health Data Science Center, China Medical University Hospital for providing administrative, technical, and funding support. Ditmanson Medical Foundation Chia-Yi Christian Hospital Research Program (R112-016)
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: conception and design: Jun-Jun Yeh, Pei-Xuan Liw, Yi-Sin Wong, Husan-Min Kao, Chia-Hsun Lee, administrative support: Chia-Hung Kao. Collection and assembly of data: all authors. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University & Hospital Research Ethics Center (CMUH104-REC2-115[CR-7]). The IRB also specifically waived the consent requirement. Ditmanson Medical Foundation Chia-Yi Christian Hospital Research Program R112-016.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flow chart of selection of patients.
Additional file 2: Table S1.
Full name of ICD-9CM with immune-mediated inflammatory diseases.
Additional file 3: Table S2.
Incidence and HRs of all cancer in the colchicine cohorts compared with those in the non-colchicine cohorts by Cox proportional hazard models with time-dependent exposure covariates.
Additional file 4: Table S3.
Incidence and HRs of cancer in the colchicine cohorts compared with those in the non-colchicine cohorts by Cox proportional hazard models with time-dependent exposure covariates in immune-mediated inflammatory diseases -chronic colitis in young adult
Additional file 5: Table S4a.
The crude HR and aHR for the individual comorbidity in the colchicine use and non-colchicine use among the immune-related cohort by Cox proportional hazard model with time-dependent covariates in propensity-score-matched cohorts.
Additional file 6: Table S4b.
The crude HR and aHR for the individual medication in the colchicine use and non-colchicine use among the immune-related cohort by Cox proportional hazard model with time-dependent covariates in propensity-score-matched cohorts.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yeh, JJ., Liw, PX., Wong, YS. et al. The effect of colchicine on cancer risk in patients with immune-mediated inflammatory diseases: a time-dependent study based on the Taiwan’s National Health Insurance Research Database. Eur J Med Res 29, 245 (2024). https://doi.org/10.1186/s40001-024-01836-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01836-1