Abstract
Background
Roxadustat is an oral hypoxia inducing factor-prolyl hydroxylase inhibitor (HIF-PHI) that regulates iron metabolism in patients with chronic kidney disease (CKD) primarily by reducing hepcidin levels and mobilizing internal iron stores. More data are needed to demonstrate the efficacy of roxadustat in regulating iron metabolism in patients with peritoneal dialysis (PD) compared with erythropoiesis stimulating agents (ESAs).
Methods
This prospective cohort study enrolled PD patients with a mean hemoglobin level of 60–100 g/L. All subjects were randomized into two groups at a ratio of 2:1 the roxadustat group (106 cases), and the ESA group (53 cases). The primary endpoint was the change in the iron biomarker levels and the proportion of patients with absolute iron deficiency and functional iron deficiency.
Results
Compared with ESAs, roxadustat significantly decreased hepcidin level (difference, − 20.09 ng/mL; 95% CI, − 30.26 to − 9.92), attenuated the increase in serum soluble transferrin receptor (sTFR) level (difference, − 7.87 nmol/L; 95% CI, − 12.11 to − 3.64), and reduced the proportion of patients with functional iron deficiency (roxadustat, 11.43%; ESA, 33.33%). There was no significant difference in safety of the two groups over the duration of the study.
Conclusions
Compared with ESA group, roxadustat group showed significant differences in all iron biomarker levels except serum ferritin (sFt) and transferrin saturation (TSAT). These results suggest that roxadustat was superior to ESAs as a therapy for iron metabolism in PD patients.
Trial registration: This study completed Chinese Clinical Trial Registration on March 4, 2022 (registration number: ChiCTR2200057231).
Graphical abstract

Similar content being viewed by others
Background
The prevalence of anemia increases with the progression of CKD [1]. Renal anemia is associated with dysregulation of oxygen sensing by the malfunctioning kidneys, leading to inadequate synthesis of erythropoietin (EPO), iron deficiency, and inflammation [2]. Iron deficiency especially functional iron deficiency due to chronic inflammation is one of the major causes of anemia in CKD patients [3]. Abnormal iron metabolism in CKD patients is mediated by hepcidin, a key regulator of iron uptake and mobilization that is downregulated in hypoxia [4, 5]. Because PD patients do not have venous blood loss problems, less clinical focus is given to anemia than hemodialysis patients, but the data suggest that among PD patients, the prevalence of iron deficiency anemia (IDA) was reported to be between 16 and 23% [6]. The inflammatory status of CKD patients changes the actual levels of these parameters by affecting transferrin and hepcidin, reducing TSAT and increasing sFt levels [7]. Therefore, sTFR, which is not affected by inflammation, may provide more information than TSAT and sFt to reflect iron metabolism in CKD patients [8, 9].
The clinical application of ESAs can increase hemoglobin concentration without the risk of transfusion-related iron overload, substantially improving patients' quality of life [10]. However, ESAs further deplete circulating iron, leading to the need for additional iron supplementation as the primary treatment for CKD anemia [11]. Meanwhile, several clinical problems have been reported, including ESAs hyporesponsiveness [12] and increased risk of cardiovascular events and death [13, 14].
By simulating hypoxic environment, roxadustat effectively inhibits HIF-PHD activity and increases the accumulation of HIF [15], thereby inducing the expression of EPO, EPO receptors, and proteins required for iron metabolism [16, 17]. The identification of HIF-PHI provides a new therapeutic means for the treatment of renal anemia [18, 19]. We now report the results of a 24-week, cohort study involving PD patients to analyze the effect of roxadustat on iron metabolism in PD patients by comparing changes in iron biomarker levels including sTFR.
Methods
Study design and population
This trial evaluated the efficacy and safety of roxadustat in regulating iron metabolism in PD patients through 24 weeks of observation. The eligible patients who agreed to participate in the study were numbered according to the time of visit to the hospital, and were divided into roxadustat group and ESA group with a ratio of 2:1 by random number table. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY-KL39-01) and completed Chinese Clinical Trial Registration (registration number: ChiCTR2200057231), and all the enrolled patients signed an informed consent form. Eligible patients ranged in age from 18 to 75, had received stable PD for > 6 weeks, did not receive ESAs, roxadustat, and iron supplementation within 6 weeks before enrollment, and met the clinical diagnosis criteria of renal anemia as stipulated in the Chinese Expert Consensus on the Diagnosis and Treatment of Kidney Anemia (2018 Revision). Exclusion criteria included severe organic dysfunction of the heart, liver, lung, and brain, a change in the dialysis method, transfusion, and intravenous iron.
Calculation sample size
Sample sizes (N1 = 34; N2 = 68) for the roxadustat and ESA groups were calculated using PASS 15.0 software based on a multicenter randomized controlled clinical trial conducted in China with α of 0.05 and efficacy of 1 − β of 90% [20]. A total of 159 patients were included considering an estimated drop-out rate of 30%.
Study drug administration
Patients in the roxadustat group were treated with roxadustat capsules [Enambojin (China) Pharmaceutical Technology Development Co., LTD., Sinopharm H20180024 (50 mg), H20180023 (20 mg)], administered three times a week at 70 mg (< 45 kg), 100 mg (45 to < 60 kg) or 120 mg (≥ 60 kg). The dose was adjusted according to a preset dosing ladder as follows: 20, 40, 50, 70, 100, 120, 150, and 200 mg. The maximum dose was 2.5 mg/kg. If the patient's hemoglobin increase was greater than 20 g/L within 2 weeks and the hemoglobin value was greater than 90 g/L, the dose was lowered by one step. Only one dose reduction over 4 weeks was recommended when hemoglobin rose too rapidly. Patients in the ESA group were treated with generic epoetin alpha [Sansheng Pharmaceutical Co., LTD., S19980073 (2000 IU), S19980074 (3000 IU), S19980072 (4000 IU), S20010001 (10000 IU)] at 75–100 IU/kg/week. The maximum dose of each adjustment was 30 IU/kg/week. The initial treatment target was an increased hemoglobin level of 10–20 g/L per month, and subsequent adjustments would be made according to the patient's hemoglobin level, speed of hemoglobin change, and treatment response. Oral iron can be accepted when STAT ≤ 20% or sFt ≤ 100 µg/L while intravenous iron is not permitted during the study. The use of statins and phosphate binders was allowed.
End points
The primary efficacy endpoints were the changes of iron biomarker levels from baseline to week 24 including hepcidin, sTFR, serum iron (SI), sFt, TSAT, total iron binding capacity (TIBC), and the proportion of patients with absolute iron deficiency (defined as TSAT < 20% and sFt < 100 ng/mL) and functional iron deficiency (defined as TSAT < 20% and sFt ≥ 100 ng/mL). Secondary efficacy endpoints were as follows: the hemoglobin levels at weeks 8 and 24, the proportion of patients who achieved the treatment target (defined as a mean hemoglobin level greater than or equal to 100 g/L but less than 120 g/L), the mean changes from baseline in lipid metabolism levels, and the incidence of hyperkalemia, metabolic acidosis and peritonitis.
Statistical analysis
All the data were processed with SPSS 25.0. Measurement data with a normal distribution were expressed as means ± standard deviation, and comparisons between groups were analyzed using independent sample t-tests. Nonnormally distributed data were expressed as medians and interquartile ranges. The enumeration data were expressed as percentages, and the Chi-squared test was used to compare groups. We used the mixed-effects repeated-measures model to analyze the mean changes from baseline in iron biomarker levels, hemoglobin levels and lipid metabolism levels over week 24. Before analyzing the above parameters, logarithmic transformation of the values of sFt and EPO were carried out to make them meet the normal distribution. Safety was monitored by assessment of changes in blood pressure and the incidence of hyperkalemia, metabolic acidosis, and peritonitis during treatment. Bitailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the patients
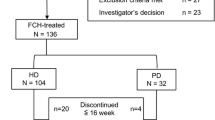
From June 2021 through April 2022, 159 patients underwent randomization (roxadustat, 106; ESA, 53). Of these patients, 106 completed the 24-week study (roxadustat, 70; ESA, 36) (Fig. 1). Median duration of PD therapy was 83.50 (40.00,120.75) and 90.00 (36.50, 209.50) weeks, respectively. The most common type of peritoneal transport was low mean transport (roxadustat, 41; ESA, 22), followed by high mean transport (roxadustat, 25; ESA, 11). The most common primary diagnoses were chronic glomerulonephritis (roxadustat, 39; ESA, 21), hypertension (roxadustat, 30; ESA, 14), and diabetes (roxadustat, 18; ESA, 9). All the patients had hemoglobin levels greater than 60 g/L and less than 100 g/L. The baseline characteristics of the patients were similar in the two groups (Table 1).
Patient disposition. Among 391 PD patients regularly followed up, 159 eligible patients were grouped [roxadustat group, n = 106 (66.67%); ESA group, n = 53 (33.33%)]. A total of 106 patients completed 24-week treatment [roxadustat group, n = 106 (66.67%); ESA group, n = 53 (33.33%)], whereas 20.75 and 11.32% of patients were lost to follow-up and 13.21%, and 20.75% discontinued intervention in the roxadustat and ESA groups, respectively
Iron biomarker levels
During the 24-week study, 2 patients (roxadustat, 1; ESA, 1) stopped intervention because of the intravenous iron. 48 patients (45.28%) in the roxadustat group received oral iron therapy compared with 38 (71.70%) in the ESA group.
At week 8, the hepcidin levels of roxadustat and ESA groups decreased from baseline by 12.46 ± 24.48 ng/mL and 1.78 ± 21.31 ng/mL, respectively (Fig. 2A). The difference between groups was − 10.68 ng/mL (95% CI, − 19.26 to − 2.11). At week 24, the change from baseline was − 24.77 ± 22.77 ng/mL in the roxadustat group and − 4.68 ± 28.91 ng/mL in the ESA group. The difference between groups was − 20.09 ng/mL (95% CI, − 30.26 to − 9.92) (Fig. 2B). There were no remarkable changes in the hepcidin levels were observed in the ESA group; in the roxadustat group, the baseline was significantly higher than week 8 (P < 0.001) and week 24 (P < 0.001). At week 8, the difference of sTFR levels between groups was − 5.86 nmol/L (95% CI, − 9.73 to − 1.99), and the additions from baseline were 3.02 ± 10.14 nmol/L and 8.87 ± 11.44 nmol/L, respectively (Fig. 2C). At week 24, the additions were 8.64 ± 9.99 nmol/L in the roxadustat group and 16.51 ± 11.21 nmol/L in the ESA group (Fig. 2D).
Hepcidin levels (A) and mean change from baseline by treatment (roxadustat versus ESAs) at week 8 and week 24 (B). sTFR levels (C) and mean change from baseline by treatment (roxadustat versus ESAs) at week 8 and week 24 (D). sTFR denotes serum soluble transferrin receptor. *Significant differences between groups (P < 0.05). **Significant differences between groups (P < 0.01)
In the roxadustat group, the SI and TSAT levels were clinically stable, and the changes from baseline were 0.47 ± 7.12 µmol/L (95% CI, − 1.22 to 2.17) and − 3.26 ± 15.88% (95% CI, − 7.04 to 0.53), respectively, with an increase in the TIBC, the change from baseline was 7.13 ± 9.63 µmol/L (95% CI, 4.83 to 9.42) and a decrease in the logsFt, the change from baseline was − 0.11 ± 0.40 µmol/L (95% CI, − 0.01to − 0.20). In the ESA group, the SI and TIBC were clinically stable, and the changes from baseline were − 2.11 ± 7.75 µmol/L (95% CI, − 4.73 to 0.51), and 2.81 ± 9.93 µmol/L (95% CI, − 0.55 to 6.17), with a decrease in TSAT and logsFt, the changes from baseline were − 7.85 ± 16.72% (95% CI, − 13.50 to − 2.19), and − 0.19 ± 0.43 ng/mL (95% CI, − 0.33 to − 0.04) (Table 2). At week 24, 22.86% of the roxadustat group and 22.22% of the ESA group had absolute iron deficiency, with no significant differences between groups (P = 0.956). At the same time, we observed that while the percentage of patients with functional iron deficiency remained stable in the roxadustat group (P = 0.982), ESA group increased significantly (P = 0.002) (Fig. 3A).
Hemoglobin levels
At baseline, the logEPO level was 0.77 ± 0.30 mIU/mL in the roxadustat group and 0.78 ± 0.31 mIU/mL in the ESA group. At week 24, the increase from baseline was 0.13 ± 0.42 mIU/mL and 0.78 ± 0.62 mIU/mL in the roxadustat and ESA groups. The difference between groups was -0.65 mIU/mL (95% CI, − 0.85 to − 0.45). Although the increase of logEPO levels in roxadustat group was much smaller than that in ESA group, no significant difference was observed in the improvement of anemia in PD patients between two groups. At week 24, hemoglobin levels increased 23.89 ± 17.87 g/L in roxadustat group and 19.73 ± 20.67 g/L in ESA group. The difference between groups was 4.16 g/L (95% CI, − 3.51 to 11.83). The percentage of patients with a hemoglobin level greater than or equal to 100 g/L but less than 120 g/L was 44.29% in the roxadustat group, and 47.22% in the ESA group in week 24 (Fig. 3B). The mean dose of roxadustat was 111.86 ± 12.77 mg at baseline, 106.71 ± 31.56 mg at week 8, and 94.29 ± 48.02 mg at week 24 (Fig. 4).
Lipid metabolism levels
At week 24, the mean decreases in the lipid levels in the roxadustat group were as follows: 0.26 ± 0.98 mmol/L in the total cholesterol level (treatment difference, − 0.28 mmol/L; 95% CI, − 0.72 to 0.16), 0.39 ± 0.78 mmol/L in the low-density lipoprotein level (treatment difference, − 0.28 mmol/L; 95% CI, − 0.64 to 0.07), 0.10 ± 0.22 mmol/L in the high-density lipoprotein level (treatment difference, − 0.06 mmol/L; 95% CI, − 0.16 to 0.05), and 0.19 ± 0.55 mmol/L in the triglyceride level (treatment difference, − 0.59 mmol/L; 95% CI, − 1.07 to − 0.12).
Adverse events and safety
The changes in the mean arterial pressure from baseline to week 24 were 1.67 ± 17.13 mmHg in the roxadustat group and 3.82 ± 11.68 mmHg in the ESA group (difference, − 2.14 mmHg; 95% CI, − 8.45 to 4.16). Although no difference was found in the proportion of patients who did not achieve the control goal (roxadustat, 64.29%; ESA, 69.44%), a higher proportion of patients in the ESA group increased or adjusted blood pressure medication (roxadustat, 25.47%; ESA, 47.17%).
Roxadustat did not increase the incidence of hyperkalemia (P = 0.651), and significantly reduced the incidence of metabolic acidosis (P < 0.001). The mean changes in the potassium levels were as follows: at week 8, a change of − 0.01 ± 0.62 mmol/L (95% CI, − 0.14 to 0.13) in the roxadustat group and − 0.25 ± 0.72 mmol/L (95% CI, − 0.47 to 0.03) in the ESA group; at week 24, a change of − 0.03 ± 0.59 mmol/L (95% CI, − 0.11 to 0.17) in the roxadustat group and − 0.14 ± 0.72 mmol/L (95% CI, − 0.38 to 0.11) in the ESA group. In the roxadustat group, 3.77% of patients had with hyperkalemia (> 5.50 mmol/L) at baseline, 2.27% at week 8, and 1.43% at week 24. The ESA group's percentages were 9.43% at baseline, 2.27% at week 8, and 8.33% at week 24. In the roxadustat group, the proportion of patients with bicarbonate levels less than 22 mmol was 48.11% at baseline, 26.41% at 8 weeks, and 18.57% at 24 weeks. The ESA group's percentage was 45.28% at baseline, 39.59% at week 8 and 30.56% at week 24.
High-sensitivity C-reactive protein levels in the roxadustat (P = 0.421) and ESA groups (P = 0.367) remained stable over the course of the study. 25 cases of peritonitis were identified during the 24-week study (roxadustat, 14; ESA, 11). The severity of peritonitis was mild/moderate and was resolved with antibiotic treatment, and no case resulted in the discontinuation of roxadustat and ESAs.
Discussion
In this 24-week clinical study of PD patients, roxadustat significantly reduced the rise of sTFR and reduced the occurrence of functional iron deficiency by lowering hepcidin levels, although there was no significant difference in the incidence of absolute iron deficiency between the two groups. In addition, roxadustat improved anemia and lipid metabolism without increasing the incidence of hyperkalemia, metabolic acidosis, and peritonitis.
Since there were 22% of patients who had insufficient iron reserve state the number. The roxadustat group still had significantly improved iron metabolism levels in PD patients, with a smaller proportion of oral iron patients. We suggest that the decreased hepcidin levels and increased iron availability associated with roxadustat may have contributed to these findings [21]. Elevated hepcidin levels due to persistent inflammation [5] are often observed in patients with CKD [12, 22]. During the 24 weeks of the study, the hepcidin levels were significantly reduced in patients treated with roxadustat, resulting in reduced degradation of ferroportin (FPN) [23] and increased iron absorption and release to avoid the occurrence of functional iron deficiency [24]. The TIBC levels increased significantly in the roxadustat group but not in patients receiving ESAs, and this increase may be a direct result of stabilizing HIF levels with roxadustat. The gene-encoding transferrin is a HIF target with good properties because its enhancer region contains two HIF binding sites [25]. At week 8 of the study, the roxadustat group showed a more significant reduction in sFt than the ESA group, indicating increased iron utilization. In addition to its inhibitory effect on hepcidin, HIF also induces the expression of heme oxygenase-1, ceruloplasmin, FPN, and transferrin receptors [26], all of which are essential for iron cycling.
Clinically, TSAT (an index of iron utilization state) < 20% and sFt (an index of iron storage state) < 100 ng/mL is diagnosed as absolute iron deficiency in PD patients. Functional iron deficiency is characterized by TSAT < 20% and normal or elevated sFt levels [27]. Notably, the specificities of sFt and TSAT are very poor, and the results are affected by various factors, especially inflammation [7]. Therefore, to better analyze the iron metabolism level of patients, this parameter must be supplemented with high-sensitivity C-reactive protein and nutrition index tests. sTFR is mainly derived from the FPN on the juvenile erythroid membrane surface and mediates iron-containing transferrin into the cells [28]. Unlike other iron parameters, it is not affected by inflammation, trauma, stress or other factors [8, 9], and the sTFR concentration increases rapidly in the early stage of iron deficiency, leading to an early diagnosis of IDA [29]. The concentration of sTFR can reflect the demand of cells for iron and is a good indicator of functional iron deficiency [30, 31]. In recent years, many studies have shown that elevated sTFR levels were associated with the high prevalence of cardiovascular diseases [32, 33]. In the 24th week, although the proportion of patients with absolute iron deficiency did not increase significantly, the sTFR level increased significantly, suggesting that iron deficiency occurred in PD patients with the increase in iron utilization, and appropriate iron supplementation may be necessary.
Because roxadustat mediates the transition from aerobic to anaerobic metabolism, the immediate consequence of increased glycolysis is tissue acidification and lactic acid overproduction. The acidosis reaction leads to the release of potassium ions from cells, resulting in hyperkalemia [34]. In a phase III study conducted in China involving CKD patients on dialysis, patients treated with roxadustat were more likely to develop hyperkalemia compared with ESAs patients [35]. We found that the incidence of hyperkalemia and metabolic acidosis in this study was inconsistent with previous reports. We speculate that this may be related to the use of diuretics and bicarbonate supplements [36].
CKD patients often suffer from chronic inflammation due to excessive production and retention of urinary toxins, abnormal intestinal flora, and changes in the integrity of intestinal barrier. Inflammation blocks the output and absorption of iron that bacteria need to survive by stimulating the expression of hepcidin [37], which is thought to be a defense mechanism against infection [38]. In this study, roxadustat significantly reduced hepcidin levels without increasing high-sensitivity C-reactive protein level and the incidence of peritonitis.
It should be noted that this study has the following limitations: first of all, this trial is a single-center study, and these participants may not be generalizable to other populations. Secondly, we believe that the use of diuretics and bicarbonate supplements reduced the incidence of hyperkalemia and metabolic acidosis in roxadustat group, but we regret that the use of drug was not counted during the study. Finally, this study only studied the changes in patients’ condition after 24 weeks of treatment, which may not be long enough to observe the safety of roxadustat. The risks of angiogenesis and cancer associated with HIF have been demonstrated, and further studies are needed before firm conclusions can be drawn.
Conclusion
In conclusion, this 24-week prospective cohort study comparing the efficacy of roxadustat and ESAs in PD patients showed the benefit of roxadustat in improving iron metabolism. In addition to its inhibitory effect on hepcidin, roxadustat also induces the expression of molecules required for iron circulation. Thus, the increased iron consumption observed in the roxadustat group may be attributable not only to enhanced hematopoietic production, but also to improved iron utilization efficiency. Information on the differences in the effects of roxadustat and ESAs on iron metabolism could help in selecting appropriate treatment options for PD patients. Roxadustat displayed a manageable safety with no increased risk of the incidence of hyperkalemia, metabolic acidosis and peritonitis.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- CKD:
-
Chronic kidney disease
- ESAs:
-
Erythropoiesis stimulating agents
- EPO:
-
Erythropoietin
- FPN:
-
Ferroportin
- HIF-PHI:
-
Hypoxia inducing factor-prolyl hydroxylase inhibitor
- IDA:
-
Iron deficiency anemia
- PD:
-
Peritoneal dialysis
- SI:
-
Serum iron
- sFt:
-
Serum ferritin
- sTFR:
-
Serum soluble transferrin receptor
- TIBC:
-
Total iron binding capacity
- TSAT:
-
Transferrin saturation
References
Shaikh H, Hashmi MF, Aeddula NR. Anemia of chronic renal disease. In: StatPearls. StatPearls Publishing, Treasure Island (FL): 2022.
Fishbane S, Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis. 2018;71:423–35.
Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397:233–48.
Nemeth E, Ganz T. Hepcidin and iron in health and disease. Annu Rev Med. 2022. https://doi.org/10.1146/annurev-med-043021-032816.
Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Zafar MU, Badimon JJ. Prolyl hydroxylase inhibitors: a new opportunity in renal and myocardial protection. Cardiovasc Drugs Ther. 2022;36:1187–96.
Perlman RL, Zhao J, Fuller DS, et al. International anemia prevalence and management in peritoneal dialysis patients. Perit Dial Int. 2019;39:539–46.
Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287:153–70.
Hou L, Lu J, Jiang X, Guo X, Ma C, Cheng X. Analytical evaluation of three soluble transferrin receptor measurement systems for diagnosis of iron deficiency anemia: a retrospective study. J Clin Lab Anal. 2020;34: e23342.
Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106:1606S-1614S.
Miura T, Sato T, Yano T, et al. Role of erythropoiesis-stimulating agents in cardiovascular protection in CKD patients: reappraisal of their impact and mechanisms. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-022-07321-3.
Weir MR. Managing anemia across the stages of kidney disease in those hyporesponsive to erythropoiesis-stimulating agents. Am J Nephrol. 2021;52:450–66.
Gluba-Brzózka A, Franczyk B, Olszewski R, Rysz J. The influence of inflammation on anemia in CKD patients. Int J Mol Sci. 2020;21:725.
Locatelli F, Del Vecchio L, De Nicola L, Minutolo R. Are all erythropoiesis-stimulating agents created equal? Nephrol Dial Transplant. 2021;36:1369–77.
Souza E, Cho KH, Harris ST, Flindt NR, Watt RK, Pai AB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a paradigm shift for treatment of anemia in chronic kidney disease? Expert Opin Investig Drugs. 2020;29:831–44.
Dhillon S. Roxadustat: first global approval. Drugs. 2019;79:563–72.
Locatelli F, Del Vecchio L. Hypoxia-inducible factor-prolyl hydroxyl domain inhibitors: from theoretical superiority to clinical noninferiority compared with current ESAs? J Am Soc Nephrol. 2022;33:1966–79.
Voit RA, Sankaran VG. Stabilizing HIF to ameliorate anemia. Cell. 2020;180:6.
McCallum W, Weiner DE. HIF-PHIs for anemia management in CKD: potential and uncertainty ASCEND. Clin J Am Soc Nephrol. 2022;17:1255–8.
Sugahara M, Tanaka T, Nangaku M. Future perspectives of anemia management in chronic kidney disease using hypoxia-inducible factor-prolyl hydroxylase inhibitors. Pharmacol Ther. 2022;239: 108272.
Chen N, Hao C, Peng X, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–10.
Kaplan J. Roxadustat and anemia of chronic kidney disease. N Engl J Med. 2019;381:1070–2.
Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50.
Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131:899–910.
Pagani A, Nai A, Silvestri L, Camaschella C. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31:456–68.
Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27:281–98.
Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron deficiency anemia in chronic kidney disease. Acta Haematol. 2019;142:44–50.
Schwartz AJ, Das NK, Ramakrishnan SK, et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest. 2019;129:336–48.
Babitt JL, Eisenga MF, Haase VH, et al. Controversies in optimal anemia management: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 2021;99:1280–95.
Sierpinski R, Josiak K, Suchocki T, et al. High soluble transferrin receptor in patients with heart failure: a measure of iron deficiency and a strong predictor of mortality. Eur J Heart Fail. 2021;23:919–32.
Daude S, Remen T, Chateau T, et al. Comparative accuracy of ferritin, transferrin saturation and soluble transferrin receptor for the diagnosis of iron deficiency in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:1087–95.
Weidmann H, Bannasch JH, Waldeyer C, et al. Iron metabolism contributes to prognosis in coronary artery disease: prognostic value of the soluble transferrin receptor within the atherogene study. J Am Heart Assoc. 2020;9: e015480.
Zhu S, Liu C, Zhao C, et al. Increased serum soluble transferrin receptor levels were associated with high prevalence of cardiovascular diseases: insights from the national health and nutrition examination survey 2017–2018. Front Cell Dev Biol. 2022;10: 874846.
Zielniok K, Burdzinska A, Paczek L. Roxadustat for anemia in patients with chronic kidney disease. N Engl J Med. 2020;383: e3.
Chen N, Hao C, Liu BC, et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–22.
Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI study. J Nephrol. 2019;32:989–1001.
Zhang DL, Wu J, Shah BN, et al. Erythrocytic ferroportin reduces intracellular iron accumulation, hemolysis, and malaria risk. Science. 2018;359:1520–3.
Wunderer F, Traeger L, Sigurslid HH, Meybohm P, Bloch DB, Malhotra R. The role of hepcidin and iron homeostasis in atherosclerosis. Pharmacol Res. 2020;153: 104664.
Acknowledgements
We would like to extend our sincere gratitude to The Affiliated Hospital of Xuzhou Medical University for providing us with a conducive and comfortable platform during our study. We also deeply appreciate our dear colleagues for their reliable, tireless support and assistance.
Funding
This study was supported by the National Natural Science Foundation of China (82270731, 82000703); the Jiangsu Provincial Natural Science Foundation (BK20211054); a project of Qing Lan of Jiangsu Province; a project of Jiangsu Provincial Post Graduate Innovation Plan (KYCX21_2701, KYCX22_2903); Science and technology development fund of Affiliated Hospital of Xuzhou Medical University (XYFC2020001; XYFY2020038); Xuzhou key R & D Program (Social Development) (KC20160); Xuzhou Medical leading Talent training Project (XWRCHT20210038); Beanstalk talent of Affiliated Hospital of Xuzhou Medical University; and a project of Practice and Innovation Plan of Jiangsu Province (SJCX21_1147).
Author information
Authors and Affiliations
Contributions
Conception and design were by XZ, RJ, ZZ and LJ; all authors provided critical intellectual content, contributing to the analysis and interpretation of data and drafting of the manuscript, AR participated in grammar modification, and DS did the overall design, provided the funds and critically revised the important intellectual content in the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY-KL39-01). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee. All the enrolled patients signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Jia, R., Zheng, Z. et al. Effect of roxadustat on iron metabolism in patients with peritoneal dialysis: a real-world 24-week study. Eur J Med Res 28, 489 (2023). https://doi.org/10.1186/s40001-023-01465-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01465-0