Abstract
Background
An elevated systemic immune-inflammation index (SII) is associated with higher mortality in patients with coronary artery disease and other diseases. However, the potential of SII for predicting mortality in the general population has been underexplored. Therefore, this study aimed to analyze the relationship between the SII and all-cause, cardiovascular disease, and cardiocerebrovascular disease mortality in the general population.
Methods
This study involved 26,855 participants (≥ 18 years) from the National Health and Nutrition Examination Survey 1999–2014 who were grouped according to the SII tertiles. Survival differences between the groups were analyzed using log-rank tests and Kaplan–Meier plots. Furthermore, multivariate Cox regression and restricted cubic spline analyses were used to examine the relationship between the SII and all-cause, cardiovascular, and cardio-cerebrovascular mortality.
Results
Overall, 1947 (7.425%) participants died following an average follow-up of 87.99 ± 54.04 months. Among these, 325 (1.210%) deaths were related to cardiovascular diseases and 392 (1.459%) to cardio-cerebrovascular mortality. Kaplan–Meier analysis revealed statistically significant differences in all-cause, cardiovascular, and cerebrovascular mortality between the SII tertiles (log-rank test: all P < 0.001). Multi-adjusted models showed that participants in the highest tertile of SII had a higher risk of death from all-cause (hazard ratio [HR] = 1.48, 95% confidence interval [CI] 1.48–1.48) and cardiovascular mortality (HR = 1.60, 95% CI 1.60–1.61) compared with those in the lowest tertile. In addition, the restricted cubic spline curve indicated a nonlinear association between SII and all-cause mortality (P < 0.001), with threshold value of SII at 18.284. There was a 15% decrease in the risk of all-cause mortality for each twofold change in SII on the left flank (HR = 0.85, 95% CI 0.69–1.05) and a 42% increase (HR = 1.42, 95% CI 1.23–1.64) on the right flank of the inflection point. In addition, the risk of cardiovascular mortality increased nonlinearly by 39% per twofold change in SII (HR = 1.39, 95% CI 1.07–1.81). There was also a nonlinear increase in the risk of cardio-cerebrovascular mortality per twofold change in SII (HR = 1.29, 95% CI 1.00–1.66).
Conclusions
In the general population, the SII was significantly associated with all-cause, cardiovascular, and cardio-cerebrovascular mortality, regardless of the established risk factors.
Similar content being viewed by others
Introduction
Nearly one-third of all deaths worldwide are caused by cardiovascular disease (CVD) [1, 2]. In the United States (US), this accounts for 17% of all health expenditures [2, 3]. Therefore, effective mortality prediction tools are essential for preventing and treating CVD as soon as possible [4]. The burden of CVD and disability-adjusted life years is largely attributed to stroke and ischemic heart disease [5], and atherosclerosis is the major cause of these diseases [6]. Atherosclerosis is associated with thrombosis, oxidative stress, and endothelial damage. [7, 8]. Recent research has shown that the immune system has a profound relationship with inflammation during the development of atherosclerosis [8, 9]. The cells of the immune system comprise lymphocytes, neutrophils, monocytes, and macrophages, which play different roles in atherosclerosis development. For example, neutrophils activate macrophages, promote monocyte recruitment, and initiate cytotoxicity at various stages of atherosclerosis. In contrast, lymphocytes regulate inflammation and reduce atherosclerosis [10,11,12]. In addition, platelets adhere to vessel walls, aggregate leukocytes, and initiate atherosclerosis before invading plaques [13,14,15,16].
Recently, various cost-effective and verified inflammatory and immune indicators have been examined. The neutrophil–lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) offer better predictions of CVD outcomes than single cells [16,17,—18]. A new systemic immune-inflammation index (SII), calculated as (neutrophil × platelet)/lymphocyte, has been introduced for CVD, which provides a more comprehensive reflection of immunity and inflammation [19, 20]. SII can be easily obtained by routine whole blood count test (the most commonly used clinical test). This marker was proposed by Hu et al. [21]. In the field of cancer, SII has a better predictive value for prognosis compared to other inflammatory factors [22, 23]. Studies have also indicated that SII may be crucial in CVD prognosis and mortality [24].
In some studies, the SII has been associated with CVD risk. However, inconsistent results have been obtained, highlighting the importance of early detection and intervention for CVD. Therefore, we aimed to investigate the clinical significance of the SII in predicting all-cause, cardiovascular, and cardio-cerebrovascular mortality in the adult population. Further, we aimed to assess the effectiveness of the SII as an affordable and accessible CVD risk indicator.
Methods
Study population
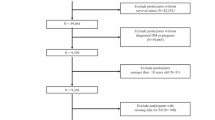
The population for this study was from the National Health and Nutrition Survey (NHANES) survey. Informed consent was obtained from all participants, and the NCHS Ethics Review Board approved the study protocol. Additional file 1 (Table S1) that shows the baseline characteristics of the included population and the follow-up missing population. Overall, 82,091 participants from the surveys conducted between 1999 and 2014 were selected for this study. Exclusion criteria were as follows: (a) individuals aged < 18 years (n = 0); (b) individuals whose peripheral lymphocyte, neutrophil, and platelet counts were not available (n = 14,990); and c) individuals who were lost to follow-up (n = 40,246). Consequently, the final analysis was conducted on 26,855 participants. Figure 1 shows a flowchart of the selection of the study population.
Exposure
The NHANES Laboratory/Medical Technologists Procedures Manual (LPM) describes the procedures for collecting and processing blood specimens. The complete blood count (CBC) parameters were determined using the Coulter® method for counting and sizing, as well as an automatic mixing and dilution device for sample processing. Hemoglobinometry was performed using a single-beam photometer, while VCS technology was used for differential analysis of WBCs. The three simultaneous measurements conducted included individual cell volume (V), high-frequency conductivity (C), and laser light scattering (S). The Scattergrams plot the cells based on these three parameters.
Under established procedures, all blood specimens were withdrawn in the morning following a 9-h fast. CBC for all blood samples was obtained using Beckman Coulter MAXM. Three simultaneous measurements, comprising V, C, and S were used to sort the neutrophils and lymphocytes from the white blood cells. The number of thrombocytes was determined by multiplying the Plt histogram by the calibration constant, and expressing it as n × 103 cells/µL. The SII was calculated as (neutrophils × platelets)/lymphocyte [21].
Outcomes
The NCHS data linkage website provides detailed information on mortality status derived from the NHANES-linked National Death Index records [25]. Study outcomes, including all-cause, cardiovascular, and cardio-cerebrovascular mortality, were defined according to the 10th Revision of the International Classification of Diseases (ICD-10) [26]. All -cause death is the primary observation outcome event, while cardiovascular death and cardio-cerebrovascular death are secondary observation outcome events. The follow-up period was recorded from December 31, 2015, until the date of participation.
Covariates
Demographic information, such as age, sex, and ethnicity, was collected through interview questionnaires. Participants who consumed 12 or more drinks in the last 12 months were classified as drinkers, while those who had smoked at least 100 cigarettes in their lifetime were classified as smokers [27]. The questionnaires also asked participants to self-report medical comorbidities such as hypertension, heart failure, diabetes mellitus, coronary heart disease, cancer, and stroke. Other covariates included hemoglobin, platelets, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, albumin, glycohemoglobin, blood urea nitrogen, creatinine, uric acid, cholesterol, and glucose levels.
Statistical analysis
The study participants were classified into three groups based on their SII tertile: low (< 391,809.52), medium (391,809.52–611,840), and high (> 611,840) SII groups. Continuous variables were expressed as means ± standard deviations, while categorical variables were expressed as frequencies with percentages. One-way ANOVA was used to analyze the differences in categorical or continuous variables among the SII tertiles. Kaplan–Meier plots and log-rank tests were used to determine the differential survival rates of all-cause, cardiovascular, and cardio-cerebrovascular mortality according to SII tertiles.
Multivariate Cox regression models were used to examine the relationship between the SII and all-cause, cardiovascular, and cardio-cerebrovascular mortality with hazard ratios (HRs) and 95% confidence intervals (CIs). Model 1 was unadjusted, and model 2 was adjusted for age, sex, ethnicity, educational level, marital status, smoking status, and drinking status. Model 3 was further adjusted for diabetes mellitus (DM), hypertension, heart failure (HF), coronary heart disease (CHD), stroke, cancer, body mass index (BMI), and levels of hemoglobin, glycohemoglobin, platelets, blood urea nitrogen, creatinine, uric acid, cholesterol, glucose, LDL-C, HDL-C, triglycerides, and albumin.
A restricted cubic spline (RCS) regression model was used to investigate the nonlinear relationship between the SII (per twofold change) and all-cause, cardiovascular, and cardiocerebrovascular mortality. The threshold point was determined for nonlinear relationships using a two-piece Cox regression model. Further investigation of the effect of the SII on death risk among men and women was conducted using sex-specific models. Empower(R) (X&Y Solutions, Inc., MA, USA) and Stata (version 14.0) were used for statistical analysis. Statistical significance was set at P-value < 0.05.
Results
Characteristics of participants at baseline
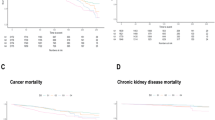
Table 1 compares the baseline characteristics of the 26,855 participants included in this study. The participants had an average age of 45.674 ± 17.238 years, and included 48.268% male participants. The average follow-up period was 7.33 ± 4.50 years, during which 1995 (7.43%) deaths occurred, including 325 (1.210%) and 392 (1.459%) caused by cardiovascular and cardio-cerebrovascular mortality, respectively. Most baseline covariates were statistically significant, except for uric acid, blood urea nitrogen, and HDL-C levels (P < 0.05). Kaplan–Meier curves for all-cause mortality showed worse outcomes with increase in the SII (log-rank P < 0.001; Fig. 2A). The fully adjusted Cox regression model (Table 2) showed HRs (95% CI) of 1.018 and 1.479 for participants in the medium and highest tertiles, respectively, compared with those in the lowest tertile. Based on the RCS model (Fig. 3A), the association between the SII and all-cause mortality was nonlinear and U-shaped (P < 0.001).
Restricted cubic spline curves of relations between SII with all-cause (A), cardiovascular mortality (B) and cardio-cerebrovascular mortality (C). Analysis was adjusted for age, gender, ethnicity, education, marital status, smoker, drinker, DM, hypertension, HF, CHD, stroke, cancer, BMI, hemoglobin, glycohemoglobin, platelets, blood urea nitrogen,uric acid, creatinine, cholesterol glucose, LDL-C, HDL-C, triglyceride, albumin. The solid and dashed lines symbolize the hazard ratios and corresponding 95% confidence intervals, respectively
The results of the two-piecewise Cox regression analysis are shown in Table 3. To fit the models, the SII was log2-transformed because of its skewed distribution. The P-values for the logarithmic likelihood ratio test were < 0.001 for the one-line Cox regression model and the two-piecewise regression model. A threshold value of 18.284 was determined for the SII. On the left and right flanks of the inflection point, a twofold change in SII resulted in a 15% decrease (HR = 0.85, 95% CI 0.69–1.05) and a 42% increase (HR = 1.42, 95% CI 1.23–1.64), respectively (see Additional file 1).
The association between SII and cardiovascular mortality
The Kaplan–Meier plot (Fig. 2B) showed that increased SII values were associated with reduced survival in CVD (log-rank P = 0.00077). When adjusted for all covariates (Table 2), the HR and CIs of cardiovascular mortality for those in the medium and highest tertiles were 1.33 (1.32–1.33) and 1.60 (1.60–1.61), respectively.
The RCS curve indicated a nonlinear association between SII and cardiovascular mortality (P for non-linearity = 0.071; Fig. 3B).
The association between SII and cardio-cerebrovascular mortality
In the Kaplan–Meier plot for cardiocerebrovascular disease (Fig. 2C), the higher SII values were associated with reduced survival (log-rank P = 0.00018). After adjusting for all covariates (Table 2), the HR and 95% CIs of cardio-cerebrovascular mortality were 1.21 (1.21–1.22) and 1.40 (1.39–1.40) for individuals in the medium and highest tertiles, respectively.
Figure 3C shows that the SII was nonlinearly associated with cardio-cerebrovascular mortality (P for non-linearity = 0.136).
Subgroup analysis
Table 4 shows that the HR and 95% CIs of cardiovascular mortality for women in the medium and highest tertiles were 3.906 (3.89–3.93) and 3.575 (3.56–3.595), respectively. In the same tertiles, the cardiovascular mortality HR and 95% CIs were 0.688 (0.68–0.69) and 1.24 (1.24–1.25), respectively.
Additionally, Fig. 4A shows a nonlinear relationship between the SII and all-cause mortality in women (P for nonlinearity = 0.011) and men (P for nonlinearity = 0.012). Figure 4B shows a nonlinear relationship between the SII and cardiovascular mortality for women (P for nonlinearity = 0.150) and men (P for nonlinearity = 0.372). Finally, Fig. 4C indicates that a higher SII was nonlinearly associated with an increased risk of cardio-cerebrovascular mortality in both women (P for nonlinearity = 0.123) and men (P = 0.555).
Restricted cubic spline curves of relations between SII and mortality in different sex groups. A all-cause mortality; B cardiovascular mortality; C cardio-cerebrovascular mortality. Analysis was adjusted for age, gender, ethnicity, education, marital status, smoker, drinker, DM, hypertension, HF, CHD, stroke, cancer, BMI, hemoglobin, glycohemoglobin, platelets, blood urea nitrogen, creatinine,uric acid, cholesterol glucose, LDL-C, HDL-C, triglyceride, albumin
Discussion
This study examined whether the SII could predict long-term outcomes in the general population. Increase in the SII appeared to be significantly related to cardiovascular and cardiocerebrovascular mortality, while it was nonlinearly related to all-cause mortality. The SII was associated with the lowest risk of death at a threshold of 18.28. Similar association patterns were observed in women and men. Although men and women showed comparable associations with all-cause mortality, women exhibited a stronger association with cardiovascular and cardiocerebrovascular mortality. Previous studies have confirmed that sex differences in CVD are attributable to the comprehensive expression of genetic and hormonal differences between men and women [28].
Recently, several studies have reported that the SII can predict CVD's severity, complications, and mortality [29]. Specifically, researchers found that the SII level was an independent prognostic indicator of poor prognosis at 3 months, which was related to the severity of stroke [30]. Furthermore, SII was significantly correlated with cerebral venous thrombosis. Xu et al. [31] reported that SII increased the total stroke risk, while Zhang et al. [32] found a significant correlation between SII and cerebral venous thrombosis. In multiple regression analysis, the degree of SII was a strong independent predictor.
Compared to other biological indicators such as PLR, NLR, or C-reactive protein, SII possesses unique advantages, making it a better predictor of coronary heart disease [33]. The SII is more stable than the blood cell count alone, which can be influenced by dehydration and fluid overload [33, 34]. This study’s results are consistent with previous research demonstrating a positive relationship between SII and cardiovascular mortality. Each twofold increase in the SII resulted in 20.4%, 21.8%, and 16.3% increases in all-cause, cardiovascular, and cardiocerebrovascular mortality, respectively.
The SII was also found to be significantly associated with all-cause mortality in several specific diseases, such as pancreatic [35], oral cavity squamous cell carcinoma [36], lung cancer [37], gastrointestinal cancer [38], urinary system cancer [39]. This study found a significant association between a higher SII and an increased risk of all-cause mortality over an average follow-up period of 88 months.
In part of the curve (SII > 18.28), all-cause mortality increased with increasing SII, and there was a positive correlation between the SII and cardiovascular mortality. This may be because of the significant association between inflammatory and immune status, as reflected by SII and CVD risk. Endothelial activation and platelet coordination are closely related, and recent research has shown a close relationship between cardiovascular mortality, platelet number, and platelet aggregation ability. Combining platelets with fibrin gives rise to a coronary thrombus, which is a crucial player in ACS pathophysiology [40]. In addition to causing blood clots, platelets also deliver mediators contributing to local inflammation [41]. Similarly, neutrophils secrete inflammatory mediators that can cause vessel wall degradation and endothelial dysfunction [42].
In addition, neutrophils interact with platelets, proteolyze coagulation factors, and release prothrombin molecules, activating the inflammatory response [43]. In addition to regulating inflammation, lymphocytes have anti-atherothrombotic properties [44].
Our study provides novel insights by demonstrating a nonlinear association between the SII and all-cause mortality, although the mechanism underlying this association is unclear. A reasonable explanation could be that participants with a lower SII had lower platelet counts. Suppose the platelet count decreases to a certain extent, bleeding symptoms may occur, which could be mild, such as in the skin and mucous membrane areas, or more serious and life-threatening, such as bleeding in the gastrointestinal or intracranial cavities [45, 46]. This may be one of the reasons for the U-shaped relationship between all-cause mortality and the SII.
Furthermore, we observed that the percentages of women and cancer patients in the group with a higher SII were higher, indicating that sex and cancer may explain the increased mortality risk associated with an increase in SII. Further research is needed to confirm the relationship between the SII increase and the risk of all-cause, cardiovascular, and cardio-cerebrovascular mortality.
Strengths and limitations of the study
Our study, which had a large research population and a long follow-up period, allowed us to better understand the relationship between the SII and all-cause mortality rate of the general population and cardiovascular and cerebrovascular deaths. Furthermore, we used the RCS model to analyze the nonlinear relationship between the SII and all-cause mortality. However, the study has some limitations, such as using self-reported data for complications and living habits, which may have memory bias. Additionally, we only collected the baseline value of the SII, which may not reflect changes in the SII during follow-up. Although we adjusted for many confounding factors, other variables may have influenced the results.
Conclusions
In summary, this study showed that the SII was independently related to all-cause, cardiovascular, and cardio-cerebrovascular mortality in the general population. These findings suggest that the SII could be useful for identifying individuals at risk of poor clinical outcomes. Considering that the SII is easy and inexpensive to acquire, it could be a convenient tool for clinicians to stratify patient risks and plan preventive and treatment strategies.
Availability of data and materials
The data of this study are publicly available on the NHANES website.
Abbreviations
- SII:
-
Systemic immune-inflammation index
- NLR:
-
Neutrophil-lymphocyte ratios
- PLR:
-
Platelet-lymphocyte ratios
- LPM:
-
Laboratory/Medical Technologists Procedures Manual
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- ACS:
-
Acute coronary syndrome
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for Health Statistics
- ICD:
-
International Classification of Diseases
- DM:
-
Diabetes mellitus
- HF:
-
Heart failure
- CHD:
-
Coronary heart disease
- BMI:
-
Body mass index
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- RCS:
-
Restricted cubic spline
- CAD:
-
Coronary artery disease
- NIHSS:
-
National Institutes of Health Stroke Score
References
Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–45. https://doi.org/10.1093/eurheartj/ehw334.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–492. https://doi.org/10.1161/CIR.0000000000000558.
Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. https://doi.org/10.1161/CIR.0b013e31820a55f5.
Liu C, Du L, Wang S, Kong L, Zhang S, Li S, Zhang W, Du G. Differences in the prevention and control of cardiovascular and cerebrovascular diseases. Pharmacol Res. 2021;170: 105737. https://doi.org/10.1016/j.phrs.2021.105737.
Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–88. https://doi.org/10.1016/S0140-6736(18)32203-7.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–46. https://doi.org/10.1161/CIRCRESAHA.115.307611.
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Herz. 2019;44(2):107–20. https://doi.org/10.1007/s00059-019-4790-y.
Merei B. Atherosclerotic plaque adhesion strength and its role in plaque rupture, Doctoral dissertation. University of South Carolina. 2017.
Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–33. https://doi.org/10.1038/s41586-021-03392-8.
Paquissi FC. The role of inflammation in cardiovascular diseases: the predictive value of neutrophil-lymphocyte ratio as a marker in peripheral arterial disease. Ther Clin Risk Manag. 2016;27(12):851–60. https://doi.org/10.2147/TCRM.S107635.
Abdolmaleki F, Gheibi Hayat SM, Bianconi V, Johnston TP, Sahebkar A. Atherosclerosis and immunity: a perspective. Trends Cardiovasc Med. 2019;29(6):363–71. https://doi.org/10.1016/j.tcm.2018.09.017.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51.
Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Min Cardioangiol. 2007;55:821.
Mass berg S, Brand K, Grüner S, Page S, Müller E, Müller I, et al. Acritical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–96. https://doi.org/10.1084/jem.20012044.
Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, et al. Platelets induce differentiation of human CD34 + progenitor cells into foam cells and endothelial cells. FASEB J. 2006;20:2559–61. https://doi.org/10.1096/fj.06-6265fje.
Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14(5):573–7. https://doi.org/10.1586/14779072.2016.1154788.
Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34(10):719–28.
Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. https://doi.org/10.1097/00062752-200701000-00011.
Li M, Li Z, Wang Z, Yue C, Hu W, Lu H. Prognostic value of systemic immune-inflammation index in patients with pancreatic cancer: a meta-analysis. Clin Exp Med. 2022;22(4):637–46. https://doi.org/10.1007/s10238-021-00785-x.
Hu T, Wang J, Xiao R, Liao X, Liu M, Sun Z. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;8(9): 933913. https://doi.org/10.3389/fcvm.2022.933913.
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. https://doi.org/10.1158/1078-0432.CCR-14-0442.
Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, Liu L. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. https://doi.org/10.1038/s41598-019-39150-0.
Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(15):8002. https://doi.org/10.3390/ijms22158002.
Zhou Y-X, Li W-C, Xia S-H, Xiang T, Tang C, Luo J-L, et al. Predictive value of the systemic immune inflammation index for adverse outcomes in patients with acute ischemic stroke. Front Neurol. 2022;13: 836595. https://doi.org/10.3389/fneur.2022.836595.
National Center for Health Statistics, Centers for Disease Control and Prevention. The linkage of national center for health statistics survey data to the National Death Index—2015 Linked Mortality File (LMF): methodology overview and analytic considerations. Hyattsville: National Center for Health Statistics. Office of Analysis and Epidemiology; 2015.
Li J, Covassin N, Bock JM, Mohamed EA, Pappoppula LP, Shafi C, et al. Excessive daytime sleepiness and cardiovascular mortality in US adults: a NHANES 2005–2008 follow-up study. Nat Sci Sleep. 2021;13:1049–59. https://doi.org/10.2147/NSS.S319675.
Liao S, Wu N, Gong D, Tang X, Yin T, Zhang H, et al. Association of aldehydes exposure with obesity in adults. Ecotoxicol Environ Saf. 2020;201: 110785. https://doi.org/10.1016/j.ecoenv.2020.110785.
Shufelt CL, Pacheco C, Tweet MS, Miller VM. Sex-specific physiology and cardiovascular disease. Adv Exp Med Biol. 2018;1065:433–54. https://doi.org/10.1007/978-3-319-77932-4_27.
Zhang X, Ding R, Li H, Liu Y, Ou W, Hu J, et al. An association between inflammation and cerebral venous thrombosis: a retrospective study. J Stroke Cerebrovasc Dis. 2021;30: 106084. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106084.
Weng Y, Zeng T, Huang H, Ren J, Wang J, Yang C, et al. Systemic immune-inflammation index predicts 3-month functional outcome in acute ischemic stroke patients treated with intravenous thrombolysis. Clin Interv Aging. 2021;16:877–86. https://doi.org/10.2147/CIA.S311047.
Xu M, Chen R, Liu L, Liu X, Hou J, Liao J, et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis. 2021;323:20–9. https://doi.org/10.1016/j.atherosclerosis.2021.02.012.
Liu Y, Ye T, Chen L, Jin T, Sheng Y, Wu G, et al. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron Artery Dis. 2021;32:715–20. https://doi.org/10.1097/MCA.0000000000001037.
Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short-and long-term mortality after Non ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–6. https://doi.org/10.1016/j.amjcard.2010.03.062.
Tekesin A, Tunç A. Inflammatory markers are beneficial in the early stages of cerebral venous thrombosis. Arq Neuropsiquiatr. 2019;77:101–5. https://doi.org/10.1590/0004-282x20190001.
Shui Y, Li M, Su J, Chen M, Gu X, Guo W. Prognostic and clinicopathological significance of systemic immune-inflammation index in pancreatic cancer: a meta-analysis of 2365 patients. Aging (Albany NY). 2021;13(16):20585–97. https://doi.org/10.18632/aging.203449.
Hung SP, Chen PR, Ho TY, Chang KP, Chou WC, Lee CH, Wu YY, Chen PJ, Lin CH, Chou YC, Fan KH, Lin CY, Huang BS, Tung-Chieh Chang J, Wang CC, Tsang NM. Prognostic significance of the preoperative systemic immune-inflammation index in patients with oral cavity squamous cell carcinoma treated with curative surgery and adjuvant therapy. Cancer Med. 2021;10(2):649–58. https://doi.org/10.1002/cam4.3650.
Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a 19 promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine (Baltimore). 2019;98(3): e13788. https://doi.org/10.1097/MD.0000000000013788.
Zhang Y, Lin S, Yang X, Wang R, Luo L. Prognostic value of pretreatment systemic immune-inflammation index in patients with gastrointestinal cancers. J Cell Physiol. 2019;234(5):5555–63. https://doi.org/10.1002/jcp.27373.
Li X, Gu L, Chen Y, Chong Y, Wang X, Guo P, He D. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med. 2021;53(1):1827–38. https://doi.org/10.1080/07853890.2021.1991591.
Rawish E, Nording H, Münte T, Langer HF. Platelets as mediators of neuroinflammation and thrombosis. Front Immunol. 2020;6(11): 548631. https://doi.org/10.3389/fimmu.2020.548631.
Khodadi E. Platelet function in cardiovascular disease: activation of molecules and activation by molecules. Cardiovasc Toxicol. 2020;20(1):1–10. https://doi.org/10.1007/s12012-019-09555-4.
Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9(1):231. https://doi.org/10.3390/cells9010231.
Shirakawa K, Sano M. Neutrophils and neutrophil extracellular traps in cardiovascular disease: an overview and potential therapeutic approaches. Biomedicines. 2022;10(8):1850. https://doi.org/10.3390/biomedicines10081850.
Chistiakov DA, Orekhov AN, Bobryshev YV. Immune-inflammatory responses in atherosclerosis: role of an adaptive immunity mainly driven by T and B cells. Immunobiology. 2016;221(9):1014–33. https://doi.org/10.1016/j.imbio.2016.05.010.
Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308–15. https://doi.org/10.1182/blood-2014-05-578336.
Neunert C, Noroozi N, Norman G, Buchanan GR, Goy J, Nazi I, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457–64. https://doi.org/10.1111/jth.12813.
Acknowledgements
The authors thank the staff and the participants of the NHANES study for
their valuable contributions.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
WH contributed to data collection, analysis and writing of the manuscript. BXF contributed to study design and writing of the manuscript. WH and BXF and NHY contributed to revise the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics review board of the National Center for Health Statistics approved all NHANES protocols and written informed consents were obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline characteristics of the included population and the follow-up missing population.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Nie, H., Bu, G. et al. Systemic immune-inflammation index (SII) and the risk of all-cause, cardiovascular, and cardio-cerebrovascular mortality in the general population. Eur J Med Res 28, 575 (2023). https://doi.org/10.1186/s40001-023-01529-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01529-1