Abstract
Background
In-stent restenosis (ISR) has been shown to be correlated with inflammation. This study aimed to examine the relationship between systemic immune-inflammation index (SII, an innovative inflammatory biomarker) and ISR in acute coronary syndrome (ACS) patients after drug-eluting stent (DES) implantation.
Methods
Subjects who were diagnosed with ACS and underwent DES implantation were enrolled retrospectively. All individuals underwent follow-up coronary angiography at six to forty-eight months after percutaneous coronary intervention (PCI). SII was defined as [(platelet count × neutrophil count)/lymphocyte count], and Ln-transformed SII (LnSII) was carried out for our analysis. Multivariate logistic regression analysis was employed to assess the association between LnSII and DES-ISR.
Results
During a median follow-up period of 12 (11, 20) months, 523 ACS patients who underwent follow-up angiography were included. The incidence of DES-ISR was 11.28%, and patients in the higher LnSII tertile trended to show higher likelihoods of ISR (5.7% vs. 12.1% vs. 16.0%; P = 0.009). Moreover, each unit of increased LnSII was correlated with a 69% increased risk of DES-ISR (OR = 1.69, 95% CI 1.04–2.75). After final adjusting for confounders, a significant higher risk of DES-ISR (OR = 2.52, 95% CI 1.23–5.17) was found in participants in tertile 3 (≥ 6.7), compared with those in tertiles 1–2 (< 6.7). Subgroup analysis showed no significant dependence on age, gender, body mass index, current smoking, hypertension, and diabetes for this positive association (all P for interaction > 0.05).
Conclusion
High levels of SII were independently associated with an increased risk of DES-ISR in ACS patients who underwent PCI. Further prospective cohort studies are still needed to validate our findings.
Similar content being viewed by others
Background
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation is the main revascularization strategy for patients with acute coronary syndrome (ACS). However, despite continued improvement in stent technology and anti-platelet therapy, in-stent restenosis (ISR) remains an important issue that limits the clinical safety and efficacy of DES. Previous studies have shown that the cumulative incidence of DES-ISR in real-world practice reaches up to 10% at 5 years following PCI [1, 2]. ISR may lead to the recurrence of adverse cardiovascular outcomes, such as myocardial infarction and sudden cardiac death. Hence, early identification of accurate and convenient biomarkers of DES-ISR has important clinical significance for ACS patients.
Inflammation has been well studied as a potential cardiovascular risk factor [3]. Accumulated evidence suggests that inflammatory reactions are correlated with atherosclerotic cardiovascular diseases, such as arterial hypertension, ischemic stroke, and coronary artery disease (CAD) [4,5,6]. Notably, a growing number of studies also show that inflammation plays a pivotal role in the genesis and advancement of ISR [7, 8]. Inflammatory factors could induce endothelial regeneration and proliferation, leading to restenosis [9, 10]. Indeed, many studies have demonstrated that inflammatory indices such as high-sensitivity C-reactive protein, neutrophil/lymphocyte ratio (NLR), and eosinophil cationic protein are associated with a high risk of ISR [11, 12]. As a result, the correlation between inflammation and ISR has garnered escalating scholarly interest.
The systemic immune-inflammation index (SII) is an innovative and integrated inflammatory biomarker based on three types of immune cells (platelets, neutrophils, and lymphocytes). Many studies have confirmed its high prognostic values in chronic heart failure, ischemic stroke, and CAD, which suggests potential implications for cardiovascular disease (CVD) [13,14,15]. Moreover, SII had better performance than platelet/ lymphocyte ratio (PLR) and NLR in predicting the poor outcomes of ACS and the hemodynamically severe obstruction of chronic coronary syndrome (CCS) [16, 17]. Thus, SII may be a superior and comprehensive indicator of local immune responses and systemic inflammation [18]. Studies have shown that blood cell parameters (such as PLR, NLR, and platelet distribution width) are correlated with an increased risk of ISR in patients with angina pectoris and coronary chronic total occlusion lesions [12]. However, there are no studies exploring the relationship between SII and DES-ISR, especially in ACS patients.
Taken together, it is of great significance to examine the relationship between SII, an economical and superior indicator, and ISR for evaluating intravascular conditions and improving the prognosis of CAD more effectively. To address this knowledge gap, we therefore designed this research to investigate the association between SII and ISR in ACS patients after DES-based PCI.
Methods
Study population
From January 2019 to October 2020, subjects who were diagnosed with ACS and underwent DES implantation in the Second Affiliated Hospital of Nanchang University were enrolled retrospectively. Inclusion criteria were: (1) age over 18 years old; (2) diagnosed as ACS; and (3) received follow-up coronary angiography (CAG) between six and forty-eight months after PCI. The indication for follow-up CAG is a comprehensive assessment by the clinician based on the patient's condition, typically one year after the procedure. Meanwhile, subjects meeting any of the following criteria were eliminated: (1) severe hepatic and renal dysfunction; (2) acute/chronic inflammatory disease; (3) treatment of the culprit lesion with a bare metal stent or balloon angioplasty; and (4) combined with malignant tumors or a life expectancy of < 6 months.
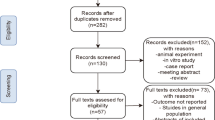
Totally, 545 patients with ACS who satisfied the inclusion and exclusion criteria were enrolled at first; after excluding individuals with missing data on SII (n = 22), 523 eligible patients were included in our final analysis. This study was performed in line with the Declaration of Helsinki, and Ethic Committee approval was obtained from the Second Affiliated Hospital of Nanchang University. All participants provided their written informed consent upon admission.
Data collection
Demographic and clinical features, including gender, age, smoking status, body mass index (BMI), past medical history, angiographic findings, laboratory tests, and medication at discharge (secondary prevention strategies), were obtained from the medical system by trained personnel. Peripheral blood samples were collected (before coronary angiography) after overnight fasting (> 8 h) for laboratory examinations. Then, serum uric acid (SUA), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), homocysteine (Hcy), total cholesterol (TC), triglycerides (TG), low-density lipoprotein-C (LDL-C), and high-density lipoprotein-C (HDL-C) were measured with standard assays. The SUA was determined by a direct enzyme method (Medical Co., Ltd., Ningbo, China), the FBG was determined with the hexokinase/glucose-6-phosphate dehydrogenase method (Biote Co., Ltd., Nanchang, China), and the Hcy was determined by enzymatic methods using test kits (AUSA Co., Ltd., Shenzhen, China). The TC was measured using the enzymatic colorimetric method (Medical Co., Ltd., Ningbo, China), the TG was measured using the GPO-POD method (Beckman Coulter, Suzhou, China), and the LDL-C and HDL-C were measured by direct homogeneous assay methods using detergents (Medical Co., Ltd., Ningbo, China). All biochemical parameters were measured in the central laboratory of the Second Affiliated Hospital of Nanchang University using an automated analyzer (Olympus AU 2700).
The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation: eGFR = 142 × min (serum creatinine/κ, 1)α × max (serum creatinine/κ, 1)−1.200 × 0.9938age × 1.012 (if female) (κ: female = 0.7, male = 0.9; α: female = -0.241, male = -0.302) [19]. BMI was calculated as weight (kg) divided by height squared (m2), and current smoking was defined as the daily use of one or more cigarettes for at least 1 year. Hypertension was defined as a blood pressure (BP) ≥ 140/90 mmHg or the use of anti- hypertensive drugs. Hyperlipidemia was defined as a fasting TG ≥ 2.26 mmol/L, TC > 6.22 mmol/L, LDL-C > 4.14 mmol/L, or receiving lipid-lowering drugs. Diabetes was defined as a FBG > 7.0 mmol/L, random blood glucose > 11.1 mmol/L, or the use of glucose-lowering medication. Besides, chronic kidney disease was confirmed based on the presence of kidney damage or an eGFR < 60 ml/min per 1.73 m2 for at least 3 months.
Exposure variable and outcomes
The complete blood counts were measured by trained medical personnel using automated hematology analyzing devices. Lymphocyte, platelet, and neutrophil counts were employed for our analysis. Accordingly, SII (× 103 cells/μl) as an exposure variable was calculated as [(platelet count × neutrophil count)/lymphocyte count] [18].
The outcome measure of this study was the incidence of ISR. It was defined as stenosis of a segment inside the stent or its 5-mm edges with a diameter stenosis of more than 50% [20, 21]. All individuals underwent follow-up CAG between six and forty-eight months after the successful baseline PCI. Then, two independent and experienced cardiologists who were not aware of the study's purpose interpreted the CAG results. Besides, all subjects received current guidelines-recommended secondary prevention for CAD.
Statistical analysis
All statistical analyses were performed using Empower software (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA) and R version 3.4.3 (http://www.R-project.org, The R Foundation). Based on the data distribution, continuous variables are described as means (standard deviations) or medians (interquartile ranges). Comparisons among groups were conducted by one-way analysis of variance or the Kruskal-Wallis test. Categorical variables are described as numbers (percentages), and comparisons among groups were conducted by the chi-squared test or Fisher’s exact 2 × 2 test.
Because SII had a strongly skewed distribution, the Ln-transformed SII (LnSII) was used for our analysis. Multivariate logistic regression analysis was employed to assess the relationship between LnSII and DES-ISR in different models. Model 1 was not adjusted for covariates; model 2 was adjusted for age, gender, and BMI; and model 3 was adjusted for age, gender, BMI, TC, LDL-C, hypertension, diabetes, length of stents, and minimal stent diameter. The covariates were selected based on the matched odds ratio (OR) changed at least 10% when added to this model [22]. We also converted LnSII into tertiles for sensitivity analyses. Furthermore, a generalized additive model and a fitted smoothing curve were used to explore the shape of the curve or the linear relationship between LnSII and DES-ISR. Subgroup analysis stratified by age (< 65/ ≥ 65 years), gender (male/female), BMI (< 25/ ≥ 25 kg/m2), current smoking (yes/no), hypertension (yes/no) and diabetes (yes/no) was also performed. Besides, a receiver operating characteristic (ROC) curve was used to assess the ability of PLR, NLR, and SII to identify DES-ISR in patients with ACS. Additionally, we used multiple imputation (MI), based on 5 replications and the Markov-chain Monte Carlo method in the SAS MI procedure, to account for missing data on TC, LDL-C, Hcy, HbA1c, and smoking status. Besides, the sample size was determined based on the power analysis. A two-tailed P value < 0.05 was considered significant.
Results
Baseline characteristics
The study included 523 patients with ACS undergoing follow-up CAGs over a median follow-up period of 12 (11, 20) months. The average age of the overall cohort was 65.70 ± 10.33 years, and 397 (75.91%) were male. According to the LnSII tertiles, subjects were divided into three subgroups. As illustrated in Table 1, patients in the highest LnSII subgroup were more likely to have higher FPG, TC, triglycerides, LDL-C, and homocysteine; to have a higher proportion of hypertension prevalence and the use of oral hypoglycemic drugs; and to have a lower level of serum albumin (all P < 0.05). Although the proportions of current smoking, hyperlipidemia, and diabetes were higher in the T3 subgroup than in the other two subgroups, the difference was not statistically significant.
Additionally, the baseline characteristics of non-ISRs (N = 464) and ISRs (N = 59) were summarized in Additional file 1: Table S1. Compared with the non-ISR subgroup, subjects in the ISR subgroup had elevated concentrations of FPG, TC, and LDL-C, as well as statistically significant differences in eGFR and serum albumin (all P < 0.05). In regards to the procedure details, ISR patients had higher proportions of chronic total occlusions, a higher number of stents, and a smaller diameter of stent (all P < 0.05).
Association of SII with in-stent restenosis
The incidence of DES-ISR was 11.28% (59/523) for the overall population, and subjects in the higher LnSII tertile trended to show higher likelihoods of DES-ISR (5.7% vs. 12.1% vs. 16.0%, P = 0.009; Fig. 1A). Additionally, LnSII was also significantly higher in the ISR subgroup than in the non-ISRs (6.66 ± 0.70 vs. 6.44 ± 0.75; Fig. 1B), with a statistical difference (P = 0.036).
The results of multivariable logistic regression for the impacts of LnSII on DES-ISR are shown in Table 2. A positive association between LnSII (per 1-unit increase) and DES-ISR was detected (Model 1: OR = 1.45, 95% CI 1.02–2.05, P = 0.037; Model 2: OR = 1.77, 95% CI 1.17–2.66, P = 0.006). Moreover, in the case of fully adjusted covariates, the results demonstrated that each unit of increased LnSII was correlated with a 69% increased risk of DES-ISR (Model 3: OR = 1.69, 95% CI 1.04–2.75, P = 0.034). When LnSII was analyzed as a categorical variable, the association persisted in different models (Table 2). After final adjusting for confounders in Model 3, a significant higher risk of DES-ISR (OR = 2.52, 95% CI 1.23–5.17, P = 0.011) was found in participants in tertile 3 (≥ 6.7), compared with those in tertiles 1–2 (< 6.7). Using imputation data, we reanalyzed the relationship between LnSII and DES-ISR and did not find any qualitative differences (Additional file 1: Table S2).
Moreover, smooth curve fitting showed no non-linear relationship between LnSII and DES-ISR in the entire population (Fig. 2).
Subgroup analysis
To evaluate the robustness of the relationship between LnSII and DES-ISR, subgroup analyses were further performed. As demonstrated in Fig. 3, none of the stratifications, including age, gender, BMI, current smoking, hypertension, and diabetes, significantly affected the positive association between LnSII and DES-ISR (all P for interaction > 0.05). These results indicated that the positive association was similar in different subgroups and could be appropriate for various population settings as well.
Subgroup analysis for the association between LnSII and DES-ISR. All presented covariates were adjusted (as Model 3) except the corresponding stratification variable. None of the stratifications, including gender, age, BMI, current smoking, hypertension, and diabetes, significantly affected the positive association between LnSII and DES-ISR
ROC curves between SII and other systemic immune indices
The ROC curve was employed to assess the ability of NLR, PLR, and SII to identify DES-ISR in ACS patients. As presented in Additional file 1: Figure S1, the area under the curve (AUC) of SII for the identification of DES-ISR was 0.600 (95% CI 0.529–0.672), higher than that of NLR (0.589, 95% CI 0.511–0.668) and PLR (0.577, 95% CI 0.500–0.655). The optimal cut-off value of SII to detect DES-ISR was > 417.9 (× 103 cells/μl), with 86.4% sensitivity and 34.5% specificity (P < 0.05). These findings indicate that SII is superior to other systemic immune indices.
Discussion
To our knowledge, this is the first report to investigate the association between SII and ISR in patients with ACS undergoing DES-based PCI. The results demonstrated that SII was significantly associated with an increased risk of DES-ISR after adjusting for covariates including demographics, CVD risk factors, angiographic features, and others, presenting a nearly linear dose-response relationship. The highest LnSII subgroup (≥ 6.7 × 103 cells/μl) was 2.52-fold correlated with DES-ISR as compared with the lowest LnSII subgroup (< 6.7 × 103 cells/μl). Moreover, the ROC curve showed that SII had better identification performance for DES-ISR than other systemic immune indices (NLR and PLR). These findings indicated that SII may be a promising predictor for evaluating ISR after DES-PCI in ACS patients.
First proposed by Hu et al. in 2014, SII was initially used to assess the immune-inflammatory status and the risk of mortality in patients with malignancies [18, 23, 24]. However, accumulating evidence suggested that SII was also related to adverse clinical outcomes in diseases other than malignancies, particularly atherosclerotic cardiovascular disease [25,26,27,28]. For example, Candemir et al. examined the association between SII and CAG findings in 669 patients with stable angina pectoris. The results indicated that SII was significantly associated with the severity of CAG and high SYNTAX scores [26]. Another retrospective cohort study indicated that high SII levels could predict high 30- and 90-day mortalities as well as a high risk of major cardiovascular adverse events (MACEs) in patients with congestive heart failure [27]. Recently, Zheng et al. carried out a retrospective study of 887 myocardial infarction patients, which showed that SII ≥ 636 was an independent risk factor for intra-stent thrombosis after coronary stent implantation [28]. However, no relevant data regarding the effect of SII on ISR after DES-based PCI is known. The present study offered a timely opportunity to evaluate the dose-response relationship between SII and ISR, revealing that SII was also an independent risk factor for DES-ISR in patients with ACS. Our subjects included patients 48 months after PCI, suggesting that SII may have potential long-term predictive value. Based on our findings, timely interventions for patients with elevated SII levels may reduce the incidence of DES-ISR.
By integrating three circulating immune cells, consisting of neutrophils, platelets, and lymphocytes, SII can assess the systemic immune and inflammatory status. An increase in the level of SII indicates a high immune-inflammatory status in the human body, which may correlate with the development of multiple inflammation-related diseases [29,30,31,32]. A cross-sectional study of 22,290 individuals from the 1999–2010 National Health and Nutrition Examination Survey found that the OR for hypertension prevalence per In-transformed increment in SII was estimated at 1.115 (95% CI 1.045–1.188) [33]. Moreover, recent evidence also indicates that higher levels of SII are correlated with a higher risk of hyperlipidemia (OR = 1.02; 95% CI 1.00–1.04) and diabetes (OR = 2.024; 95% CI 1.297–3.157) [34, 35]. Consistent with most research, our current work revealed that elevated SII was correlated with increased levels of FPG, TC, triglycerides, LDL-C, and homocysteine, a decreased level of serum albumin, and a high prevalence of hypertension, which may have contributed to the development of ISR [36,37,38,39]. As expected, SII, either as a continuous or categorical variable, was independently related to an increased risk of DES-ISR. Thus, taking SII into consideration may have important clinical implications for optimizing the early risk stratification of ISR in ACS patients.
Since SII is derived from lymphocyte, neutrophil, and platelet counts, it may be considered a modified and powerful combination of NLR and PLR [23, 40]. Indeed, the ROC curve showed that SII had better identification performance for DES-ISR than NLR and PLR (AUC: 0.600 vs. 0.589 and 0.577). However, the exact mechanisms underlying the association of SII with DES-ISR are unknown, and the following explanations can be considered. The increase in SII suggests either a relative increase in neutrophil and platelet counts or a relative decrease in lymphocyte counts. Neutrophils could lead to oxidative stress and endothelial dysfunction by releasing large amounts of myeloperoxidase and nicotinamide adenine dinucleotide phosphate oxidase [41]. Meanwhile, platelets interacted with neutrophils and lymphocytes to induce monocyte adhesion and transport, release inflammatory factors (such as interleukin-6 and tumor necrosis factor alpha), and ultimately promote local inflammation [42]. The interaction of inflammation and platelet activation promotes the formation of neointima and atherosclerosis, which in turn leads to the development of ISR [42, 43]. Besides, our study and previous evidence indicate that SII is closely associated with multiple cardiometabolic risk factors (such as FPG, triglycerides, and serum albumin) [44, 45], which may also contribute to this relationship.
Some strengths of our study can be identified. This study explores for the first time the relationship between SII and DES-ISR in ACS patients and reveals a nearly linear dose-response relationship. Although the findings showed a relatively high rate of ISR (11.28%) at 1 year follow-up, this may be related to the higher proportion of acute ST-segment elevation myocardial infarction (47.3%, data not shown) and current smoking (45.9%) [46, 47]. Moreover, we adjusted for many potential confounders to produce more reliable findings. We also handled SII as both a continuous and categorical variable, which reduced the contingency in the data analysis and improved the robustness of the results. Besides, the subgroup analyses indicated that the positive SII-ISR association was similar in various population settings. Hence, SII can be used as an inexpensive and practical method to screen for DES-ISR risk in patients with ACS in clinical settings, especially in underdeveloped areas.
Limitations
Despite that, there were certain limitations to our study. First, this is a single-center and retrospective study with a small sample size, which may cause some deviations in the results. Second, this study enrolled only patients with ACS, which affects the generalizability of the findings to CCS patients. Third, although SII had better identification performance for DES-ISR than other novel systemic indices (NLR and PLR), we did not evaluate its superiority compared to traditional inflammatory markers such as C-reactive protein. We also did not consider the immune responses of patients after recovering from COVID-19, even though all patients tested negative for COVID-19 at admission. Last, we assessed SII only once after admission and did not collect information on the changes in SII during follow-up.
Conclusion
Our study indicated that SII was significantly and positively associated with the risk of DES-ISR in ACS patients after successful PCI, presenting a nearly linear dose–response relationship. Moreover, SII had better identification performance for DES-ISR than other systemic immune indices, including NLR and PLR. Despite that, further prospective cohort studies are still needed to validate our findings.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ACS:
-
Acute coronary syndrome
- PCI:
-
Percutaneous coronary intervention
- CAG:
-
Coronary angiography
- DES:
-
Drug-eluting stent
- ISR:
-
In-stent restenosis
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- NLR:
-
Neutrophil/lymphocyte ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- SII:
-
Systemic immune-inflammation index
- BMI:
-
Body mass index
- eGFR:
-
Estimated glomerular filtration rate
- FBG:
-
Fasting blood glucose
- SUA:
-
Serum uric acid
- HbA1c:
-
Hemoglobin A1c
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- LDL-C:
-
Low-density lipoprotein-C
- HDL-C:
-
High-density lipoprotein-C
- Hcy:
-
Homocysteine
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
- MI:
-
Multiple imputation
- AUC:
-
Area under the curve
- MACEs:
-
Major cardiovascular adverse events
- CCS:
-
Chronic coronary syndrome
References
Erdogan E, Bajaj R, Lansky A, Mathur A, Baumbach A, Bourantas CV. Intravascular imaging for guiding in-stent restenosis and stent thrombosis therapy. J Am Heart Assoc. 2022;11: e26492. https://doi.org/10.1161/JAHA.122.026492.
Kokkinidis DG, Waldo SW, Armstrong EJ. Treatment of coronary artery in-stent restenosis. Expert Rev Cardiovasc Ther. 2017;15:191–202. https://doi.org/10.1080/14779072.2017.
Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. https://doi.org/10.1186/1741-7015-11-117.
Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation. 2003;107:499–11. https://doi.org/10.1161/01.cir.0000052939.59093.45.
Jayedi A, Rahimi K, Bautista LE, Nazarzadeh M, Zargar MS, Shab-Bidar S. Inflammation markers and risk of developing hypertension: a meta-analysis of cohort studies. Heart. 2019;105:686–92. https://doi.org/10.1136/heartjnl-2018-314216.
Kelly PJ, Lemmens R, Tsivgoulis G. Inflammation and stroke risk: a new target for prevention. Stroke. 2021;52:2697–706. https://doi.org/10.1161/STROKEAHA.121.034388.
Drachman DE, Simon DI. Inflammation as a mechanism and therapeutic target for in-stent restenosis. Curr Atheroscler Rep. 2005;7:44–9. https://doi.org/10.1007/s11883-005-0074-5.
Liang S, Aiqun M, Jiwu L, Ping Z. Tlr3 and tlr4 as potential clinical biomarkers for in-stent restenosis in drug-eluting stents patients. Immunol Res. 2016;64:424–30. https://doi.org/10.1007/s12026-015-8685-6.
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. https://doi.org/10.1038/nm0195-27.
Isner JM. Still more debate over vegf. Nat Med. 2001;7:639–41. https://doi.org/10.1038/88966.
Niccoli G, Dato I, Imaeva AE, et al. Association between inflammatory biomarkers and in-stent restenosis tissue features: an optical coherence tomography study. Eur Heart J Cardiovasc Imag. 2014;15:917–25. https://doi.org/10.1093/ehjci/jeu035.
Wang Z, Liu C, Fang H. Blood cell parameters and predicting coronary in-stent restenosis. Angiology. 2019;70:711–8. https://doi.org/10.1177/0003319719830495.
Wang Z, Qin Z, Yuan R, et al. Systemic immune-inflammation index as a prognostic marker for advanced chronic heart failure with renal dysfunction. ESC heart fail. 2023;10:478–91. https://doi.org/10.1002/ehf2.14217.
Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (sii) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50: e13230. https://doi.org/10.1111/eci.13230.
Zhang F, Niu M, Wang L, et al. Systemic-immune-inflammation index as a promising biomarker for predicting perioperative ischemic stroke in older patients who underwent non-cardiac surgery. Front aging neurosci. 2022;14: 865244. https://doi.org/10.3389/fnagi.2022.865244.
Karadeniz FÖ, Karadeniz Y, Altuntaş E. Systemic immune-inflammation index, and neutrophilto-lymphocyte and platelet-to-lymphocyte ratios can predict clinical outcomes in patients with acute coronary syndrome. Cardiovasc J Afr. 2023;34:1–7. https://doi.org/10.5830/CVJA-2023-011.
Erdoğan M, Erdöl MA, Öztürk S, et al. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14(16):1553–61. https://doi.org/10.2217/bmm-2020-0274.
Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin cancer res. 2014;20:6212–22. https://doi.org/10.1158/1078-0432.CCR-14-0442.
Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin c-based equations to estimate gfr without race. N Engl J Med. 2021;385:1737–49. https://doi.org/10.1056/NEJMoa2102953.
Xu X, Pandit RU, Han L, Li Y, Guo X. Remnant lipoprotein cholesterol independently associates with in-stent restenosis after drug-eluting stenting for coronary artery disease. Angiology. 2019;70:853–9. https://doi.org/10.1177/0003319719854296.
Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–73. https://doi.org/10.1016/j.jacc.2014.02.545.
Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826–32. https://doi.org/10.1056/NEJM200012213432501.
Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261–72. https://doi.org/10.3748/wjg.v23.i34.6261.
Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273:532–41. https://doi.org/10.1097/SLA.0000000000003370.
Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (sii), system inflammation response index (siri) and risk of all-cause mortality and cardiovascular mortality: a 20 year follow-up cohort study of 42,875 us adults. J Clin Med. 2023. https://doi.org/10.3390/jcm12031128.
Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between systemic immune-inflammation index (sii) and the severity of stable coronary artery disease. Angiology. 2021;72:575–81. https://doi.org/10.1177/0003319720987743.
Tang Y, Zeng X, Feng Y, et al. Association of systemic immune-inflammation index with short-term mortality of congestive heart failure: a retrospective cohort study. Front Cardiovasc Med. 2021;8: 753133. https://doi.org/10.3389/fcvm.2021.753133.
Zheng PG, Chen P, Wang LJ, Zhang N. The association of the systemic immune-inflammation index and stent thrombosis in myocardial infarction patients after coronary stent implantation-a retrospectively study. J Thorac Dis. 2023;15:1726–33. https://doi.org/10.21037/jtd-23-363.
Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36:635–47. https://doi.org/10.1016/j.cjca.2020.01.013.
Plutzky J. Inflammation in atherosclerosis and diabetes mellitus. Rev Endocr Metab Disord. 2004;5:255–9. https://doi.org/10.1023/B:REMD.0000032414.17672.5c.
Lazzerini PE, Capecchi PL, Selvi E, et al. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev. 2007;6:503–9. https://doi.org/10.1016/j.autrev.2007.03.008.
Yilmaz Y, Kelesoglu S, Elcik D, Ozmen R, Kalay N. Predictive values of systemic immune-inflammation index in new-onset atrial fibrillation following coronary artery bypass grafting. Braz J Cardiovasc Surg. 2023;38:96–103. https://doi.org/10.21470/1678-9741-2021-0278.
Xu JP, Zeng RX, Zhang YZ, et al. Systemic inflammation markers and the prevalence of hypertension: a nhanes cross-sectional study. Hyperten Res. 2023;46:1009–19. https://doi.org/10.1038/s41440-023-01195-0.
Mahemuti N, Jing X, Zhang N, et al. Association between systemic immunity-inflammation index and hyperlipidemia: a population-based study from the nhanes (2015-2020). Nutrients. 2023. https://doi.org/10.3390/nu15051177.
Chen G, Tan C, Liu X, Wang X, Tan Q, Chen Y. Associations between systemic immune-inflammation index and diabetes mellitus secondary to pancreatic ductal adenocarcinoma. J Clin Med. 2023. https://doi.org/10.3390/jcm12030756.
Tocci G, Barbato E, Coluccia R, et al. Blood pressure levels at the time of percutaneous coronary revascularization and risk of coronary in-stent restenosis. Am J Hypertens. 2016;29:509–18. https://doi.org/10.1093/ajh/hpv131.
Fang Y, Lin M, Chen L, Yang C, Liu A. Association between ldl/hdl ratio and in-stent restenosis in patients with acute coronary syndrome after stent implantation. Biomark Med. 2022;16:673–80. https://doi.org/10.2217/bmm-2021-1089.
Zhu Y, Liu K, Chen M, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20:137. https://doi.org/10.1186/s12933-021-01332-4.
Bicciré FG, Pastori D, Tanzilli A, et al. Low serum albumin levels and in-hospital outcomes in patients with ST segment elevation myocardial infarction. Nutr Metab Cardiovasc Dis. 2021;31(10):2904–11. https://doi.org/10.1016/j.numecd.2021.06.003.
Liu X, Guan G, Cui X, Liu Y, Liu Y, Luo F. Systemic immune-inflammation index (sii) can be an early indicator for predicting the severity of acute pancreatitis: a retrospective study. Int J Gen Med. 2021;14:9483–9. https://doi.org/10.2147/IJGM.S343110.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013. https://doi.org/10.1126/science.1230719.
Chandrasekar B, Tanguay JF. Platelets and restenosis. J Am Coll Cardiol. 2000;35:555–62. https://doi.org/10.1016/s0735-1097(99)00596-3.
Demirtas K. Inflammation and in-stent restenosis. Angiology. 2018;69:89. https://doi.org/10.1177/0003319717722103.
Qin Z, Li H, Wang L, et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: a population-based study. Front Immunol. 2022;13: 863640. https://doi.org/10.3389/fimmu.2022.863640.
Xiao S, Wang X, Zhang G, et al. Association of systemic immune inflammation index with estimated pulse wave velocity, atherogenic index of plasma, triglyceride-glucose index, and cardiovascular disease: a large cross-sectional study. Med inflamm. 2023. https://doi.org/10.1155/2023/1966680.
Luo D, Yang X, Hu X, et al. Non-stenting strategy is not inferior to stent implantation in patients with acute ST-segment elevated myocardial infarction and high thrombus burden and intermediate stenotic culprit lesion. Ann Palliat Med. 2021;10:10849–60. https://doi.org/10.21037/apm-21-2612.
Zhang J, Zhang Q, Zhao K, Bian YJ, Liu Y, Xue YT. Risk factors for in-stent restenosis after coronary stent implantation in patients with coronary artery disease: a retrospective observational study. Medicine. 2022;101: e31707. https://doi.org/10.1097/MD.0000000000031707.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
FX and YQW conceived and designed the study. ZZY, YRX, and ZJW contributed to data collection and statistical analysis. FX drafted the manuscript. YQW had primary responsibility for the final content of the manuscript. All authors interpreted the results, and reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the Declaration of Helsinki, and Ethic Committee approval was obtained from the Second Affiliated Hospital of Nanchang University.
Competing interests
There are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline characteristics of patients with and without ISR. Table S2. Multiple-imputation analysis which is based on 5 replications and the Markov-chain Monte Carlo method in the SAS multiple imputation procedure†. Figure S1. The ROC curve of SII, NLR and PLR for the identification of DES-ISR in patients with ACS. Abbreviations: ROC, receiver operator characteristic; SII, systemic immune inflammation index; NLR, neutrophil/lymphocyte ratio; PLR, platelet to lymphocyte ratio; DES, drug-eluting stent; ISR, in-stent restenosis; ACS, acute coronary syndrome.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, F., Yu, Z., Xiong, Y. et al. Systemic immune-inflammation index and in-stent restenosis in patients with acute coronary syndrome: a single-center retrospective study. Eur J Med Res 29, 145 (2024). https://doi.org/10.1186/s40001-024-01736-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01736-4